Abstract
Background
The methacholine challenge test quantifies airway hyper-responsiveness, which is measured by the provocative concentration of methacholine causing a 20% decrease in forced expiration volume in 1 second (PC20). The dose–response effect of inhaled corticosteroids (ICS) on PC20 has been inconsistent and within-patient variability of PC20 is not well established.
Objectives
To determine the effect of high- vs low-dose ICS on PC20 and within-patient variability in those with repeated measurements of PC20.
Methods
A randomized, double-masked, crossover trial was conducted in patients with asthma on controller medications with PC20 of 8 mg/mL or lower (n = 64) to evaluate the effect of high-dose (1,000 μg/d) vs low-dose (250 μg/d) fluticasone for 4 weeks on PC20. In addition, the variability of PC20 was assessed in participants who underwent 2 or 3 PC20 measurements on the same dose of ICS (n = 27) over a 4-week interval.
Results
Because there was a significant period effect, dose comparison of the change in PC20 was assessed in the first treatment period. There was no significant difference in the change in PC20 for high- vs low-dose ICS (39% vs 30% increase, respectively; P = .87). The within- and between-participant variances for log PC20 were 0.84 and 0.96, respectively, with an intra-class correlation of 0.53, and 37% of participants had more than 2 doubling dose changes in PC20 in those with repeated measurements.
Conclusion
The effect of ICS on PC20 is not dose dependent at fluticasone levels of 250 and 1,000 μg/d. Interpersonal variability for PC20 is large. A lack of precise measurements should be taken into account when interpreting any change in PC20.
Introduction
The methacholine challenge test (MCT) is one of the most commonly used methods to assess and quantify airway hyper-responsiveness (AHR), a key feature of asthma. The result of this test is usually expressed as the provocative concentration of methacholine that causes a 20% decrease in forced expiration volume in 1 second (FEV1; PC20). PC20 is frequently used as a tool for diagnosing asthma and as an entry criterion or outcome measurement in clinical research.1–3 The authors previously reported that 23% of patients with physician-diagnosed asthma on active controller treatment have a negative MCT result and that the sensitivity of the test varies with race and presence of atopy.4
One reason for the decreased sensitivity of MCT may be the increasing use of high-potency inhaled corticosteroids (ICS) in the treatment of asthma. Although high-potency ICS can decrease AHR,5–8 the results of studies investigating the dose–response relation between ICS and PC20 have been inconsistent.8,9 In addition, the American Thoracic Society guidelines10 for MCT state that the 95% confidence interval for short-term (1- to 8-week) repeat testing for methacholine PC20 is within ±1.5 doubling doses. This information is based on older and smaller studies before the widespread use of high-potency ICS. Therefore, the authors investigated the effect of high- vs low-dose ICS on PC20 in a randomized crossover trial and assessed the variability of the MCT for patients on a stable dose of high-potency ICS in a subgroup of those participants.
Methods
Participant Selection
The study was the second part of a 2-phase clinical study. The first phase was a cross-sectional study of patients with asthma and nonasthmatic healthy controls to measure the sensitivity and specificity of the MCT, which is described elsewhere.4 Participants in the second phase were recruited from participants with asthma who completed the first phase and who met the eligibility criteria. Inclusion criteria for the second phase were age 12 to 69 years, physician-diagnosed stable asthma, current treatment for asthma within the preceding 12 months with regular use of asthma controller medications, no asthma exacerbation during the prior 4 weeks, an FEV1 of at least 70% predicted before using a bronchodilator, a positive MCT result (methacholine PC20 ≤8 mg/mL) during phase 1, successful completion of the fluticasone run-in period demonstrating adequate adherence, an Asthma Control Questionnaire (ACQ)11 score lower than 1.5, using fewer than 16 puffs per week of a rescue β agonist during the final 2 weeks of the run-in period, and no asthma exacerbation during the previous 2 weeks. Study medication adherence was monitored through the use of diary cards filled out by the participants at each visit. In addition to medication use, participants recorded the morning peak expiratory flow rate using a Mini-Wright Peak Flow Meter (Clement Clarke International, Ltd, Harlow, Essex, United Kingdom), symptoms, and rescue medication use on the same diary card. Medication adherence was defined as self-report of taking the full assigned dose of ICS. The study was approved by the institutional review board at each participating center and all participants provided written informed consent. The trial was registered at www.clinicaltrials.gov (identifier, NCT00705341).
Study Design
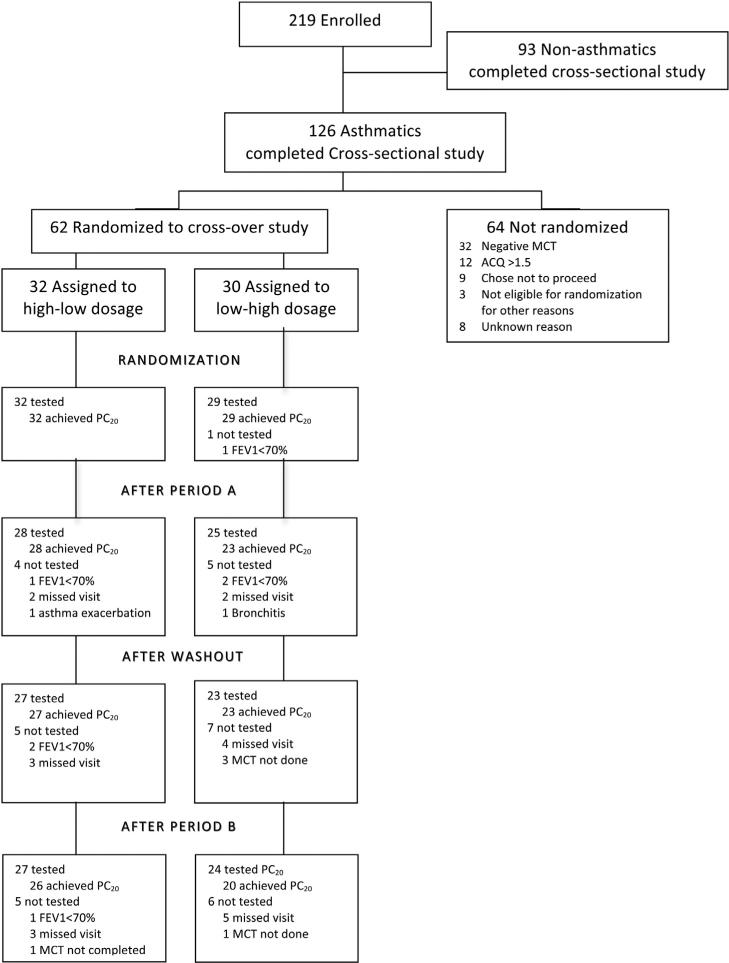
The study was conducted at 15 centers in the American Lung Association Asthma Clinical Research Centers from December 2008 until May 2010. The study was a randomized, double-masked, crossover trial designed to evaluate the effect of high- vs low-dose ICS on AHR. The participant enrollment and study schema are shown in Figures 1 and eFigure 1. The trial began with a 4-week run-in period on low-dose fluticasone (Flovent Diskus, 250 μg/d; GlaxoSmithKline, Brentford, Middlesex, United Kingdom). After completing the run-in period, eligible participants (N = 62) were randomly assigned to 1 of 2 treatment sequences: low-dose (LD) fluticasone (250 μg/d) in the first 4-week period (period A) followed by high-dose (HD) fluticasone (1,000 μg/d) in the second 4-week period (period B), or the reverse dose order. There was a 4-week washout interval between periods A and B during which the participants were maintained on LD fluticasone. The MCT was performed before and after each treatment period. A subset of study participants (n = 27) completed 2 (n = 6) or 3 (n = 21) MCTs during 3 consecutive visits at 4-week intervals while receiving a stable dose of 250 μg/d of fluticasone, which provided an opportunity to assess MCT variability. The reasons for a missing MCT included an FEV1 less than 70% or a missed visit, which are documented in Figure 1.
Figure 1.
Enrollment, randomization, and follow-up of study participants. ACQ, Asthma Control Questionnaire; FEV1, forced expiration volume in 1 second; MCT, methacholine challenge test; PC20, provocative concentration of methacholine causing a 20% decrease in forced expiration volume in 1 second.
Method of MCT
The 5-breath dosimeter method (Koko dosimeter, nSpire Health, Longmont, Colorado) was used according to published guidelines from the American Thoracic Society,10 as previously described.4 Briefly, the test sequence included 11 steps: diluent only, 0.03125, 0.0625, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and 32.0 mg/mL. All medications and foods that might interact with methacholine were held before the test, including short-acting bronchodilators (6 hours), long-acting bronchodilators (24 hours), leukotriene modifiers (24 hours), antihistamines (48 hours), and theophylline (48 hours). Quality control measures were implemented centrally by the data coordinating center, including the calibration of each nebulizer cup to deliver 9 μL ± 10% per 0.6-second actuation and certification of the technicians who performed the MCT.
Statistical Analysis
Comparisons between randomized groups were performed using Kruskal-Wallis tests for continuous variables and χ2 tests or Fisher exact tests for categorical variables. For plots and summary statistics, PC20 was imputed to be 48 mg/mL (1.5 times the highest dose inhaled) for those who did not exhibit a 20% decrease in FEV1 at the maximum concentration tested (32 mg/mL). For all other analyses, multiple imputation12 was used to adjust for the uncertainty in the unobserved PC20 for these participants. Sensitivity analyses applying a range of imputation models were performed to assess the robustness of the estimates. Repeated measurement log-linear generalized estimating equation models were used to assess the dose effect on PC20. Variability of the MCT was measured using between- and within-subject variances to construct an intraclass correlation coefficient (ICC) for participants on a stable ICS dose. P values less than .05 (2-sided) were considered statistically significant.
Results
Characteristics of Study Participants
Sixty-two participants with mild asthma on a single controller medication (30% receiving ICS alone) or combination controller therapy (60%) were included (Table 1). The participants’ overall asthma control at enrollment was suboptimal, with an average ACQ of 1.0 (n = 26 [42%] with a score ≤0.76, defined as well controlled).13 Over the course of the entire trial, medication adherence was high, with slightly higher rates for patients randomized to HD and then LD ICS compared with those randomized to LD and then HD ICS (93% vs 90%, P < .0001); however, the magnitude of the difference was small. During the run-in phase and phase A, the adherence rates were 94% and 89%, respectively, and did not differ significantly between groups.
Table 1.
Participant characteristics
| Total (N = 62) | Order of ICS |
||
|---|---|---|---|
| HD/LD (n = 32) | LD/HD (n = 30) | ||
| General characteristics | |||
| Male patients, n (%) | 22 (35) | 13 (41) | 9 (30) |
| Race/ethnicity, n (%)a | |||
| White | 35 (56) | 19 (59) | 16 (53) |
| African American | 19 (31) | 7 (22) | 12 (40) |
| Latino/Hispanic | 7 (11) | 6 (19) | 1 (3) |
| Other | 1 (2) | 0 (0) | 1 (3) |
| Age at enrollment (y), mean ± SD | 39.2 ± 15.4 | 40.6 ± 15.3 | 37.8 ± 15.7 |
| Former smoker, n (%) | 6 (10) | 4 (13) | 2 (7) |
| BMI (kg/m2), mean ± SD | 27.7 ± 6.2 | 28.2 ± 6.6 | 27.2 ± 5.9 |
| Other conditions, n (%) | |||
| Eczema | 6 (10) | 4 (13) | 2 (7) |
| Sinusitis | 7 (11) | 3 (9) | 4 (13) |
| Hay fever/rhinitis | 39 (63) | 20 (63) | 19 (63) |
| GERD | 14 (23) | 7 (22) | 7 (23) |
| Food allergy | 10 (16) | 6 (19) | 4 (13) |
| Family history of asthma, n (%) | 40 (65) | 19 (59) | 21 (70) |
| Atopy | |||
| Positive allergy skin test results, median (range) | 3 (0–14) | 3 (0–14) | 4 (0–14) |
| ≥1 positive allergy skin test reaction, n (%) | 49 (82) | 23 (72) | 26 (93) |
| Lung function, mean ± SD | |||
| Percentage of predicted FEV1 (before BD) | 86.6 ± 12.7 | 89.4 ± 12.9 | 83.6 ± 11.9 |
| Percentage of predicted FVC (before BD) | 94.4 ± 11.9 | 96.3 ± 12.4 | 92.4 ± 11.1 |
| Asthma characteristics | |||
| Age of onset (y), median (range) | 15.5 (1–53) | 15.5 (1–53) | 15.5 (1–52) |
| Asthma treatment at enrollment, n (%) | |||
| ICS/LABA combination | 37 (60) | 22 (69) | 15 (50) |
| ICS (no combination) | 19 (30) | 9 (28) | 10(33) |
| No ICS | 6 (10) | 1 (3) | 5 (17) |
| Anti-leukotriene | 13 (21) | 4 (13) | 9 (30) |
| Asthma control | |||
| Use of daily SABA, n (%) | 12 (19) | 8 (25) | 4 (13) |
| ACQ, mean ± SD | 0.6 ± 0.4 | 0.7 ± 0.4 | 0.6 ± 0.5 |
| Urgent care visits in 12 mo, n (%) | 15 (24) | 8 (25) | 7 (23) |
| Prednisone bursts in 12 mo, n (%) | 12 (19) | 7 (22) | 5 (17) |
Abbreviations: ACQ, 7-item Asthma Control Questionnaire; BD, bronchodilator; BMI, body mass index; FEV1, forced expiration in 1 second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; HD/LD, high-dose and then low-dose inhaled corticosteroids; ICS, inhaled corticosteroids; LABA, long-acting β agonist; LD/HD, low-dose and then high-dose inhaled corticosteroids; SABA, short-acting β agonist.
Race/ethnicity was determined by self-identification to best descriptive category.
Influence of Dose of ICS on PC20
Participants were randomized to LD ICS followed by HD ICS (LD/HD, n = 30) or HD ICS followed by LD ICS (HD/LD, n = 32). The demographic and asthma characteristics were similar between these groups at randomization (Table 1).
There was a significant period effect (difference in the percentage change in PC20 for HD and LD ICS depending on the order in which the doses were administered; test of interaction, P = .019; Table 2). When HD ICS was administered first, the PC20 was slightly lower after HD ICS than after LD ICS (1.19 vs 1.25 mg/mL); conversely, in the other group, the PC20 was higher after HD ICS than after LD ICS (4.31 vs 2.04 mg/mL). To remove the effect of the order in which the treatments were received and decrease the potential influence of treatment carryover, insufficient follow-up time, and loss to follow-up, the HD and LD MCT results were compared exclusively during period A, ignoring period B, and the results were analyzed as parallel groups. There was no difference in the percentage of participants with a positive MCT result at the end of period A (89% vs 88%, P > .99). In addition, there was no difference in the change in PC20 for HD vs LD ICS (39% vs 30% increase, P = .87). Sensitivity analyses with a range of alternate PC20 imputation structures produced similar results.
Table 2.
Summary of PC20 measurements at each follow-up visit
| Randomization | After period A | After washout | After period B | |
|---|---|---|---|---|
| HD then LD ICS (n = 32) | on LD ICS (run-in) | after HD ICS | on LD ICS (washout) | after LD ICS |
| PC20 | ||||
| N | 32 | 28 | 27 | 26 |
| Geometric mean (raw PC20 range) | 0.90 (0.11–7.13) | 1.19 (0.10–26.92) | 0.64 (0.008–14.49) | 1.25 (0.008–48.00) |
| PC20 >32 mg/mLa | 0 | 0 | 0 | 1 |
| Missing | 0 | 4 | 5 | 5 |
| LD then HD ICS (n = 30) | on LD ICS (run-in) | after LD ICS | on LD ICS (washout) | after HD ICS |
| PC20 | ||||
| N | 29 | 23 | 23 | 20 |
| Geometric mean (raw PC20 range) | 1.56 (0.09–7.16) | 2.04 (0.16–48.00) | 1.39 (0.14–30.55) | 4.31 (0.32–48.00) |
| PC20 >32 mg/mLa | 0 | 2 | 0 | 4 |
| Missing | 1 | 5 | 7 | 6 |
Abbreviations: HD, high-dose; ICS, inhaled corticosteroid; LD, low-dose; PC20, provocative concentration of methacholine causing a 20% decrease in forced expiration in 1 second.
The number of participants who did not achieve PC20 at 32 mg/mL is listed and the multiple imputation was used in the calculation of the geometric mean of PC20 to include data from as many participants as possible in the analysis.
MCT Variability
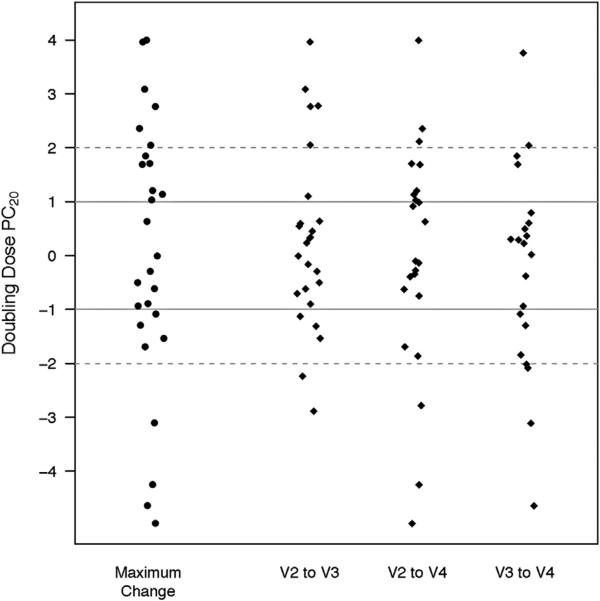
The inter- and intra-subject variabilities of PC20 were evaluated during 3 consecutive visits for 27 participants who completed 2 (n = 6) or 3 (n = 21) MCTs after the initial positive MCT result at entry (methacholine PC20 ≤8 mg/mL). These tests were conducted at 4-week intervals while participants received a stable dose of 250 μg/d of fluticasone. Over this 12-week period across multiple MCTs, the within- and between-subject variances for log PC20 were 0.84 and 0.96, respectively, resulting in an ICC of 0.53. Furthermore, without change in the ICS dose, 74% of participants had more than 1 doubling dose change in their PC20 and 37% had more than 2 doubling dose changes (Fig 2).
Figure 2.
Change in provocative concentration of methacholine causing a 20% decrease in forced expiration volume in 1 second (PC20) between visits in a subset of participants who underwent a methacholine challenge test on a stable dose of inhaled corticosteroids. Twenty-seven participants who were assigned to receive low-dose and high-dose inhaled corticosteroids underwent 2 (n = 6) or 3 (n = 21) PC20 measurements at 4-week intervals while receiving 250 μg/d of fluticasone during the run-in phase (visits 1 to 2 [V1 to V2]), the low-dose treatment phase (visits 2 to 3 [V2 to V3]), and the washout period (visits 3 to 4 [V3 to V4]). The methacholine challenge test was performed at the end of each interval (V2, V3, and V4). This figure shows the change in PC20 (measured in doubling doses) between each visit and the maximal change for each participant.
Factors Associated with Increased Variability
The association of various participant characteristics at baseline with variance of PC20 was examined. Older age, defined as older than 50 vs 50 years or younger, was associated with greater variance (difference in location 2.23, 95% confidence interval 1.62–2.59, P = .0001) and the presence of atopy was associated with lower variance (difference in location –2.18, 95% confidence interval –2.58 to –0.23, P = .0228). There was no significant difference in the variability for participants providing 2 and 3 measurements (P = .84). Other risk factors that were examined but did not have a significant impact on variability included sex, race, body mass index greater than 30 kg/m2, a family history of asthma, predicted FEV1 greater than 400%, and an ACQ score lower than or equal to 0.76; all had minimal impact (absolute difference ranges 0.010–0.075, P = 0.55–0.98). Use of a prednisone burst (difference 0.36, P = .17) or an urgent care visit (difference –0.22, P = .20) in the past 12 months or peak flow greater than 400 (difference –0.23, P = .39) had a larger effect on variability but were not statistically significant. There was no significant difference in the variation based on season of enrollment (P = .74), but the sample size available for comparison was small.
Discussion
In the present study, the authors made several and clinically important observations about PC20 measured in MCT: (1) change to a dose of a high-potency ICS over a short period had little effect on AHR in participants already taking LD ICS, (2) there is a large amount of intra-subject variability with MCT in the same patients on a stable dose of ICS, and (3) age and atopy are associated with MCT variability.
The authors did not observe a dose-dependent change in PC20 when comparing LD with HD ICS in participants with asthma over a 4-week interval. This is in agreement with a meta-analysis including 11 studies in steroid-naive patients with mild asthma that did not show a dose–response effect,7 but conflicts with a meta-analysis of 25 placebo-controlled trials evaluating the dose–response of ICS on AHR in patients with asthma with varying degrees of disease severity that concluded that higher doses of ICS produced greater improvement in AHR.8 The lack of a consistent dose-dependent pattern of change in PC20 in the present study could be because the treatment period with ICS was too short to affect AHR. The authors chose a treatment period of 4 weeks based on the fact that the initial ICS effect on PC20 can be seen as soon as 1 week.14,16 It is also possible the authors did not detect an effect of HD ICS on AHR in patients with mild persistent asthma owing to a ceiling effect, such as having limited room for improvement in those who have only mild disease. Similarly, the dose-dependent nature of the relation may be observable only for lower doses of ICS than were used in this study, with a plateau in the effect at levels of ICS at or above what was defined as a low dose. An ideal scientific design would have been to take the participants completely off their ICS during the run-in and washout periods. However, this was not possible because the present study population involved those with mild to moderate asthma in whom daily ICS treatment was indicated as standard of care. Moreover, the large degree of intra-subject variability may have prevented detection of a dose-dependent effect of ICS on PC20.
Although this study was not specifically designed to evaluate the reproducibility of PC20 measurements, the authors were able to make observations on the variability of PC20 in a subset of participants who underwent multiple MCTs on a stable low dose of ICS. A meta-analysis evaluated the reproducibility of measurements in bronchial challenge and found that the ICC was higher than 0.9 in short-term studies (<4 months), but many of the studies included were small.17 However, a larger degree of intra-subject variability was seen in the present study, including 27 participants with asthma: the ICC was 0.53 and more than one third of participants had a change in their PC20 of at least 2 doubling doses. The authors speculate that this large degree of intra-subject variability of MCT may have contributed to the decreased sensitivity of MCT observed in the authors’ previous study4 and to the lack of a dose-dependent change in PC20 in the present study. This variability could be due to methodologic issues with the MCT. However, the authors believe this is unlikely because they had instituted quality control procedures with centralized certification and calibration of nebulizer cups and standardization of equipment and techniques to ensure that the MCT was performed in a standardized fashion. This variability also could be due to changes in the participants’ clinical conditions. The authors could not detect a reason for the variation in PC20 from baseline characteristics; markers of asthma control and severity at baseline (ACQ score, FEV1, and peak flows) were similar for participants with high and low MCT variability. Although the present study included those with stable asthma and excluded those with poorly controlled asthma, many did not have well-controlled asthma. Therefore, day-to-day subclinical disease instability from various environmental exposures is a possible cause of this variability in PC20. Further exploration of the effect of season, which was not feasible in this study because of the small sample, also would be valuable. Further, it is likely that AHR is not a static characteristic of asthma and varies with time; after all, the periodic nature of the syndrome is a defining feature of asthma.
The authors also observed that older patients with asthma had greater variance and those with atopy had lower variance in PC20. The authors are not aware of any other studies that evaluated risk factors for variation in PC20 in 1 patient. Recent studies have identified various different phenotypes of asthma and those with adult-onset asthma may have increased risk of health care usage,18 which could be due in part to the variability of AHR. The authors were surprised that those with atopy had less variability in the serial PC20 measurements. This observation is in contrast with studies showing the association of the presence of an allergic condition with AHR19 and exposure to allergens with worsening in AHR.20 Because this association analysis was performed only in a small group of patients, full assessment of the reproducibility of PC20 in patients with asthma and the risk factors associated with variation awaits further study involving more subjects with a wide range of methacholine sensitivity and asthma severity.
There are several limitations to this study. First, some participants did not complete all MCT measurements. Efforts were made to decrease the impact of missing data using robust analytic techniques (generalized estimating equation and multiple imputation) and the focus on only period A. Second, the treatment effect was greater during the second period for the 2 doses of ICS. This may be due to an insufficient duration for the treatment and/or the washout period. A washout time of 4 weeks for ICS was chosen based on studies reporting exacerbations occurring after this period (indicating disappearance of treatment effect).21,22 Nevertheless, a differential effect was observed due to the ordering of doses. The authors attempted to mitigate the influence of the period effect by considering only the first treatment period. However, the power to detect a difference in the change in PC20 with a parallel design is much less than with a crossover design. Third, the study included patients with mostly mild persistent asthma on long-term asthma treatment, so the result may not apply to those with more severe asthma or those who do not require controller medications.
In summary, in patients with physician-diagnosed asthma on controller medication, no dose-dependent change was observed in PC20 between 250- and 1,000-μg/d ICS doses of fluticasone. A large amount of intra-subject variability of MCT was observed in patients with asthma on a stable dose of ICS, which decreases the precision of estimates of change in PC20. This low-precision PC20 measurement would need to be taken account when using PC20 values for asthma diagnosis or when using PC20 as an outcome measurement in clinical studies.
Supplementary Material
Acknowledgments
Members of the research group for the MethaCholine bronchopro-vocation - influence of high potency Inhaled corticoSteroids in asthma (MeCIS) study were N.A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Bertrand, RN, RPFT (principal clinic coordinator), M. Atik, MD (coordinator), Baylor College of Medicine, Houston, Texas; L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), A. Dugdale, C Foss, D. Jaggers, R. Newton (coordinators), Duke University Medical Center, Durham, North Carolina; L. Smith (principal investigator), J. Moy, E. Naureckas, (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, M. Morley, V. Zagaja (coordinators), Illinois Consortium, Chicago, Illinois; M. Busk (principal investigator), P. Puntenney (principal clinic coordinator), N. Busk, J. Hutchins (coordinators), Indiana University, Asthma Clinical Research Center, Indianapolis, Indiana; W.R. Summer (principal investigator), G. Meyaski (principal clinic coordinator), A. Antoine (coordinator), Louisiana State University Health Sciences Center, Ernest N. Morial Asthma, Allergy, and Respiratory Disease Center, New Orleans, Louisiana; R. Katial (principal investigator), H. Currier (principal clinic coordinator), National Jewish Health, Denver, Colorado; J. Lima (principal investigator), K. Blake, J. Lang (co-principal investigators), M. McRae (principal clinic coordinator), Nemours Children's CliniceUniversity of Florida Consortium, Jacksonville, Florida; J. Reibman (principal investigator), E. DiMango, L. Rogers (co-principal investigators), K. Carapetyan (principal clinic coordinator, at New York University), E. Simpson, N. Surinder (clinic coordinators at Columbia University), New York University-Columbia University Consortium, New York, New York; A.J. Dozor (principal investigator), S. Krishnan, (co-investigator), I. Gherson (principal clinic coordinator), New York Medical College, New York, New York; R. Cohen (principal investigator), R. Ramdeo (principal clinic coordinator), North Shore–Long Island Jewish Health System, New Hyde Park, New York; C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky (co-principal investigators), S.M. Burns (principal clinic coordinator), L.V. Griffes, J.E. Lippmann (coordinators), University of Vermont, Burlington, Vermont; J. Mastronarde (principal investigator), J. Parsons (co-principal investigator), K. McCoy, C. Benninger (co-investigator), J. Drake (principal clinic coordinator), R. Compton, D. Cosmar (coordinators), Ohio State University Medical Center-–Nationwide Children’s Hospital, Columbus, Ohio; A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida), D. Miller, M. Grandstaff, P Rebolledo (coordinators), University of Miami at Miami–University of South Florida, Tampa, Florida; G. Salzman (principal investigator), D. Pyszczynski (co-principal investigator), P. Haney (principal clinic coordinator), University of Missouri–Kansas City School of Medicine, Kansas City, Missouri; M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), J. Tarsi (principal clinic coordinator), S. De Martino (coordinator), St. Louis Asthma Clinical Research Center of Washington University, St Louis, Missouri; S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator), T. Greene (principal clinic coordinator) K. Kinninger (clinic coordinator), University of California, San Diego, San Diego, California; W. Bailey (research group chair), University of Alabama, Birmingham, Alabama; R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), A. Adler, D. Amend-Libercci, M. Daniel, A. Lears, G. Leatherman, C. Levine, D. Nowakowski, N. Prusakowski, S. Roettger, D. Shade, E. Sugar, C. Wei, Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore, Maryland. Steering Committee: W. Bailey (chair), M. Busk, M. Castro, R. Cohen, A. Dozor, N. Edelman, N. Hanania, J. Holbrook, C. Irvin, R. Katial, E. Lancet, J. Lima, J. Mastronarde, J. Reibman, G. Salzman, L. Smith, W. Summer, J. Sundy, A. Wanner, S. Wasserman, R. Wise. ALA Data and Safety Monitoring Board: V. Chinchilli (chair), P. Lanken, C.R. Rinaldo, D. Tashkin. Project Office, American Lung Association, New York, New York: N. Edelman (scientific consultant), E. Lancet, S. Rappaport. American Lung Association Scientific Advisory Committee: N. Schachter (chair), L.A. Baggott, W.C. Bailey, A.L. Brannen, M. Castro, B. Christman, A. Chung, R.M. Donaldson, C. Holloway, T.A., Mahr, J.A. Neubauer, J. Samet, E. Swenson, D. Upson, D. Weiss, R. Wise.
Disclosures: Dr Sumino has received funding from the American Lung Association. Dr Castro's institution received a grant from the American Lung Association Asthma Clinical Research Centers and he is a board memberof the American Lung Association. Dr Irvin as principal investigator received grants from the American Lung Association and National Institutes of Health and participated in a leadership group for the American Lung Association. Dr Wise served on the clinical end-point committee of GlaxoSmithKline, the Data Monitoring Committee of Merck, and as a consultant to AstraZeneca. Dr Kaminsky as co-investigator received funding from the American Lung Association.
Disclaimer: Methapharm and GlaxoSmithKline played no role in the design, conduct, data analysis, or interpretation of the study.
Funding: This work was supported by the American Lung Association and drugs were donated by Methapharm (Provocholine) and GlaxoSmithKline (Ventolin HFA and Flo-vent). Dr Sumino received grant 1KM1CA156708-01 from the National Institutes of Health.
Footnotes
A list of participants in the American Lung Association Asthma Clinical Research Centers is available in the “Acknowledgments” section.
Clinical Trials Registration: ClinicalTrials.gov, identifier NCT00705341.
Supplementary Data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.anai.2014.01.013.
References
- 1.Sutherland ER, King TS, Icitovic N, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mastronarde JG, Anthonisen NR, Castro M, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–1499. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129:S65–S87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumino K, Sugar EA, Irvin CG, et al. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol. 2012;130:69–75. e6. doi: 10.1016/j.jaci.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Brannan JD. Bronchial hyperresponsiveness in the assessment of asthma control: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(suppl):11S–17S. doi: 10.1378/chest.10-0231. [DOI] [PubMed] [Google Scholar]
- 6.Foresi A, Mastropasqua B, Chetta A, et al. Step-down compared to fixed-dose treatment with inhaled fluticasone propionate in asthma. Chest. 2005;127:117–124. doi: 10.1378/chest.127.1.117. [DOI] [PubMed] [Google Scholar]
- 7.Reddel HK, Belousova EG, Marks GB, et al. Does continuous use of inhaled corticosteroids improve outcomes in mild asthma? A double-blind randomised controlled trial. Prim Care Respir J. 2008;17:39–45. doi: 10.3132/pcrj.2008.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Grunsven PM, van Schayck CP, Molema J, et al. Effect of inhaled corticosteroids on bronchial responsiveness in patients with “corticosteroid naive” mild asthma: a meta-analysis. Thorax. 1999;54:316–322. doi: 10.1136/thx.54.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie GP, Fowler SJ, Lipworth BJ. Dose response of inhaled corticosteroids on bronchial hyperresponsiveness: a meta-analysis. Ann Allergy Asthma Immunol. 2003;90:194–198. doi: 10.1016/S1081-1206(10)62140-0. [DOI] [PubMed] [Google Scholar]
- 10.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing—1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 12.Dai JY, Ruczinski I, LeBlanc M, et al. Imputation methods to improve inference in SNP association studies. Genet Epidemiol. 2006;30:690–702. doi: 10.1002/gepi.20180. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Lim S, Jatakanon A, John M, et al. Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 15.van Rensen EL, Straathof KC, Veselic-Charvat MA, et al. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999;54:403–408. doi: 10.1136/thx.54.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erin EM, Zacharasiewicz AS, Nicholson GC, et al. Rapid effect of inhaled ciclesonide in asthma: a randomized, placebo-controlled study. Chest. 2008;134:740–745. doi: 10.1378/chest.07-2575. [DOI] [PubMed] [Google Scholar]
- 17.Chinn S, Schouten JP. Reproducibility of non-specific bronchial challenge in adults: implications for design, analysis and interpretation of clinical and epidemiological studies. Thorax. 2005;60:395–400. doi: 10.1136/thx.2004.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewitt DJ. Interpretation of the “positive” methacholine challenge. Am J Ind Med. 2008;51:769–781. doi: 10.1002/ajim.20631. [DOI] [PubMed] [Google Scholar]
- 20.Duong M, Gauvreau G, Watson R, et al. The effects of inhaled budesonide and formoterol in combination and alone when given directly after allergen challenge. J Allergy Clin Immunol. 2007;119:322–327. doi: 10.1016/j.jaci.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Marabini A, Cardinalini G, Severini C, et al. Is normal bronchial responsiveness in asthmatics a reliable index for withdrawing inhaled corticosteroid treatment? Chest. 1998;113:964–967. doi: 10.1378/chest.113.4.964. [DOI] [PubMed] [Google Scholar]
- 22.Castro M, Bloch SR, Jenkerson MV, et al. Asthma exacerbations after glucocorticoid withdrawal reflects T cell recruitment to the airway. Am J Respir Crit Care Med. 2004;169:842–849. doi: 10.1164/rccm.200208-960OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.