Abstract
Objective
Generalized social anxiety disorder (GSAD) is characterized by excessive fear of public scrutiny and reticence in social engagement. Previous studies have probed the neural basis of GSAD often using static, non-interactive stimuli (e.g., face photographs) and have identified dysfunction in fear circuitry. We sought to investigate brain-based dysfunction in GSAD during more real-world, dynamic social interactions, focusing on the role of reward-related regions that are implicated in social decision-making.
Methods
Thirty-six healthy individuals (HC) and 36 individuals with GSAD underwent fMRI scanning while participating in a behavioral economic game (‘Trust Game’) involving iterative exchanges with fictive partners who acquire differential reputations for reciprocity. We investigated brain responses to reciprocation of trust in one’s social partner, and how these brain responses are modulated by partner reputation for repayment.
Results
In both HC and GSAD, receipt of reciprocity robustly engaged ventral striatum, a region implicated in reward. In HC, striatal responses to reciprocity were specific to partners who have consistently returned the investment (‘cooperative partners’), and were absent for partners who lack a cooperative reputation. In GSAD, modulation of striatal responses by partner reputation was absent. Social anxiety severity predicted diminished responses to cooperative partners.
Conclusion
These results suggest abnormalities in GSAD in reward-related striatal mechanisms that may be important for the initiation, valuation, and maintenance of cooperative social relationships. Moreover, this study demonstrates that dynamic, interactive task paradigms derived from economics can help illuminate novel mechanisms of pathology in psychiatric illnesses in which social dysfunction is a cardinal feature.
Introduction
Generalized social anxiety disorder (GSAD), also known as generalized social phobia, is characterized by an exaggerated and pervasive fear, and avoidance of scrutiny by others. GSAD is very common [1], typically originates prior to adolescence, foretells significant functional impairment and psychiatric comorbidity including other anxiety, mood and substance abuse/dependence disorders, and rarely remits without treatment [2–4]. Individuals with GSAD exhibit a number of cognitive biases/distortions relevant to initiation and maintenance of ongoing social relationships [5] including attention and memory bias for signals of threat [6–8], misattribution and/or expectation bias for negative outcomes [9–11]. Previous studies have probed the neural basis of GSAD primarily using tasks with non-interactive stimuli (e.g., face photographs), and have implicated fear-related neurocircuitry, especially the amygdala [12]. However, the neurocircuitry of GSAD in the context of a more real-world dynamic, social interaction remains largely unexplored.
In parallel, the emerging fields of neuroeconomics and social cognitive neuroscience [13–15] are increasingly uncovering the brain mechanisms involved in motivation to trust in social partners, form social networks, keep track of reputations, and experience rewards from others who are cooperative and fair [16; 17]. Accumulating evidence suggests that the brain’s reward system, and in particular ventral striatum (vSTR), plays a key role in supporting cooperative social relationships [13; 18]. VSTR plays a critical role in reward and motivation generally [19; 20], but also displays preferential sensitivity for outcomes that are fair and equitable between social partners [18; 21]. VSTR is also implicated in decoding the reputations of others based on prior actions [22], and using this reputational information to bias subsequent emotions and social decisions [16; 17; 22; 23]. In a previous report with healthy participants [24], we used the Trust Game, an economic exchange game that mimics real life decisions in that gains from cooperation must be balanced against risks from partners’ defection, and demonstrated that vSTR reward signals are modulated by partner reputation. In particular, we found vSTR responses to partner reciprocation are amplified with social partners with a prior reputation for cooperation versus partners with a reputation for defection, and other groups have confirmed these findings [25; 26]. These results suggest vSTR modulation by partner reputation might be a potentially important mechanism for initiating and sustaining cooperative relationships. If so, the natural question arises whether this mechanism might be dysregulated in GSAD and other neuropsychiatric illnesses in which social dysfunction is a cardinal feature.
Using a social neuroeconomics approach, we presented 36 adults with GSAD and 36 matched healthy control (HC) individuals with a Trust Game modified into an iterative format for functional magnetic resonance imaging (fMRI). We sought to uncover abnormalities in GSAD in vSTR-mediated reward functioning during ongoing dynamic social exchange. Based on prior data and theory, we predicted that relative to psychiatrically healthy controls, the GSAD group would exhibit differences in VSTR responses to reciprocity and/or reputation.
Method
Subjects
Thirty-six right-handed individuals with GSAD (21 females; mean age and SD 27.0 ± 7.6 years) and 36 right-handed HCs (22 females; mean age and SD 29.5 ± 8.3 years) participated in this study. After the nature of the procedures was explained, all subjects provided informed consent. Social anxiety disorder, generalized subtype, was assessed with Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria as confirmed by the Structured Clinical Interview for DSM-IV and Liebowitz Social Anxiety Scale (LSAS, mean 76.9 ± 16.7). Entry criteria and other clinical characteristics of GSAD participants are described in the Supplement.
Trust Game Task
The fMRI task involved an event-related design (Fig. 1) previously detailed [24]. Participants played the role of an investor [“Decision Maker 1” (DM1)] who must decide whether or not to invest 20 monetary units (MU) to a trustee partner [“Decision Maker 2” (DM2)]. If the investor chooses to ‘keep’ the money (not invest), the money is evenly split and each person receives 10 MU with certainty. But if the investor chooses to invest the money (‘trust’), the money is doubled (40 MU) and the trustee can then choose to either ‘reciprocate’ by sending back half the money to the investor, or ‘defect’ by retaining the entire amount thereby sending nothing back to the participant (0 MU). A key manipulation was that investors played in repeated interactions with three different fictive partner types, each associated with different tendencies for reciprocity which were unknown to the participant at the start of the experiment. Unbeknownst to the participants, the frequency of reciprocity was actually fixed at the following frequencies: 1) ‘COOPERATIVE’ partner = 75%; 2) ‘UNCOOPERATIVE’ partner = 25%; and 3) ‘NEUTRAL’ partner = 50%. This task manipulation forces participants to learn the tendencies of their partners to reciprocate (based on prior interactions with that partner) in order to maximize personal gains. An additional ‘COMPUTER’ partner was included, which did not require real-time learning of reputation (participants were told ahead of time this partner reciprocates 50% of the time), and served as a non-social control.
Figure 1.
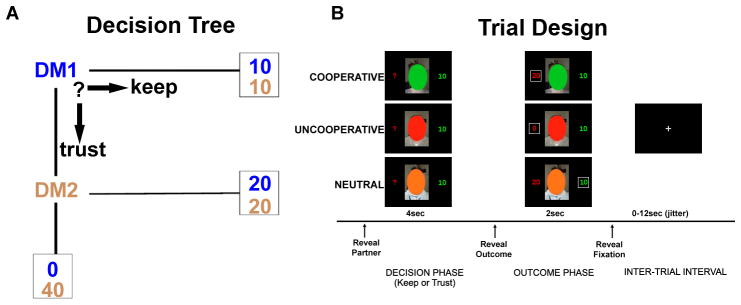
A) Decision tree; and B) Exemplar trial design showing 4 potential outcomes TRUST-Reciprocate ;TRUST-Defect; KEEP-{Reciprocate}; KEEP-{Defect}. Payoff shown within box represents subjects’ actual payoff, while the other payoff represents what would have been received if alternative action were chosen (hypothetical payoff).
At the start of each trial (Fig. 1B), participants viewed one of three different obscured face photographs representing a DM2 type or they viewed an image of a computer. The color of the oval designated the type of DM2 and participants were not aware of the mapping between color of oval and type of DM2 at the start of the experiment – they had to learn the mapping during the task. The DM2/computer image appeared for 4 s during which the participants were instructed to make their choice (KEEP or TRUST) by button-press. In real-time and based on the subject’s own decision/choice, feedback was provided immediately in the form of a DM2/computer image reappearing for 2 s along with information about the participant’s choice, as well as the DM2’s actual (in instances of DM1 TRUST) or hypothetical (in instances of DM1 KEEP) response.
There were a total of 80 trials equally representing the 3 types of DM2s and the computer (i.e., 20 trials of each). Trials were semi-randomly presented with a 0–12 seconds jittered intertrial trial interval, and distributed evenly across 4 runs. After the experimental session was complete, participants were paid according to the actual outcomes accumulated over 80 trials of the task. In addition, subjects completed a post-scan subjective rating questionnaire of “trustworthiness”, one rating for each type of DM2 (“How much do you trust this person?”) on a Likert scale of 1–10, anchored by the following descriptors (1, not at all trustworthy; 10, extremely trustworthy).
Image Acquisition and Processing
Scanning was performed with BOLD (Blood Oxygenation-Level Dependent)-sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric; Milwaukee, WI, LX 8.3, Neuro-optimized gradients); see Supplement for details. Preprocessing steps were implemented using Statistical Parametric Mapping 5 software (SPM5; Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). Preprocessing followed conventional procedures: 1) slice time correction; 2) spatial realignment; 3) normalization to the Montreal Neurologic Institute (MNI) template; 4) spatial smoothing with a 8mm kernel; 5) high-pass temporal filtering (128s). After preprocessing, statistical analyses were performed at the individual and group level using the general linear model (GLM). Regressors represented each partner type (COOPERATIVE, UNCOOPERATIVE, NEUTRAL, computer) and the 4 types of outcomes: 1) TRUST decisions in which one’s partner actually Reciprocates (‘TRUST-Reciprocate’) or 2) Defects (‘TRUST-Defect’), and 3) KEEP decisions in which hypothetically one’s partner would have reciprocated (‘KEEP-{Reciprocate}’) or 4) would have defected (‘KEEP-{Defect}’); here, curly parentheses represent the DM2’s hypothetical choice had the participant chosen to TRUST. Regressors of interest (condition effects) were generated from the onset of the outcome being revealed and were convolved with the HRF. In the second-level analysis, subjects were treated as a random effect and images were thresholded using a voxelwise threshold of p < 0.001 uncorrected with a minimum cluster size of 237 voxels. This threshold was chosen using AlphaSim [27] to correspond to a false positive rate of p < 0.05, corrected for multiple comparisons across the whole brain.
Prior evidence suggest that the vSTR is most sensitive to the relative difference between positive and negative outcomes [17] and between ‘High-Fairness’ and ‘Low-Fairness’ outcomes [18]. Therefore, we were most interested in the differential activation to actual outcomes (real monetary units won or lost) that reflected instances when the partner reciprocated compared to those when the partner defected as represented by the contrast Reciprocate>Defect following TRUST decisions. We were less interest in hypothetical outcomes, because {Reciprocate} and {Defect} outcomes following KEEP decisions do not represent real gains or real losses, since the participant received 10 MUs regardless of partner responses. As such, we would not have expected vSTR activation between these two fictive outcomes. Thus, first, in order to measure the brain response to reciprocity, we searched the entire brain for activations to positive vs. negative partner feedback (Reciprocate>Defect) following participant decision to TRUST. Second, given our prior finding of modulation of the response to reciprocity by partner type [24], we performed whole-brain ANOVAs in the TRUST-Reciprocate > TRUST-Defect contrast with partner type as the factor for HC and GSAD groups to identify regions in which response to partner reciprocity differed according to partner reputation.
Given our a priori hypotheses about the vSTR specifically, we extracted parameter estimates (β weights, a.u.) for the TRUST-Reciprocate > TRUST-Defect contrast for each individual subject in HC and GSAD groups from an anatomical region of interest (ROI) encompassing the entire ventral and lateral region of the striatum derived the anatomical atlas from Tzourio-Mazoyer and colleagues [28]. The resulting β weights represent activation averaged across the entire anatomical vSTR ROI, which were then analyzed with t-tests. Second, in order to examine how vSTR activation to reciprocity varied as a function of partner type, we used this same anatomical ROI and extracted parameter estimates for each individual subject from the contrast of positive and negative feedback (TRUST-Reciprocate vs. TRUST-Defect) for each partner type, which were then analyzed with ANOVAs and follow-up t-tests. Additionally, to examine functional-clinical relevance of vSTR function within the GSAD group, we computed Pearson product-moment correlations between these β weights and social anxiety symptom severity as measured by the LSAS.
Results
Investment behavior in relation to partner type
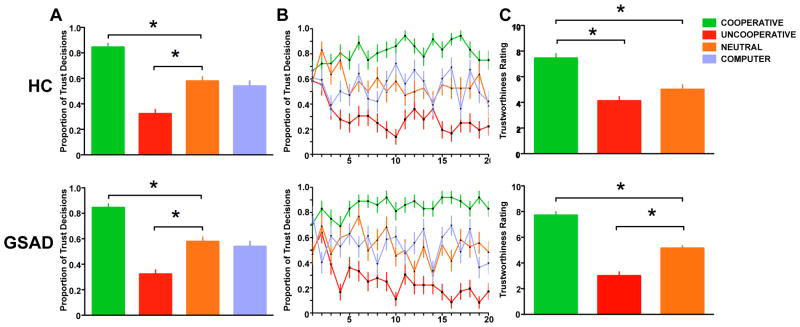
Participants in both groups accurately associated each DM2 type with the corresponding likelihood for reciprocity and adjusted their TRUST vs. KEEP choice accordingly, as indicated by participants’ differential investment behavior according to partner type (Main Effect of Partner: F(2,140) = 116.397, p < 0.001), which did not differ between HC and GSAD participants F(1,70) = 0.371, p = 0.54 (Fig. 2A). Learning occurred rapidly (Fig. 2B), with differential investing based on partner type observed on average by the fifth trial, and stabilizing thereafter. The subjective rating of “trustworthiness” for each DM2 partner type was consistent with investment behavior, showing a significant main effect of DM2 partner type (HC: F[2,52] = 18.1, p < 0.001; GSAD: F[2,68] = 64.2, p < 0.001); subsequent t-tests revealed that in both HC and GSAD subjects perceived COOPERATIVE (> NEUTRAL > UNCOOPERATIVE) partners as most ‘trustworthy’ based on subjective ratings collected after scanning (all t-tests: p < 0.05; Fig. 2C).
Figure 2.
Behavioral results for healthy participants (Top Panel) and individuals with GSAD (Lower Panel). A) Participants in both groups chose to Trust COOPERATIVE more often than UNCOOPERATIVE, NEUTRAL, and COMPUTER partners (COOPERATIVE > NEUTRAL = COMPUTER > UNCOOPERATIVE, *p < 0.05); B) Trial-to-trial trust behavior (proportion ‘Trust’ decisions collapsed across subjects) for each partner type over 20 trials during the fMRI experiment; C) Participants in both groups perceived COOPERATIVE partners to be more ‘trustworthy’ than UNCOOPERATIVE and NEUTRAL partners based on subjective ratings collected after fMRI scan (*p < 0.05).
Brain response to reciprocity following TRUST and KEEP decisions
In whole-brain, voxel-wise neuroimaging analysis of the TRUST-Reciprocate > TRUST-Defect contrast, we observed robust activations in bilateral vSTR, a region known to signal reward and pleasure [29–31], in both HC and GSAD groups (Fig. 3; Table 1). In addition to vSTR, additional activations were observed in healthy participants in inferior occipital gyrus, medial frontal gyrus/orbitofrontal cortex, precentral gyrus, and cerebellum; and in GSAD participants in inferior occipital gyrus, medial frontal gyrus/orbitofrontal cortex, middle and superior frontal gyrus, precuneus, and cerebellum (Table 1). In the reverse contrast of TRUST-Defect > TRUST-Reciprocate, no significant activations were observed in either HC or GSAD groups. In the between group contrast of HC versus GSAD groups in the TRUST-Reciprocate > TRUST-Defect contrast, no differences between groups were observed.
Figure 3.
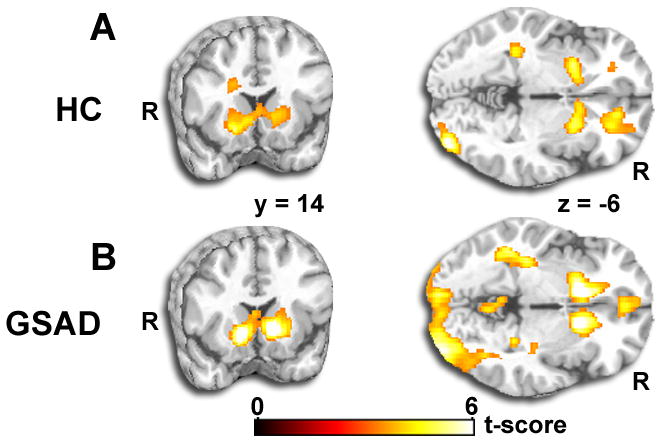
Whole-brain results for healthy participants (Top Panel) and individuals with GSAD (Lower Panel). Both groups demonstrated discrete and robust activation to positive reciprocity (TRUST-Reciprocate > TRUST-Defect contrast) in bilateral ventral striatum. Results displayed on a canonical T1 brain template (all activations are displayed at whole-brain voxel-wise p < 0.05, corrected for multiple comparisons).
Table 1. Results for regions of interest analyses.
Results for anatomical regions of interest in left and right striatum.
| Healthy Controls (n=36) | GSAD (n=36) | |||||||
|---|---|---|---|---|---|---|---|---|
| Left Striatum | Right Striatum | Left Striatum | Right Striatum | |||||
| Outcome | β | β | β | β | ||||
| T-R | 0.18 (.06) | 0.09 (.06) | 0.12 (.05) | 0.08 (.06) | ||||
| T-D | −0.07 (.07) | −0.13 (.07) | −0.12 (.08) | −.15 (.09) | ||||
| Outcome Comparisons | T score | p value | T score | p value | T score | p value | T score | p value |
| T-R > T-D | 3.55 | <0.001 | 3.31 | <0.01 | 3.54 | <0.001 | 3.13 | <0.01 |
| T-R > T-D by Partner Type | β | β | β | β | ||||
| C | 0.38 (.09) | 0.34 (.08) | 0.24 (.10) | 0.30 (.09) | ||||
| U | −0.08 (.15) | −0.08 (.14) | 0.30 (.18) | 0.27 (.18) | ||||
| N | −0.02 (.14) | −0.05 (.12) | 0.15 (.09) | 0.18 (.09) | ||||
| Com | 0.15 (.15) | 0.18 (.14) | 0.22 (.14) | 0.16 (.18) | ||||
| Partner Type Comparisons | T score | p value | T score | p value | T score | p value | T score | p value |
| C > U | 3.12 | <0.01 | 2.82 | <0.05 | −0.17 | n.s. | .28 | n.s. |
| C > N | 3.01 | <0.01 | 2.72 | <0.05 | 0.90 | n.s. | .73 | n.s. |
| U > N | −0.67 | n.s. | −0.80 | n.s. | 1.96 | 0.05 | .91 | n.s. |
β = average activation in arbitrary units within the anatomic ROI; T-R = TRUST-Reciprocate; T-D = TRUST-Defect; C = Cooperative; U = Uncooperative; N = Neutral; Com = Computer.
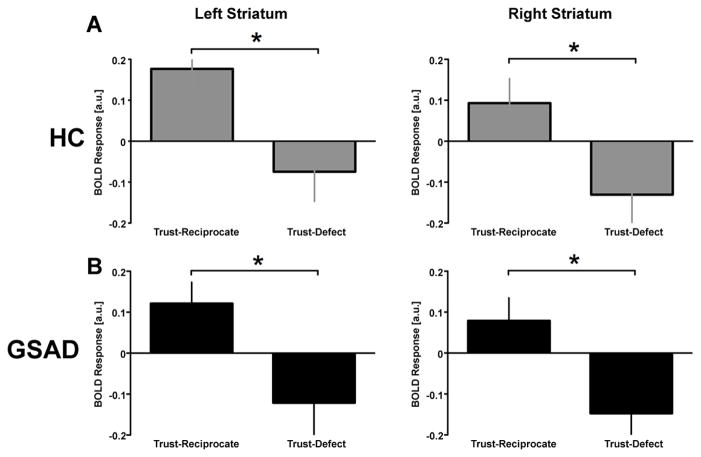
To clarify and complement the whole-brain analyses, we also extracted β weights and analyzed the nature and direction of activation for the TRUST-Reciprocate and TRUST-Defect conditions separately from anatomically-derived vSTR ROIs. Between-outcomes t-tests of these parameter estimates revealed significantly greater vSTR activation following TRUST trials in which the partner reciprocates compared to trials in which the partner defects in both HC and GSAD groups (Fig. 4 and Table S2), but that these activations did not differ between groups. In sum, these results show a robust vSTR response to positive feedback (Reciprocate > Defect) similarly in both healthy individuals and individuals with GSAD.
Figure 4.
Ventral striatum region of interest analysis for healthy participants (Top Panel) and individuals with GSAD (Lower Panel). In both groups, left and right ventral striatum exhibit a positive response (‘activation’) to reciprocity following trust decisions (TRUST-Reciprocate) and a negative response (‘deactivation’) to defection following trust decisions (TRUST-Defect) (TRUST-Reciprocate > TRUST-Defect, *p < 0.05).
Brain responses to reciprocity by partner type
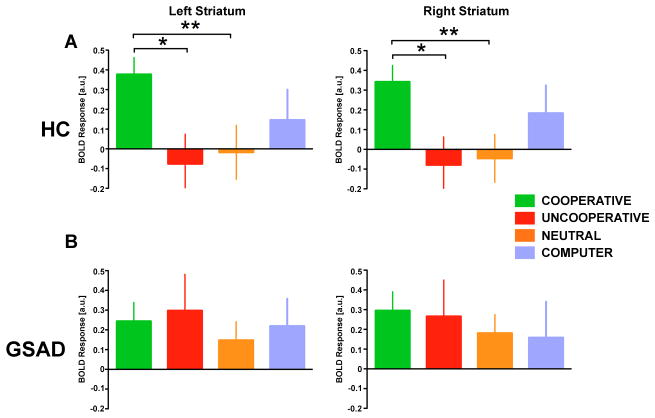
Given our previous finding that partner reputation modulates vSTR activation in the TRUST-Reciprocate > TRUST-Defect contrast [24], we next examined how these vSTR responses are modulated by partner reputations for cooperation associated with different DM2 partner types. Specifically, we extracted parameter estimates of activation for each of the four DM2 partner types from the anatomical vSTR ROIs for the TRUST-Reciprocate > TRUST-Defect contrast. In healthy participants, a one-way ANOVA showed a significant main effect of partner type on striatal responses (F[5,115] = 5.48, p < 0.001). Follow-up t-tests revealed significantly greater vSTR activation to COOPERATIVE than to UNCOOPERATIVE (p < 0.05) and NEUTRAL (p < 0.05) partners bilaterally (Figure 5; Table S2), as previously reported in [24]. In GSAD, a one-way ANOVA revealed the main effect of partner type was not significant (F[5,115] = 5.48, p = 0.274). Follow-up t-tests revealed a significant difference between responses to UNCOOPERATIVE versus NEUTRAL partners in left striatum only (p = 0.05); otherwise there were no significant differences between any partner types in either striatum (Figure 5; Table S2). We next tested the Group × Partner Type interaction in striatal responses. This revealed an interaction with trend-level significance (F [5,255] = 3.08, p = 0.12) suggesting that striatal responses to human partners differed across the two groups. Follow-up tests for Group × Partner Type interactions examined differences between the groups in striatal responses to pairs of partners. These tests found trend-level evidence that HC versus GSAD differed in terms of responses to COOPERATIVE versus UNCOOPERATIVE partners and COOPERATIVE versus NEUTRAL partners (COOPERATIVE vs. UNCOOPERATIVE: p = 0.14; COOPERATIVE vs. NEUTRAL: p = 0.11). More specifically, healthy participants were more responsive to COOPERATIVE partners than UNCOOPERATIVE and NEUTRAL partners, while GSAD did not exhibit this difference.
Figure 5.
Ventral striatum region of interest analysis for healthy participants (Top Panel) and individuals with GSAD (Lower Panel) stratified by partner type. In healthy participants, activation in both left and right ventral striatum in response to partner reciprocation compared to partner defection following TRUST decisions is selective for COOPERATIVE partners (COOPERATIVE > UNCOOPERATIVE and COOPERATIVE > INDIFFERENT, *p < 0.05, ** p < 0.01. In individuals with GSAD, activation in both left and right ventral striatum was not modulated by partner type.
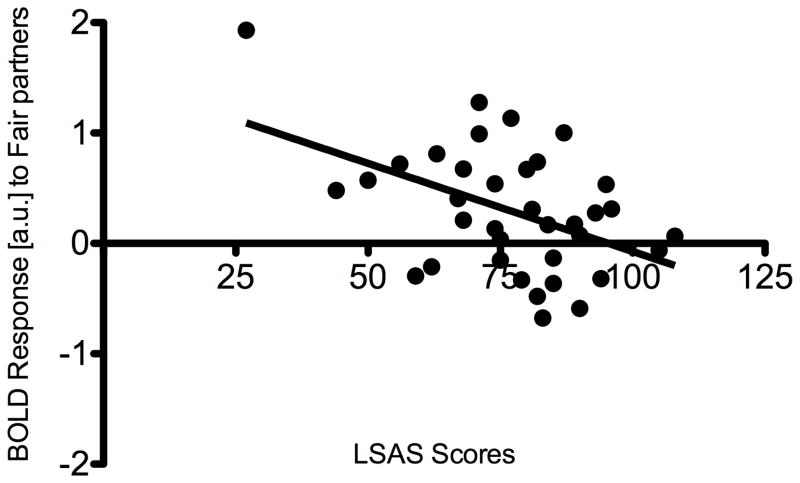
Finally, given our finding that reputation modulated responses to reciprocation in the HC group but not in the GSAD group, we examined if vSTR function in the GSAD group would relate to clinical state. Within the GSAD group, we correlated brain responses to reciprocation (>defection) for each partner type with scores on the LSAS, a measure of social anxiety severity. We found that among individuals with GSAD, greater symptom severity was associated with diminished vSTR responses to COOPERATIVE partners (right: r = −0.47, p = 0.004; left: r = −.268, p = 0.12; Figure 6).
Figure 6.
In participants with GSAD, higher LSAS scores are associated with reduced responses in right striatum to cooperative partners, r = −0.47.
Discussion
Using event-related fMRI and a novel neuroeconomic task in healthy and GSAD groups, we investigated differences in the neural correlates of reciprocity during iterative economic exchanges with fictive partners who develop different reputations for repaying (or not repaying) the investment entrusted to them. We previously demonstrated that in healthy individuals, vSTR activates to reciprocation from partners who have consistently returned the investment (‘cooperative’ partners), and is absent for partners who lack a reputation for cooperation [24], suggesting that this might be an important mechanism for initiating and maintaining cooperative social relationships. Here, we show that in individuals with GSAD, this modulation of vSTR reward signals by partner reputation is absent. These findings extend previous studies that have investigated GSAD using primarily static, non-interactive stimuli. In addition, they show that neuroeconomics could be an important, informative new paradigm for elucidating novel behavioral and brain mechanisms in GSAD during more ecologically valid dynamic, ongoing social interactions.
Human beings are prosocial animals who face multiple challenges in navigating highly complex social landscapes [32]. One challenge consists in identifying cooperative partners with whom to form long-term social relationships, while avoiding uncooperative individuals who show a proclivity to defect [33], and the brain’s striatal reward center has increasingly been implicated in these functions [13; 15]. We have previously shown that people’s preference for fair and equitable outcomes [18; 21], and their ability to track the reputations of partners based on prior actions [16; 17; 22; 23] interact within the striatal reward system [24]. In particular, vSTR responses to outcomes involving reciprocation are enhanced with partners with a prior reputation for reciprocation compared to partners who lack such a reputation, and this finding has been confirmed by other groups [25; 26]. These consistent results suggest that vSTR modulation by partner reputation may be an important mechanism to support the formation and maintenance of cooperative relationships by providing an enhanced automatic, internal reward signal in the context of positive outcomes that occur specifically with cooperative partners. This can be advantageous evolutionary, since these partners have demonstrated a track record of cooperation, and will presumably produce positive reciprocal outcomes in the future. In other words, this could represent neural mechanism for differentiating the value of those who have shown to be cooperative and trustworthy from those who have gained the opposite reputation.
In the present study, we found evidence that in GSAD, the modulation of vSTR reward signals by partner reputation is absent – striatal reward signals to reciprocation did not differ according to whether one’s partner had a prior reputation for cooperation or lacked such a reputation (Figure 5). That is, our results suggest that individuals with GSAD show a similar vSTR reward response to a positive reciprocity outcome (i.e., TRUST-Reciprocate) from all types of partners, regardless of the partner’s prior proclivity, or lack thereof, for reciprocity. Interestingly, the extent of symptom severity in GSAD participants negatively predicted responses to cooperative partners in both right (Figure 6) and left striatum. In particular, those with more social anxiety symptoms mounted the least vSTR response, suggesting that the processing of social reward from trustworthy partners may be related to severity of the illness. Of note, we did not find that partner defection activated fear-related neurocircuitry in amygdala and insula in either HC or GSAD groups (Table S2), consistent with our prior findings [24] and the findings of other groups [16; 17; 34], highlighting a potentially distinctive role for vSTR in dynamic social decision-making. These results thus add to a mounting body of neuroimaging evidence that suggests that in addition to well-known abnormalities in threat processing circuits located in amygdala and insula [12], GSAD is also associated with abnormalities in striatal-mediated reward function [35–38].
Previous neuroimaging studies have shown that socially shy adults [36] and behaviorally inhibited adolescents [37; 38] exhibit enhanced sensitivity to rewards in the striatum. These findings have been interpreted by these authors in terms of hypersensitivity to receipt of contingent rewards, i.e., rewards whose receipt is not guaranteed but rather depends on one’s choosing the correct actions [37], or to a more non-specific state of generally increased arousal to incentive cues [38]. Applying a similar interpretation to the present study, it may be that in healthy individuals, rewards from partners without a prior history of reciprocation lose their salience. In individuals with GSAD, however, perhaps due to a generalized state of hyperarousal, these rewards continue to remain inappropriately salient. This is consistent with behavioral findings that GSAD individuals inappropriately remain tense and hyperaroused during social interactions more broadly [39; 40]
An alternative hypothesis interprets the results of the present study in terms of deficits in being able to differentiate others based on their prior actions and/or in being able to use this discriminative information to generate implicit biasing signals during social decision-making. The ability to rapidly and accurately detect and discriminate social partners in terms of prosocial reputation [33] is a useful skill that might facilitate better choices of social partners, and thus an enhanced sense of confidence and trust in partners that have been selected. We had previously shown that GSAD subjects fail to engage the dorsal medial prefrontal cortex when ‘mentalizing’, the socio-cognitive process of making inferences about the motives and intentions of social partners to guide appropriate behaviors, during the Trust Game [41]. Here, the absence of modulation of reward signals by partner reputation in GSAD may further reflect reduced ability to discriminate reputations of social partners in GSAD or to generate learning signals useful for biasing subsequent decisions to cooperate or defect. Resulting impaired social decision-making might in turn contribute to more uncertainty, anxiety, and distrust during social situations. It is noteworthy, however, that GSAD participants did not differ from healthy individuals in terms of investment behavior according to partner type indicating they decoded reputations appropriately and used reputations to guide behavioral choices (Figure 2). It may be though that the striatal signals detected in this study represent largely automatic and implicit biases, consistent with prior studies that implicate striatal regions in implicit learning processing. Thus it may be that these striatal signals did not affect investment decisions in this relatively simple investment task, but these signals may nonetheless play a more important role in formation and maintenance of relationships in more complex, ecologically realistic situations, where reliance on automatic implicit cues is thought to be far more prevalent and necessary [42]. Indeed, one of the only other neuroimaging studies to examine striatal dysfunction in GSAD uncovered deficits in striatal implicit learning processing [35]. Further studies are needed to investigate whether the pattern of striatal responses to partner reciprocity in GSAD uncovered in the current study would affect investment behavior in social contexts where implicit decision biases are known to be more important.
In summary, this study extends previous work by using a dynamic, interactive neuroeconomic task to investigate ventral striatal reward processing during social-decision-making. We found that in healthy individuals, the brain’s reward center selectively responds to monetary rewards received from partners with a reputation for cooperative play (and not to partners who lack a cooperative reputation), while in GSAD, the modulation of striatal reward signals by reputation was entirely absent. This study provides new insights into the brain basis of social dysfunction in GSAD, and demonstrates that dynamic, interactive task paradigms derived from economics can help illuminate mechanisms contributing to pathology in psychiatric disorders in which social dysfunction and/or socio-cognitive decision making deficits are cardinal features.
Supplementary Material
Acknowledgments
This research was supported by NIH grant MH076198 and the Brain Research Foundation Seed Grant to K.L.P. C.S.’s effort was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000433, and NIH grant AA020297.
References
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371(9618):1115–25. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 3.Ruscio AM, Brown TA, Chiu WT, et al. Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med. 2008;38(1):15–28. doi: 10.1017/S0033291707001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneier FR. Clinical practice. Social anxiety disorder. N Engl J Med. 2006;355(10):1029–36. doi: 10.1056/NEJMcp060145. [DOI] [PubMed] [Google Scholar]
- 5.Alden LE, Taylor CT. Interpersonal processes in social phobia. Clin Psychol Rev. 2004;24(7):857–82. doi: 10.1016/j.cpr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behav Res Ther. 2003;41(11):1325–35. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 7.Foa EB, Gilboa-Schechtman E, Amir N, Freshman M. Memory bias in generalized social phobia: remembering negative emotional expressions. Journal of Anxiety Disorders. 2000;14(5):501–19. doi: 10.1016/s0887-6185(00)00036-0. [DOI] [PubMed] [Google Scholar]
- 8.Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. J Abnorm Psychol. 2004;113(1):160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch CR, Clark DM. Information-processing bias in social phobia. Clinical Psychology Review. 2004;24(7):799–825. doi: 10.1016/j.cpr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Stopa L, Clark DM. Social phobia and interpretation of social events. Behav Res Ther. 2000;38(3):273–83. doi: 10.1016/s0005-7967(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 11.Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry. 2002;51(1):92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 12.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18(2):159–65. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36(2):265–84. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 15.Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci. 2007;11(10):419–27. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 16.King-Casas B, Tomlin D, Anen C, et al. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308(5718):78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 17.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8(11):1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 18.Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychol Sci. 2008;19(4):339–47. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 19.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 20.O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–66. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Tabibnia G, Lieberman MD. Fairness and cooperation are rewarding: evidence from social cognitive neuroscience. Ann N Y Acad Sci. 2007;1118:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- 22.Krueger F, McCabe K, Moll J, et al. Neural correlates of trust. Proc Natl Acad Sci U S A. 2007;104(50):20084–9. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer T, Kiebel SJ, Winston JS, et al. Brain responses to the acquired moral status of faces. Neuron. 2004;41(4):653–62. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- 24.Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proc Natl Acad Sci U S A. 2010;107(29):13099–104. doi: 10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. J Neurosci. 2012;32(26):9045–52. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fareri DS, Chang LJ, Delgado MR. Effects of direct social experience on trust decisions and neural reward circuitry. Front Neurosci. 2012;6:148. doi: 10.3389/fnins.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward B. Simultaneous inference for fMRI data. 2000 From http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- 28.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 30.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 31.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Richerson PR, Boyd R, Henrich J. Cultural evolution of human cooperation. In: Hammerstein P, editor. Genetic and Cultural Evolution of Cooperation. Cambridge, MA: MIT Press; 2003. pp. 373–404. [Google Scholar]
- 33.Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437:1291–1270. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- 34.King-Casas B, Sharp C, Lomax-Bream L, et al. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321(5890):806–10. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sareen J, Campbell DW, Leslie WD, et al. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biol Psychiatry. 2007;61(3):396–404. doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Hardin MG, Perez-Edgar K, Guyer AE, et al. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Pers Individ Dif. 2006;40(4):699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20(8):1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beidel DC, Turner SM, Dancu CV. Physiological, cognitive and behavioral aspects of social anxiety. Behaviour Research and Therapy. 1985;23(2):109–117. doi: 10.1016/0005-7967(85)90019-1. [DOI] [PubMed] [Google Scholar]
- 40.Borkovec TD, Stone NM, O’Brien GT, Kaloupek DG. Evaluation of a clinically relevant target behavior for analog outcome research. Behavior Therapy. 1974;5(4):503–513. [Google Scholar]
- 41.Sripada CS, Angstadt M, Banks S, et al. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport. 2009;20(11):984–9. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deutsch R, Strack F. Duality Models in Social Psychology: From Dual Processes to Interacting Systems. Psychological Inquiry. 2006;17(3):166–172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.