Abstract
Background
It is known that expanded epicardial fat is associated with atrial fibrillation (AF). However, infiltrated intraatrial fat has not been previously quantified in individuals at risk as determined by the ARIC AF risk score.
Methods
Patients in sinus rhythm (N=90, age 57±10y; 55 men [63.2%]), in 3 groups at risk of AF as determined by the ARIC AF risk score [low (≤11 points; n=15), moderate (12–18 points; n=40), high (≥19 points; n=23) risk of AF], and paroxysmal AF (n=12) underwent cardiac magnetic resonance study. Intraatrial and epicardial fat was analyzed with a Dark-blood DIR-prepared Fat-Water-separated sequence in the horizontal longitudinal axis. OsiriX DICOM viewer (Geneva, Switzerland) was used to quantify the intraatrial fat area. Width of the cephalad portion of the interatrial septum was measured at the level of the fossa ovalis.
Results
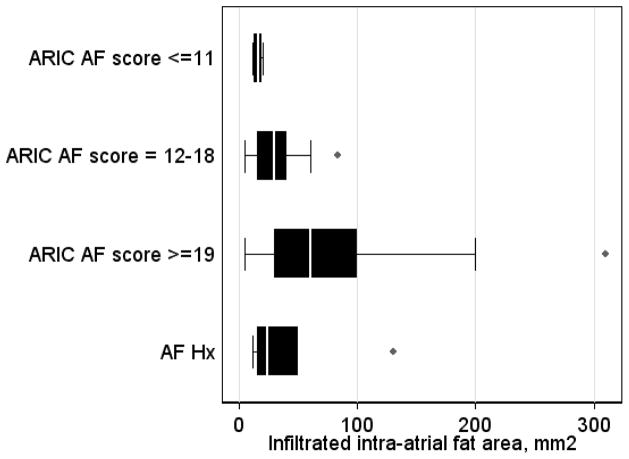
Intraatrial fat monotonically increased with growing AF risk in study groups (low AF risk 16±4 vs. moderate AF risk 32±18 vs. high AF risk 81±83 mm2; ANOVA P=0.012). Log-transformed intraatrial fat predicted ARIC AF risk score in multivariate ordered probit regression after adjustment for sex, race, left and right atrial area indices, and body mass index (β-coefficient 0.50 [95%CI 0.03–0.97]; P=0.037), whereas epicardial fat did not. Interatrial septum width showed similar association (3.0±1.4 vs. 5.0±1.8 vs. 7.1±2.7 mm; ANOVA P<0.001; adjusted β-coefficient 2.80 [95%CI 1.19–4.41]; P=0.001).
Conclusions
Infiltrated intraatrial fat characterizes evolving substrate in individuals at risk of AF.
Keywords: atrial fibrillation, cardiac magnetic resonance, intraatrial infiltrated fat, risk score
Introduction
Atrial fibrillation (AF) is the most common arrhythmia1. The estimated prevalence of AF increases with age, from 1% in general population to 8% in those older than 80y1. AF is a major risk factor for serious cardiovascular events, such a stroke, heart failure (HF) and premature death2. Arterial remodeling with fibrosis and dilatation, hypertension and diastolic heart failure predispose to AF3–5. The aging population and the rising prevalence of chronic heart disease lead to a 66% increase in hospital admissions for AF. In spite of advances in radiofrequency ablation for the treatment of AF, the high recurrence of AF (up to 40%) during the first year after pulmonary vein isolation (PVI) procedure6 supports the notion that primary prevention of AF, if successful, would be the most efficient strategy to decrease AF burden for patients and healthcare providers. NIH identified primary prevention of AF as especially important priority for future research7. However, mechanisms of AF are not completely understood, and data characterizing AF substrate early in the continuum of structural heart disease are lacking.
Epicardial adipose tissue (EAT) has recently emerged as a factor associated with paroxysmal8 and persistent AF9, and with AF recurrence after PVI10. In the Framingham Heart Study (FHS) higher EAT volumes were associated with higher odds of prevalent AF11. Mechanisms of EAT effect on the myocardium were studied in experiments, which confirmed paracrine properties of EAT and release of pro-inflammatory and pro-fibrotic substances (adipo-fibrokines)12. At the same time, autopsy studies have shown that EAT can infiltrate myocardium13. However, until recently there was no imaging modality available for in-vivo assessment of infiltrated intraatrial fat. In 2009 Kellman et al14 developed novel fat-water-separated MRI imaging approach, which enabled assessment of infiltrated intraatrial fat. While EAT was shown associated with prevalent AF, the myocardial substrate that predisposes to AF in patients at AF risk but without diagnosed AF remains largely unknown. We hypothesized that infiltrated intraatrial fat is associated with AF risk as determined by the AF risk score.
Methods
Study Population
We analyzed the data of an ongoing prospective observational cohort study Personalized Risk Identification and Management for Arrhythmias and Heart Failure by ECG and MRI (PRIMERI). Study was approved by the Johns Hopkins Institutional Review Board, and all study participants signed informed consent upon entering the study. Study included patients of the Johns Hopkins Hospital, who have had signs of structural heart disease on 12-lead ECG (wide spatial QRS-T angle ≥105° and/or the Selvester QRS score15 ≥5). Exclusion criteria were age above 70 years, left ventricular ejection fraction (LV EF) ≤ 35%, or high risk of non-cardiac death due to concomitant non-cardiac diseases. The Johns Hopkins Hospital database was screened and study candidates were invited to participate.
At enrollment detailed medical history was collected, 12-lead ECG was recorded at rest by the Marquette MAC 5000 ECG system with 12SL TM algorithm (GE Medical Systems, Milwaukee, WI), and cardiac magnetic resonance (CMR) study was performed. In addition, Holter ECG (GE Medical Systems, Milwaukee, WI) was recorded during 30–45 min at rest and during 6-min walk.
Risk of AF assessment: the ARIC AF risk score calculation
AF risk was assessed by the Atherosclerosis Risk In Communities (ARIC) AF risk score, which was calculated as described by Chamberlain et al16. ARIC AF risk score was selected since the PRIMERI study population was bi-racial, and included participants of similar to ARIC study age range. Nonetheless, due to minor age differences between PRIMERI and ARIC study populations, we applied slightly modified ARIC AF risk score. ARIC AF risk score was developed for individuals of 45–65 years of age, and was assigned a maximum of 8 points for individuals 60–64 years. We assigned 10 points for study participants ≥ 65 years of age.
Study participants were categorized into 4 groups. Patients with a documented history of paroxysmal AF comprised the AF group. Study participants without AF history were separated into 3 groups, based on ARIC AF risk score. Low AF risk group included subjects scoring ≤ 11 points, who had a 10-year predicted probability of developing AF16 of <5%. As shown previously, patients scoring ≥ 19 points had >24% predicted probability of developing AF within 10 years16, they comprised high AF risk group. Individuals scoring in between these two groups, (12–18 points) comprised moderate AF risk group.
Atrial CMR Analysis
Patients underwent CMR with a 1.5-T scanner (Avanto, Siemens Healthcare, Erlangen, Germany) on the same day as the ECG recordings. All CMR functional analyses were performed on dedicated workstations with ARGUS (Siemens Healthcare, PA) by 3 experienced observers who were blinded to the results of the ECG analysis (NM, PR, CL). LVEF, LVEDV, LVESV and LV mass were calculated using Simpson’s rule and its values normalized by body surface area (BSA) calculated with the Mosteller equation. The LA and the right atria (RA) areas were measured at their maximum size in the end-systolic phase of the ventricles using the 4-chamber view, and normalized by BSA17.
The presence of interatrial fat was assessed with a Dark-blood DIR-prepared Fat-Water-separated sequence14;18 in the horizontal longitudinal axis (4 chamber view). The typical sequence parameters were: band width = 977 Hz/pixel; TE = 1.53, 3.76, 5.99, and 8.22 ms; TR ≈ 11 ms; flip angle = 12°; image matrix = 256 × 180; ECG triggered every RR interval with views per segment = 15; and breath-hold duration = 13 heartbeats, including one initial heartbeat discarded for transition to steady state. The multiecho GRE sequence incorporated a double inversion recovery dark blood preparation. The multiecho GRE sequence incorporated a dark blood preparation. OsiriX DICOM viewer (Geneva, Switzerland) was used to quantify the area of the structure of interest (Figure 1). The width of the cephalad portion of the interatrial septum was measured in this study at the level of the fossa ovalis, as described by Shirani et al19. Epicardial fat area was measured in the same horizontal longitudinal axis.
Figure 1.
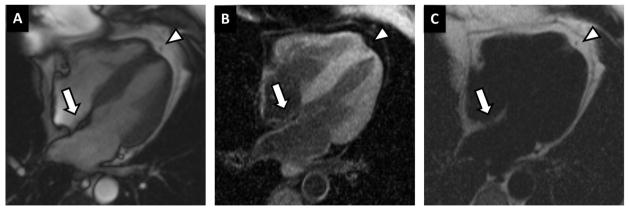
Cardiac magnetic resonance (CMR) fat image by Fat-Water DB IR GRE sequence. Representative example of fat infiltration in the inter-atrial septum using a dedicated CMR sequence that allows fat and water imaging in separate. (A) Regular functional steady-state free precession (SSFP) sequence; (B) “Water phase” – fat signal is suppressed; (C) “Fat phase” – water signal is suppressed. A fat deposit can be observed in the inter-atrial septum (arrows), with an increased signal in the “fat phase” and decreased in the “water phase”. The robust fat-water separation obtained with this sequence can be observed in the pericardial fat (arrow heads) imaging.
Statistical Analysis
STATA 12 (StataCorp LP, College Station, TX) was used for statistical analysis. Normally distributed variables were compared across study groups using a one-way ANOVA. Categorical variables were compared by Pearson’s chi-square test. Pairwise correlations were studied between normally-distributed variables. Intraatrial fat area was log-transformed to normalize distribution. Ordered probit regression analysis was performed to study association between ARIC AF risk score (outcome, range 1–27) and log-transformed intraatrial fat, or interatrial septum width (predictors), adjusted by LA area index, RA area index, epicardial fat, body mass index, sex and race. Multiple linear regression analysis was employed to determine if intraatrial fat predicts interatrial septum width after adjustment for age, sex, race, and BMI.
Results
Study population
We analyzed data of 90 patients (mean age 57±10 years). About half of the study population was comprised of men [n=55 (61%)], and whites [n=53 (59%)]. Screening QRS score indicated the presence of myocardial scar20 (5.4±2.1), and wide spatial QRS-T angle (114±38) point to high likelihood of the presence of an underlying structural heart disease21;22.
Clinical characteristics of patients at low, moderate, and high risk of AF
History of paroxysmal AF was known in 12 patients (Table 1). Successful PVI procedure was performed in 5 patients (41%), in whom amount of interatrial fat seemed to be smaller, as compared to AF patients without ablation procedures history (35±18 mm2 after PVI vs. 45±57 mm2 without PVI), although our study was not powered or designed to compare interatrial fat before and after PVI.
Table 1.
Clinical characteristics of study participants
| ARIC AF risk score ≤11 (n=15) | ARIC AF risk score 12–18 (n=40) | ARIC AF risk score ≥19 (n=23) | ANOVA P value | Paroxysmal AF (n=12) | |
|---|---|---|---|---|---|
| Age (SD),y | 54.0(8.4) | 59.2(8.4) | 62.8(9.0) | 0.013 | 57.6(12.4) |
| Females,n(%) | 12(80.0) | 17(42.5) | 1(4.3) | <0.0001 | 2(16.7) |
| Blacks,n(%) | 11(73.3) | 17(42.5) | 3(13.0) | 0.001 | 4(33.3) |
| Hypertension,n(%) | 9(60.0) | 26(65.0) | 21(91.3) | 0.023 | 7(58.3) |
| Hypertension Hx(SD),y | 1.5(3.2) | 3.2(5.5) | 5.8(7.3) | 0.068 | 5.6(8.3) |
| Diabetes mellitus,n(%) | 4(26.7) | 11(27.5) | 4(18.2) | 0.416 | 2(16.8) |
| Diabetes mellitus history (SD), y | 1.8(5.0) | 2.8(5.6) | 0.9(2.0) | 0.292 | 0.7(1.6) |
| Body mass index(SD),kg/m2 | 28.9(7.5) | 31.7(7.6) | 32.3(6.8) | 0.343 | 30.6(8.0) |
| History of MI,n(%) | 1(6.7) | 5(12.5) | 8(34.8) | 0.035 | 4(33.3) |
| History of PCI/CABG,n(%) | 1(6.7) | 9(22.5) | 13(56.5) | 0.021 | 4(33.3) |
| NYHA class ≥ II,n(%) | 3(20.0) | 9(22.5) | 9(39.1) | 0.293 | 5(41.7) |
| Systolic blood pressure (SD),mmHg | 137.2(18.9) | 147.3(19.7) | 159.6(27.0) | 0.017 | 136.4(14.5) |
| Diastolic blood pressure (SD),mmHg | 88.3(16.4) | 86.6(9.3) | 91.6(12.5) | 0.357 | 78.1(12.8) |
| eGFR,ml/min | 64.7(1.3) | 64.3(3.3) | 65(0) | 0.535 | 65(0) |
| Glucose (SD),mg/dL | 109.0(50.7) | 119.1(39.2) | 141.3(70.0) | 0.170 | 124.5(79.8) |
| Beta-blockers,n(%) | 3(20.0) | 10(25.0) | 16(69.6) | 0.001 | 6(50.0) |
| ACE-I or ARBs,n(%) | 8(53.3) | 14(35.0) | 11(47.8) | 0.385 | 4(33.3) |
| TZD,n(%) | 1(6.7) | 11(27.5) | 5(21.7) | 0.249 | 1(8.3) |
| Statins,n(%) | 5(33.3) | 21(52.5) | 18(78.3) | 0.019 | 8(66.7) |
| Current or former smoker,n(%) | 7(46.7) | 21(52.5) | 11(57.8) | 0.817 | 7(58.3) |
MI=myocardial infarction; NYHA=New York Heart Association heart failure class; ACE-I= angiotensin-converting-enzyme inhibitor.
Relatively low predicted 10-years AF risk (<5%) as determined by the presence of ≤ 11 points, was found in the lowest quartile of the risk score distribution in our study population (n=15). The highest quartile of the risk score distribution (≥ 19 points) in our study characterized 23 participants with predicted probability of AF > 24%. Two middle quartiles of ARIC AF risk score (12–18 points, 40 participants) predicted wide range of AF risk probability, 5–24%.
As expected, the clinical characteristics of patients in the AF group were remarkably similar to clinical characteristics of participants in the high AF risk group (Table 1). Anticipated monotonically increasing prevalence of AF risk factors (age, male sex, white race, history of myocardial infarction and revascularization procedures, use of antihypertensive medication) was observed in individuals at growing AF risk. P wave duration on 12-lead ECG was significantly longer in patients at high AF risk (Table 2). There were no statistically significant differences in LV volumes, LVEF, LV mass, LA and RA indices, and epicardial fat across study groups (Table 2).
Table 2.
ECG and structural parameters of the heart atria and ventricles.
| ARIC AF risk score ≤11 (n=15) | ARIC AF risk score 12–18 (n=40) | ARIC AF risk score ≥19 (n=23) | ANOVA P value | Paroxysmal AF (n=12) | |
|---|---|---|---|---|---|
| QRS score(SD) | 4.0(2.0) | 4.8(2.2) | 5.7(2.1) | 0.062 | 4.0(2.6) |
| Spatial QRS-T angle(SD),deg | 119.6(33.1) | 106.9(37.9) | 110.5(39.5) | 0.561 | 124.0(25.3) |
| P duration(SD),ms | 106.7(9.8) | 110.0(13.4) | 118.5(10.9) | 0.007 | 115(13.1) |
| PQ interval(SD),ms | 168.0(28.1) | 172.6(29.2) | 184.7(25.5) | 0.150 | 187.0(53.5) |
| Heart rate(SD),bpm | 65.2(11.4) | 65.7(10.5) | 66.6(10.6) | 0.923 | 63.0(9.8) |
| QRS duration(SD),ms | 98.4(22.4) | 97.1(18.0) | 113.1(25.8) | 0.019 | 106.3(20.6) |
| P-axis(SD),deg | 48.3(22.1) | 45.7(21.7) | 50.3(52.7) | 0.876 | 49.1(26.6) |
| QTc duration(SD),ms | 437.3(28.0) | 424.0(23.2) | 432.5(24.3) | 0.156 | 424.2(20.9) |
| LVEF(SD),% | 57.3(9.1) | 63.4(8.8) | 57.7(9.7) | 0.036 | 56.6(8.8) |
| LV mass index(SD),g/m2 | 63.8(13.9) | 66.5(23.0) | 70.1(11.4) | 0.643 | 64.2(10.9) |
| LVESVI (SD),ml/m2 | 32.5(11.9) | 25.1(10.4) | 28.3(11.9) | 0.112 | 34.1(15.4) |
| LVEDVI (SD),ml/m2 | 74.1(12.8) | 64.1(14.3) | 66.1(19.9) | 0.137 | 76.9(24.6) |
| Right atrium area index (SD),cm2/m2 | 9.4(1.6) | 8.7(3.0) | 9.0(2.8) | 0.780 | 9.9(3.3) |
| Left atrium area index(SD),cm2/m2 | 11.9(2.6) | 10.1(2.2) | 10.1(2.3) | 0.064 | 11.2(2.9) |
| Epicardial fat(SD),cm2 | 13.3(4.4) | 14.0(6.7) | 17.5(5.5) | 0.114 | 13.3(3.0) |
| Interatrial fat(SD),mm2 | 16(4) | 32(18) | 81(83) | 0.012 | 40.7(41.9) |
| Log-transformed interatrial fat(SD) | −1.87(0.27) | −1.34(0.67) | −0.69(1.10) | 0.021 | −1.25(0.87) |
| Atrial septum width(SD),mm | 3.0(1.4) | 5.0(1.8) | 7.1(2.7) | <0.0001 | 5.4(2.8) |
LVEF=left ventricular ejection fraction; LVEDVI=left ventricular end diastolic volume index; LVESVI=left ventricular end systolic index.
Infiltrated intraatrial fat and interatrial septum width in patients at low, moderate, and high risk of AF
Amount of intraatrial fat monotonically increased from low AF risk patients, to moderate AF risk patients, and then increased further to high AF risk patients (Figure 2). Adjusted ordered probit regression analysis showed that increase in infiltrated intraatrial fat resulted in increase in the odds of having a higher ARIC AF risk score (Table 3).
Figure 2.
Box-plot of infiltrated intraatrial fat area in groups of patients at AF risk and patients with paroxysmal AF. Median (white vertical line crossing the box) and interquartile range [IQR] (box). Whiskers specify the adjacent values, defined as the most extreme values within 1.5 IQR of the nearer quartile.
Table 3.
Multivariate ordered probit regression models predicting the ARIC atrial fibrillation risk score in individuals without AF history.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| Predictor | β-Coefficient (95% CI) | P | β-Coefficient (95% CI) | P |
| Log-transformed intraatrial fat | 0.50(0.03–0.97) | 0.037 | - | |
| Interatrial septum,mm | - | 1.80(1.19–4.41) | <0.001 | |
| Left atrium area index,cm2/m2 | 0.20(−0.19 to 0.23) | 0.849 | 0.15(0.001–0.30) | 0.049 |
| Right atrium area index,cm2/m2 | 0.11(−0.08 to 0.30) | 0.270 | 0.08(−0.05 to 0.21) | 0.227 |
| Body mass index,kg/m2 | 0.005(−0.07 to 0.74) | 0.897 | 0.04(−0.01 to 0.095) | 0.096 |
| Epicardial fat area, cm2 | −0.018(−0.08 to 0.44) | 0.573 | −0.01(−0.06 to 0.04) | 0.722 |
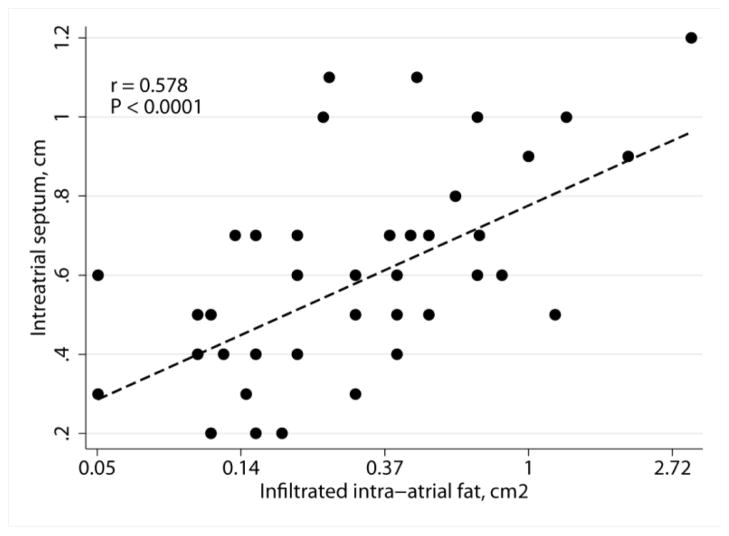
Similarly, strong association between AF risk and width of interatrial septum was found (Table 2). Widening of interatrial septum was associated with increase in the odds of having a higher ARIC AF risk score (Table 3). Moreover, strong correlation between intraatrial fat and interatrial septum width was observed (Figure 3), which was confirmed in multiple linear regression analysis after adjustment for age, race, sex, and BMI [β-coefficient 0.15 (95%CI 0.06–0.24); P=0.002].
Figure 3.
Scatterplot of the interatrial septum width in cm (Y) against intraatrial fat area in cm2 (X) with linear fitted line.
Discussion
Our study revealed important findings. We showed that in individuals without AF history, monotonically increasing amounts of infiltrated intraatrial fat were independently associated with the 10-year predicted probability of developing AF as determined by the ARIC AF risk score16. Thus, infiltrated intraatrial fat characterizes evolving substrate of per-clinical AF early in a continuum of structural heart disease. This observation opens a new avenue for mechanistic pathophysiological studies that are needed in order to explain mechanisms of fat infiltration of atria, and to develop therapies for prevention of fat infiltration.
In addition, we showed that the width of the interatrial septum strongly correlates with infiltrated intraatrial fat and therefore, may serve as its surrogate. While visualization and quantification of intraatrial fat requires special CMR procedure, measurement of interatrial septum width could be easily done on any 4-chamber CMR image. Future investigations of interatrial septum width in large epidemiological studies might improve our understanding of AF substrate development before AF onset.
ARIC AF risk score and early pre-clinical AF substrate
Several clinical AF risk scores exist. Framingham Heart Study (FHS) AF risk score was developed to predict 10-year AF risk in middle-aged to elderly whites5, and was successfully validated23. Another risk score for AF incidence was developed in a prospective biracial community based cohort16, Atherosclerosis Risk In Communities (ARIC). Recently, AF risk factors were identified in pooled analysis of 3 large cohorts (FHS, ARIC and the Cardiovascular Health Study [CHS]) and were externally validated in the Age, Gene and Environment—Reykjavik study (AGES) and the Rotterdam Study (RS)24. We choose ARIC AF risk score over FHS AF risk score because our study population is (1) bi-racial, and (2) relatively young (57±10 years). In addition, the age at which significant cardiac murmur developed (FHS risk factor) was unknown in our study. ARIC AF risk score16 was developed in a population study and is based on risk factors, commonly measured in clinical practice. Very few of our study participants were 65–70 years of age, whom we added two additional risk points for their age, as CHARGE-AF consortium demonstrated monotonically increasing AF risk for every 5 years of age24. Importantly, the use of ARIC AF risk score for the first time revealed an evolving pre-clinical AF substrate, as presented by the infiltrated intraatrial fat.
Infiltrated intraatrial fat: role in AF
Atrial fibrosis and fibrotic atrial cardiomyopathy25 is associated with clinically manifest AF. Recent experimental study12 provided evidence that secreted by EAT adipo-fibrokine Activin A can induce fibrosis of the myocardium. Importantly, experiments showed that fat tissue infiltrates myocardium and in situ elicit paractine effect of EAT secretome on the neighboring myocardium. Results of our study constitute the first evidence that in humans infiltrated intraatrial fat represents early pre-clinical AF substrate in individuals at AF risk. We speculate that the timely prevention of fat infiltration of atria might prevent AF development, and should be considered as a future therapy target.
More than a hundred years ago fatty heart was considered to be a common cause of cardiac death26. Pathologists described intra-myocardial fat and fibrofatty infiltration many years ago27, and the fact that adipocytes can infiltrate myocardium, is well known. However, this fact did not attract attention as until very recently there was no tool available for in-vivo fat infiltration assessment. Kellman et. al. first developed the methodology of in-vivo imaging of the infiltrated fat14;18, which was used in this study. Our study underscores the importance of infiltrated fat imaging and quantification. At the same time we acknowledge that measurement of intraatrial fat requires special CMR techniques and is time consuming. Our finding of strong correlation between intraatrial fat and interatrial septum width on a standard 4-chamber CMR view suggests that the simple measure of interatrial septum width might serve as a marker of the degree of adipose tissue infiltration of the atria. Future studies are needed to test this hypothesis.
Role of interatrial septum in atrial arrhythmogenesis
Conditions, affecting interatrial septum, are known to be associated with a higher rate of supraventricular arrhythmia. Massive adipose tissue infiltration of the interatrial septum28 was shown to be associated with atrial arrhythmias, requiring antiarrhythmic medications29. High prevalence of atrial arrhythmia (25–50%) was noticed in patients with atrial septal aneurysm30. Many abnormalities of the interatrial septum are considered to be of unknown clinical significance. It remains unknown whether specific features of interatrial septum itself, or associated abnormalities are related to the pathogenesis of atrial arrhythmia. Development of the interatrial septum has been intensely studied31. However, the exact mechanism for the genesis of the adipose tissue in the interatrial septum is unknown. Further studies are needed to compare fibrofatty infiltration of interatrial septum and other areas of atrial myocardium, both in AF patients and in subjects at risk of AF.
In addition, other mechanisms other than fat infiltration pathological processes in interatrial septum are plausible. Hypertrophy of the interatrial septum, with subsequent fibrosis, might contribute to interatrial septum thickening, as well. Unlike LV, LA myocardium is thin and does not sustain hypertrophy long-term. Future studies are needed to explore possible interaction between intraatrial fat infiltration and atrial myocytes hypertrophy.
While our study was not designed to compare interatrial fat before and after PVI, we have found that AF patients with PVI history have smaller amounts of interatrial fat, as compared to AF patients without PVI history. We speculate that interatrial septum puncture facilitates local fibrosis, which plays a role of confounding factor in this study.
Limitations
Several limitations of this study should be acknowledged. First, we did not measure the EAT volumetrically, which might explain absence of associations between ARIC AF risk score and epicardial fat in our study. Previously FHS study analysis showed that epicardial fat is associated with prevalent AF11. However, the goal of this study was quantification of intraatrial fat, but not epicardial fat. At the same time, intraatrial fat in this study was not measured volumetrically as well. Meaningful findings of our study suggest that measurement of intraatrial fat area might be sufficient. However, future study with volumetric measurement of intraatrial fat is needed to validate our approach. Secondly, this study population is small, and the statistical power of the study is limited. Moreover, patients with AF history, and patients at AF risk in our study differed largely in their clinical characteristics, and therefore, likely in the substrate of AF development, too. However, unique CMR technique was used in this study, which for the first time allowed quantification of infiltrated intraatrial fat. Third, we used slightly modified ARIC AF risk score in order to determine AF risk in study participants. However, applied in this study AF risk score was similar to recently reported CHARGE AF consortium risk score24, which was developed in the largest to date sample of general population and therefore provided optimized AF risk stratification. Importantly, our study was cross-sectional. Future prospective studies are needed to validate association between interatrial infiltrated fat, and AF development. Lastly, AF in our study was diagnosed based on carefully collected past medical history, review of medical record, recorded 12 lead ECG and 30–45 min Holter ECG. We recognize that silent paroxysmal AF might be missed, as study was not designed for 30-days ECG monitoring or implantation of a loop recorder. Future studies are needed to determine association between silent AF and interatrial fat.
Acknowledgments
Financial support & relationships with industry: Study was partially supported by Cardiac Translational Research Implementation Program (C-TRIP) grant from NIH/National Heart, Lung and Blood Institute #P20HL101397 and the Leducq Foundation.
Footnotes
Clinical Trial Registration Information at www.clinicaltrials.gov: NCT01353131.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DC, Yolton RL, Reinke AR, Kohl P, Lundy-Ekman L. The dizzy patient: a review of etiology differential diagnosis and management. J Am Optom Assoc. 1995;66:545–558. [PubMed] [Google Scholar]
- 4.Rosen MR. Mechanisms of cardiac arrhythmias: focus on atrial fibrillation. J Gend Specif Med. 2001;4:37–47. [PubMed] [Google Scholar]
- 5.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel policy procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart lung and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin S, Yong H, Lim H, Na J, Choi C, Choi JI, Kim S, Kim J, Kim E, Park S, Rha SW, Park C, Seo H, Oh D, Kim YH. Total and Interatrial Epicardial Adipose Tissues Are Independently Associated With Left Atrial Remodeling in Patients With Atrial Fibrillation. J CARDIOVASC ELECTROPHYSIOL. 2011;22:647–655. doi: 10.1111/j.1540-8167.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 9.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial Fat Is Independently Associated With Human Atrial Fibrillation. J Am Coll Cardiol. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Tsao HM, Hu WC, Wu MH, Tai CT, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Sheu MH, Chang CY, Chen SA. Quantitative Analysis of Quantity and Distribution of Epicardial Adipose Tissue Surrounding the Left Atrium in Patients With Atrial Fibrillation Effect of Recurrence After Ablation. The American Journal of Cardiology. 2011;107:1498–1503. doi: 10.1016/j.amjcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial Fat Is Associated With Prevalent Atrial Fibrillation/Clinical Perspective. Circulation: Arrhythmia and Electrophysiology. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venteclef N, Guglielmi V, Balse E, Gaborit B+, Cotillard Al, Atassi F, Amour J, Leprince P, Dutour A, Cl+¬ment K, Hatem SpN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European Heart Journal. 2013 doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 13.Tansey DK, Aly Z, Sheppard MN. Fat in the right ventricle of the normal heart. Histopathology. 2005;46:98–104. doi: 10.1111/j.1365-2559.2005.02054.x. [DOI] [PubMed] [Google Scholar]
- 14.Kellman P, Hernando D, Shah S, Zuehlsdorff S, Jerecic R, Mancini C, Liang ZP, Arai AE. Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn Reson Med. 2009;61:215–221. doi: 10.1002/mrm.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. Arch Intern Med. 1985;145:1877–1881. [PubMed] [Google Scholar]
- 16.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellman P, Hernando D, Arai AE. Myocardial Fat Imaging. Curr Cardiovasc Imaging Rep. 2010;3:83–91. doi: 10.1007/s12410-010-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirani J, Roberts WC. Clinical Electrocardiographic and Morphologic Features of Massive Fatty Deposits (“Lipomatous Hypertrophy”) in the Atrial Septum. J Am Coll Cardiol. 1993;22:226–238. doi: 10.1016/0735-1097(93)90839-s. [DOI] [PubMed] [Google Scholar]
- 20.Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G, Marban E, Weiss RG, Tomaselli GF, Wagner GS, Wu KC. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–336. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tereshchenko L, Han L, Cheng A, Marine JE, Spragg D, Sinha S, Dalal D, Calkins H, Tomaselli GF, Berger RD. Comparison of Predictive Value of the Spatial Qrs-T Angle and Its Variability for Risk Stratification of Ventricular Arrhythmia. J Am Coll Cardiol. 2011;57:E147. [Google Scholar]
- 22.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, Pencina MJ, D’Agostino RB, Sr, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially and Geographically Diverse Population: the CHARGE-AF Consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Morton JB, Sanders P, Kalman JM. Long-term effects of catheter ablation for lone atrial fibrillation: Progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012;9:473–480. doi: 10.1016/j.hrthm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Osler W. The Principles and Practice of Medicine Designed for Use of Practitioners and Students of Medicine. D. Appleton; New York: 1892. [Google Scholar]
- 27.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal excessive (cor adiposum) subepicardial adipose tissue its clinical significance its effect on electrocardiographic QRS voltage. The American Journal of Cardiology. 1995;76:414–418. doi: 10.1016/s0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 28.Heyer CM, Kagel T, Lemburg SP, Bauer TT, Nicolas V. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence imaging findings and clinical symptoms. Chest. 2003;124:2068–2073. doi: 10.1378/chest.124.6.2068. [DOI] [PubMed] [Google Scholar]
- 29.Hutter AM, Jr, Page DL. Atrial arrhythmias and lipomatous hypertrophy of the cardiac interatrial septum. Am Heart J. 1971;82:16–21. doi: 10.1016/0002-8703(71)90156-6. [DOI] [PubMed] [Google Scholar]
- 30.Mügge A, Daniel WG, Angermann C, Spes C, Khandheria BK, Kronzon I, Freedberg RS, Keren A, Dennig K, Engberding R, Sutherland GR, Vered Z, Erbel R, Visser CA, Lindert O, Hausmann D, Wenzlaff P. Atrial Septal Aneurysm in Adult Patients: A Multicenter Study Using Transthoracic and Transesophageal Echocardiography. Circulation. 1995;91:2785–2792. doi: 10.1161/01.cir.91.11.2785. [DOI] [PubMed] [Google Scholar]
- 31.Rojas CA, El-Sherief A, Medina HM, Chung JH, Choy G, Ghoshhajra BB, Abbara S. Embryology and Developmental Defects of the Interatrial Septum. American Journal of Roentgenology. 2010;195:1100–1104. doi: 10.2214/AJR.10.4277. [DOI] [PubMed] [Google Scholar]