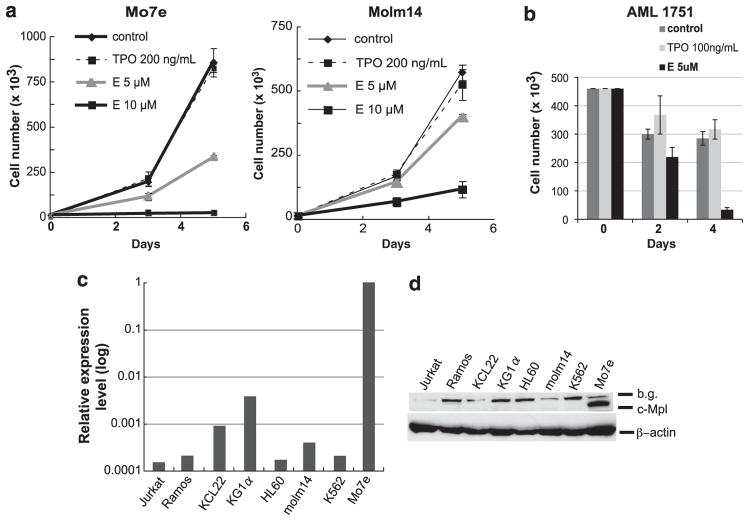
Figure 1.
(a) Cell growth analyses of Mo7e and Molm14. A total number of 1000 to 20 000 cells in 250 μl culture medium were seeded in triplicate wells in a 96-well plate and treated with E (5 or 10 μM, supplied by GlaxoSmithKline, Collegeville, PA, USA) or rhTPO (200 ng/ml, R&D Systems, Minneapolis, MN, USA) as a control. The numbers of viable cells were counted using a hemocytometer with trypan blue exclusion on days 3 and 5. Experiments were repeated at least three times with consistent results. Data are shown as the mean±s.d. Mo7e cell line requires cytokines (TPO or granulocyte-macrophage colony-stimulating factor (GM-CSF)) for its proliferation and is maintained with 5 ng/ml of GM-CSF (Immunex Corporation, Seattle, WA, USA). (b) Cell growth analyses of primary AML cells. The proliferation assays from a representative primary AML sample are shown. Primary samples were obtained from the Stem Cell and Xenograft Core facility at the University of Pennsylvania School of Medicine. All samples were collected in accordance to the federal and University guidelines, and provided to us pathologically annotated without patient personal information. Available clinical information about AML’s is provided in Supplementary Table 1. A total number of 100 000 to 450 000 cells were cultured in the presence of E (5 μM), rhTPO (100 ng/ml) or in medium alone. The numbers of viable cells were counted on indicated days and data are shown as the mean±s.d. of three replicates. (c) Relative c-Mpl mRNA expression levels in various leukemia cell lines; Jurkat, Ramos, KCL22, KG1α, HL60, Molm14, K562 and Mo7e. qRT–PCR using a SYBR Green reagent was performed using β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as reference genes to normalize mRNA expression in different cell lines. The sequences of primers are as follows. c-Mpl, Forward: 5′-TTTCTCCCGAACATTTGAGG-3′; reverse: 5′-GTGCAGCGGAAAGAAGAGAC-3′. β-Actin, Forward: 5′-AAACTGGAACGGTGAAGGTG-3′; reverse: 5′-AGAGAAGTGGGGTGGCTTTT-3′. GAPDH, Forward: 5′-GACAGTCAGCCGCATCTTCTT-3′; reverse: 5′-CCAATACGACCAAATCCGTTGA-3′. The two-step PCR method was performed according to the following protocol: Enzyme activation at 95 °C, 10 min, 40 cycles of denaturing step at 95 °C for 15 s and annealing/extension step at 60 °C for 1 min. c-Mpl mRNA expression in Mo7e is denoted as 1, and the data are presented on a log scale. (d) c-Mpl protein expression in leukemia cell lines. Cell pellets of each cell line were lysed in ice-cold lysis buffer (50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) containing a cocktail of phosphatase inhibitors and protease inhibitors (Pierce, Rockford, IL, USA). Protein lysates were separated using SDS-polyacrylamide gel electrophoresis and were transferred onto polyvinylidene fluoride membrane. Transferred proteins were probed with the following primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies: anti-c-Mpl antibody (Millipore, Billerica, MA, USA, no.06-944), anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA), HRP -conjugated anti-rabbit immunoglobulinG (IgG; Cell Signaling, Danvers, MA, USA, no.7074) and HRP-conjugated anti-mouse IgG (Cell Signaling, no.7076). Protein–antibody signals were detected using enhanced chemiluminescence detection reagent (GE Healthcare, Piscataway, NJ, USA).