Abstract
We describe a bead-based, multiplexed, oligonucleotide ligation assay (OLA) performed on the Luminex flow cytometer. Differences between this method and those previously reported include the use of far fewer beads and the use of a universal oligonucleotide for signal detection. These innovations serve to significantly reduce the cost of the assay, while maintaining robustness and accuracy. Comparisons are made between the Luminex OLA and both pyrosequencing and direct sequencing. Experiments to assess conversion rates, call rates, and concordance across technical replicates are also presented.
Keywords: single nucleotide polymorphism, genotyping, Luminex
Introduction
Single nucleotide polymorphisms (SNPs) represent a major source of genetic variation in human beings; the NCBI database dbSNP contains nearly 12 million unique SNPs, which correlates to an average of 1 SNP every 250 base pairs. Due to their frequency, SNPs are an important type of marker used in association studies of human disease, and can themselves induce functional changes that contribute to disease and drug response variability [1, 2]. In a relatively short time, SNP genotyping technologies have evolved from rudimentary and simplexed gel-based assays to highly multiplexed arrays designed to simultaneously interrogate more than 1 million SNPs. Ultra-high throughput array-based genotyping technologies, such as those offered by Illumina [3] and Affymetrix [4], are utilized in genome-wide studies requiring genotyping of hundreds of thousands of SNPs in hundreds (or low thousands) of samples. However, fine-mapping, candidate gene, and other targeted genetic studies often require genotyping of dozens or hundreds of SNPs in thousands of samples, and ultra-high throughput methods are not ideal for these applications. Though too numerous to list in their entirety, popular medium-throughput commercial methods include technologies such as Biotage’s pyrosequencing [5], Third Waves’s Invader assay [6], ABI’s TaqMan SNP assay [7], and ABI’s SNPplex platform [8]. Drawbacks of commercial genotyping platforms include equipment costs and the need to purchase proprietary reagents. The genotyping method presented here utilizes the Luminex flow cytometer, which is within the budget of many academic labs, and is a relatively open source platform readily amenable to “home-brew” assays not requiring expensive and proprietary reagents. Additionally, unlike many SNP genotyping platforms, the Luminex flow cytometer is one of the few instruments capable of quantitating both proteins and nucleic acids (DNA, mRNA, and miRNA) [9–12], providing it with uses beyond the SNP genotyping assay presented here.
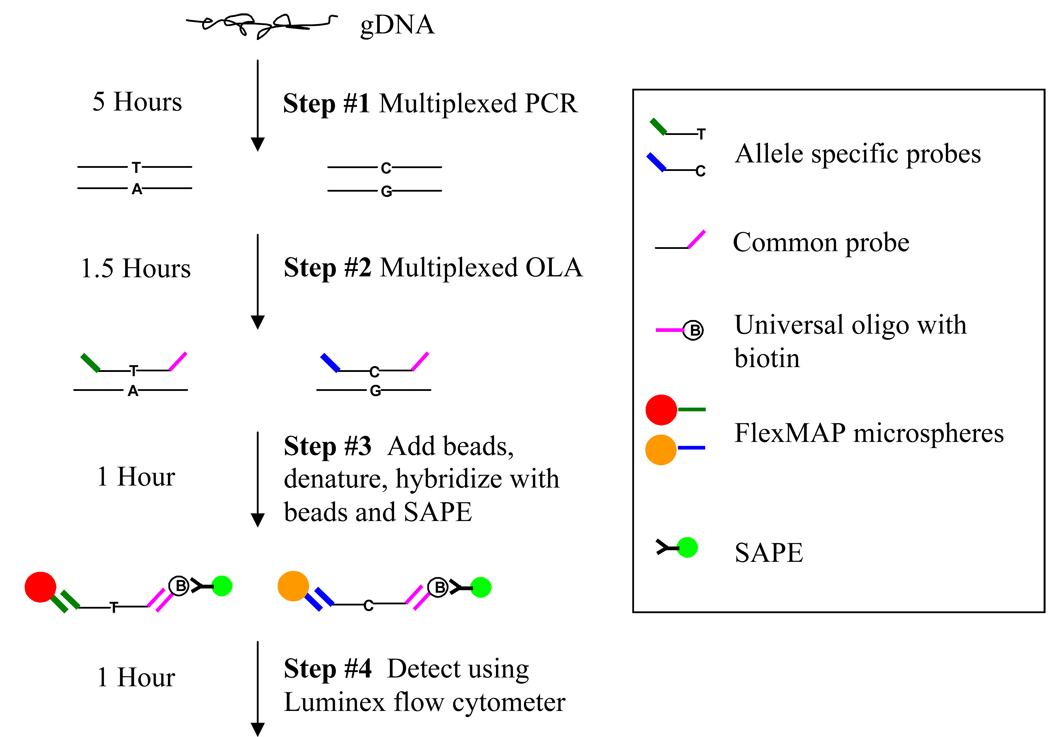
Our method uses the robust chemistry of the oligonucleotide ligation assay (OLA) in conjunction with the Luminex flow cytometry platform (Figure 1) [13–18]. The assay is performed in 96-well plate format and has an upper multiplex capability of 50 SNPs per well, making it suitable for a moderate number of SNPs in a large number of samples. Two key modifications make this method less expensive and easier to perform than similar published methods. First, we use fewer beads than are traditionally used in quantitative Luminex assays [19–22]. The most significant expense of typical Luminex-based assays is the polystyrene beads used. Typical recommendations for SNP typing assays are to use 2500 input beads while counting 100 beads, but our experience indicates that these numbers can be substantially reduced, with a significant savings in cost per genotype.
Figure 1. Overview of Luminex OLA assay.
The assay consists of four steps; 1) Multiplexed PCR 2) multiplexed OLA 3) hybridization with beads and SAPE 4) Detection using the Luminex flow cytometer. Steps 2–4 take place in the same 96-well plate.
Second, a universal biotinylated oligonucleotide is employed for signal quantification. For a biallelic SNP the OLA assay requires two allele specific oligonucleotides and a common oligonucleotide. Traditionally, the common oligonucleotide is labeled (during synthesis) with a detector molecule (i.e. biotin), which allows for quantification of the OLA product. In contrast, we synthesize the common oligonucleotide to have an attached sequence which is complementary to a universal oligonucleotide double-labeled with biotin, thus saving on the cost of synthesizing biotin-labeled common oligonucleotides for each SNP assay. In addition to being less expensive, the method presented here does not employ typical wash or centrifugation steps, making it simple to perform.
Materials and Methods
Oligonucleotide primer and probe sets
The multiplex PCR primer selection program used in this study was an enhanced version of the program that was used to create 1000+ plex PCRs for parallel genotyping [23]. Amplicon size in multiplexed PCRs is generally below 400 base pairs, though we have successfully genotyped from amplicons as large as 1200 base pairs. PCR primers were ordered in 96-well plate format (IDT, Coralville, Iowa) and purified using standard desalting.
The OLA requires two allele-specific and one common probe for each SNP being assayed. Allele-specific probe pairs consist of a 5’ tag sequence and a 3’ locus specific portion which differs only at the terminal position of the probe. Each allele specific probe contains a unique 24-base FlexMAP™ tag (Luminex® Corporation, Austin, TX) at the 5’ end to allow hybridization to a reverse complement anti-tag coupled to a unique FlexMAP™ microsphere. Common probes contain a locus specific portion at the 5’ end, a universal capture sequence at the 3’ end, and are 5’ phosphorylated by the manufacturer (Integrated DNA Technologies, Coralville, IA). The universal capture sequence is the reverse complement of a doubly biotinylated “universal oligonucleotide” which is included in the bead hybridization step. For the universal capture sequence we employed the “tag” of FlexMAP™ bead 100 and the “universal oligonucleotide” sequence is the anti-tag of bead 100; for this reason, FlexMAP™ bead 100 is not utilized in our assays. All OLA probe sets are designed so that the locus-specific portion of both the common and allele-specific probes have a melting temperature of approximately 64 C. Module 1 of the HyTher server was used to determine melting temperature (http://ozone3.chem.wayne.edu/). OLA probe sets were ordered in 96-well plate format and purified using standard desalting. All primer and probe sets are contained in Supplementary Material 1 – Primer and Probe Sets.
Genomic DNA Samples and Multiplex PCR Reaction
Samples previously genotyped in our laboratory were used in this study [24, 25]. Genomic DNA was isolated from whole blood or cell lines. All PCR reactions presented in this paper were fully multiplexed; for example, the 19 SNP assay presented involved a 19-plex PCR. We have performed as high as 40-plex PCR reactions (data not shown). Amplifications were performed in a solution (30 µl) containing 1.5 U AmpliTaq Gold DNA polymerase (Applied Biosystems); 1:10 dilution of 10X Buffer II (supplied with AmpliTaq Gold); 200 µM of each dNTP; 166 nM (5 pmol) of each primer; 2.5 mM MgCl; and 40 ng genomic DNA. Reactions were initially heated at 94 °C for 10 minutes, followed by 40 cycles of 94 °C for 40 seconds, 60 °C for 30 seconds, ramping from 60 °C to 65 °C using fifty 0.1 °C two second cycles, and 72 °C for 2 minutes. Reactions were completed at 72 °C for 10 minutes and 20 °C for 5 minutes. All reactions were performed in 96-well plates.
Oligonucleotide Ligation Reaction
All OLA reactions presented in this paper were fully multiplexed; for example, the 19 SNP assay presented involved a 19-plex OLA. We have performed as high as 40-plex OLA reactions (data not shown). OLA reactions were performed in a solution (15 µl) containing 20 mM Tris/HCl buffer pH 7.6; 25 mM KOAc; 10 mM MgOAc; 1 mM NAD+; 10 mM DTT; 0.1% Triton X-100; 10 nM (150 fmol) of each OLA probe; 2 µl of multiplexed PCR product; and 3 U of Taq DNA ligase (New England Biolabs, Beverly, MA). Reactions were initially heated for 1 minute at 95 °C, followed by 32 thermal cycles of 95 °C for 15 seconds (denaturation) and 58 °C for 2 minutes (annealing/ligation). Reactions were then cooled to 4 °C, and were used immediately in the hybridization step or stored at −20 °C for up to 1 week before proceeding with the hybridization step. All reactions were performed in 96-well plates.
Hybridization of OLA Products to Microspheres and Universal Oligonucleotide
50 µl of TMAC hybridization solution (3 M tetramethylammonium chloride, 50 mM Tris-HCl, pH 8.0, 3 mM EDTA, pH 8.0, 0.10% SDS) containing 20nM (1000 fmol) of universal oligonucleotide and 200 beads (unless otherwise noted) from each FlexMAP™ microsphere set was added directly to a well containing a completed 15 µl OLA reaction. Note that TMAC hybridization solution should be warmed to 37 °C prior to use as the SDS falls out of solution at room temperature. Hybridization reactions were denatured at 95 °C for 90 seconds and hybridized at 37 °C for 20 minutes.
Fluorescent Labeling and Flow Cytometric Analysis
Following hybridization, 6 µl of TMAC hybridization solution containing 180 ng streptavidin-R-phycoerythrin (Molecular Probes – Invitrogen Corporation, Carlsbad, CA) was added directly to the well containing the hybridization reaction. Labeling reactions were incubated at 37 °C for 40 minutes. Care must be taken to avoid exposing SA-PE to excessive amounts of light. Unless otherwise noted, 20 of each FlexMAP™ bead included in the multiplexed assay were sorted and quantitated using a Luminex 100 or Luminex 200 flow cytometer. The Luminex XY plate should be heated to 37 °C for the duration of bead sorting.
Direct sequencing and pyrosequencing
Semiautomated fluorescent direct sequencing was performed on the Beckman CEQ 8000 instrument. Pyrosequencing assays were performed as simplexed reactions on the automated PSQ HS96A platform [26, 27]. PCR primers were designed using Primer3 (Whitehead Institute) and the sequencing primer used for the Pyrosequencing assay was designed using the Pyrosequencing SNP Primer Design Software v1.0. PCR reactions contained 40 ng of template DNA, 0.5 U Taq polymerase, 0.03 µM of each primer, 0.1 mM of dNTP, 3.0 mM of MgCl2, 50mM of KCl, 10mM of Tris—HCl (pH 9.0), and 0.1% Triton X-100 in a 20 µl volume. A touchdown amplification program was used. After 8 minutes at 95°C, 15 cycles were done at 94°C for 30 s, annealing for 20 seconds, and at 72°C for 20 seconds, with the annealing temperature starting at 60°C and decreasing by 0.5°C for each cycle. This was followed by 35 cycles at 94°C for 20 seconds, at 52°C for 20 seconds, at 72°C for 30 seconds, and then a final extension step at 72°C for 15 minutes.
Genotype calling for OLA/Luminex assay
A semi-automated method of assessing cluster quality and designating genotypes was applied to each SNP assay. Raw data is outputted as the median fluorescence intensity (MFI) for each allele. As an initial filter, data where neither allele has an MFI greater than 1000 (using the high PMT setting when calibrating the Luminex flow cytometer) were removed from analysis. This filter is based on our observation that the cluster quality (described below) decreases significantly for assays where the majority of MFI values for both alleles are below 1000. After this initial data cleaning step the raw data from both alleles was converted to a single number, the allelic ratio, as follows:
Next, using the program Cluster 3.0 (http://bonsai.ims.u-tokyo.ac.jp/~mdehoon/software/cluster/software.htm) k-means clustering was applied to the allelic ratio to designate one of three genotypes [28]. The following settings were assigned in the Cluster 3.0 graphical user interface: (1) Organize genes (2) k = 3 (3) Iterations = 100 (4) k-Means (5) Similarity metric = Euclidean distance. Resulting genotype assignments were then used to generate Silhouette scores for assessment of cluster quality [29]. SNP assays resulting in Silhouette scores less than 0.70 were considered failed assays when assessing conversion rate. As a final step, manual inspection of scatter plots was performed by expert technicians. Data points not clearly belonging to one of three clusters were considered failed genotypes when assessing call rates. For assessment of concordance with other SNP genotyping methods, all Luminex OLA calls were made blinded to the results obtained from those other methods.
Results
As shown in Figure 1, the SNP genotyping assay can be divided into four steps: 1) multiplexed PCR, 2) multiplexed OLA, 3) hybridization/labeling 4) and detection using the Luminex flow cytometer. For an individual SNP the OLA involves a pair of allele specific primers and a 5’ phosphorylated common primer, with tag and capture sequences utilized in downstream hybridization and labeling reactions. If an allele is present, Taq DNA ligase facilitates ligation between the allele specific and common oligonucleotides, using PCR product as a template. The OLA product is then simultaneously hybridized to FlexMAP™ beads and the universal oligonucleotide, followed by labeling with streptavidin-phycoerythrin (SA-PE). The Luminex flow cytometer is then used to sort the FlexMAP™ beads and quantitate the signal from each unique bead, thereby allowing determination of the absence or presence of each allele. Of note, different signal to noise ratios are achieved with different SA-PE conjugates. Five SA-PE conjugates were tested, one from Invitrogen/Molecular Probes and four from ProZyme (PJ31S, PJ70S, PJ35S, PJ37S) with the best signal to noise ratio achieved with the Invitrogen/Molecular Probes SA-PE (data not shown).
A control experiment was performed to demonstrate that counting as few as 20 beads allows one to distinguish a positive and negative population of beads. In order to mimic an OLA reaction, we used a synthesized OLA product as a positive control and unligated OLA probes as a negative control (see Supplementary Material – Primer and Probe Sets). Sixteen replicate wells were assessed for each of four conditions: low beads/positive control, low beads/negative control, high beads/positive control, and high beads/negative control. Each of the four conditions was performed in a separate quadrant of the same 96-well plate, and positive and negative wells were alternated within each quadrant. Table 1 shows that inter-well CVs and range of MFIs are very similar when counting 20 beads versus 300, and that positive and negative MFIs differ by approximately an order of magnitude, providing a signal to noise ratio more than sufficient for accurate genotype calling. Importantly, inter-well CV and MFI were based on the median values obtained from each number of beads counted (20 or 300), and not the mean. Inter-well and intra-well CVs calculated using the mean were higher when counting low versus high number of beads (data not shown), likely due to a small percentage of bead carryover that occurs with the Luminex flow cytometer.
Table 1.
Inter-well CV and MFI range using low and high number of beads in control experiment
| 200 input/20 count | Positive control | Negative control |
|---|---|---|
| Avg. inter-well CV | 2% | 26% |
| Avg. MFI | 21,968 | 192 |
| MFI range | 21,003–22,842 | 62–299 |
| 5000 input/300 count | Positive control | Negative control |
| Avg. inter-well CV | 2% | 20% |
| Avg. MFI | 22,619 | 162 |
| MFI range | 21,496–23,022 | 115–205 |
Note: Median fluorescence intensity (MFI) was assessed using the high PMT setting of the Luminex 100 flow cytometer. Inter-well CVs were calculated manually and are based on the median.
The Luminex-based OLA assay, using 200 input beads and counting 20, was compared to two other genotyping methods to assess validity (see Supplementary Material – Primer and Probe Sets). First, eight SNPs were compared to direct sequencing in fourteen human DNA samples representing both homozygotes and heterozygotes, and concordance with direct sequencing was 100%. Second, a comparison was made between the multiplexed Luminex OLA and simplexed pyrosequencing. We compared 1471 genotypes from seven SNPs (Table 2), and concordance between Luminex OLA and pyrosequencing was excellent, averaging 99.52%, with the lowest concordance for any SNP at 98.80 %.
Table 2.
Pyrosequencing concordance
| SNP | concordant calls | % concordant |
|---|---|---|
| rs56387268 | 231/232 | 99.60% |
| rs1123005 | 287/289 | 99.30% |
| rs11806859 | 168/170 | 98.80% |
| rs12122048 | 58/58 | 100.00% |
| rs4657179 | 290/290 | 100.00% |
| rs4657187 | 167/168 | 99.40% |
| rs905720 | 263/264 | 99.62% |
| Total | 1464/1471 | 99.52% |
To assess robustness when counting 20 beads, replicate genotyping of an 8-plex assay was performed in 84 human genomic DNA samples (see Supplementary Material – Primer and Probe Sets). Two separate PCR amplifications were performed, and an OLA was performed in duplicate on each PCR product, for a total of four OLA assays on the same 8 SNPs in the same 84 individuals. 2685 of 2688 genotypes were “callable”, with a concordance of 100% between the 99.9% of genotypes that were callable (Table 3).
Table 3.
Call rates of technical replicates
| Assay | call rate | % call rate |
|---|---|---|
| OLA 1 – PCR 1 | 670/672 | 99.7% |
| OLA 2 – PCR 1 | 672/672 | 100% |
| OLA 3 – PCR 2 | 671/672 | 99.9% |
| OLA 4 – PCR 2 | 672/672 | 100% |
| Total | 2685/2688 | 99.9% |
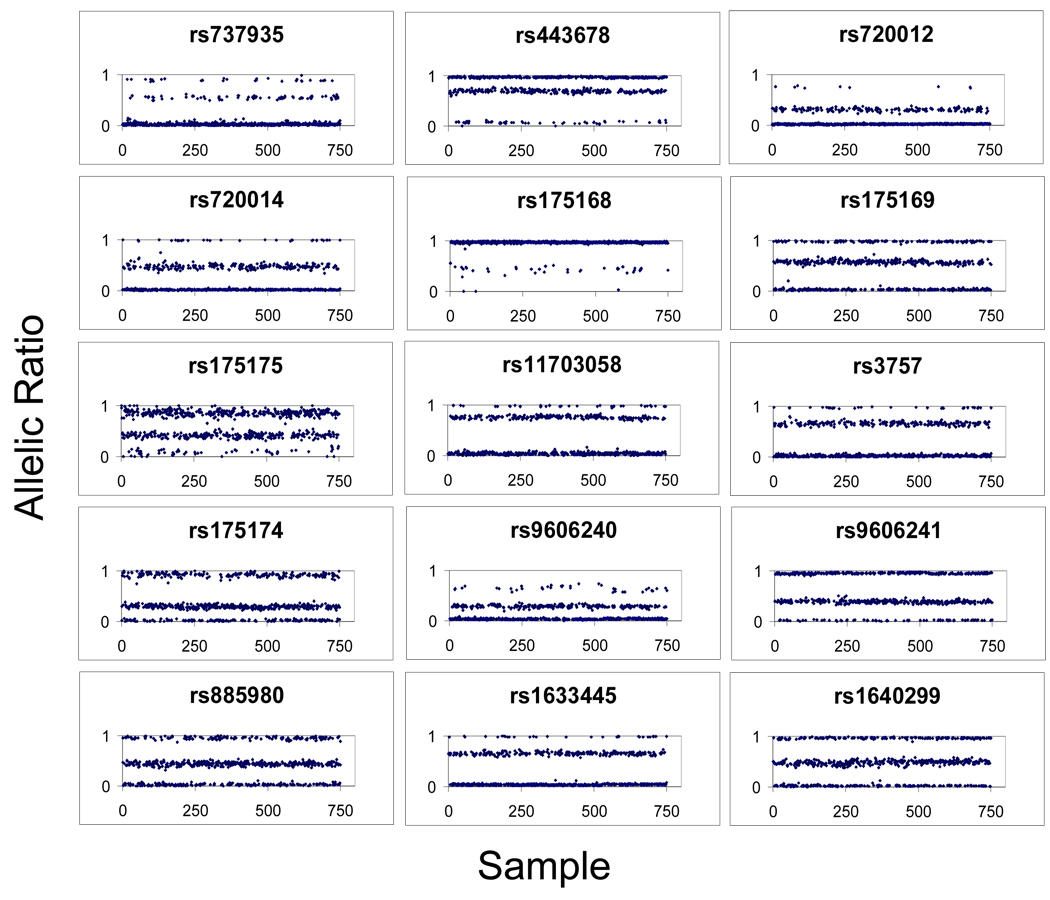
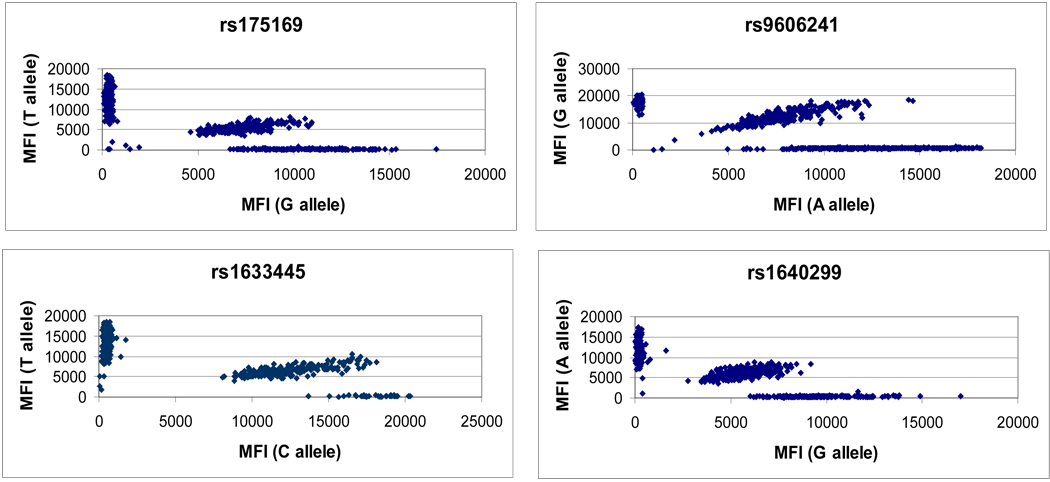
A 19-plex assay is presented in order to demonstrate typical results obtained from “first-pass” genotyping of 750 samples (see Supplementary Material – Primer and Probe Sets). Fifteen of nineteen assays were converted, with four failing due to the majority of MFI values falling below 1000. The approximately 80% conversion rate observed in this 19-plex assay is typical of the results observed across dozens of similar multiplexed panels, though more recent work indicates that this conversion rate can be increased by predicting nucleic acid interactions at the OLA step and carefully dividing multiplexed panels accordingly. Figure 2 shows allelic ratios (Y-axis) plotted against samples (X-axis) for the 15 working assays within the 19-plex assay. Figure 3 shows representative raw data corresponding to the allelic ratios for four of the SNPs presented in Figure 2. Table 4 gives the complete set of call rates and silhouette scores for the 15 working assays. 11,224 of 11,250 genotypes were callable (first-pass call rate 99.77 %), and the average silhouette score is >0.90.
Figure 2. Genotype clusters for 15-plex assay.
Individual figures represent the allelic ratios (X axis) produced from a first pass genotyping of 750 human genomic DNA samples (Y axis). Variability in allelic ratios is observed for different SNP assays, generally with skewing of heterozyote clusters, though homozygote clusters can also be skewed (i.e. rs9606240). Nonetheless, for each SNP genotype clusters are robust (see Table 4 for silhouette scores).
Figure 3. Raw data of 4 representative SNP assays.
Each scatterplot represents the median fluorescence intensity (MFI) of allele 1 and allele 2 for four SNPs. The scatterplots represent the raw data from which allelic ratios (figure 2) were calculated.
Table 4.
Call rates and silhouette scores for 15-plex assay
| Calls made | Percent callable | Silhouette score | |
|---|---|---|---|
| rs737935 | 746/750 | 99.5% | 0.938 |
| rs443678 | 750/750 | 100.0% | 0.932 |
| rs720012 | 750/750 | 100.0% | 0.876 |
| rs720014 | 750/750 | 100% | 0.916 |
| rs175168 | 748/750 | 99.7% | 0.914 |
| rs175169 | 748/750 | 99.7% | 0.942 |
| rs175175 | 744/750 | 99.2% | 0.777 |
| rs11703058 | 748/750 | 99.7% | 0.921 |
| rs3757 | 749/750 | 99.9% | 0.931 |
| rs175174 | 745/750 | 99.3% | 0.845 |
| rs9606240 | 750/750 | 100.0% | 0.821 |
| rs9606241 | 750/750 | 100.0% | 0.946 |
| rs885980 | 746/750 | 99.5% | 0.910 |
| rs1633445 | 750/750 | 100.0% | 0.952 |
| rs1640299 | 750/750 | 100.0% | 0.912 |
| Total or average | 11224/11250 | 99.77% | 0.902 |
Discussion
We have presented a method for multiplexed SNP genotyping on the Luminex platform that is accurate, robust, and significantly less inexpensive than previously published methods. Differences between this method and previously published Luminex-based methods include the use of significantly fewer beads, use of a universal biotinylated oligonucleotide for signal quantification, and no wash or centrifugation steps. We assessed concordance with both direct sequencing and pyrosequencing to establish validity, and observed 100% and >99% concordance respectively. Additional experiments with separate multiplexed assays demonstrated complete concordance across technical replicates and high call rates (>99%). Additionally, we presented a 19-plex assay in which 15 of 19 assays were converted. It should be noted that secondary SNPs within OLA probe binding sites can negatively impact conversion rates [17], and careful assay design requires knowledge of secondary SNPs. Cross-hybridization between nucleic acids present within single-tube, multiplexed OLA reaction can also reduce conversion rates (data not shown). Bioinformatics approaches which predict unwanted nucleic acid interactions could be used to rationally partition multiplexed OLA reactions and increase the conversion rate.
The cost is approximately $0.085 per genotype when performing both the PCR and OLA reactions in a 15-plex format (Table 5). The cost of other SNP genotyping methods is difficult to estimate, as innovations continue to drive down the cost of established methods and individual users find ways to achieve economies of scale. For example, while service-based TaqMan SNP genotyping costs are approximately $0.50 per genotype, advances in microfluidics are likely to significantly drive down this cost (http://www.fluidigm.com/). In general, per genotype costs of $0.085 are on the low-end of informal estimates of other medium throughput genotyping technologies, and our method should be useful to academic labs on limited budgets as well to core facilities requiring flexibility in performing targeted genetic studies. While the PCR step of our assay is time consuming (5 hours) due to specialized cycling parameters required for the multiplexed PCR reaction, workflows can be established that result in approximately 40,000 genotypes per day. In practice, we perform multiple PCR and OLA reactions in parallel, and can then analyze one plate per hour on the Luminex flow cytometer, with each 96-well plate producing 4,800 genotypes when multiplexing at 50 SNP assays per well.
Table 5.
Reagent costs per genotype
| Reagent | Cost ($) |
|---|---|
| FlexMAP™ microspheres | 0.0560 |
| PCR + OLA oligos | 0.0200 |
| Taq DNA ligase | 0.0030 |
| SA-PE | 0.0020 |
| 96-well plate | 0.0030 |
| Hybridization buffer | 0.0002 |
| Sheath fluid | 0.0007 |
| Total | $0.0849 |
Note: Calculations show the cost of each reagent per genotype generated. Cost of each reagent assumes that 15 SNPs are multiplexed at both the PCR and OLA steps; reagent costs decrease as multiplexing increases. Cost of PCR and OLA oligonucleotides assumes a sample size of 2000; oligonucleotide costs decrease as sample size increases, given that only a small portion of a typical OLA oligonucleotide order is utilized to genotype 2000 samples.
For this study, a combination of k-means clustering, Silhouette scores, and manual inspection of raw data was used for genotype calling (outlined in Materials and Methods). While this provides a method for semi-automated genotype calling, k-means clustering requires manual inspection of the raw data to ensure that the algorithm arrives at a reasonable solution. In this study there were two SNPs for which k-means clustering resulted in genotype designations which clearly did not agree with visual inspection of scatterplots. It appeared in both cases that high density genotype clusters were incorrectly divided, a problem for k-means clustering that has been noted elsewhere [30]. In both cases, running the k-means algorithm multiple times eventually resulted in a visually “correct” solution. Alternatively, we found that we could resolve the problem by splitting data from 750 samples into two separate data sets containing allelic ratios from 375 samples each. Neither of these solutions are ideal and refinements to the clustering algorithm, such as careful seeding of k centers, may prove to be useful [31].
No reports directly assess the “bead count” issue when performing SNP genotyping assays on the Luminex platform. One previous report has assessed the variability in measurements when counting different numbers of beads using limiting amounts of SA-PE or analyte, and concluded that under the appropriate conditions counting as few as ten beads can distinguish two populations [32]. The appropriate conditions included a high amount of SA-PE (or analyte) relative to bead number. The conditions of our assay utilize massively saturating amounts of SA-PE (equivalent to 1.5 × 10^8 molecules per bead) and analyte, so it is not surprising that we can clearly and robustly distinguish two populations when counting as few as 20 beads (and perhaps less). Of note, run times on the Luminex flow cytometer are impacted by the number of input beads and the proportion of those input bead one attempts to count. A typical 96-well plate assay using 5000 input beads (per well) and counting 100 beads has an approximately 30 minute run time, while using 200 input beads and counting 20 requires approximately 45 minutes; the degree of multiplexing does not dramatically affect run times. Issues of machine time-out begin to appear when trying to use less than 200 beads while still counting 20, or when trying to count more than 20 beads with only 200 input beads. Also, their may be a small percentage of well-to-well bead carryover using the Luminex flow cytometer. Using the median, rather than the mean, ensures that this carryover will not significantly affect the MFI and subsequent genotype calls when counting 20 beads. It is important to point out that we have only assessed using low bead counts for a qualitative, OLA SNP genotyping assay. In quantitative assays, where minimizing intra and inter-well variance is paramount, it may not be appropriate to use bead counts as low as 20. Using low bead counts may also not be appropriate for assays utilizing the direct hybridization format, where the non-target DNA strand will be available to compete with the probe-coupled microsphere for annealing to the target strand.
Supplementary Material
Acknowledgements
We thank Jared Hayter, Jaime Simone, and Irina Tereschenko for technical assistance. This work was supported by funding from grants R01MH062440, R01MH070366, R01MH080429, R01MH080429 and U24MH068457 from the National Institute of Mental Health and NARSAD and the Staglin Family Music Festival Schizophrenia Research Award.
Footnotes
Statement of Competing Interests
The authors declare no competing interests.
Additional data files
The sequence (and modifications) of all primer and probe sets used in this study are contained within “Supplementary Material – Primer and Probe Sets”.
References
- 1.Giacomini KM, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81(3):328–345. doi: 10.1038/sj.clpt.6100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas FJ, McLeod HL, Watters JW. Pharmacogenomics: the influence of genomic variation on drug response. Curr Top Med Chem. 2004;4(13):1399–1409. doi: 10.2174/1568026043387638. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson KL, et al. Whole-genome genotyping. Methods Enzymol. 2006;410:359–376. doi: 10.1016/S0076-6879(06)10017-8. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy GC, et al. Large-scale genotyping of complex DNA. Nat Biotechnol. 2003;21(10):1233–1237. doi: 10.1038/nbt869. [DOI] [PubMed] [Google Scholar]
- 5.Alderborn A, Kristofferson A, Hammerling U. Determination of single-nucleotide polymorphisms by real-time pyrophosphate DNA sequencing. Genome Res. 2000;10(8):1249–1258. doi: 10.1101/gr.10.8.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyamichev V, et al. Polymorphism identification and quantitative detection of genomic DNA by invasive cleavage of oligonucleotide probes. Nat Biotechnol. 1999;17(3):292–296. doi: 10.1038/7044. [DOI] [PubMed] [Google Scholar]
- 7.Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14(5–6):143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 8.Tobler AR, et al. The SNPlex genotyping system: a flexible and scalable platform for SNP genotyping. J Biomol Tech. 2005;16(4):398–406. [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar SA, et al. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAP system. J Microbiol Methods. 2003;53(2):245–252. doi: 10.1016/s0167-7012(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 11.Flagella M, et al. A multiplex branched DNA assay for parallel quantitative gene expression profiling. Anal Biochem. 2006;352(1):50–60. doi: 10.1016/j.ab.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, et al. Small interfering RNA and gene expression analysis using a multiplex branched DNA assay without RNA purification. J Biomol Screen. 2005;10(6):549–556. doi: 10.1177/1087057105277414. [DOI] [PubMed] [Google Scholar]
- 13.Landegren U, et al. A ligase-mediated gene detection technique. Science. 1988;241(4869):1077–1080. doi: 10.1126/science.3413476. [DOI] [PubMed] [Google Scholar]
- 14.Iannone MA, et al. Multiplexed single nucleotide polymorphism genotyping by oligonucleotide ligation and flow cytometry. Cytometry. 2000;39(2):131–140. [PubMed] [Google Scholar]
- 15.McNamara DT, et al. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg. 2006;74(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- 16.Mehlotra RK, et al. Prevalence of CYP2B6 alleles in malaria-endemic populations of West Africa and Papua New Guinea. Eur J Clin Pharmacol. 2006;62(4):267–275. doi: 10.1007/s00228-005-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald SJ, et al. A low-cost open-source SNP genotyping platform for association mapping applications. Genome Biol. 2005;6(12):R105. doi: 10.1186/gb-2005-6-12-r105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunbar SA. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin Chim Acta. 2006;363(1–2):71–82. doi: 10.1016/j.cccn.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bortolin S, et al. Analytical validation of the tag-it high-throughput microsphere-based universal array genotyping platform: application to the multiplex detection of a panel of thrombophilia-associated single-nucleotide polymorphisms. Clin Chem. 2004;50(11):2028–2036. doi: 10.1373/clinchem.2004.035071. [DOI] [PubMed] [Google Scholar]
- 20.Pickering JW, et al. Flow cytometric assay for genotyping cytochrome p450 2C9 and 2C19: comparison with a microelectronic DNA array. Am J Pharmacogenomics. 2004;4(3):199–207. doi: 10.2165/00129785-200404030-00007. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JD, et al. Flow cytometric platform for high-throughput single nucleotide polymorphism analysis. Biotechniques. 2001;30(3):661–666. 668–669. doi: 10.2144/01303dd04. [DOI] [PubMed] [Google Scholar]
- 22.Ye F, et al. Fluorescent microsphere-based readout technology for multiplexed human single nucleotide polymorphism analysis and bacterial identification. Hum Mutat. 2001;17(4):305–316. doi: 10.1002/humu.28. [DOI] [PubMed] [Google Scholar]
- 23.Wang HY, et al. A genotyping system capable of simultaneously analyzing > 1000 single nucleotide polymorphisms in a haploid genome. Genome Res. 2005;15(2):276–283. doi: 10.1101/gr.2885205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brzustowicz LM, et al. Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am J Hum Genet. 2004;74(5):1057–1063. doi: 10.1086/420774. Epub 2004 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saviouk V, et al. Association of synapsin 2 with schizophrenia in families of Northern European ancestry. Schizophrenia Research. 2007;96(1#x02013;3):100–111. doi: 10.1016/j.schres.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadian A, et al. Single-nucleotide polymorphism analysis by pyrosequencing. Anal Biochem. 2000;280(1):103–110. doi: 10.1006/abio.2000.4493. [DOI] [PubMed] [Google Scholar]
- 27.Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281(5375):363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 28.Eisen MB, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovmar L, et al. Silhouette scores for assessment of SNP genotype clusters. BMC Genomics. 2005;6(1):35. doi: 10.1186/1471-2164-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huentelman MJ, et al. SNiPer: improved SNP genotype calling for Affymetrix 10K GeneChip microarray data. BMC Genomics. 2005;6:149. doi: 10.1186/1471-2164-6-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur D, Vassilvitskii S. k-means++ The Advantages of Careful Seeding. 2007 Symposium on Discrete Algorithms. 2007 [Google Scholar]
- 32.Jacobson JW, et al. Analysis of individual data from bead-based assays ("bead arrays") Cytometry A. 2006;69(5):384–390. doi: 10.1002/cyto.a.20293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.