Abstract
Background
Generalized social anxiety disorder (gSAD) is characterized by exaggerated amygdala reactivity to social signals of threat, but if and how the amygdala interacts with functionally and anatomically connected prefrontal cortex (PFC) remains largely unknown. Recent evidence points to aberrant amygdala connectivity to medial PFC in gSAD at rest, but it is difficult to attribute functional relevance without the context of threat processing. Here, we address this by studying amygdala-frontal cortex connectivity during viewing of fearful faces and at rest in gSAD patients.
Methods
Twenty patients with gSAD and 17 matched healthy controls (HCs) participated in functional magnetic resonance imaging of an emotional face matching task, and a resting state task. Functional connectivity and psychophysiological interaction analysis were used to assess amygdala connectivity.
Results
Compared to HCs, gSAD patients exhibited less connectivity between amygdala and the rostral anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) while viewing fearful faces. gSAD patients also showed less connectivity between amygdala and rostral ACC at rest in the absence of fearful faces. DLPFC connectivity was negatively correlated with LSASFear.
Conclusions
Task and rest paradigms provide unique and important information about discrete and overlapping functional networks. In particular, amygdala coupling to DLPFC may be a phasic abnormality, emerging only in the presence of a social predictor of threat, whereas amygdala coupling to the rostral ACC may reflect both phasic and tonic abnormalities. These findings prompt further studies to better delineate intrinsic and externally-evoked brain connectivity in anxiety and depression in relation to amygdala dysfunction.
Keywords: Amygdala, ACC, DLPFC, Connectivity, Social Phobia, PPI
INTRODUCTION
Generalized social anxiety disorder (gSAD) is a common psychiatric disorder characterized by excessive and pervasive fear of the potential for scrutiny by others during social situations [1; 2]. It emerges early, foretells significant psychiatric co-morbidity, and leads to substantial occupational and social impairment [2]. The disorder is thought to manifest from an underlying attention and memory bias for social signals of threat (e.g., angry/fearful faces) [3] and an exaggerated fear response during anticipation [4] and perception of social evaluative threat [5].
Prominent in most brain models of gSAD is the amygdala [6], which plays a key role in fear responses [7], social information processing [8], and the perception of salient emotional stimuli [9]. Evidence of a link between amygdala hyperactivity and symptom severity suggests it is a core deficit in the pathophysiology of gSAD [10]. Although less commonly implicated [11], abnormalities within the prefrontal cortex (PFC) are hypothesized to underlie gSAD patients’ failure to effectively modulate amygdala reactivity, consequently leading to enhanced anxiety, social threat perception, and/or reticence to engage in social interactions [12]. Consistent with this hypothesis, gSAD patients show atypical reactivity to the detection of social signals of threat in areas such as the anterior cingulate cortex (ACC) [10; 13], and dorsal and ventral medial PFC (mPFC) [14]. Given their strong reciprocal structural connections with the amygdala [15], discrete areas of the PFC are well-positioned to play a significant role in the regulation of fear and emotional responding. In particular, it has been suggested that engagement of dorsal PFC may reflect voluntary, conscious appraisal-regulation functions whereas ventral PFC may reflect more implicit (less effortful, less conscious) processes of emotion regulation [16; 17].
These cognition-emotion interactions suggest a dynamic interplay between the prefrontal cortex and the amygdala. Recently, advances in neuroimaging analysis techniques have permitted the examination of amygdala-frontal networks at rest (‘resting-state functional connectivity’, rsFC). In the absence of an overt task to isolate cognitive and/or emotional function, assessment of rsFC allows the study of disturbance of networks at baseline, which may allow for interpretation of a broader (e.g., more pervasive) deficit leading to diverse levels of psychopathology. For example, resting state studies have shown that gSAD patients exhibit aberrant patterns of amygdala-ventral mPFC, amygdala-cingulate and frontal-frontal cortex connectivity compared to healthy controls’ networks (e.g. [18–20]).
However, beyond recognizing that evidence of disturbed connectivity from amygdala to mPFC/ACC isolated to the resting state may relate to an intrinsic brain disorganization or disconnection, it is difficult to interpret its functional relevance in relation to underlying abnormal perception of social signals of threat and/or anxiety responses in gSAD. While studies of gSAD using cognitive-emotional probes have implicated PFC dysfunction, few have directly examined how PFC dysfunction relates to amygdala reactivity [21–23]. Thus, much relevant to gSAD, a disorder characterized by exaggerated amygdala reactivity to social evaluative threat, it is important to analyze both task and resting state data together to better delineate if disturbances in amygdala-frontal networks are present only on task (i.e., in the presence of overt social threat processing), present only at rest (i.e., independent of task and in the absence of overt social threat processing), or present both on task and at rest. Convergent and divergent findings across both data sets would lead to a more refined and comprehensive brain model of gSAD. In particular, such approaches may disambiguate if amygdala-frontal disorganization represents a tonic (spontaneous, task-independent) versus phasic (evoked, task-dependent) phenomena.
Here, we examined the amygdala-frontal FC of thirty-seven participants (20 gSAD patients and 17 healthy controls) from whom we collected BOLD signal fluctuations using functional magnetic resonance imaging (fMRI) during both a task involving social signals of threat (e.g. fearful faces) and the resting state. Based on prior data and theory, we hypothesized that gSAD patients would exhibit aberrant (less) connectivity between amygdala and dorsal portions of the PFC (dorsal/rostral ACC, dorsal mPFC, dorsolateral PFC [DLPFC]), areas implicated in conscious appraisal-regulatory functions, observable particularly during the viewing of fearful faces, whereas aberrant (increased) amygdala connectivity to more ventral portions of PFC (ventral/subgenual ACC, ventral medial PFC, orbitofrontal cortex [OFC]), implicated in experiential functions, would be observed only at rest.
MATERIALS AND METHODS
Participants
Twenty right-handed patients with gSAD and 17 matched healthy controls (HC) participated in the study. Psychiatric diagnostic classification of participants was based on administration of the Structured Clinical Interview for DSM-IV (SCID-IV). gSAD participants were additionally verified to have gSAD based on the clinician-administered Liebowitz Social Anxiety Scale (LSAS). HCs were required to be free of prior or current psychiatric disorder. Trained clinicians, including a board-certified psychiatrist (K.L.P.) conducted all clinical assessments. All participants provided written informed consent and the study was approved by the institutional review boards at both the University of Michigan and the University of Chicago.
Table 1 shows the participants’ demographic and clinical characteristics. Two gSAD patients were on a selective serotonin reuptake inhibitor with no change in medication or dosage for at least 8 weeks prior to the scan. All other participants were free of psychoactive medications at the time of scanning and urine toxicology screens were negative for all participants on scan day. No patients had a major depressive episode or substance abuse within a six-month period prior to scanning. Some gSAD patients had psychiatric co-morbidity (n=3 with current specific phobia, one of whom also had current generalized anxiety disorder; n=1 had current obsessive-compulsive disorder; and n=1 with current panic disorder); of note, for all patients, gSAD was the primary, most clinically salient diagnosis at the time of study entry.
Table 1.
Participant demographic and clinical characteristics
| Group Mean (SD) | ||||
|---|---|---|---|---|
| gSAD | Control | t value | p value | |
| Age | 25.95 (5.39) | 25.71 (7.15) | 0.12 | 0.907 |
| Gender | 9 M / 11 F | 7 M / 10 F | 0.06a | 0.815 |
| LSAS | 79.35 (15.41) | 7.94 (7.05) | 18.56 | < 0.001 |
| BDI | 14.35 (8.33) | 0.82 (1.07) | 7.19 | < 0.001 |
| STAI-T | 46.45 (11.88) | 26.00 (3.04) | 7.42 | < 0.001 |
χ2 analysis.
LSAS, Liebowitz Social Anxiety Scale; BDI, Beck Depression Inventory; STAI-T, Spielberger State-Trait Anxiety Inventory - Trait
Experimental Tasks
All participants performed both the emotional face matching task (EFMT) and the resting state scan (RS) following conventional procedures previously described by our and other groups in healthy and gSAD subjects [19; 24; 25]. The EFMT, a variant of the task originally described by Hariri et al. [26], is designed to isolate amygdala response to social signals of threat. In brief, this task involved photographs from a validated set of face stimuli [27] presented in a block-design during which participants view a trio of faces and select one of two faces (bottom) that expressed the same emotion (happy, fearful or angry) as the target face (top). The identity of all three faces was always different, and an equal number of male and female faces were used in the task. The face matching task was interspersed with an identical geometric shape matching task. There were a total of 18 blocks in the task, three of each of the three emotions, and a corresponding nine blocks of shape matching. Each block lasted 20 seconds with five presentations of either faces or shapes per block. The order of emotion blocks was counterbalanced across participants, however the task always began with face matching and alternated with shape matching.
The RS scan is designed to probe intrinsic connectivity patterns at rest; subjects were instructed to fixate on a crosshair on a blank gray screen, relax, and let their mind wander without falling asleep for 5 minutes.
Functional MRI Parameters
Images were acquired on two identical 3.0T GE Signa scanners using the standard radiofrequency head coil and associated software (LX 8.3, Neuro-optimized gradients, General Electric). Seven participants (4gSAD, 3HCs) were scanned on one scanner while the remaining participants were scanned on an identical scanner at a different institution. There was no difference between image acquisition parameters or processing steps between scanners. Whole-brain functional MRI scans were acquired using a T2-weighted reverse spiral gradient-recall echo sequence (TR=2000ms, TE=25ms, 64×64 matrix, flip angle of 77°, FOV=240mm, 3.75mm2 inplane voxels) with 30 contiguous 5mm axial slices per volume.
Functional MRI Data Analysis
Data was preprocessed and analyzed using Statistical Parametric Mapping 5 (SPM5; http://www.fil.ion.ucl.ac.uk/spm). The first four volumes from each task run and the first eight volumes from each resting run were discarded to allow for T1 equilibration effects. Images were realigned to correct for motion, corrected for errors in slice timing, spatially transformed to standard MNI space using the echo-planar imaging template provided with SPM5, resampled every 2mm using sinc interpolation and smoothed with an 8mm full-width-half-maximum Gaussian kernel to decrease spatial noise prior to statistical analysis. Translational movement in millimeters (x,y,z) and rotational motion in degrees (pitch, roll, yaw) was calculated based on the SPM5 parameters for motion correction. None of the participants had movement greater than 2mm translation or 2° rotation.
In order to extract signal from a region of interest (ROI) that is robust, yet unbiased to any one particular emotional expression (fearful face, happy face) or to any one particular group (gSAD, HC), we used a ‘functional’ localizer ROI approach that also takes into account anatomical constraints [28]. We defined an amygdala seed derived from the functional activation of the “all faces” vs. “all shapes” task contrast of the second level GLM with a threshold of p<0.001. Results from the conjunction of task activation from both groups were confined within the AAL defined anatomical amygdala [29]. The resulting right amygdala ROI ‘seed’ was 696mm3 in volume and the left amygdala ROI ‘seed’ was 1008mm3 in volume.
To examine amygdala-frontal connectivity to fearful faces, we employed conventional steps using Psycho-Physiological Interaction (PPI) analysis [30]. PPI analysis allows us to isolate the context-dependent coupling (‘PPI-FC’) pattern during the task of perceiving social signals of threat and comparing 2 face stimuli directly allows us to de-confound non-salient features that are common to both types of stimuli. For the PPI analysis, the interaction term of the amygdala seed timeseries with the task parameters (fearful vs. happy) was the variable of interest. The timeseries of the seed itself as well as the task covariate and the six movement parameters were all included as effects of no interest.
rsFC was implemented using conventional methods previously described [31]. Importantly, we used the same right and left amygdala seeds for both of the two (PPI-FC and rsFC) connectivity analyses. In brief, the resting data was first bandpass filtered between frequencies of 0.008 to 0.1 to limit the analysis to resting state frequencies of interest [32]. The seed ROI timeseries was used as a covariate of interest in a first level model for each participant to provide whole-brain correlation values. The six motion parameters and the global signal were covariates of no interest.
All second-level analyses for between-group results consisted of random effects models. For between-group comparisons (two samples t-test) we set a whole-brain voxel-wise significance threshold for peak voxel significance at p<0.05, cluster-level corrected for multiple comparisons across the entire brain (cluster volume > 28048mm3, for the PPI-FC analysis; cluster volume > 25512mm3 for the rsFC analysis); these cluster-level thresholds were calculated using Monte-Carlo simulations (AFNI AlphaSim, http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). After multiple comparison correction, the AAL toolbox for SPM was used to further identify the surviving clusters by computing the volume of each cluster overlapping the anatomical regions defined by the AAL. Within significant clusters, we searched for those PFC regions a priori hypothesized to exert group differences in amygdala connectivity (ACC, mPFC, DLPFC, OFC). Results are given as peak Z-score of the cluster and volume of each a priori region within those significant clusters. To clarify group differences, beta (β) weights (an estimate of connectivity strength in arbitrary units) were extracted from 5mm-radius spheres surrounding the peak voxel within the a priori region.
Post-hoc Correlation Analysis
We conducted post-hoc Pearson’s correlations between symptom severity measures and the connectivity beta weights from peak ROIs described above. Beta weights were correlated with LSAS total score as well as the LSASFear and LSASAvoidance subscales. Correlations were Bonferroni corrected for multiple comparisons leading to a significance threshold of p<0.008.
RESULTS
Behavioral Results
Both groups performed the EFMT well, achieving means of >90% accuracy and reaction time <2000ms on average per trial. Overall, there was no main effect of group or group x emotion interaction on accuracy (gSAD patients M = 94.99, SE = 0.75; HCs M = 94.88, SE = 0.64) or reaction time (gSAD patients M = 1471.84, SE = 46.50; HCs M = 1400.63, SE = 54.17; all ps > 0.05).
Functional MRI Results
Task Activations
The EFMT is designed to detect between group differences in amygdala activity [10; 14], and thus we restrict our report of activations to between group results. gSAD patients showed greater right amygdala activity than healthy controls in the fearful versus happy faces contrast (p < 0.05, cluster extent > 200 voxels). There were no differences in amygdala activity between gSAD patients and controls when viewing angry faces versus happy faces even at this low threshold, thus we restricted our subsequent connectivity analysis to fearful versus happy faces. There were no group differences in activation seen in the anterior cingulate; however, gSAD patients did show greater activity than healthy controls in DLPFC regions (p<0.05, cluster extent > 1000; Table S1).
Connectivity
We report and discuss here group differences in amygdala connectivity to discrete frontal brain areas for which we had an a priori hypothesis, namely within the ACC, mPFC, OFC and DLPFC (Table 2). We display the findings as: 1) between-groups whole-brain voxel-wise t-maps; and 2) mean (S.E.M.) extracted β-weights for each group to clarify the within-group connectivity driving the group differences (Figure 1). Of note, using scanner as a covariate of no interest to remove any potential confound in the data made no change to the results described.
Table 2.
Between Group Differences in Amygdala Connectivity to Fearful Faces and at Rest in a priori Areas
| Scan | Region | Cluster Z | MNI Coordinate
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Volume (mm3) | |||||
| Right Amygdala: | |||||
| Threat Connectivity | 3.46 | ||||
| ACC | 649 | 14 | 38 | 22 | |
| DLPFC | 860 | 44 | 46 | 4 | |
| Left Amygdala: | |||||
| Threat Connectivity | 4.44 | ||||
| ACC | 82 | 8 | 20 | 22 | |
| DLPFC | 47 | −24 | −6 | 48 | |
| Right Amygdala: | |||||
| Rest Connectivity | 3.39 | ||||
| ACC | 772 | −2 | 8 | 24 | |
| Left Amygdala**: | |||||
| Rest Connectivity | 3.78 | ||||
| ACC | 650 | −4 | 34 | −6 | |
Cluster-level significance set at p< 0.05, whole brain corrected for multiple comparisons. Within each significant cluster, Z-score and associated a priori region are noted along with Montreal Neurological Institute (MNI) atlas coordinates of peaks. Of note, the volume is specifically that contained within the anatomically defined a priori region, not the total cluster volume. ACC, Anterior Cingulate Cortex; DLPFC, Dorsolateral Prefrontal Cortex.
Although this ACC region did not fall within a cluster that survived correction for multiple comparisons, given its similarity in extent and location to the pattern observed in relation to the right amygdala, we include it here for completeness.
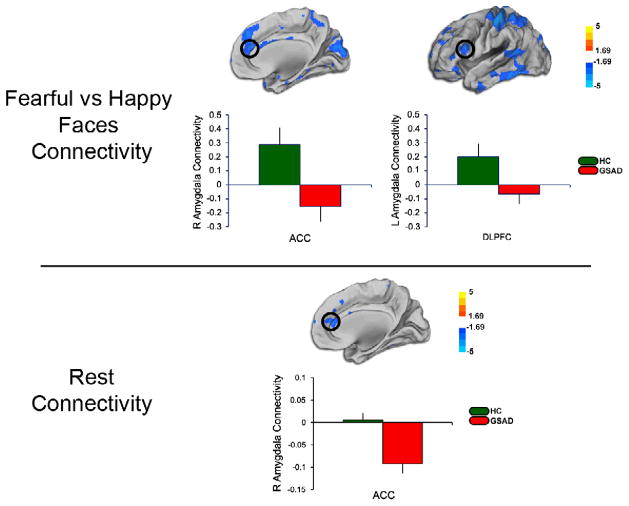
Figure 1.
Top panel shows results from whole-brain voxel-wise statistical t-map of less amygdala connectivity to the rostral ACC and DLPFC during viewing of fearful faces minus happy faces; bar graphs show extracted measure of connectivity within each group. Bottom panel shows results from whole-brain voxel-wise statistical t-map of less amygdala connectivity to the rostral ACC during rest; bar graphs show extracted measure of connectivity within each group. Color scale reflects t-score. GSAD, Generalized Social Anxiety Disorder; HC, Healthy Control
Fearful vs. Happy Face Connectivity (PPI-FC)
From both the left and right amygdala, within the medial frontal wall, gSAD patients exhibited less connectivity to rostral ACC during viewing of fearful minus happy faces than HCs (Figure 1, Table 2). From both the left and right amygdala, at the lateral prefrontal wall, gSAD patients exhibited less connectivity to bilateral DLPFC during viewing of fearful minus happy faces than HCs (Figure 1, Table 2). In contrast, we did not observe any a priori areas in the medial or lateral PFC that showed greater connectivity to either left or right amygdala in the gSAD group compared to HCs. We did not observe group differences in amygdala connectivity to mPFC or OFC during perception of fearful (vs. happy) faces.
Rest Connectivity (rsFC)
From both the left and right amygdala, within the medial frontal wall, gSAD patients exhibited less connectivity with rostral ACC than HCs (Figure 1, Table 2); of note, only the ACC cluster connected to right amygdala exhibited a group difference significant for cluster-level correction for multiple comparisons. We did not observe group differences in amygdala connectivity with DLPFC, mPFC, or OFC at rest. In order to eliminate concern about the global signal regression introducing false negatives into our results [33], a rsFC analysis without the use of global signal regression was conducted and yielded similar results (Table S4).
Overlap between Rs-FC and PPI-FC
We explored the overlap between the ACC connectivity findings for both the PPI-FC and the rsFC using a conjunction analysis. The connectivity findings showed a shared volume of 3456mm3, indicating that the ACC cluster showing hypo-connectivity to amygdala during threat perception overlaps approximately 40% with the ACC cluster showing hypo-connectivity to amygdala at rest.
Post-hoc Correlation Analysis
We found that right amygdala connectivity with right DLPFC in the PPI-FC analysis was significantly correlated with the LSASFear subscale symptom measure in gSAD patients (r=−0.595, p=0.006). No other connectivity measures showed significant correlations with LSAS total score, LSASFear subscale or LSASAvoidance subscale.
Additional Analysis
In order to account for potentially confounding effects, connectivity analyses (rsFC and PPI) were re-conducted excluding the gSAD patients on medications, the gSAD patients with comorbid psychiatric disorders, and the seven participants scanned at a different institution, all of which yielded similar results.
DISCUSSION
To the best of our knowledge, this is the first study to directly examine and report amygdala functional connectivity engaged by an emotional/cognitive task and at rest in the same group of gSAD patients in comparison to healthy controls. Using two complementary analyses of functional connectivity to examine aberrant patterns shared across tasks, we confirmed our hypothesis that gSAD patients would exhibit less connectivity between amygdala and dorsal-rostral areas of the PFC, specifically the ACC and DLPFC during viewing of fearful minus happy faces. Interestingly, we also observe less amygdala to rostral ACC connectivity in gSAD patients at rest. Thus aberrant amygdala-ACC connectivity may exist even at rest in the absence of a social signal of threat, whereas aberrant amygdala-DLPFC connectivity may be revealed only during social threat perception. Contrary to our hypotheses, we did not observe group differences in amygdala connectivity to ventral portions of the PFC at rest.
Group Differences in Activity versus Connectivity
Group differences in activity to the EFMT were found in the amygdala and DLPFC for gSAD patients greater than healthy controls on the fearful versus happy faces contrast. Interestingly, while gSAD patients showed greater activity in DLPFC, indicating what initially might have been interpreted as greater explicit emotion regulation in the presence of a social signal of threat [34], connectivity between amygdala and DLPFC was decreased in gSAD patients as compared to healthy controls which has previously been interpreted as less emotion regulation [12; 22]. These seemingly conflicting results indicate the importance of both activation and connectivity analyses as activity within a single region may be high, but communication between different brain regions may be interrupted in a given disorder.
Additionally, no activation differences were found in ACC during the task, while significant differences were found between groups for the connectivity analyses. ACC is known to play an important role in regulating activity within the amygdala [16; 21], and thus although activity in this region may not differ, differences in connectivity between amygdala and ACC indicate dysregulation of the limbic system which may underlie the symptamatology of gSAD. Both the findings in DLPFC and ACC underscore the need for both activation and connectivity analyses in future studies, as one provides information about individual regions of the brain, and the other provides information about how networks may be communicating with each other. Differences in one analysis alone may not provide a complete picture of changes in brain function within a given disorder.
Amygdala-Frontal Connectivity Abnormalities Across Fearful Faces and At Rest
In gSAD patients, we observed less amygdala connectivity to the frontal cortex that was localized in the same general rostral ACC area during both fearful minus happy faces and at rest. More specifically, gSAD patients demonstrated an inverse pattern of connectivity to HCs. This finding suggests that aberrant amygdala-ACC connectivity in gSAD may exist at baseline even in the absence of any detection of social threat in the environment, and that the pattern reflects both phasic and tonic abnormality in this circuit. Rostral ACC is thought to provide feedback to amygdala by modulating the extent to which it responds to social evaluative threat and other salient emotional signals [21]. Failure to recruit rostral ACC in the regulation of a provoked or unprovoked anxiety state may lead to persistent amygdala activity often seen in gSAD across a variety of social-emotional threat-related tasks [11; 14] due to decreased attentional control and an increased attentional bias for threat[35]. The decreased connectivity seen across rest and task potentially illustrates the increased vigilance for threatening information seen in gSAD patients in the absence of a threat cue (i.e. at rest)[35], and their decreased emotion regulation and increased response to threat when a threatening cue is present (i.e. during viewing of fearful faces)[5].
Recently, Hahn et al. [19] also demonstrated reduced amygdala connectivity to rostral ACC/ventral mPFC in social phobic patients at rest. It should be noted, however, that using different connectivity analytic approaches (‘effective connectivity’ as determined by Granger causality analysis, GCA, and independent component analysis, ICA), Liao et al. [18; 25] observed increased amygdala to ventral mPFC or ACC connectivity in subjects with social anxiety disorder during the resting state. Of note, the patient sample in those studies had a lower mean LSAS score (almost 30 points lower than the current cohort), and many of those subjects may not have the more severe, pervasive, generalized subtype of social phobia seen in our participant population. Additionally, as noted by the authors [25], findings from the ICA or GCA approaches may be interpreted to reflect global functional connectivity or directional influence across all voxels in the brain, whereas findings from a seed-based connectivity method such as the one employed here may be interpreted to reflect local undirected functional connectivity. Thus, the differences in aims and analytic approach to resting state data may account for some of the differences reported between studies.
Nevertheless, our finding here indicates that the deficit in amygdala-ACC connectivity may be relevant to a brain model of gSAD involving abnormalities that both persist at the baseline state, and during social signals of threat. The current finding of group differences is also consistent with evidence in generalized anxiety disorder in which decreased connectivity between rostral ACC and amygdala is associated with a failure of implicit emotion regulation [36], and with major depressive disorder, where patients exhibit decreased amygdala to ACC connectivity in response to emotion processing [37]. These results indicate a possible commonality across anxiety disorders, and perhaps across both anxiety and depressive disorders. Similar aberrant connectivity patterns at rest and during task indicate that this differential connectivity between fear generating (amygdala) and fear regulating (rACC) regions may underlie the pervasive attention, interpretive, and memory bias for threatening information and persistent negative self-reflection seen in patients with gSAD [35].
Amygdala-Frontal Connectivity Abnormalities Specific to Fearful Faces
In gSAD patients, we observed less amygdala to DLPFC connectivity during viewing of fearful minus happy faces but not at rest. Again, gSAD patients show an inverse pattern of connectivity to HCs. The current finding of reduced connectivity of amygdala with DLPFC in gSAD patients is similar to the findings of Danti et al. [22] and Goldin et al. [12]. Lateral PFC, known for its broad role in cognition, is involved in explicit emotion regulation [34]. Therefore, aberrant connectivity between amygdala and this region could result in the maladaptive response of a regulation region when (and only when) social signals of threat are present. This pattern of decreased connectivity with the DLPFC only during task but not during rest supports the notion of impaired effortful engagement of this area in gSAD, perhaps leading to their increased responsiveness to threat[5]. Interestingly, decreased amygdala and DLPFC coupling was observed only when gSAD patients were viewing fearful faces, the increased cognitive resources needed to regulate emotions provoked by these threatening cues seem to be impaired in gSAD patients, potentially leading to the increased amygdala activity seen in multiple studies [11; 14]. This data further supports the role of parallel, complementary analyses of both PPI-FC and rsFC and considering brain function across more than just one task.
Amygdala-Frontal Connectivity Abnormalities and gSAD Symptomatology
A behavioral measure of anxiety quantified by the LSASFear was negatively correlated with right amygdala connectivity with DLPFC during viewing of fearful faces. These results indicate that as the severity of the disorder increases, connectivity between amygdala and DLPFC decreases, presumably leading to increased amygdala activity and decreasing emotion regulation. This decrease in emotion regulation may underlie gSAD patients’ increased response to threatening social information[5]. Recently Liao et al. [18] have shown a correlation between amygdala connectivity and the LSASAvoidance subscale during the resting state while our findings are specific to during task. Correlations between symptom measures and connectivity seen in multiple studies indicates that amygdala connectivity during task and rest needs to be further studied as a potential tool for understanding the anxiety symptoms seen in gSAD.
Limitations and Future Directions
The current findings should be considered in the context of several noteworthy limitations. Two of our gSAD patients were on medication at the time of study participation and additionally five patients had comorbid anxiety disorders. While all analyses for this study were run excluding these patients and the results remained the same, the sample size may have introduced the risk of false negatives. Additionally, the current analysis and discussion were confined to detection of specific clusters exhibiting group differences that were localized to areas of the brain that are known to be functionally and anatomically connected to the amygdala (albeit inclusive of large regions of medial and lateral PFC), and hence findings can only be interpreted in the context within this limited set of a priori regions. The use of a block fMRI design and a PPI-based connectivity analysis prohibited a more comprehensive examination of effective connectivity, including exploiting the trial-to-trial temporal dynamics of amygdala activity to ascertain the directional influence of amygdala on specific PFC regions and vice versa. Additionally, while the current task has been validated as a tool for detecting and understanding differential amygdala activity between groups it focuses on face emotion perception, does not embody all aspects of emotion processing, and does not require direct emotional engagement when viewing the faces, and therefore interpretations of emotion processing in general or emotion regulation due to the fearful faces are limited. Future studies are needed to replicate and extend the current results and to address these important, unanswered questions.
Conclusion
The major contribution of the approach described is the use of functional connectivity analysis in gSAD and healthy controls for both a task involving perception of social threat and the resting state to begin to elucidate the extent to which amygdala hyper-reactivity in gSAD is due to intrinsic prefrontal disorganization versus exaggerated activation in response to external emotionally salient signals. Our results indicate that task and rest paradigms each provide unique and important information about brain function in gSAD patients. While abnormalities emerged in lateral PFC regions only in the presence of fearful faces, those in medial PFC regions were evident across the fearful faces and rest states, suggesting that amygdala coupling to DLPFC may be a phasic abnormality whereas its coupling to the medial PFC may reflect both phasic and tonic abnormalities. Patients with the generalized form of social anxiety disorder studied here show a constant attention bias for threatening information [35]; we believe the dysregulated amygdala connectivity with ACC seen during both threat perception and task may be related to this altered attention state both in the presence and absence of threat-related cues. Dysregulated amygdala connectivity with DLPFC is seen only during the direct perception of threatening information and may be related to a decrease in emotion regulation and the increase in response to threatening social information seen in gSAD[5], especially as this particular finding was correlated with the LSASFear subscale, indicating a potential relationship with increased fear intensity symptoms. These findings prompt further studies to better delineate the functional relevance of amygdala-frontal networks and similar approaches that account for task-dependent and task-independent patterns of intrinsic and externally-evoked brain connectivity in health and disease.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institutes Health, National Institute of Mental Health Patient-Oriented Career Development Award K23MH076198 (to KLP). KEP was supported by National Institute of Mental Health Training Grant 5T32MH014279. HK was supported by UL1RR024986 from the National Center for Research Resources (NCRR).
Footnotes
Financial Disclosure: All authors report no competing interests.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Stein M, Stein D. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 3.Clark D, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51(1):92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47(2):85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- 5.McTeague L, Lang P, Laplante M-C, et al. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65(5):374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas-Ferrari MC, Hallak JEC, Trzesniak C, et al. Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(4):565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41(10):1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 9.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the neurosciences. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 10.Phan K, Fitzgerald D, Nathan P, Tancer M. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldin PR, Manber T, Hakimi S, et al. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66(2):170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amir N, Klumpp H, Elias J, et al. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Stein MB, Goldin PR, Sareen J, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 15.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyurak A, Gross J, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition and emotion. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao W, Qiu C, Gentili C, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One. 2010;5(12):e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Chen H, Qiu C, et al. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magnetic Resonance Imaging. 2011;29(5):701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Goldin PR, Manber-Ball T, Werner K, et al. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biological Psychiatry. 2009;66(12):1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danti S, Ricciardi E, Gentili C, et al. Is Social Phobia a “Mis-Communication” Disorder? Brain Functional Connectivity during Face Perception Differs between Patients with Social Phobia and Healthy Control Subjects. Front Syst Neurosci. 2010;4:152. doi: 10.3389/fnsys.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyer AE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of general psychiatry. 2008;65(11):1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labuschagne I, Phan KL, Wood A, et al. Oxytocin Attenuates Amygdala Reactivity to Fear in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao W, Chen H, Feng Y, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52(4):1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, Tessitore A, Mattay VS, et al. The Amygdala Response to Emotional Stimuli: A Comparison of Faces and Scenes. NeuroImage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 27.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–43. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 28.Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 30.Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 31.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 32.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR, American journal of neuroradiology. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 33.Fox M, Zhang D, Snyder A, Raichle M. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochsner K, Gross J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Bögels S, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical psychology review. 2004;24(7):827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carballedo A, Scheuerecker J, Meisenzahl E, et al. Functional connectivity of emotional processing in depression. Journal of Affective Disorders. 2011;134(1–3):272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.