Abstract
γδ T cells have been recognized as effectors with immunomodulatory functions in cellular immunity. These abilities enable them to interact with other immune cells, thus having the potential for treatment of various immune-mediated diseases with adoptive cell therapy. So far, the interactions between γδ T cell and other immune cells have not been well defined. Here we will discuss the interactivities among them and the perspective on γδ T cells for their use in immunotherapy could be imagined. The understanding of the crosstalk among the immune cells in immunopathology might be beneficial for the clinical application of γδ T cell.
1. Introduction
γδ T cell accounts for a small group, which is less than 10% of the T cell pool in healthy human individuals [1]. Strong evidence demonstrates that γδ T cell participates as part of both innate and adaptive immunity. On activation, these cells can expand markedly and display various effector functions in immune responses. For example, chemokines and inflammatory cytokines release, potent cytolytic activity against tumor or microbial pathogens, and immunologic memory generation. These characteristics may contribute to the cell-cell contact manner of γδ T cell with other immune cells.
Empirical studies demonstrate that γδ T cells recognize transformed cells, microbial or tumor-expressed antigens, and then develop the immune surveillance functions [2]. It is clear that γδ T cells are able to respond to pathogen-associated molecular patterns of infection and autoimmunity. Virtually, their functions are not limited to antitumor or antiviral actions but also involved in modulating immune system homeostasis [3]. And this homeostasis may depend on the cross-reactivities between γδ T cells and their neighbour immune cells [4]. Selective stimulation of γδ T cells in vivo for antitumor therapy was accompanied by unexpected expansion of natural killer cells (NK cells) in a clinical trial [5]. It cannot be clearly distinguished whether the antitumor effect is produced by anyone of these two cells or there exists a synergy effect between them. The cell-cell interactions between γδ T cell and other immune cells are largely unknown and therefore, it is hard to assess their roles for the example above.
In recent clinical studies, suppressive regulatory T cells (Tregs) have been infused into patients to control the activation of alloreactive T lymphocytes after allogeneic haematopoietic stem cell transplantation (AHSCT) [6, 7]. Adoptive transfer of different immune cell subsets for treating cancer and/or immune-mediated diseases is increasingly being tested in clinical trials. The challenge for this therapy is how to efficiently exert regulatory effects on the target cells. As described above, γδ T cell plays an important role in immune response and thus has the potential for such immune-based therapies. Therefore this raises the question how the γδ T cell communicates with other immune cells. Understanding their crosstalk may be beneficial for the development of immunotherapeutic strategies.
2. γδ T Cell and αβ T Cell
T lymphocytes express either αβ or γδ T cell receptor heterodimers. Previous works have revealed the similarities between γδ T cell and the more populous αβ T cell in some aspects, such as cytolysis [8] and secretion of multiple cytokines [9]. These properties of γδ T cells permit them to regulate many types of immune response and cellular activities, including those of the predominant subsets-αβ T cells. A variety of studies show that Vδ2+ T cells act like professional antigen-presenting cells (APCs) to take up and present antigens (Ags) to human αβ T cells [10, 11], as well as in some mouse γδ T cells [12]. This capacity for Ag presentation by γδ T cells is considered to be a cooperative way in immune defense. Furthermore, the isopentenyl pyrophosphate- (IPP-) activated Vδ2+ T cells can promote proliferation and differentiation of naïve CD8+ αβ T cells [8] and even enhance the interferon (IFN)-γ production from autologous colonic αβ T cells [13]. However, all of these results are derived from in vitro experiments. Still, little is known about whether these cell-cell interactivities can be investigated under both ex vivo and in vivo conditions.
From a mouse model, γδ T cell depletion by anti-γδ T cell receptor (TCR) monoclonal antibody GL3 followed by concomitant elevated numbers of αβ T cells was described [14]. Likewise, the CD8+ T cell-mediated liver damage in Listeria-infected TCRδ −/− mice could be prevented by transferred with γδ T cells, and this effect may depend upon the ability of γδ T cells to reduce tumour necrosis factor (TNF)-α secretion or expansion of CD8+ T cells [15]. Obviously, there is homeostatic competition between αβ T cells and γδ T cells in vivo, and the IL-15 production and trans-presentation by dendritic cells (DCs) may be one possible mechanism for this activity [16]. Nevertheless, we cannot conclude that γδ T cells only have immunosuppressive effects on αβ T cells in vivo. An interesting result shows that inactivation and/or depletion of Vγ4+ T cells in the complete Freund's adjuvant- (CFA-) treated mice lead(s) to significant decreased number of αβ T cells as well as reduced TNF-α and IFN-γ production [17]. These results provide the concept that the modulation effects of γδ T cell on αβ T lymphocyte are mysterious. There has been no explanation so far for such discrepancy.
By studying the αβ lymphocytes, it has been found that CD8+ αβ T cells potently inhibit γδ T cells expansion and compete for essential cytokine stores when both of them are cotransferred into TCRβ −/−/δ −/− mice [4]. Similar results are seen in the CD4+CD25+ regulatory αβ T cells, a subset of αβ T cells, and they also have the capacity to suppress the expansion and functions of γδ T cells [18]. However, when adoptive αβ T cells (or CD4 T cells) were transferred into TCRβ −/− mice, these cells positively restored interleukin-17+ (IL-17+) γδ T cells generation, thereby implicating the supportive role of CD4+ T cells for IL-17+ γδ T cells [19]. Taken together, these data demonstrate that the reciprocal effects between γδ T cells and αβ T cells are debatable. The possible reasons for the contradictory data may be the different functions of subsets of γδ T cells, and the use of heterogeneous or homologous T lymphocytes also induces various immune responses.
3. γδ T Cell and B Cell
In the γδ T cell/B cell coculture experiments, the amounts of some immunoglobulins production increased remarkably [20, 21]. It is reported that γδ T cells also can collaborate with B cells to support the germinal center formation [22, 23]. In addition, an in vivo finding showed that TCRα −/− mice still efficiently developed normal germinal center and produced immunoglobulins (Igs), which thus prompts the hypothesis that γδ T cells might provide help for B cells [24]. In fact, not only the activation of immune responses but also the promotion of B cell maturation by γδ T cell can be investigated in human [25]. It is clear that γδ T cell is responsible, at least in part, for support of B cell functions. And recent investigations suggest that production of great amounts of cytokines from γδ T cells may influence B cell responses in humoral immunity [26, 27].
Although most studies state the promotion of B cell activities by γδ T cell, the limitation has been found under some circumstances. When mice received stimulation with ovalbumin (OVA) repeatedly, their splenic γδ T cells can inhibit the primary IgE production [28]. These regulatory functions are speculated to be mediated by different γδ T cells. For example, innate Vγ1+ T cells, including Vγ1Vδ5+ subsets, can enhance IgE responses, whereas acquired Vγ4+ T cells repress the αβ T cell-dependent antigen-specific IgE responses [29]. Collectively, these findings suggest that γδ T cells have complex immunomodulatory functions upon B lymphocytes similar to that on αβ T cells and this phenomenon may be related to the subsets of γδ T cells.
To understand whether the B cell can affect γδ T cell, multiple studies are attempting to disclose their interactions. It is reported that allogeneic Epstein-Barr virus- (EBV-) transformed B cell lines augment the proliferation of Vδ1+ T cells [30]. Further insights into the mechanism indicate that the isolated human peripheral blood B lymphocytes can induce proliferative response of Vδ1+ T cells, and it may be attributed to the expression of B7 and CD39 molecules on the surface of activated B cells [31]. Because γδ T cells undergo immune response independent of major histocompatibility complex (MHC) molecules, mutant EBV-transformed B cell lines lacking MHC molecules still can present the bacterial phosphoantigens (PAgs) to γδ T cells for other T cells activation [32, 33]. Accordingly, rational combination of these two cell types for immunotherapies would be considerable.
4. γδ T Cell and NK Cell
Similar to another lymphoid cell-NK cell, γδ T cell exerts immune functions in the antibody-independent and non-MHC-dependent manners. Although the NK-cell-like functions of γδ T cell are well characterized, the interactivity between γδ T cell and NK cell remains enigmatic. Chapoval et al. showed that γδ T cells were indispensable for regulation of NK-cell antitumor responses [34]. Afterwards they further identified that, in the TCR-δ −/− mutant mice, early IFN-γ production seriously decreases in the listeriosis-infected group, and they also confirmed that NK cells were the critical producer of IFN-γ [35]. Maniar et al. have described a similar result that zoledronic acid-activated γδ T cells can lead to enhancement of NK cell-mediated tumor cytotoxicity [36]. Recently, the DC-like cell-dependent NK cell cytokine production has been found to be controlled by γδ T cells [37]. Thus these results manifest that lack of γδ T cells may lead to impaired NK cells activation.
In turn, when stimulated by the antigens from M. tuberculosis, activated NK cells can improve γδ T cells proliferation [38]. However, under in vivo condition, NK cells play a suppressive role in regulating γδ T cells expansion in the absence of αβ T cells [4]. Such phenomenon demonstrates that, different from the in vitro condition, the interplay between NK cells and γδ T cells may lie on the competitive advantages of the cells under in vivo condition. Actually, NK cells or γδ T cells use different mechanisms to mediate cytotoxicity functions, so the combination of them for immunotherapy would be considered. Understanding of their correlation may provide the concept for how to enhance the antitumor functions by immune cells.
5. γδ T Cell and Monocyte/Macrophage
Monocytes play a role in immune defense and after travelling to tissues, they mature and differentiate into macrophage populations. At the sites of infection/inflammation, circulating γδ T cells and monocytes are rapidly recruited to eliminate infected or transformed cells. Several investigations demonstrate that they do not function alone but affect each other. It is previously shown that microbe-responsive Vγ9Vδ2 T cells can induce monocytes differentiating into inflammatory DCs, which further results in production of inflammatory mediators and antigen-presenting functions of these differentiated monocytes [39]. In turn, some evidence suggests that M. tuberculosis-infected monocytes are potent in inducing γδ T proliferation [40] and processing the M. tuberculosis for mycobacterial antigen-specific CD4+ αβ and γδ T cells [41]. After being incubated with M. tuberculosis Bacillus Calmette-Guérin (BCG), infected monocytes promote the cytotoxic activity of γδ lymphocytes [42]. Additionally, the N-BP drug zoledronic acid-treated monocytes can effectively trigger activation of γδ T cells [43]. But in atopic dermatitis (AD) patients, once contacted with activated monocytes, NK and γδ + T cells specifically undergo apoptosis associated with reduction of type 1 cytokine production [44]. It seems that different pathological states of monocytes may account for the fate of γδ T cells.
In the case of macrophage, when incubated with Mycobacterium tuberculosis-derived products, macrophages release chemokines to efficiently recruit γδ T cells as well as regulating the latter's function [45]. As for γδ T cell, different subsets may be responsible for their specific functions. In the mice infected with influenza A virus, Vγ6Vδ1+ T cells might contribute to the initial recruitment of macrophages whereas Vγ4+ and Vγ1+ T cells mediate elimination of macrophages [46]. For preventing the hyperinflammatory responses, γδ T cells exert cytotoxic activity against activated macrophages [47]. Similarly, human or avian influenza virus-infected macrophages were killed by PAg-expanded γδ T cells for virus clearance [48]. However, human peripheral Vγ9Vδ2 T cells have been shown to efficiently induce TNF-α and IL-1β production of BCG-infected macrophages [49]. Obviously, different subtypes of γδ T cells have their specific functions to regulate the activities of macrophages.
6. γδ T Cell and DC
Unlike mouse γδ T cells, human γδ T cells distribute in the peripheral blood, spleen, lymph nodes, or the intraepithelial lymphocytes in intestine [50], but rare in human skin [51]. Various subsets of γδ T cells scatter in a disparate anatomic location and assume distinct functions [52]. The γδ1+ T cell population abundant in lymphoid tissues markedly blocks the maturation and inhibits the function of DCs [53]. Interestingly, the Vδ2+ T cells, which consist of most human peripheral blood γδ T cells, seem to react in another way for DCs. When cocultured with Vδ2+ T cells isolated from human blood samples, the maturation of immature DCs (iDCs) is shown to be potentiated [54], and the consistent phenomenon has been found in mice [55]. Furthermore, γ9δ2TCR-transduced αβ T cells efficiently promote the maturation of DCs [56]. Apart from the induction of maturation of DCs, Vγ9Vδ2 T cells have been identified for their ability for restoring Brucella-infected DCs function [57]. It is also reported that γδ T cells enhance DCs activation for the production of IL-12 [58]. The possible mechanism of γδ T cell for the induction of DC activation may be due to recognition of the cell-surface molecules or inflammatory cytokines, such as CD1 [59], CD86 [60], CD40 [58], and IFN-γ [61]. However, a recent study found that the immunosenescence could be induced in the DCs by γδ Treg cells [62]. Therefore, it is plausible that diverse effects of γδ T cells on DCs may rely on different γδ subsets.
It is investigated that DCs induce the cell cytokine production of freshly isolated Vγ9Vδ2 T cells [54], and they have the potent capacity to expand peripheral blood Vδ2+ T cells [63] or support the development of Vγ4+ γδ T cells from the spleen [64]. Moreover, mycobacteria can induce Vδ2 T cell antitumour responses indirectly via a specific subset of DCs [65]. These in vitro studies suggest that the modulation effect of DC on γδ T cell is definite no matter what type of the γδ T cell is. In tuberculosis patients, M. tuberculosis-infected DCs selectively induced expansion of phenotypically immature, central memory-type Vγ9Vδ2 T cells [66]. Even though, in the patients with multiple myeloma (MM), zoledronic acid-treated DCs are potent in activating autologous γδ T cells [67], the above-mentioned findings, not only ex vivo but also in vivo study, are all suggestive of the promotion effects of DCs on γδ T cells regardless of the latters' subtype.
7. γδ T Cell and Granulocyte
During inflammatory or infectious processes, granulocytes and γδ T cells promptly accumulate at the site of inflamed tissues and eradicate the bacteria, virus, or transformed cells. It is established that γδ T cells partially resemble the granulocytes in cytotoxic activities against pathogens. Understanding of their cross-reactivity in the immune response might pave the way for immunotherapies. Neutrophils constitute most of the granulocytes; hence the investigations on the activities of granulocytes are focused on this population. HMB-PP stimulated Vγ9/Vδ2 T cells have been reported to induce neutrophil survival and activation depending on the number of γδ T cells [68]. Similarly, IPP or zoledronic acid-stimulated peripheral blood Vγ9Vδ2 T cells were observed to induce phagocytosis and migration of neutrophils [69], as well as granules release from activated granulocytes [70]. In addition, limbal epithelial γδ T cells [71] and hepatic IL-17A+CD3+ γδ TCR+ cells [72] have the capacity to regulate early infiltration or accumulation of neutrophils.
However, discrepant result reported that, in a mouse model of sepsis, there was a reverse association of the cell number between γδ T cells and neutrophils, and γδ T cells rapidly killed lipopolysaccharide- (LPS-) treated neutrophils [73]. At the presence of LPS—the major component of the membrane of Gram-negative bacteria—hypertonic saline leads to the increased elimination of inflammatory neutrophils by γδ T cells [74]. These controversial results have not yet been well explained so far, and it seems to be attributed to the status of neutrophils. Unfortunately, the function of neutrophils on γδ T cells has rarely been reported and the interaction between them should be further clarified.
8. γδ T Cell and Mesenchymal Stromal Cell
Mesenchymal stem cells (MSCs) are a minor subset residing in several tissues, generally isolated from bone marrow. The ability of MSC to regulate other major immune cell populations has been demonstrated in numerous studies, but only a few studies elaborate the interplay between γδ T cells and MSCs. The immunomodulatory activities of MSCs on T cells have been further investigated since it was confirmed that MSCs could inhibit the T lymphocytes proliferation either in mixed lymphocyte reactions or under the stimulation of polyclonal activators [75]. As recently reported, human MSCs inhibit the proliferation and immune responses of Vγ9Vδ2 T cells through prostaglandin E2 (PGE2) [76]. In addition, MSCs can suppress the expansion of Vδ2+ T cells without affecting the functions of cytotoxicity or antigen processing/presentation to CD4+ T cells by Vδ2+ T cells [77, 78]. The inhibitory role of MSC on γδ T cell appears to be determined by the MSC-derived molecules [79]. It is interesting to note that when infection or organ injury develops, γδ T cells increase the recruitment of MSCs to the site of infection [80]. Immunosuppressive effect of MSC is supposed to contribute to restoring tissue homeostasis whereas γδ T cell exerts defending functions against pathogens and tumors. Therefore, the use of MSC or γδ T cell in adoptive immunotherapy should carefully be taken into consideration for their interaction.
9. Concluding Remarks
A growing set of data has clearly identified that γδ T cells play different roles in immune responses, thus providing a promising candidate for treatment of many diseases. More recently, γδ T cells are making their way into clinical trials. In this review, the interactions between γδ T cell and other immune cells have been discussed (Figure 1), and there is the prospect that γδ T cells are potential for treating immune-mediated diseases. Unfortunately, current experimental data is almost derived from ex vivo studies or animal models, and there are extremely limited observations of the interaction between γδ T cells and other immune cells in human body. This would impede the application of γδ T cells for clinical therapeutics in the future. Therefore, more preclinical and clinical investigations of γδ T cells are needed for making effective strategies to harness immune responses in various diseases.
Figure 1.
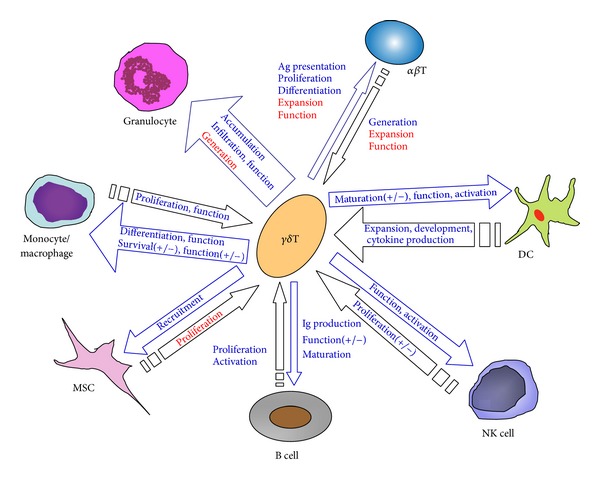
Interactions between γδ T cell and other immune cells. The positive effects are depicted in blue font, while the negative effects are in red. The mathematical symbol (+/−) indicates that the contradictory effects are observed between γδ T cell and other immune cells. MSC: mesenchymal stem cell; DC: dendritic cell; NK cell: natural killer cell; Ag: antigen; Ig: immunoglobulin.
By studying the effects of γδ T cells on other immune cells, γδ T cells reveal a dual role so that it is not definite whether they would display active or suppressive function in immune activities. That γδ T cells are prone to be the positive or negative effectors may depend on their subtype or internal homeostasis. In this regard, strategies to regulate the interaction of these cells should be targeted on specific subsets as well as environmental factors. With regard to the modulation of other immune cells on γδ T cell, most of them have the role of supporting the function of γδ T cell except the inhibitive effects from MSC. Thus, the balance between γδ T cells and their neighbour immune cells should be considered sufficiently in the adoptive cell therapies for treating cancer or immune-related diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81230014, 81200338, 81170526, and 81270640) and the Natural Science Foundation of Zhejiang Province, China (Y2110152).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Williams N. T cells on the mucosal frontline. Science. 1998;280(5361):198–200. doi: 10.1126/science.280.5361.198. [DOI] [PubMed] [Google Scholar]
- 2.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gd T cells to immunology. Nature Reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11(1):57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 4.French JD, Roark CL, Born WK, O’Brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunzmann V, Smetak M, Kimmel B, et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. Journal of Immunotherapy. 2012;35(2):205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 8.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 9.Sardinha LR, Elias RM, Mosca T, et al. Contribution of NK, NK T, γδ T, and αβ T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi. Infection and Immunity. 2006;74(4):2031–2042. doi: 10.1128/IAI.74.4.2031-2042.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ cells. Science. 2005;309(5732):264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 11.Brandes M, Willimann K, Bioley G, et al. Cross-presenting human γδ T cells induce robust CD8+ αβ T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, Cui Y, Shao H, et al. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. Journal of Neuroimmunology. 2008;203(1):3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy NE, Bashir Z, Vossenkämper A, et al. Proinflammatory Vδ2+T cells populate the human intestinal mucosa and enhance IFN-γ production by colonic αβ T cells. The Journal of Immunology. 2013;191(5):2752–2763. doi: 10.4049/jimmunol.1202959. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann SH, Blum C, Yamamoto S. Crosstalk between α/β T cells and γ/δ T cells in vivo: activation of α/β T-cell responses after γ/δ T-cell modulation with the monoclonal antibody GL3. Proceedings of the National Academy of Sciences of the United States of America . 1993;90(20):9620–9624. doi: 10.1073/pnas.90.20.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing γδ T cells protect the liver from Listeria-elicited, CD8+ T cell-mediated injury. European Journal of Immunology. 2008;38(8):2274–2283. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- 16.Do J-S, Min B. IL-15 produced and trans-presented by DCs underlies homeostatic competition between CD8 and γδ T cells in vivo . Blood. 2009;113(25):6361–6371. doi: 10.1182/blood-2008-12-192997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roark CL, Huang YF, Jin NY, et al. A canonical Vγ4Vδ4+γδ T cell population with distinct stimulation requirements which promotes the Th17 response. Immunologic Research. 2013;55(1–3):217–230. doi: 10.1007/s12026-012-8364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves-Sousa N, Ribot JC, DeBarros A, Correia DV, Caramalho Í, Silva-Santos B. Inhibition of murine γδ lymphocyte expansion and effector function by regulatory αβ T cells is cell-contactdependent and sensitive to GITR modulation. European Journal of Immunology. 2010;40(1):61–70. doi: 10.1002/eji.200939715. [DOI] [PubMed] [Google Scholar]
- 19.Do J-S, Visperas A, O’Brien RL, Min B. CD4 T cells play important roles in maintaining IL-17-producing γδ T-cell subsets in naive animals. Immunology and Cell Biology. 2012;90(4):396–403. doi: 10.1038/icb.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo . Proceedings of the National Academy of Sciences of the United States of America. 2009;106(20):8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen L, Pao W, Wong FS, et al. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. The Journal of Experimental Medicine. 1996;183(5):2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Marinova E, Han J, Tan T-H, Han S. Cutting edge: γδ T cells provide help to B Cells with altered clonotypes and are capable of inducing Ig gene hypermutation. The Journal of Immunology. 2003;171(10):4979–4983. doi: 10.4049/jimmunol.171.10.4979. [DOI] [PubMed] [Google Scholar]
- 23.Wen L, Hayday AC. γδ T-cell help in responses to pathogens and in the development of systemic autoimmunity. Immunologic Research. 1997;16(3):229–241. doi: 10.1007/BF02786392. [DOI] [PubMed] [Google Scholar]
- 24.Wen L, Pao W, Wong FS, et al. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. The Journal of Experimental Medicine. 1996;183(5):2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermijlen D, Ellis P, Langford C, et al. Distinct cytokine-driven responses of activated blood γδ T cells: insights into unconventional T cell pleiotropy. The Journal of Immunology. 2007;178(7):4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annual Review of Immunology. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 27.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nature Reviews Immunology. 2002;2(5):336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 28.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265(5180):1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Jin N, Roark CL, et al. The influence of IgE-enhancing and IgE-suppressive γδ T cells changes with exposure to inhaled ovalbumin. The Journal of Immunology. 2009;183(2):849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hacker G, Kromer S, Falk M, Heeg K, Wagner H, Pfeffer K. Vδ1+ subset of human γδ T cells responds to ligands expressed by EBV- infected Burkitt lymphoma cells and transformed B lymphocytes. The Journal of Immunology. 1992;149(12):3984–3989. [PubMed] [Google Scholar]
- 31.Orsini DLM, van Gils M, Kooy YMC, et al. Functional and molecular characterization of B cell-responsive Vδ1+ γδ T cells. European Journal of Immunology. 1994;24(12):3199–3204. doi: 10.1002/eji.1830241243. [DOI] [PubMed] [Google Scholar]
- 32.Fisch P, Malkovsky M, Kovats S, et al. Recognition by human V(γ)9/V(δ)2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 33.Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3(4):495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 34.Chapoval AI, Fuller JA, Kremlev SG, Kamdar SJ, Evans R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. The Journal of Immunology. 1998;161(12):6977–6984. [PubMed] [Google Scholar]
- 35.Ladel CH, Blum C, Kaufmann SHE. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by γ/δ T lymphocytes. Infection and Immunity. 1996;64(5):1744–1749. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniar A, Zhang X, Lin W, et al. Human γδ T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood. 2011;118(10):2743–2751. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Zheng X, Li B, Wei H, Tian Z. Human NK cells positively regulate γδ T cells in response to Mycobacterium tuberculosis. The Journal of Immunology. 2006;176(4):2610–2616. doi: 10.4049/jimmunol.176.4.2610. [DOI] [PubMed] [Google Scholar]
- 39.Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. A rapid crosstalk of human γδ T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathogens. 2009;5(2) doi: 10.1371/journal.ppat.1000308.e1000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human γΔ T cells by monocytes infected with live Mycobacterium tuberculosis. Journal of Clinical Investigation. 1991;87(2):729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaji KN, Boom WH. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ αβ and γδ T cells: role of particulate antigen. Infection and Immunity. 1998;66(1):98–106. doi: 10.1128/iai.66.1.98-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γδ T cells by aminobisphosphonate antigen. The Journal of Immunology. 2001;166(9):5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 43.Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. British Journal of Haematology. 2009;144(2):245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katsuta M, Takigawa Y, Kimishima M, Inaoka M, Takahashi R, Shiohara T. NK cells and γδ + T cells are phenotypically and functionally defective due to preferential apoptosis in patients with atopic dermatitis. The Journal of Immunology. 2006;176(12):7736–7744. doi: 10.4049/jimmunol.176.12.7736. [DOI] [PubMed] [Google Scholar]
- 45.Ferrero E, Biswas P, Vettoretto K, et al. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on γδ cells. Immunology. 2003;108(3):365–374. doi: 10.1046/j.1365-2567.2003.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by γ/δ + T cells. The Journal of Experimental Medicine. 1990;172(4):1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tramonti D, Rhodes K, Martin N, Dalton JE, Andrew E, Carding SR. γδT cell-mediated regulation of chemokine producing macrophages during Listeria monocytogenes infection-induced inflammation. Journal of Pathology. 2008;216(2):262–270. doi: 10.1002/path.2412. [DOI] [PubMed] [Google Scholar]
- 48.Qin G, Mao H, Zheng J, et al. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. Journal of Infectious Diseases. 2009;200(6):858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer CT, Abate G, Sakala IG, et al. Granzyme A produced by γδ2T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLOS Pathogens. 2013;9(1) doi: 10.1371/journal.ppat.1003119.e1003119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucy RP, Chen C-LH, Cihak J, Losch U, Cooper MD. Avian T cells expressing γδ receptors localize in the splenic sinusoids and the intestinal epithelium. The Journal of Immunology. 1988;141(7):2200–2205. [PubMed] [Google Scholar]
- 51.Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR δ repertoire in normal human skin is restricted and distinct from the TCR δ repertoire in the peripheral blood. Journal of Investigative Dermatology. 2001;116(2):275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 52.Bonneville M, O’Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10(7):467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 53.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang R-F. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-Hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vγ9Vδ2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunology, Immunotherapy. 2010;59(7):1109–1120. doi: 10.1007/s00262-010-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang H, Welte T, Zheng X, et al. γδ T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunology and Medical Microbiology. 2010;59(1):71–80. doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcu-Malina V, Heijhuurs S, van Buuren M, et al. Redirecting αβT cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood. 2011;118(1):50–59. doi: 10.1182/blood-2010-12-325993. [DOI] [PubMed] [Google Scholar]
- 57.Ni M, Martire D, Scotet E, Bonneville M, Sanchez F, Lafont V. Full restoration of Brucella-infected dendritic cell functionality through Vg9Vd2 T helper type 1 crosstalk. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043613.e43613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue SI, Niikura M, Takeo S, et al. Enhancement of dendritic cell activation via CD40 ligand-expressing gd T cells is responsible for protective immunity to Plasmodium parasites. Proceedings of the National Academy of Sciences of the United States of America. 109(30):12129–12134. doi: 10.1073/pnas.1204480109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leslie DS, Vincent MS, Spada FM, et al. CD1-mediated γ/δ T cell maturation of dendritic cells. The Journal of Experimental Medicine. 2002;196(12):1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated γδ T cells: role of CD86 and inflammatory cytokines. The Journal of Immunology. 2005;174(1):252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 61.Devilder M-C, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-γ responses of human Vγ9Vδ2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. The Journal of Immunology. 2009;183(6):3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 62.Ye J, Ma CL, Hsueh EC, et al. Tumor-derived gd regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. The Journal of Immunology. 2013;190(5):2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cabillic F, Toutirais O, Lavoué V, et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vγ9Vδ2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunology, Immunotherapy. 2010;59(11):1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook L, Miyahara N, Jin N, et al. Evidence that CD8+ dendritic cells enable the development of γδ T cells that modulate airway hyperresponsiveness. The Journal of Immunology. 2008;181(1):309–319. doi: 10.4049/jimmunol.181.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate γδ T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunology, Immunotherapy. 2012;61(4):535–547. doi: 10.1007/s00262-011-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of Vγ9Vδ2 T cells by Mycobacterium tuberculosis-infected dendritic cells. The Journal of Immunology. 2010;185(3):1770–1776. doi: 10.4049/jimmunol.1000966. [DOI] [PubMed] [Google Scholar]
- 67.Castella B, Riganti C, Fiore F, et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9Vδ2 T cells, αβ CD8+ T cells, regulatory T cells, and dendritic cells. The Journal of Immunology. 2011;187(4):1578–1590. doi: 10.4049/jimmunol.1002514. [DOI] [PubMed] [Google Scholar]
- 68.Davey MS, Lin C-Y, Roberts GW, et al. Human neutrophil clearance of bacterial pathogens triggers anti-microbial γδ T cell responses in early infection. PLoS Pathogens. 2011;7(5) doi: 10.1371/journal.ppat.1002040.e1002040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caccamo N, La Mendola C, Orlando V, et al. Differentiation, phenotype, and function of interleukin-17-producing human vγ9vδ2 T cells. Blood. 2011;118(1):129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 70.Agrati C, Cimini E, Sacchi A, et al. Activated Vγ9Vδ2 T cells trigger granulocyte functions via MCP-2 release. The Journal of Immunology. 2009;182(1):522–529. doi: 10.4049/jimmunol.182.1.522. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Burns AR, Rumbaut RE, Smith CW. γδ T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. American Journal of Pathology. 2007;171(3):838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang XF, Sun R, Wei HM, Tian ZG. High-mobility group box 1 (HMGB1)-toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of gamma delta T cells with macrophages. Hepatology. 2013;57(1):373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 73.Hirsh MI, Hashiguchi N, Chen Y, Yip L, Junger WG. Surface expression of HSP72 by LPS-stimulated neutrophils facilitates γδT cell-mediated killing. European Journal of Immunology. 2006;36(3):712–721. doi: 10.1002/eji.200535422. [DOI] [PubMed] [Google Scholar]
- 74.Hirsh MI, Hashiguchi N, Junger WG. Hypertonic saline increases γδT cell-mediated killing of activated neutrophils. Critical Care Medicine. 2008;36(12):3220–3225. doi: 10.1097/CCM.0b013e31818f238e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicola MD, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 76.Martinet L, Fleury-Cappellesso S, Gadelorge M, et al. A regulatory cross-talk between Vγ9Vδ2 T lymphocytes and mesenchymal stem cells. European Journal of Immunology. 2009;39(3):752–762. doi: 10.1002/eji.200838812. [DOI] [PubMed] [Google Scholar]
- 77.Petrini I, Pacini S, Petrini M, Fazzi R, Trombi L, Galimberti S. Mesenchymal cells inhibit expansion but not cytotoxicity exerted by gamma-delta T cells. European Journal of Clinical Investigation. 2009;39(9):813–818. doi: 10.1111/j.1365-2362.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 78.Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and γδ T cells or invariant natural killer T cells. Stem Cells. 2009;27(3):693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 79.Shi M, Liu Z-W, Wang F-S. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clinical and Experimental Immunology. 2011;164(1):1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tschöp J, Martignoni A, Goetzman HS, et al. γδ T cells mitigate the organ injury and mortality of sepsis. Journal of Leukocyte Biology. 2008;83(3):581–588. doi: 10.1189/jlb.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]


