Abstract
Background
Alcohol consumption is prevalent in late adolescence, however little is known about its effect on sleep in this group. In mature adults, alcohol decreases sleep onset latency (SOL) and sleep efficiency (SE) and increases wake after sleep onset (WASO). It also increases slow wave sleep (SWS) and decreases REM sleep in the first half of the night, with the inverse occurring in the second half. Alcohol’s effect on sleep during late adolescence is of interest given that this age group shows both dramatic increases in alcohol consumption, and significant developmental changes in the central nervous system. This study examined the effect of alcohol on sleep architecture in women and men aged 18–21 years and whether previously reported sleep architecture effects may have been as an artificial result of changes to sleep cycle length.
Methods
24 (12 female) healthy 18–21 year old light social drinkers (19.1±1.0yrs) underwent two conditions: pre-sleep alcohol (Target BAC 0.10%) and placebo administered under controlled conditions, followed by standard polysomnography.
Results
In the alcohol condition, mean breath alcohol concentration (BAC) at lights out was 0.084 ±0.016%. Time in bed, total sleep time and sleep onset latency (all p>.05) did not differ between conditions. However, there was less REM (p=.011) and more stage 2 sleep (p=.035) in the alcohol condition. Further, alcohol increased SWS (p=.02) and decreased REM sleep (p<.001) in the first half of the night and disrupted sleep in the second half, with increased WASO (interaction: p=.034), and decreased SE (p=.04) and SWS (p=.01) and no REM sleep rebound in the second half of the night (p=.262). Additionally, alcohol had no effect on sleep cycle length (p=.598).
Conclusions
The results were broadly consistent with the adult literature with the novel extension that half night sleep architecture effects could not be attributed to changes in sleep cycle length. However, alcohol did not reduce SOL, or result in a REM rebound following reduced REM in the first half of the night. The results suggest that the effects of alcohol on sleep are modified by sleep’s prevailing developmental stage.
Keywords: Alcohol, Late Adolescence, Sleep, Sleep Architecture, Sleep Onset Latency, REM rebound
INTRODUCTION
Alcohol consumption is particularly prevalent in late adolescence with studies finding that ninety percent of students consume alcohol by the age of 20 and up to 43 percent of young people report binge drinking (Bonomo et al., 2004; Maxwell, 2008; Wechsler et al., 2000). The dramatic increase in drinking in this age group is a major social issue with excessive alcohol consumption being linked to numerous negative impacts including an increased likelihood of accidental death through events such as motor vehicle accidents and the development of alcohol use disorders in adulthood (Abbey et al., 2006; Bonomo et al., 2004; Toumbourou et al., 2009; Wechsler et al., 1994) . Alcohol use at both clinical and sub-clinical levels is commonly associated with sleep disturbance (Aldrich, 1998; Brower, 2001). Indeed, reduced sleep quality and insomnia are predictive of relapse in abstinent alcoholics (Brower et al., 1998). The relationship between alcohol use and sleep problems (particularly insomnia) is thought to be bidirectional, with disturbed sleep leading to increased alcohol use which in turn further disrupts sleep (Brower, 2003).
Late adolescence is associated with both the continued maturation of the brain, particularly within frontal regions, and with the final stages of an adolescence related reduction in slow wave sleep (SWS; delta activity) (Carskadon et al., 2001; Feinberg, 1974; Sowell et al., 2004). While the rate of fall in delta activity is maximal between the ages of 12 and 16, it is likely that the changes persist into late adolescence (Carskadon et al., 2001; Feinberg and Campbell, 2010; Feinberg et al., 2011). The changes in sleep are thought to reflect the development of neural systems, in particular myelination and synaptic pruning (Feinberg and Campbell, 2010; Sowell et al., 2004). It is plausible that the continuing neural development may put adolescents and young adults at a heightened vulnerability for alcohol related brain damage (Crews et al., 2000). Additionally because emotional maturation and maturation of the frontal cortices, which are important for executive control, is delayed relative to physical maturation adolescents are more likely to engage in risk taking behaviors, including risky alcohol use (Schwandt et al., 2010; Spear, 2002). As such, adolescence is a period of increased risk for the development of alcohol use disorders (DeWit et al., 2000; Squeglia et al., 2009; Witt, 2010). Similarly, human studies indicate that prolonged excessive alcohol consumption during adolescence is related to reduced neural functioning and cognitive deficits (Brown et al., 2000; Zeigler et al., 2005) with one study indicating greater memory impairment in younger adults than older adults (Acheson et al., 1998).
The literature on the effects of alcohol on sleep remains limited with most studies including only older adult participants (Aldrich, 1998; Roehrs and Roth, 2001). Results indicate that alcohol initially decreases sleep onset latency (SOL), consistent with its sedative pharmacologic action, whereas later in the sleep period, it decreases sleep efficiency (SE), and increases wake after sleep onset (WASO) (Landolt et al., 1996; MacLean and Cairns, 1982; Roehrs et al., 1989; Rundell et al., 1972). The sleep onset effect is thought to be a mechanism by which sleep problems lead to and perpetuate alcohol use, as the shortened sleep onset latency is perceived to be beneficial to sleep and leads individuals to consume alcohol to assist falling asleep (Roehrs et al., 1999). Alcohol’s most consistent effects on sleep architecture are an increase in SWS coupled with a decrease in REM sleep in the first half of the night, indicating an initial exaggeration of SWS dominance (and therefore suppression of REM). In studies using low to moderate doses, this may be followed inversely by an increase in REM sleep and a decrease in SWS in the second half of the night (Aldrich, 1998; Rundell et al., 1972; Stone, 1980; Williams et al., 1983). REM facilitation in the second half of the night is thought to be due to a ‘rebound effect’ resulting from first half suppression, while sleep disruption may be due to the reduced facilitation of SWS, or to the accumulation of alcohol metabolites (Feige et al., 2006; Roehrs and Roth, 2001).
The sleep architecture changes in the first and second halves of the night following alcohol consumption may either be a result of changes in the distribution of sleep stages within each sleep cycle, or a result of changes in sleep cycle length. For example, if alcohol consumption initially lengthens sleep cycles, observed changes in REM sleep may be due to REM sleep being pushed into the second half of the night by more lengthy sleep cycles, rather than an initial REM suppression. To date, there is no literature distinguishing between these two possibilities.
Despite the potential impact of alcohol on the sleep of late adolescents, only one study has examined the effect of acute alcohol consumption in this group (18–21 year olds) (Williams et al., 1983). This study tested subjects at three different alcohol doses (BACs reached .005, .054 and .081%) with five nights intervening between each session. While their findings were largely consistent with the adult literature, they did not observe a REM sleep rebound effect. However, this study was limited in size and only included female participants. In light of the importance of studying the effect of alcohol in late adolescents and given the limitations of the Williams et al. (1983) study, it is appropriate to re-visit this issue.
The present study sought to investigate the effects of acute alcohol consumption on sleep in 18–21 year old men and women. Additionally, we aimed to examine whether any observed changes in sleep architecture were due to changes in sleep cycle length, or changes in the distribution of sleep stages within each cycle. Consistent with previous findings, we hypothesized that there would be shorter SOL, increased SWS, and decreased REM sleep in the first half of the night, with increased sleep disturbance in the second half of the night. Further, consistent with the findings of Williams et al. (1983), we hypothesized that alcohol consumption would not result in a REM rebound in the second half of the night.
MATERIALS AND METHODS
Participants
Participants were 24 (12 female) volunteers (although, as described below, two participants were subsequently excluded) aged between 18 and 21 years (mean 19.1 years ±1.0 years) and a mean body mass index of 22.0 (±2.3kg/m2). Participants were recruited through advertisement. Potential participants attended a screening interview and were asked about drug use, socioeconomic status, family history of alcoholism, administered a guided 30 day retrospective drugs/medications and alcohol diary (including size, type and brand of beverages), and a structured interview to estimate total lifetime alcohol consumption (Pfefferbaum et al., 1988). Healthy participants were selected for the sleep study if they consumed less than 7 standard drinks per week on average and had no more than two occasions of consuming more than 4 standard drinks in the previous 30 days (NHMRC, 2009). Potential participants were excluded if they reported, sleep disorders, any substantial period of binge drinking in their lifetimes, current use of medications known to affect sleep, or a first degree family history of alcoholism. Data from one female participant were unavailable for analysis due to equipment failure. The protocol had prior approval of The University of Melbourne Human Subjects Ethics Committee and informed consent was obtained prior to the screening interview.
Design
The effects of alcohol consumption on sleep were studied using a single blind, repeated measures design with 2 levels (alcohol and placebo).
General Laboratory Procedures
Participants attended three non-consecutive nights at the School of Psychological Sciences Sleep Laboratory in The University of Melbourne, Australia. The first night was an adaptation night and also served to screen for occult sleep disorders as exclusion criteria. The second and third nights were counterbalanced experimental nights: one involving pre-sleep alcohol administration dosed to obtain a peak breath alcohol concentration (BAC) of 0.1% (vodka and orange juice); and one involving a placebo beverage (orange juice with a straw dipped in vodka). The dose of vodka was determined using height, weight and a total body water measurement obtained from commercially available scales (Tanita® BC541 Innerscan. Arlington Heights, IL, USA) according to the formula published in Curtain and Fairchild (2003). Total beverage volumes were adjusted for body mass such that a 70Kg person would receive 400ml- less for a lighter individual and more for a heavier one. This resulted in a range of concentrations of alcohol as a percentage of total drink volume, which were dependent on the ratio of total body water to body mass (Range men= 17.42–21.80%, women= 14.03–20.08%; Mean±SD men= 19.60 ±1.21%, women= 17.15 ±1.66%). Unsurprisingly, there was a sex difference in concentrations received reflective of the typical higher body water to body mass ratio in men compared to women [t(22)= −4.13, p< .001]. However, there was no difference in milliliters of alcohol administered per kilogram of body water [men= 1.82 ±.01ml; women= 1.82±.005ml; t(22)= −1.02, p= .320]. Female participants were tested during the mid-follicular phase of their menstrual cycle, and for those on oral contraceptives (n=4) experimental nights were scheduled during their week of placebo pills (Baker and Driver, 2007; Baker et al., 2001).
Participants were requested to refrain from alcohol for 48 hours before the sessions and from food and caffeine after lunchtime on the day of the sessions. All sessions started five hours before the participant’s normal bedtime. On arrival, participants were breathalysed using a calibrated breathalyzer (Alchosense Precision, AndatechR. Blackburn, Victoria, Australia) - all had a BAC of 0.00%- and received a standard meal. Instrumentation commenced 2 hours before bedtime and all beverages were consumed evenly over a 30 minute period, starting 1 hour before lights out. Data were recorded continuously from the participants’ normal bedtime (lights out) until natural morning awakening.
Participants were blind to experimental condition. To increase the accuracy of estimated alcohol consumption, the experimenter obtained an additional guided 30-day retrospective alcohol history on all nights. Alcoholic beverages from the diary were converted into total number of standard drinks (10g alcohol/standard drink) for each participant (NHMRC, 2009).
Measurements and Recordings
Respiratory and Body Position Equipment
A nasal pressure cannula, body position sensor, thoracic and abdominal respiratory effort bands, and finger pulse oximeter (Compumedics Ltd., Abbotsford, Victoria, Australia) assessed the presence and extent of sleep disordered breathing. Additionally, a peizoelectric vibration sensor (BRAEBON Medical Corpoation, Kanata, Ontario, Canada) was attached to the throat to detect the presence and duration of snoring. Left and right anterior tibiallis EMG was recorded to screen for periodic limb movements.
Electrophysiological measures
Eleven EEG [F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, O2 (Jasper, 1958)] electrodes, left and right eye EOG, submental (chin) EMG and ECG were recorded. Following skin preparation, EEG electrodes were attached to the scalp with Collodian (Mavidon™, Lake Worth, FL, USA) and filled with conductive gel (Signa-gel®, Parker Laboratories, Fairfield, NJ, USA). EEG electrodes were recorded to one forehead reference and subsequently digitally re-referenced to the contralateral ear (with midline sites referenced to A2).
Data Acquisition
Signals were acquired using an ambulatory sleep system (Siesta Unit, Compumedics Ltd., Abbotsford, Victoria, Australia), with data being saved to an internal data card and to a host computer via a wireless link. All signals were acquired and displayed continuously, and filtered optimally according to standard guidelines (AASM, 2007).
Data Reduction and Statistical Analyses
Data recorded on the adaptation night were visually scored for sleep disordered breathing or periodic limb movements according to AASM criteria (2007). The exclusion criteria were an apnea hypopnea index or periodic limb movement index of more than 5 events per hour. One participant was excluded on the basis of these criteria. Sleep polysomnography data were visually scored independently by two researchers according to AASM guidelines for sleep staging, EEG events, arousals, sleep stage shifts and sleep onset latency (AASM, 2007). Differences in scoring were resolved by an independent adjudicator. All scorers were blinded to experimental condition.
Sleep cycle length was scored using the following rules, based on those used by Trinder et al. (1982): 1) A cycle was defined as the time from the beginning of the NREM period (any stage of NREM sleep after sleep onset) to the end of the following REM period. 2) The end of a REM period was retrospectively indicated by a lapse in REM sleep of more than 15 minutes. 3) The first REM period was deemed to have occurred if any indication of REM sleep characteristics could be observed around the period during which it might be expected to have occurred. This method was adopted because of the well-known characteristic of young subjects to have rudimentary first REM periods which are not always reflected in an epoch being scored as REM sleep (Trinder et al., 1982).
Differences in sleep quality and architecture were assessed using 2 (sex) by 2 (alcohol vs. placebo) by 2 (first vs. second half of the night) mixed model ANOVAs on each variable. Data were also examined by sleep cycle using 2 (sex) by 2 (alcohol vs. placebo) by 4 (sleep cycle) mixed model ANOVAs on each sleep variable, as well as sleep cycle length, to evaluate whether sleep cycle length was a confounding factor for any half night effects. Cycle and condition by cycle effects were tested for sphericity and when violated, F-values were tested against Hyund-Feldt corrected degrees of freedom. 19 of the 22 participants had 4 complete sleep cycles in both alcohol and placebo conditions. To account for this, the data were analysed in two ways: once with 19 participants (excluding the three without 4 cycles) and once interpolating missing data from the remaining three participants. Missing data were substituted (one value for each of the participants) with values based on the group mean for the fourth cycle, scaled based on the relationship of each subject’s values for the first 3 cycles to the mean of the other 20 participants. When data were analyzed with and without interpolated values, no differences in the pattern of results or the number of significant effects were noted. Therefore, the interpolated dataset (with adjusted degrees of freedom) are reported in order to allow for more direct comparison with the half night analyses.
RESULTS
No main effects or interactions for sex were observed in any analysis of alcohol levels, drinking history, or sleep quality or architecture measures. Alcohol administration resulted in a mean alcohol dose of 0.828 ±0.085 g/kg (Mean ±SD) on alcohol nights. Mean BAC at lights out on alcohol nights was .084 ±016% compared with 0.00% on placebo nights. There were no differences between men and women for alcohol consumption 30 days prior to participation in the experiment [men: M(SD)= 2.35(1.63); women: 3.88(2.66) standard drinks per week; t(22)=1.7, p=.103]. Estimated lifetime consumption of alcohol also did not differ between genders [men: 3.17(4.167); women: 6.87(7.06)kg of alcohol; [t(22)=1.564, p=.132].
Sleep Quality Measures
No main effect of alcohol condition on sleep onset latency was observed [F(1,21)=0.59, p=.452]. Further, there were no effects of alcohol condition for most sleep quality variables, with the exception of WASO which was significantly higher following alcohol consumption compared to placebo [F(1,21)=5.41, p=.03] (Table 1).
Table 1.
Means (SEM) and significance levels of sleep architecture and quality measures for the first and second halves of the night for placebo and alcohol conditions.
| Placebo Condition | Alcohol Condition | Significance | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1st Half | 2nd Half | 1st Half | 2nd Half | condition | half | condition* half | |
| TST (mins) | 500.57 (16.18) | 479.80 (15.45) | ns | N/A | N/A | ||
| TIB (mins) | 551.37 (19.04) | 536.91 (18.85) | ns | N/A | N/A | ||
| SOL (mins) | 16.94 (2.85) | 14.28 (2.91) | ns | N/A | N/A | ||
| SWSL (mins) | 11.42 (1.07) | 11. 30 (2.09) | ns | N/A | N/A | ||
| REML (mins) | 95.73 (9.37) | 123.19 (9.74) | * | N/A | N/A | ||
| Arousal Index | 9.04 (0.82) | 7.02 (0.65) | 7.33 (0.77) | 10.7 (2.76) | ns | ns | * |
| WASO (mins) | 8.52 (1.60) | 25.07 (3.16) | 8.26 (1.67) | 38.36 (5.92) | * | ** | * |
| Awakenings | 7.22 (0.66) | 13.83 (0.10) | 5.48 (0.72) | 13.57 (0.92) | ns | ** | ns |
| SE (%) | 90.29 (1.16) | 90.38 (1.21) | 92.36 (0.94) | 86.2 (1.74) | ns | * | * |
| Stage 1 (%) | 3.46 (0.43) | 7.23 (0.80) | 2.57 (0.31) | 8.23 (0.77) | ns | ** | * |
| Stage 2 (%) | 39.23 (2.03) | 50.97 (2.26) | 41.72 (2.14) | 55.98 (1.53) | * | ** | ns |
| SWS (%) | 44 (2.35) | 12.12 (1.42) | 49.15 (2.49) | 8.21 (1.23) | ns | ** | ** |
| REM (%) | 13.3 (2.77) | 28.21 (1.30) | 6.56 (0.89) | 27.13 (1.48) | ** | ** | ** |
p< .05
p<.01
TST: Total Sleep Time; TIB: Time In Bed; SOL: Sleep Onset Latency; SWSL: Slow Wave Sleep Latency; REML: Rapid Eye Movement (sleep) Latency; WASO: Wake After Sleep Onset; SE: Sleep Efficiency; SWS: Slow Wave Sleep; REM: Rapid Eye Movement (Sleep).
Differences between first and second halves of the night were seen for WASO [F(1,21)=37.91, p<.001], and the number of awakenings [F(1,21)=97.17, p<.001], with both being higher leading to a lower SE [F(1,21)=6.36, p=.02] in the second half of the night. There were no effects observed for arousal duration, with the exception that arousals were shorter in the second half of the night compared with the first half overall [F(1,21)=6.81, p=.016].
Significant interactions between alcohol condition and time of night were seen for arousal index (AI) [F(1,21)=6.57, p=.018], WASO [F(1,21)=5.17, p=.034] and SE [F(1,21)=4.67, p=.042]. Paired samples t-tests indicated significantly fewer arousals in the first half of the night [t(22)=2.49, p=.01] following alcohol compared with placebo. Increased WASO [t(22)=−2.48, p=.01] and decreased SE [t(22)=2.15, p=.022] were limited to the second half of the night following alcohol consumption, indicating sleep disruption localized to the second half of the night after alcohol.
Sleep Architecture Measures
There was a significant main effect of alcohol condition for REM latency [F(1,21)=6.40, p=.019] and REM sleep as a percentage of total sleep time (%TST) [F(1,21)=16.37, p=.001], with delayed REM onset and less REM sleep overall after alcohol.
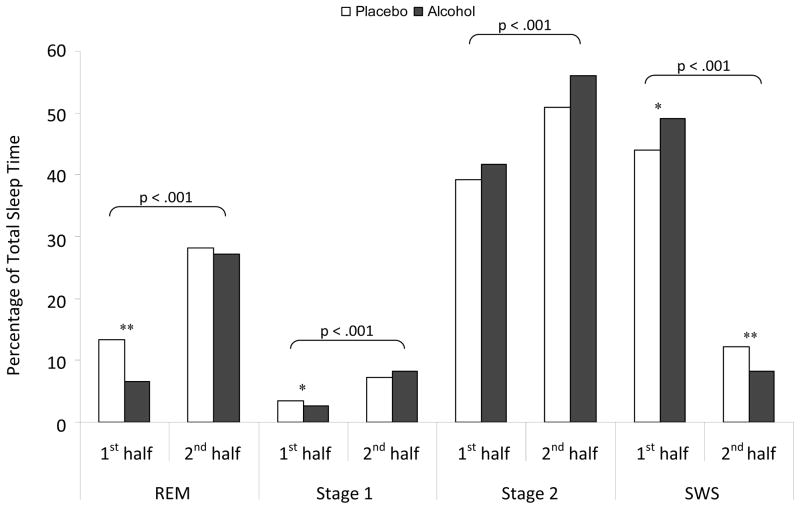
Significant time of night effects were observed for each sleep stage with less REM [F(1,21)=185.11, p<.001], stage one [F(1,21)=67.93, p<.001] and stage two sleep percentage [F(1,21)=32.24, p<.001], and more SWS percentage [F(1,21)=11.07, p=.003] in the first half of the night compared to the second half (Figure 1).
Figure 1.
Mean percentages of total sleep time spent in each sleep stage for the first and second halves of the night following alcohol (black bars) or placebo. Horizontal parenthesis indicate half night significance levels while asterixes indicate significant interaction directions as assessed by paired samples t-tests. NB: *p<.05, **p<.01
Critically, there were significant alcohol condition by half interactions for SWS [F(1,21)= 11.07, p=.003], REM [F(1,21)=8.14, p=.01] and stage one sleep percentages [F(1,21)=4.89, p=.038]. As hypothesized, there was significantly more SWS in the first half of the night [t(22)=−2.27, p=.017] and less SWS in the second half of the night [t(22)=−1.89, p=.01] in the alcohol as compared with the placebo condition. While there was less stage 1 and, as predicted, less REM sleep in the first half of the night following the alcohol condition [t(22)=2.04, p=.027; t(22)=6.76, p<.001, respectively], there was, consistent with Williams et al. (1983), no subsequent rebound in the second half of the night [t(22)=−1.42, p=.09; t(22)=0.65, p=.262, stage 1 and REM respectively].
Sleep Cycle Length
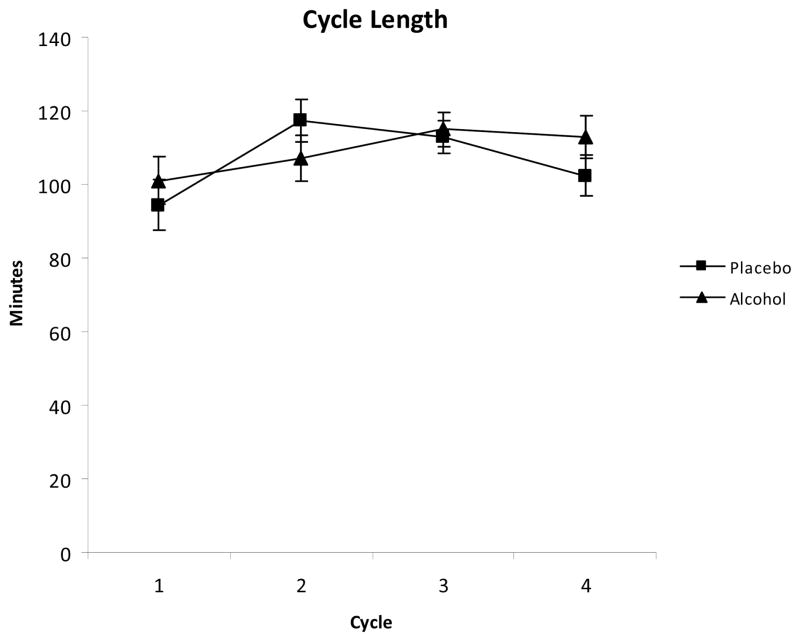
Data from an additional male subject were excluded from the cycle length analyses due to minor equipment issues that could have skewed the cycle length data. There was no effect of alcohol consumption on cycle length [F(1,17)=0.29, p=.598]. However, there was a significant main effect of sleep cycle on sleep cycle length [F(3,57)=3.73, p=.016] indicating that sleep cycle length overall tended to be shorter in the first sleep cycle than in subsequent cycles. No interaction between alcohol condition and cycle was observed [F(3,57)=1.03, p=.388] (Figure 2).
Figure 2.
Mean cycle length in minutes (± SEM) for each of the first 4 sleep cycles.
Analysis by cycle
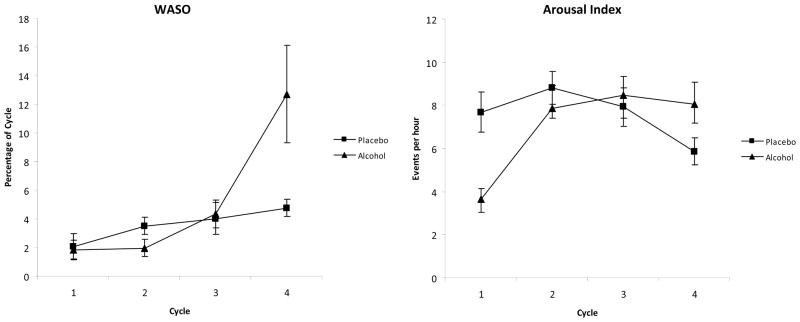
There were no main effects of alcohol condition on WASO or AI. Both variables had significant main effects of cycle (WASO: [F(1.74, 34.88)=8.20, p=.002]; AI: [F (3,57)=5.22, p=.003]) and significant alcohol condition by cycle interactions (WASO: [F(1.84, 36.8)=4.94, p=.014]; AI: [F(3,57)=6.20, p=.001]). In the alcohol condition WASO and AI were higher in the fourth sleep cycle and AI was lower in the first two sleep cycles, compared to placebo (Figure 3).
Figure 3.
Mean percentages (± SEM) of total sleep time of wake after sleep onset (WASO: left pane) and mean values for arousal index (right pane) for each of the first 4 sleep cycles.
There were no main effects of alcohol condition on any sleep architecture variable, with the exception of REM sleep [F(1,17)=21.81, p<.001] indicating less overall REM sleep following alcohol than placebo. Significant main effects of sleep cycle were observed for all sleep stages at p<.001 showing sleep cycle main effects which were consistent with half night main effects, with more stage 1, 2 and REM sleep in the later sleep cycles and more SWS in the earlier sleep cycles overall.
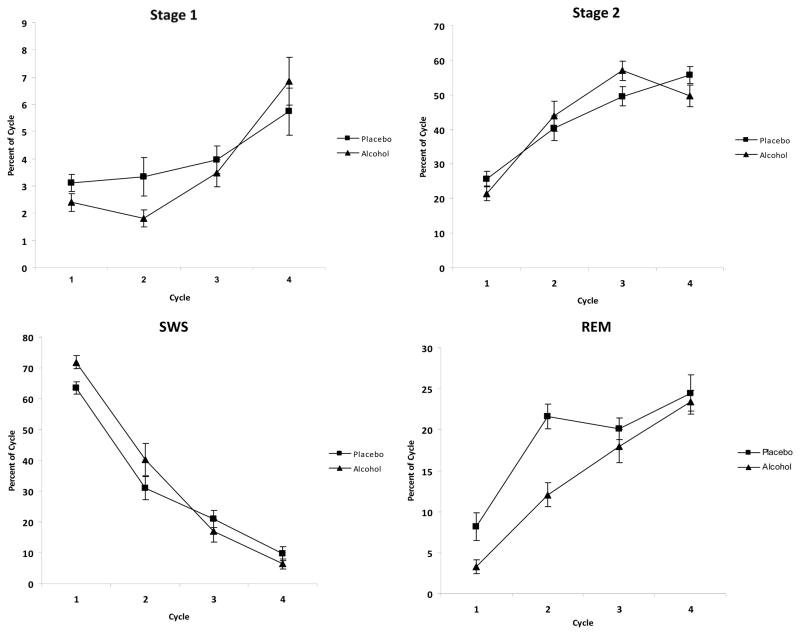
There were significant interactions between cycle and alcohol condition for SWS [F(3, 57)=4.54, p=.006] and REM sleep [F(2.73, 54.93)=3.39, p=.028]. There was more SWS and less REM sleep in the first two cycles, and less SWS with similar percentages of REM sleep in the last two cycles following alcohol consumption relative to placebo (Figure 4).
Figure 4.
Mean percentages total sleep time (± SEM) spent in stage 1 sleep (top left pane), stage 2 sleep (top right pane), SWS (bottom left pane) and REM sleep (bottom right pane) for each of the first 4 sleep cycles.
DISCUSSION
As predicted, alcohol consumption initially promoted sleep, with fewer arousals, less stage one sleep and higher levels of SWS in the first half of the night, although REM sleep was reduced and no sleep onset latency effect was observed. Later in the night, sleep was disrupted with markedly higher WASO levels, particularly during the fourth sleep cycle, and SE was lower throughout the second half of the night. Consistent with the sleep disturbance measures, SWS was lower in the second half of the night in the alcohol condition compared to placebo, while REM sleep was the same as the placebo condition failing to show a rebound effect. There were minimal whole night effects of alcohol condition on sleep quality with higher WASO levels being the only significant consequence of alcohol consumption. The sleep cycle analysis indicated that sleep cycle length was not affected by alcohol, and thus sleep architecture effects were not an artifact of alcohol changing cycle length.
While pharmacologically alcohol is a sedative, there were minimal sedative effects observed in the data. Consistent with past research, there were fewer arousals and less stage one sleep in the first half of the night (Aldrich, 1998; White, 2003), but the absence of a shortened sleep onset latency following alcohol consumption is inconsistent with previous findings in adults (Aldrich, 1998; Arnedt et al., 2011; Eckardt et al., 1998; Roehrs and Roth, 2001; White, 2003) and late adolescents (Williams et al., 1983). Despite the Williams et al. (1983) data, it is possible that the absence of a sleep onset latency effect was due to the unique developmental context of these individuals. As previously mentioned, late adolescents have dramatic changes to their sleep involving a delay in the circadian timing, which in combination with lifestyle factors often results in sleep restriction, and reduction in the amount of delta sleep. Therefore, it is possible that the current dose of alcohol had minimal effects on sleep onset latency in this younger age group. A second possibility is that the finding was due to the recruitment of light-drinkers in whom the novel effects of the high dose of alcohol administered may have been an attentional stimulus, acting as a distraction when participants were trying to fall asleep. Finally, the failure to observe a SOL effect may be due to a ‘basement effect’. We consider this unlikely as previous adult studies have shown an alcohol related reduction from a sober baseline of ~15 minutes, close to the placebo level in this study (Arnedt et al., 2011; Rundell et al., 1972). However, it is possible that SOL length may differ in a younger cohort, as Williams et al. (1983) had longer placebo SOLs (~24 minutes) and similar alcohol SOLs as in the present study. Irrespective of the mechanism, this finding is of interest and may have implications for the extent to which the perceived beneficial effects of alcohol are experienced in this age group. It must also be noted that as the current study only included light drinking young adults without sleeping problems, this does not preclude that heavy or binge drinking young adults, or those with sleeping problems such as insomnia may still exhibit the shortened SOL effect.
This study’s finding that alcohol consumption disrupts subsequent sleep and that this disruption is localized to the second half of the sleep period is consistent with previous findings (Dijk et al., 1992; Landolt et al., 1996; Rouhani et al., 1989; Williams et al., 1983; Yules et al., 1966). Inspection of sleep disruption over sleep cycles in the present study extended these findings showing that, at least in late adolescents, these effects were mostly seen in the fourth sleep cycle, indicating that sleep disruption occurs quite late in the sleep period. The current research, however, found no gender differences, contrary to results from Arnedt et al. (2011) which indicated that women experience greater sleep disruption following alcohol consumption. It must be noted, however, that Arnedt et al. (2011) had a significantly larger sample size and the present study may have been underpowered to detect gender differences.
The current sleep architecture results are broadly consistent with previous literature, supporting the contention that alcohol changes sleep architecture by initially promoting SWS and suppressing REM sleep, resulting in a subsequent decrease in SWS later in the night ( Aldrich, 1998; Rundell et al., 1972; Stone, 1980; Williams et al., 1983). This is further extended by the sleep cycle analysis which illustrated that alcohol did not affect sleep cycle length. Although SWS latency is not normally reported in the literature, the shortened latency in this sample is consistent with the promotion of SWS. Increased SWS is generally seen as indicative of more restorative sleep and is potentially interpretable as a positive consequence of alcohol, however total SWS percentage did not differ between alcohol and placebo nights, a finding consistent with recent assessment of the wider literature (Pressman, 2012). Although we did see an increase in SWS in the first half of the night, it is possible that increases in overall SWS may have been limited due to the tendency for young adults to have very high levels of SWS during normal sleep. Further, it is typically assumed that the sleep architecture changes caused by alcohol offset natural sleep stage shifts from other physiological processes (e.g. the endogenous release of cortisol and growth hormone) resulting in negative consequences (Roehrs and Roth, 2001).
The current study did not identify a REM sleep rebound in the second half of the night following alcohol consumption as is commonly reported in the adult literature (Knowles et al., 1968; MacLean and Cairns, 1982; Rundell et al., 1972). Many previous studies used moderate or low doses of alcohol and some data suggests that the REM rebound effect may be diminished or eliminated increasing doses of alcohol (Arnedt et al., 2011; Knowles et al., 1968; Roehrs, 1991; Sagawa et al., 2011; Williams et al., 1983). Indeed, the only other study of the effects of alcohol on sleep in late adolescents also failed to show a REM sleep rebound with increasing doses of alcohol, but similar to the current results did find a whole night suppression effect (Williams et al., 1983). A likely explanation for the dose effect on second half REM rebound is that, as the elimination of alcohol over time is linear, higher doses will take longer to be eliminated and will exercise a REM suppressing effect further into the night. It is also possible that developmental differences in late adolescents may interact with the effects of a high dose of alcohol (Williams et al., 1983).
Although this study represents the most comprehensive examination of the effects of alcohol consumption on sleep quality and architecture in this age group, it is not without its limitations. Firstly, although participants were blinded to condition, the study did not assess the veracity of the placebo and the high dose of alcohol administered made it unlikely that participants remained unaware of which beverage contained alcohol (MacLean and Cairns, 1982). While participant awareness of alcohol has been shown to influence subjective ratings of sleep quality, we consider it unlikely to have affected the objective measures utilized in this study, with the possible exception of sleep onset latency. However, it is nevertheless possible that expectancy effects, or participant expectation of having received the alcohol, may have influenced some objective sleep measures (Fratello, et al., 2005), particularly given the high concentrations of alcohol administered. Secondly, although participants reported they maintained a stable sleep wake pattern prior to the in laboratory studies, the current study did not measure sleeping habits prior to the adaptation night. As condition order was counterbalanced, however, it is unlikely that there were any systematic differences in pre-study sleep between conditions. Third, although there were no sex differences observed in any aspect of the data, estimated lifetime consumption of alcohol was numerically higher for women than for men, which is uncharacteristic when compared to sex differences in the pattern of consumption in the broader population. It is likely that this was due to the recruitment of light drinkers in the present study. Many of the young men in this age group were excluded for binge drinking, or breaching the light drinking criteria, and those that were included tended to be those who did not drink regularly or who had a very short drinking history. On the other hand, it was comparatively easier to recruit young women who had been drinking regularly for some time, but who drank at a responsible level and who still met the study criteria. Further to this as the current study was restricted to light drinking late adolescents without sleeping problems, the findings cannot be readily generalized to more frequent drinkers, or those with abnormal sleep. Given that many late adolescents engage in binge drinking, further studies are needed to clarify the acute effects, particularly the perceived beneficial effects, of alcohol in heavy or binge drinking cohorts, and in young people with sleeping problems (Bonomo et al., 2004; Wechsler et al., 1994). Finally, although our underlying purpose was to elucidate possible unique sleep effects of alcohol in late adolescents, the strength of interpretation of the results is to some extent limited by the restriction of data collection to this group without a direct older comparison group. Nevertheless, the effect of acute administration of alcohol on adults is well characterized in the literature, which therefore provides an effective comparison.
In conclusion, consistent with the adult literature a high dose of alcohol markedly diminishes late adolescent sleep quality in the second half of the night, specifically very late in the night, following consumption. Alcohol also changed sleep architecture, initially promoting SWS and suppressing REM sleep, resulting in decreased SWS later in the night. Additionally, there was no effect of alcohol on sleep cycle length, meaning that sleep architecture changes could not be attributed to changes in sleep cycle length and indeed reflect true within cycle changes. In contrast to previous reports in adults, we did not observe a REM sleep rebound. It remains unclear whether this was due to the high dose of alcohol used or whether alcohol affects REM sleep systems differently in late adolescents compared to adults, or an interaction of the two. Finally, we did not observe reduced sleep onset latency with alcohol in this age group despite this being consistently reported in the adult literature. Further work is needed to elucidate whether these unique findings may be attributed to the unique developmental characteristics of young adults.
Acknowledgments
Support:
The project was supported by the Australasian Sleep Association (Rob Pierce Grant) and National Health & Medical Research Council (1012195) to CLN and NIH (AA017320; AA020565) to IMC
References
- AASM. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester: 2007. [Google Scholar]
- Abbey A, Saenz C, Buck PO, Parkhill MR, Hayman LW., Jr The effects of acute alcohol consumption, cognitive reserve, partner risk, and gender on sexual decision making. J Stud Alcohol. 2006;67:113–121. doi: 10.15288/jsa.2006.67.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Aldrich MS. Effects of alcohol on sleep. In: GOMBERG EL, HEGEDUS AM, ZUCKER RA, editors. Alcohol Problems and Aging, Alcohol Problems and Aging. National Institute of Health; Bethesda: 1998. pp. 281–300. [Google Scholar]
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, Howland J. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res. 2011;35:870–878. doi: 10.1111/j.1530-0277.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442:729–737. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research and Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Seifer R. Extended nights, sleep loss, and recovery sleep in adolescents. Arch Ital Biol. 2001;139:301–312. [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Brunner DP, Aeschbach D, Tobler I, Borbely AA. The effects of ethanol on human sleep EEG power spectra differ from those of benzodiazepine receptor agonists. Neuropsychopharmacology. 1992;7:225–232. [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Feige B, Gann H, Brueck R, Hornyak M, Litsch S, Hohagen F, Riemann D. Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcohol Clin Exp Res. 2006;30:1527–1537. doi: 10.1111/j.1530-0277.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feinberg I, de Bie E, Davis NM, Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34:325–333. doi: 10.1093/sleep/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratello F, Curcio G, Ferrara M, Marzano C, Couyoumdjian A, Petrillo G, Bertini M, De Gennaro L. Can an inert sleeping pill affect sleep? Effects on polysomnographic, behavioral and subjective measures. Psychopharmacology (Berl) 2005;181:761–770. doi: 10.1007/s00213-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Jasper H. Report of committee on methods of clinical exam in EEG. Electroencephalogr Clin Neurophysiol. 1958;10:370–375. [Google Scholar]
- Knowles JB, Laverty SG, Kuechler HA. Effects on REM sleep. Q J Stud Alcohol. 1968;29:342–349. [PubMed] [Google Scholar]
- Landolt H-P, Roth C, Dijk D-J, Borbely AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. J Clin Psychopharmacol. 1996;16:428–436. doi: 10.1097/00004714-199612000-00004. [DOI] [PubMed] [Google Scholar]
- MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young men. J Stud Alcohol. 1982;43:434–444. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. Are we becoming more alike? Comparison of substance use in Australia and the United States as seen in the 1995, 1998, 2001 and 2004 national household surveys. Drug Alcohol Rev. 2008;27:473–481. doi: 10.1080/09595230802090055. [DOI] [PubMed] [Google Scholar]
- NHMRC. Australian Guidelines to reduce health risks from drinking alcohol. National Health and Medical Research Council of Australia; Canberra: 2009. [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pressman MR. Alcohol Does Not Increase Slow Wave Sleep. Alcohol Clin Exp Res. 2012;36:1474. doi: 10.1111/j.1530-0277.2012.01746.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: Self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20:279–286. doi: 10.1016/S0893-133X(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepniness and alcohol use. Alcohol Research and Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Yoon J, Roth T. Nocturnal and next-day effects of ethanol and basal level of sleepiness. Hum Psychopharm Clin. 1991;6:307–311. [Google Scholar]
- Roehrs T, Zwyghuizen-Doorenbos A, Timms V, Zorick F, Roth T. Sleep extension, enhanced alertness and the sedating effects of ethanol. Pharmacol Biochem Behav. 1989;34:321–324. doi: 10.1016/0091-3057(89)90319-5. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Tran G, Leplaideur F, Durlach J, Poenaru S. EEG effects of a single low dose of ethanol on afternoon sleep in the nonalcohol-dependent adult. Alcohol. 1989;6:87–90. doi: 10.1016/0741-8329(89)90078-5. [DOI] [PubMed] [Google Scholar]
- Rundell OH, Lester BK, Griffiths WJ, Williams HL. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26:201–218. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- Sagawa Y, Kondo H, Matsubuchi N, Takemura T, Kanayama H, Kaneko Y, Kanbayashi T, Hishikawa Y, Shimizu T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093–2100. doi: 10.1111/j.1530-0277.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Chen S, Higley JD, Suomi SJ, Heilig M, Barr CS. Alcohol response and consumption in adolescent rhesus macaques: life history and genetic influences. Alcohol. 2010;44:67–80. doi: 10.1016/j.alcohol.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. Alcohol’s effects on adolescents. Alcohol Res Health. 2002;26:287–291. [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BM. Sleep and low doses of alcohol. Electroencephalogr Clin Neurophysiol. 1980;48:706–709. doi: 10.1016/0013-4694(80)90427-7. [DOI] [PubMed] [Google Scholar]
- Toumbourou JW, Hemphill SA, McMorris BJ, Catalano RF, Patton GC. Alcohol use and related harms in school students in the USA and Australia. Health Promot Int. 2009 doi: 10.1093/heapro/dap037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder J, Stevenson J, Paxton SJ, Montgomery I. Physical fitness, exercise, and REM sleep cycle length. Psychophysiology. 1982;19:89–93. doi: 10.1111/j.1469-8986.1982.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA. 1994;272:1672–1677. [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. J Am Coll Health. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- Williams DL, MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44:515–523. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Yules RB, Freedman DX, Chandler KA. The effect of ethyl alcohol on man’s electroencephalographic sleep cycle. Electroencephalogr Clin Neurophysiol. 1966;20:109–111. doi: 10.1016/0013-4694(66)90153-2. [DOI] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]