Abstract
Background
Insomnia complaints are common in older adults and may be associated with mortality risk. However, evidence regarding this association is mixed. We thus prospectively examined whether men with insomnia symptoms had an increased risk of mortality during 6 years of follow-up.
Methods and Results
A prospective cohort study of 23,447 US men participating in the Health Professionals Follow-up Study and free of cancer, reported on insomnia symptoms in 2004 were followed through 2010. Deaths were identified from state vital statistic records, the National Death Index, family reports, and the postal system. We documented 2025 deaths during 6 years of follow-up (2004-2010). The multivariable-adjusted hazard ratios (HRs) of total mortality were 1.25 (95% confidence interval (CI):1.04-1.50) for difficulty initiating sleep, 1.09 (95%CI:0.97-1.24) for difficulty maintaining sleep, 1.04 (95%CI:0.88-1.22) for early-morning awakenings, and 1.24 (95%CI:1.05-1.46) for non-restorative sleep, comparing men with those symptoms most of the time to men without those symptoms, after adjusting for age, lifestyle factors and presence of common chronic conditions. Men with difficulty initiating sleep and non-restorative sleep most of the time had a 55% (HR:1.55; 95% CI:1.19-2.04; P-trend= 0.01) and 32% (HR:1.32; 95% CI:1.02-1.72; P-trend=0.002) increased risk of CVD mortality, respectively, relative to men without those symptoms.
Conclusion
Some insomnia symptoms, especially difficulty initiating asleep and non-restorative sleep, are associated with a modestly higher risk of mortality.
Keywords: Mortality, Sleep Disorders, Cardiovascular Outcomes, Insomnia
Insomnia, the most common sleep/wake disorder, is characterized by difficulty initiating sleep, difficulty maintaining sleep, early morning awakenings or by non-restorative sleep1,2. Inadequate or unrefreshing nighttime sleep of insomniacs is accompanied by significant distress, daytime fatigue, and the likelihood of falling asleep during the day1,3-8. Insomnia affects ten percent to one third of the general population in the United States7 depending on its definition. The total cost associated with insomnia is estimated at $92.5 to $107.5 billion annually in the US9.
Insomnia in older adults is of particular concern because it could increase risk of injury10, impaired quality of life6, cognitive impairment11, depression12 and metabolic syndrome.13 Insomnia is also associated with a moderately increased risk for cardiovascular diseases14,15. In this context, insomnia has been thought to influence total mortality, and cardiovascular mortality specifically, but regarding results to date have been inconsistent16-19. We thus examined whether men with insomnia symptoms had an increased risk of all cause and cause specific mortality in the Health Professionals Follow-up Study (HPFS), taking into account the effects of a variety of lifestyle factors and prevalent medical morbidities which are known to be associated with mortality risk. To further test our research hypothesis, we also conducted a meta-analysis including the current study with another 9 previously published studies8,20-27 regarding the association between insomnia symptoms and mortality.
MATERIALS AND METHODS
Ethics Statement
The institutional review board at Brigham and Women's Hospital and Harvard School of Public Health reviewed and approved this study, and receipt of each questionnaire implied participant's consent.
Study population
The HPFS was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40–75 years completed a mailed questionnaire about their medical history and lifestyle. Follow-up questionnaires were mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases.
In 2004, 34, 884 men responded to the 2004 questionnaire, which included questions about insomnia. We excluded men with a cancer diagnosis (other than non-melanoma skin cancer, n=7590) to reduce the potential for an effect of disease on insomnia symptoms, and men with missing values for insomnia questions (n=3847), leaving 23,447 men for this analysis.
Assessment of insomnia symptoms
In 2004, the participants in HPFS were asked how often (rarely/never, sometimes or most of the time) they: (1) “have difficulty falling asleep” (referred to as “difficulty initiating sleep” in the manuscript), (2) “have trouble with waking up during the night” (referred to as “difficulty maintaining sleep”), (3) “are troubled by waking up too early and not being able to fall asleep again” (referred to as “early-morning awakenings”), and (4) “feel really rested when waking up in the morning” (we code non-restorative sleep frequency as the opposite of feeling rested when waking up in the morning (referred to “non-restorative sleep”)). Excessive daytime sleepiness was also assessed in 2004 with a question of “get so sleepy during the day or the evening that have to take a nap”.
In addition to individual insomnia symptoms described above, we defined insomnia disorder as the combination of a nocturnal insomnia symptom and a resulting consequence, in two ways: having one or more of the three nocturnal insomnia symptoms (difficulty initiating sleep, difficulty maintaining sleep, and early morning awakenings), accompanied by 1) non-restorative sleep (“insomnia disorder A”); and 2) excessive daytime sleepiness (“insomnia disorder B”).
Ascertainment of deaths
Deaths were identified from state vital statistics records, the National Death Index, reported by the families, and the postal system28. The follow-up for death in the HPFS was at least 98% complete. Cause of death was identified from death certificates or review of medical records. In the current analysis, we evaluated all-cause mortality, and also death from cardiovascular diseases (CVD) (International Classification of Diseases, eighth revision (ICD-8) codes 390 through 458), cancer (ICD-8 codes 140 through 207), and other conditions.
Assessment of other covariates
Information on potential confounders, such as age, ethnicity, smoking status, weight, height, physical activity, marriage status, living status, medication use ( e.g., aspirin, antidepressant, tranquilizers, melatonin and antihypertensive drugs), and history of major chronic conditions {e.g., elevated total cholesterol, elevated triglyceride, hypertension, diabetes, myocardial infarction, stroke, and lower urinary tract symptoms(LUTS)29} was collected via biennial questionnaires. Information on sleep duration and snoring frequency was collected in the 2000 questionnaire. Information on food and alcohol consumption was collected every four years via a validated semi-quantitative food frequency questionnaire30.
Body mass index (BMI) was calculated as weight (kg)/height (m)2. High blood pressure was considered as either professionally diagnosed hypertension or use of antihypertensive medications. A participant was considered as having depression symptoms if he reported “sad, blue or depressed” for two weeks or longer in the past two years or regular use of antidepressant medications. The phobic anxiety status was assessed by the Crown-Crisp phobia index31. Information on LUTS was collected based on the American Urological Association symptom index (AUASI) (29). Diet quality was assessed by the Alternate Healthy Eating Index (AHEI), which is associated with a lower risk of major chronic diseases and death in this cohort32.
Statistical analyses
We used Cox proportional hazard models to calculate the hazard ratios (HRs) of mortality and their 95% confidence intervals (CIs), across categories of each insomnia symptom. For tests of trend, we assigned a numeric value of 0 to 2 to the insomnia categories (0: rarely/never have insomnia complaint; 1: sometimes; 2: most of the time) and treated it as a continuous variable.
In addition to an age-adjusted model, we also ran two multivariable adjusted models. In the first model, we simultaneously adjusted for the known risk factors of mortality, except for presence of chronic conditions and sleep related variables, because they are possible biologic intermediates in the relationship between insomnia symptoms and total mortality. The adjusted covariates in the multivariable model 1 included: age, ethnicity, smoking status, alcohol drinking, BMI, physical activity, alternate healthy eating index, marriage status, and living status. The second model further included regular use of aspirin, the Crown-Crisp phobic anxiety index, LUTS, presence of chronic conditions, use of medications, sleep duration and snoring frequency. Time-varying covariates were used to reflect the most recent information, except for ethnicity, sleep duration and snoring frequency. If data were missing at a given time point, the last observation was carried forward for 1 cycle. We also did secondary analyses by using baseline covariates in the models.
We examined the joint effect on total mortality of difficulty initiating sleep with non-restorative sleep and excessive daytime sleepiness, separately. Because insomnia could result in depression symptoms12, we also tested the joint effect between difficulty initiating sleep and depression. We tested the interaction by comparing the -2 log likelihood of the models with and without interaction term. To minimize potential residual confounding due to co-morbidities of insomnia, we conducted sensitivity analyses excluding men with prevalent cardiovascular disease, and then further excluding frequent snoring (snoring every night or most night), diabetes and Parkinson's disease at baseline. We conducted another sensitivity analysis by excluding men who reported tranquilizer use (e.g., valium and xanax) or melatonin, two groups of commonly used hypnotic medications, through the end of follow-up, because benzodiazepines can cause several adverse effects33 and thus bias the observed insomnia-mortality relationship.
Finally, we conducted a meta-analysis to combine our study with previously published studies on insomnia symptoms and total and CVD mortality. Relevant studies were identified through searches of PubMed using the keywords of (insomnia OR sleep complaints OR difficulty falling asleep OR difficulty initiating sleep OR waking up at night OR waking up early OR early-morning awakening OR difficulty maintaining sleep OR non-restorative sleep OR no restorative sleep) AND (dead OR death OR mortality OR survival) for all published studies in English by July 31th, 2013. In addition, the reference lists from the relevant publications were used to identify additional studies. We focused on individual insomnia symptoms in the meta-analysis because there was no universal definition of insomnia across previous studies16-27. In this meta-analysis, we did not include the studies 1) that reported the association between insomnia and mortality but did not provide results regarding individual insomnia symptoms; 2) that reported association but did not provide relative risk value; or 3) that did not include control individuals assessed in the same study. We identified 9 studies8,20-27 which met our criteria for the meta-analysis (Supplemental Table 1). We used the Q statistic to examine heterogeneity among the studies. We used random-effects models to calculate the pooled HR because a significant heterogeneity (P<0.1) was observed. Publication bias was assessed using the Begg's test.
We used the SAS statistical package (version 9: SAS Institute, Cary, NC) for the cohort analyses, and STATA (version 9.0; College Station, TX) for the meta-analysis.
RESULTS
Basic characteristics
In this cohort, 4.1%, 25.2%, 7.7%, 6.2% and 11.1% of men reported difficulty initiating sleep, difficulty maintaining sleep, early-morning awakenings, non-restorative sleep or excessive daytime sleepiness most of the time at baseline, respectively. The characteristics of the study population according to difficulty initiating sleep were presented in Table 1. The other three insomnia symptoms showed similar associations to these characteristics (data not shown). In general, insomnia symptoms were associated with lower physical activity, a higher BMI, and a higher prevalence of depression symptoms, hypertension, elevated total cholesterol, elevated triglycerides, diabetes, myocardial infarction and stroke.
Table 1.
Baseline characteristics of participants according to difficulty initiating sleep status*
| Difficulty Initiating Sleep |
|||
|---|---|---|---|
| Rarely or never | Sometimes | Most of the time | |
| Participants (n) | 16811 | 5666 | 970 |
| Percentage, % | 71.7 | 24.2 | 4.1 |
| Age, years | 68.5 | 68.6 | 68.7 |
| Body mass index, kg/m2 | 26.1 | 26.3 | 26.6 |
| Caucasian, % | 96.6 | 95.3 | 96.0 |
| Marriage status (married), % | 88.6 | 86.2 | 84.6 |
| Living alone, % | 8.3 | 10.5 | 11.7 |
| Past smokers, % | 53.3 | 55.1 | 55.0 |
| Current smoker, % | 3.3 | 3.9 | 3.9 |
| Alcohol, g/day | 13.4 | 12.4 | 11.6 |
| Physical activity, MET-h/week | 47.2 | 42.8 | 37.9 |
| Alternate Healthy Eating Index | 51.6 | 50.9 | 50.2 |
| Use of aspirin, % | 59.8 | 62.3 | 60.1 |
| Use of Valium or other tranquilizers, % | 3.6 | 8.3 | 18.0 |
| Use of melatonin | 2.8 | 5.1 | 11.0 |
| Sleep duration (hours/day) | 7.1 | 6.9 | 6.5 |
| Frequent snoring, % | 35.0 | 34.1 | 35.3 |
| Phobic Anxiety Index | 1.9 | 2.2 | 2.4 |
| Lower Urinary Tract Symptoms Score | 5.0 | 6.0 | 7.2 |
| Depression symptoms, %† | 7.9 | 16.1 | 30.5 |
| Elevated cholesterol, % | 58.6 | 63.0 | 70.3 |
| Elevated triglycerides, % | 41.0 | 47.5 | 56.3 |
| High blood pressure, %‡ | 53.8 | 58.2 | 68.0 |
| Diabetes, % | 9.1 | 10.7 | 14.7 |
| Myocardial infarction, % | 8.7 | 10.8 | 12.7 |
| Stroke, % | 2.8 | 3.4 | 3.6 |
Abbreviation: MET, metabolic equivalents from recreational and leisure-time activities.
Values are means or percentages and are standardized to the age distribution of the study population except age.
Depression symptom was defined as use of antidepressant medications or feel blue, sad or depressed
High blood pressure was considered as either hypertension or use of antihypertensive medications.
Insomnia symptoms and total mortality
We documented 2025 deaths during 6 years of follow-up (127, 768 person years). Men with difficulty initiating sleep and non-restorative sleep had an increased risk of total mortality compared to men without those symptoms in a dose-dependent manner (Table 2), which was independent of a variety of risk factors for mortality. In the fully adjusted models, the HRs (95% CIs) of total mortality were 1.25 (95% CI: 1.04-1.50) for men with difficulty initiating sleep most of the time and 1.24 (1.05-1.46) for men with non-restorative sleep most of the time, compared to those without those symptoms (P-trend< 0.03 for both). We found no significant associations between difficulty maintaining sleep or early-morning awakenings and total mortality (Table 2). Similarly, excessive daytime sleepiness was also significantly associated with total mortality; the multivariable-adjusted HR comparing the two extreme categories of this symptom was 1.24 (95% CI: 1.09-1.42; P-trend=0.0006). The associations between the insomnia symptoms and mortality did not materially change after further adjustment for use of tranquilizers or excessive daytime sleepiness (data not shown).
Table 2.
Hazard ratio of mortality according to insomnia symptoms
| Insomnia symptoms | Death/Person years | Hazard Ratio (95% Confidence Interval) |
||
|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | ||
| Difficulty initiating sleep | ||||
| Rarely or never | 1285/91 993 | Reference | Reference | Reference |
| Sometimes | 594/30 661 | 1.11(1.00-1.22) | 1.06(0.96-1.17) | 1.05(0.95-1.17) |
| Most of the time | 146/5114 | 1.41(1.19-1.68) | 1.27(1.07-1.52) | 1.25(1.04-1.50) |
| P for trend | 0.0001 | 0.01 | 0.03 | |
| Yes versus No§ | 1.15(1.05-1.26) | 1.09(1.00-1.20) | 1.08(0.99-1.19) | |
| Difficulty maintaining sleep | ||||
| Rarely or never | 542/38 183 | Reference | Reference | Reference |
| Sometimes | 878/57 573 | 0.99(0.89-1.10) | 1.06(0.96-1.19) | 1.08(0.97-1.21) |
| Most of the time | 605/32 012 | 1.02(0.91-1.14) | 1.09(0.97-1.23) | 1.09(0.97-1.24) |
| P for trend | 0.76 | 0.15 | 0.15 | |
| Yes versus No§ | 1.00(0.91-1.11) | 1.07(0.97-1.19) | 1.09(0.98-1.20) | |
| Early-morning awakenings | ||||
| Rarely or never | 1006/63 318 | Reference | Reference | Reference |
| Sometimes | 840/54 747 | 0.92(0.83-1.00) | 0.96(0.87-1.05) | 0.96(0.88-1.06) |
| Most of the time | 179/9703 | 1.00(0.85-1.17) | 1.02(0.87-1.20) | 1.04(0.88-1.22) |
| P for trend | 0.30 | 0.73 | 0.87 | |
| Yes versus No§ | 0.93(0.85-1.01) | 0.97(0.89-1.06) | 0.97(0.89-1.07) | |
| Non-restorative sleep | ||||
| Rarely or never | 1326/90 340 | Reference | Reference | Reference |
| Sometimes | 519/29 665 | 1.24(1.12-1.37) | 1.13(1.02-1.25) | 1.09(0.98-1.21) |
| Most of the time | 180/7763 | 1.54(1.32-1.80) | 1.35(1.15-1.58) | 1.24(1.05-1.46) |
| P for trend | <0.0001 | <0.0001 | 0.006 | |
| Yes versus No§ | 1.31(1.19-1.43) | 1.18(1.07-1.29) | 1.12(1.02-1.24) | |
Model 1 adjusted for age;
Model 2: model 1 plus ethnicity (Caucasian, yes/no), smoking status (never smoker, former smoker, or current smoker), alcohol drinking (g/d: 0, 0.1-9.9, 10.0-19.9, 20.0-29.9, and ≥30), body mass index (kg/m2: <23, 23-24.9, 25-26.0, 27-29.9, ≥30), physical activity (quintiles), alternate healthy eating index (quintile), marriage status (married, divorced/separate/single, widowed); living status (alone or not);
Model 3: model 2 plus regular use of aspirin (yes/no), the Crown-Crisp phobic anxiety index (0-1,2,3 or>3), lower urinary tract symptoms (0-6, 7-14, ≥15), feel sad, blue or depressed two more weeks (yes/no), use of antidepressant drugs (yes/no), presence of elevated total cholesterol, high blood pressure, elevated triglyceride, diabetes, myocardial infarction and stroke (each yes vs. no), sleep duration (hours: ≤5, 6, 7, 8, ≥9), and snoring frequency (every night, most night, few night per week, occasionally, once a week or less, missing).
Yes versus No: having the insomnia symptom sometimes or most of the time versus rarely/never.
The multivariable-adjusted HR of total mortality was 1.13 (95% CI: 1.03-1.25) for the insomnia disorder A, defined as having any of the three nocturnal insomnia symptoms accompanied by non-restorative sleep, and 1.13 (1.03-1.24) for the insomnia disorder B defined as having any of the three nocturnal symptoms accompanied by excessive daytime sleepiness.
We also observed that men with both difficulty initiating sleep and non-restorative sleep (Figure 1A) or both difficulty initiating sleep and excessive daytime sleepiness (Figure 1B) had the highest risk of total mortality compared to those without these symptoms. A similar pattern was observed for the combination of difficulty initiating sleep and depression symptoms (Figure 1C).
Figure 1.


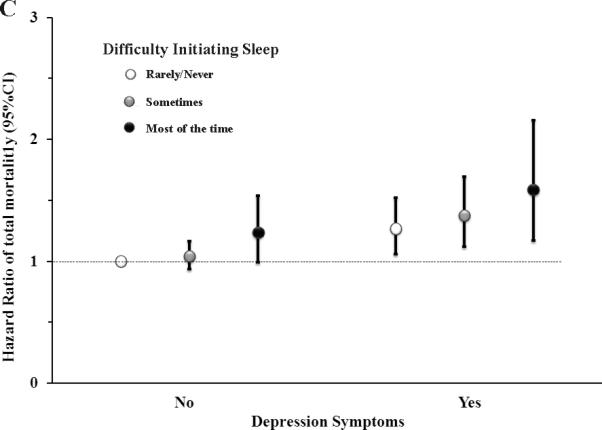
Hazard ratio of total mortality according to the joint classification of difficulty initiating sleep with non-restorative sleep (A), excessive daytime sleepiness (B) and Depression symptoms (C) *,†,‡. *Up to six years of follow-up (2004-2010) of the Health Professionals Follow-up Study;†Multivariable adjusted hazard ratio estimated from Cox proportional hazards models adjusted for age, ethnicity (Caucasian, yes/no), smoking status (never smoker, former smoker, or current smoker), alcohol drinking (g/d: 0, 0.1-9.9, 10.0-19.9, 20.0-29.9, and ≥30), body mass index (kg/m2: <23, 23-24.9, 25-26.0, 27-29.9, ≥30), physical activity (quintiles), alternate healthy eating index (quintile), marriage status (married, divorced/separate/single, widowed); living status (alone or not), regular use of aspirin (yes/no), the Crown-Crisp phobic anxiety index (0-1,2,3 or>3), lower urinary tract symptoms (0-6, 7-14, ≥15), presence of elevated total cholesterol, high blood pressure, elevated triglyceride, diabetes, myocardial infarction and stroke (each yes vs. no), sleep duration (hours: ≤5, 6, 7, 8, ≥9), snoring frequency (every night, most night, few night per week, occasionally, once a week or less, missing); feel sad, blue or depressed two more weeks, use of antidepressant drugs, non-restorative sleep and excessive daytime sleepiness, except the joint variable. ‡P for interaction was 0.98 for difficulty initiating sleep and non-restorative sleep (A), 0.96 for difficulty initiating sleep and excessive daytime sleepiness (B), and 0.72 for difficulty initiating sleep and depression symptom (C), tested by comparing the -2 log likelihood of the model including interaction term with the model that contained only the main effects.
Insomnia and cause-specific mortality
Men with difficulty initiating sleep and non-restorative sleep most of the time had a 55% (HR: 1.55; 95% CI: 1.19-2.04; P-trend= 0.01) and 32% (HR: 1.32; 95% CI: 1.02-1.72; P-trend=0.002) increased risk of CVD mortality, respectively, relative to men without those symptoms (Table 3). We did not observe significant associations between insomnia symptoms and mortality due to cancer or other causes, but these results were limited to rapidly fatal cancer, as those with prevalent cancer were excluded at baseline.
Table 3.
Risk of cause-specific mortality according to insomnia symptoms*
| Insomnia symptoms | CVD mortality (case #=741) | Cancer mortality (case #=493) | Others (case #=791) |
|---|---|---|---|
| Difficulty initiating sleep | |||
| Rarely or never | Reference | Reference | Reference |
| Sometimes | 1.06(0.89-1.25) | 0.96(0.78-1.19) | 1.11(0.95-1.30) |
| Most of the time | 1.55(1.19-2.04) | 1.04(0.69-1.58) | 1.02(0.75-1.40) |
| P for trend | 0.01 | 0.90 | 0.38 |
| Difficulty maintaining sleep | |||
| Rarely or never | Reference | Reference | Reference |
| Sometimes | 1.02(0.85-1.22) | 1.20(0.96-1.50) | 1.05(0.88-1.25) |
| Most of the time | 0.99(0.81-1.21) | 1.12(0.87-1.44) | 1.12(0.92-1.36) |
| P for trend | 0.94 | 0.37 | 0.26 |
| Early-morning awakenings | |||
| Rarely or never | Reference | Reference | Reference |
| Sometimes | 1.04(0.89-1.21) | 0.96(0.79-1.15) | 0.91(0.78-1.05) |
| Most of the time | 1.09(0.83-1.43) | 1.08(0.77-1.51) | 0.97(0.74-1.27) |
| P for trend | 0.49 | 0.99 | 0.39 |
| Non-restorative sleep | |||
| Rarely or never | Reference | Reference | Reference |
| Sometimes | 1.32(1.12-1.57) | 0.88(0.70-1.10) | 1.02(0.86-1.21) |
| Most of the time | 1.32(1.02-1.72) | 1.14(0.80-1.61) | 1.16(0.89-1.50) |
| P for trend | 0.002 | 0.92 | 0.35 |
Adjusted for age, ethnicity (Caucasian, yes/no), smoking status (never smoker, former smoker, or current smoker), alcohol drinking (g/d: 0, 0.1-9.9, 10.0-19.9, 20.0-29.9, and ≥30), body mass index (kg/m2: <23, 23-24.9, 25-26.0, 27-29.9, ≥30), physical activity (quintiles), alternate healthy eating index (quintile), marriage status (married, divorced/separate/single, widowed); living status (alone or not), regular use of aspirin (yes/no), the Crown-Crisp phobic anxiety index (0-1,2,3 or>3), lower urinary tract symptoms (0-6, 7-14, ≥15), feel sad, blue or depressed two more weeks (yes/no), use of antidepressant drugs (yes/no), presence of elevated total cholesterol, high blood pressure, elevated triglyceride, diabetes, myocardial infarction and stroke (each yes vs. no), sleep duration (hours: ≤5, 6, 7, 8, ≥9), snoring frequency (every night, most night, few night per week, occasionally, once a week or less, missing).
Sensitivity analyses
After we excluded men with prevalent cardiovascular diseases at baseline, the multivariable-adjusted HRs were 1.25 (95% CI: 0.99-1.56; P-trend=0.02) for total mortality and 1.45 (95% CI:1.02-2.06; P-trend=0.04) for CVD mortality, comparing men with difficulty initiating sleep most of the time to those without this symptom. The multivariable-adjusted HRs among men with non-restorative sleep most of the time were 1.26 (95% CI: 1.04-1.54; P-trend=0.02) for total mortality and 1.37 (95% CI: 0.98-1.91; P-trend=0.005) for CVD mortality. The significant associations between difficulty initiating sleep, non-restorative sleep and total mortality were basically unchanged even after we further excluded participants with frequent snoring, diabetes and Parkinson's disease, or when we used covariates at baseline in the models (P trend<0.05 for both). Because a previous study19 suggested that a short sleep duration insomnia (<6 hours) was associated with increased mortality risk, we also treated the covariate of sleep duration as a binary variable (<6 versus ≥6 hours) and obtained similar results (data not shown). After we excluded participants who used tranquilizers or melatonin through the follow-up, the adjusted HRs comparing the two extreme categories were 1.34 (95% CI: 1.09-1.66; P-trend=0.02) for difficulty initiating sleep and 1.21 (95% CI: 1.01-1.45; P-trend=0.01) for non-restorative sleep.
Meta-analysis
We pooled the present study and 9 other published studies8,20-27 that evaluated the associations between individual insomnia symptoms and total mortality. The pooled HRs of total mortality were 1.14 (95%CI: 1.04-1.24) for difficulty initiating sleep, 1.08 (95%CI: 0.96-1.22) for difficulty maintaining sleep, 1.00 (95%CI: 0.94-1.06) for early-morning awakenings, and 1.17 (95%CI: 1.01-1.36) for non-restorative sleep, relative to individuals without those symptoms (Figure 2). The results did not change materially after excluding two studies21,23 with low quality score (Supplemental Table 1) based on Newcastle-Ottawa Quality Assessment Scale (Figure 2). We also conducted a meta-analysis using data from present study and five published studies22,25-27,34 that examined the associations between insomnia symptoms and CVD mortality. The pooled HR of CVD mortality was 1.45 (95%CI: 95%CI: 1.09-1.93) for difficulty initiating sleep, 1.03 (95%CI: 0.89-1.17) for difficulty maintaining sleep, and 1.00 (95%CI: 0.89-1.13) for early-morning awakenings (Figure 3). However, the association between non-restorative sleep and CVD mortality was not examined previously. Further excluding the present prospective study from the meta-analyses did not change the pooled results between insomnia and total/CVD mortality materially (Supplemental Figure 1 and Supplemental Figure 2).
Figure 2.
Meta-analysis of the association between individual insomnia symptoms and total mortality *,†,‡. *Publication bias are estimated by the Begg's test, all P>0.4; †The name of the study and full list of covariates each study adjusted for were listed in Supplemental Table 1; ‡Pooled, Total = pooled relative risk for all studies listed in the Figure; Pooled sensitivity = pooled relative risk for studies excluded the two studies, e.g. Althuis, 1998 (21) and Newman, 2000 (23), with the Newcastle-Ottawa Quality Score <7 (Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed August 8, 2013.).
Figure 3.
Meta-analysis of the association between individual insomnia symptoms and cardiovascular mortality (publication bias are estimated by the Begg's test, all P>0.4, there is no other published data of non-restorative sleep and cardiovascular mortality).
DISCUSSION
In this large prospective cohort, we observed that men with difficulty initiating sleep and those with non-restorative sleep had a modest but significantly increased and dose-dependent risk of total mortality compared to men without those symptoms. The increased risk was independent of a variety of risk factors for mortality including lifestyle factors and presence of several medical morbidities.
Normal sleep continuity is considered to be important for the maintenance of cardiovascular, metabolic and immune function, physiological homeostasis and psychological balance35,36. Suboptimal sleep disturbs both circadian rhythms and other physiological systems35,36. Sleep disturbance has been shown to adversely influence metabolism and endocrine function similar to the effects of premature aging37, including reducing endogenous testosterone levels38, altering the hypothalamic pituitary adrenal axis39, and elevating markers of chronic inflammation35. Insomnia has also been associated with incident depression12, a risk factor for cardiovascular morbidity and mortality40. Thus, insomnia may increase mortality risk through effects on several biological pathways. This notion has been supported by a study conducted by Dew and colleagues16, who assessed sleep status of 184 healthy older adults via polysomnography. They found that insomnia symptoms, such as difficulty falling asleep and poor sleep efficiency, were associated with an almost doubled risk of all-cause mortality (n=66) during 12 years of follow-up16. The current study with a much larger sample size and the meta-analysis further confirmed that observation. In the present study, we controlled for multiple confounders using updated information on lifestyle risk factors and common chronic diseases over the course of follow-up. In addition, we conducted a sensitivity analysis by excluding the participants with CVD and depression, and found that the association between insomnia symptoms and mortality was basically unchanged. Though the possibility of residual confounding cannot be completely excluded, our study, together with the previous studies14,41 suggests that chronic disorders cannot totally explain the observed association between insomnia symptoms and total mortality. It is thus likely that sleep disturbances characterized by insomnia symptoms represent novel risk factors for mortality.
Several epidemiological studies have found significant associations between insomnia and CVD intermediate markers and risk factors, such as carotid intima-media thickness42; cardiorespiratory fitnes43, and Framingham risk score44. Difficulty initiating sleep was associated with 20-year incidence of myocardial infarction or coronary death among women in the Framingham Study34. Trouble initiating sleep, but not difficulty maintaining sleep or early-morning awakenings, was reported to be associated with a higher risk of CVD mortality in a cohort of middle-aged Swedish men22, the Piedmont Health Survey15,45 and the Malmo Preventive study25. Difficulty initiating sleep also appeared to have the strongest and the most robust association with acute myocardial infarction14. Biologically, it is plausible that prolonged sleep latency or reduced sleep maintenance have different effects on sleep stage distribution and associated neurohormonal activities16,46. Further, delayed sleep could also lead to alterations in circadian rhythms47, which are important for cardiovascular disease pathogenesis. Most previous epidemiological studies focused on three nocturnal insomnia symptoms: difficulty initiating sleep, difficulty maintaining sleep and early-morning awakenings 20,21,23,26,27. Non-restorative sleep, another important component of insomnia, is included in criteria of insomnia recommended by NIH1, DSM-IV48 and ICSD-II49, but few studies have addressed its potential effects on mortality8,24, because there is controversy as to whether individuals with this complaint share similar pathophysiologic mechanisms with the other nocturnal symptoms. The finding of the present cohort study and meta-analysis indicates an increased higher risk of total mortality among people with non-restorative sleep. Excessive daytime sleepiness has also been increasingly recognized as a major health hazard and has been linked to an increased risk of all-cause mortality8,23. In the Cardiovascular Health Study, women with excessive daytime sleepiness and frequent nighttime awakenings were more likely to develop congestive heart failure23. In the present study, men with the combination of difficulty initiating sleep and excessive daytime sleepiness most of the time were the most prone to total mortality. As we did not find a significant interaction on risk of mortality, it was more likely that both symptoms were independently associated with mortality.
The strengths of our study include a relatively large sample size, with a sufficient number of cases to explore the CVD and cancer-specific mortality, and detailed and repeated assessments of life style risk factors and common chronic diseases over the course of follow-up. Another important strength is the high follow-up rate. In each 2- or 4-year cycle of the HPFS survey, follow-up rates have averaged 94 percent. The follow-up for death in the HPFS is at least 98% completed.
Our study has several potential limitations. First, assessment of insomnia symptoms was based on self-report, and objective measures of sleep quality were not available in our cohort. Such perceived symptoms may reflect a negative self-view or early onset of depression, and the latter is associated with mortality40. Though we adjusted for depressed mood and use of antidepressants in the main analyses, we still could not totally rule out the confounding effects due to depression. Likewise, we employed frequent snoring as surrogate measure of obstructive sleep apnea (OSA). Frequent snoring has been shown to correlate closely with OSA in men and has been used as a surrogate of OSA in previous epidemiological studies50, though misclassification is inevitably introduced. To minimize the residual confounding effects due to imperfect measure of OSA, we excluded men with frequent snoring, and other common chronic disorders in the sensitivity analysis and generated similar results. Second, we did not ask a specific question in the HPFS regarding use of hypnotic drugs, except for benzodiazepine and melatonin, until 2008. Previous studies suggested that hypnotics were associated with several adverse health outcomes, including dementia and mortality33. However, the observed association between insomnia symptoms and mortality did not materially change after excluding men using benzodiazepine or melatonin. Further excluding those who reported hypnotic drugs in 2008 also did not change our results (data not shown). It is worth noting that if the observed associations were due to hypnotic medication use, rather than insomnia per se, we would expect to observe similar associations for all individual insomnia symptoms. However, in our cohort study and the meta-analysis, the significant associations were consistently observed only in some, but not all insomnia symptoms. In this context, although we cannot rule out the possibility of residual confounding due to hypnotic drug use, the effects are likely to be modest. Another limitation was that this cohort included mostly Caucasian men, thus these findings might not be generalizable to female or nonwhite male populations. However, in the meta-analysis including populations with diverse social and economic backgrounds, we observed similar patterns between different insomnia symptoms and mortality. It is also worth noting that only 9 published studies were included in the meta-analyses, which precludes use to conduct subgroup analysis to explore potential sources of the observed heterogeneity across studies, suggesting further works in this area should be a priority.
In conclusion, this large prospective cohort study indicates that difficulty initiating sleep and non-restorative sleep are associated with a modestly higher risk of total and CVD specific mortality and these associations persisted even after we excluded participants with cardiovascular diseases and depression. These observations were supported by our concurrent meta-analysis including results of the present study and nine previously published studies. Future research in this field is warranted, especially the long-term epidemiological studies about the association between individual insomnia symptoms and mortality, and experimental and clinical studies probing mechanisms underlying the insomnia-mortality associations. Although future studies are still needed before a firm conclusion can be reached, our study provides consistent evidence that insomnia symptoms may be an important modifiable risk factor affecting longevity.
Supplementary Material
Acknowledgments
Funding Sources: The study was supported by grant R01 NS062879-01A2 from the National Institute of Neurological Disorders and Stroke, grant P01 CA87969 from the National Cancer Institute, and NIH Transdisciplinary Research in Energetics and Cancer Center (TREC) grant (number 1U54CA155626). None of the sponsors participated in the design of study or in the collection, analysis, or interpretation of the data. None of the sponsors participated in the design of study or in the collection, analysis, or interpretation of the data.
Footnotes
Conflict of Interest Disclosures: J.W.W receives research grants from UCB (Neupro Clinical Trial), GSK (MRS imaging with restless legs syndrome (RLS) subjects) and Impax (Clinical Trial for RLS), and reports consulting relationship with Xenoport. S.R. receives multiple NIH research grants examining sleep disorders, their epidemiology and treatment and is a Board of Director for the American Academy of Sleep Medicine, a nonprofit professional society for sleep medicine. Other authors have indicated no financial conflicts of interest.
References
- 1.National Institute of Health [October 19, 2012];Insomnia. http://www.nhlbi.nih.gov/health/public/sleep/insomnia.pdf.
- 2.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, Stepanski EJ, American Academy of Sleep Medicine Work Group Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA, Brown ED, Dement WC. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 4.Carskadon MA, van den Hoed J, Dement WC. Sleep and daytime sleepiness in the elderly. J Geriatr Psychiatry. 1980;13:135–151. [PubMed] [Google Scholar]
- 5.Prinz PN, Vitiello MV, Raskind MA, Thorpy MJ. Geriatrics: Sleep disorders and aging. N Engl J Med. 1990;323:520–526. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 6.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey II. Sleep. 1999;22(Suppl 2):S354–S358. [PubMed] [Google Scholar]
- 7.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 8.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 9.Stoller MK. Economic effects of insomnia. Clin The. 1994;16:873–897. [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund PA, Coulouvrat C, Fitzgerald T, Hajak G, Roth T, Shahly V, Shillington AC, Stephenson JJ, Walsh JK. Insomnia, comorbidity, and risk of injury among insured Americans: Results from the America Insomnia Survey. Sleep. 2012;35:825–834. doi: 10.5665/sleep.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szelenberger W, Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiol Exp (Wars) 2000;60:373. doi: 10.55782/ane-2000-1356. [DOI] [PubMed] [Google Scholar]
- 12.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, Drumheller O, Reis SE. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–1640. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation. 2011;124:2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz S, McDowell Anderson W, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999;47:313–333. doi: 10.1016/s0022-3999(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 16.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF., 3rd Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 17.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 18.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28:965–971. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Fernández-Mendoza J, Bixler EO. Insomnia with short sleep duration and mortality: The Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida OP, Alfonso H, Yeap BB, Hankey G, Flicker L. Complaints of difficulty to fall asleep increase the risk of depression in later life: the health in men study. J Affect Disord. 2011;134:208–216. doi: 10.1016/j.jad.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Althuis MD, Fredman L, Langenberg PW, Magaziner J. The relationship between insomnia and mortality among community-dwelling older women. J Am Geriatr Soc. 1998;46:1270–1273. doi: 10.1111/j.1532-5415.1998.tb04544.x. [DOI] [PubMed] [Google Scholar]
- 22.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: A 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Rresearch Group. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Davis HS, Merry HR, MacKnight C, McDowell I. Sleep disturbances and mortality: results from the Canadian Study of Health and Aging. J Am Geriatr Soc. 2001;49:639–641. doi: 10.1046/j.1532-5415.2001.49125.x. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson PM, Nilsson JA, Hedblad B, Berglund G. Sleep disturbance in association with elevated pulse rate for prediction of mortality--consequences of mental strain? J Intern Med. 2001;250:521–529. doi: 10.1046/j.1365-2796.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki E, Yorifuji T, Ueshima K, Takao S, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–141. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Rod NH, Vahtera J, Westerlund H, Kivimaki M, Zins M, Goldberg M, Lange T. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300–309. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 29.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 31.Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1927. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 32.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–157. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 33.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–864. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 35.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 37.Motivala SJ. Sleep and inflammation: Psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42:141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 38.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E, Osteoporotic Fractures in Men Study Group The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–2609. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12:295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 42.Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, Ozaki N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens. 2012;25:1149–1155. doi: 10.1038/ajh.2012.107. [DOI] [PubMed] [Google Scholar]
- 43.Strand LB, Laugsand LE, Wisloff U, Nes BM, Vatten L, Janszky I. Insomnia Symptoms and Cardiorespiratory Fitness in Healthy Individuals: The Nord-Trondelag Health Study (HUNT). Sleep. 2013;36:99–108C. doi: 10.5665/sleep.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cintra F, Bittencourt LR, Santos-Silva R, Andersen M, de Paola A, Poyares D, Tufik S. The association between the Framingham risk score and sleep: a Sao Paulo epidemiological sleep study. Sleep Med. 2012;13:577–582. doi: 10.1016/j.sleep.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz S. Are sleep complaints an independent risk factor for coronary heart disease? Evidence from the Piedmont Study. Doctoral dissertation. Chapel Hill, North Carolina: University of North Carolina. Ann Epidemiol. 1998;8:384–392. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- 46.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: Scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in delayed sleep phase syndrome. J Biol Rhythms. 2009;24:313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Psychiatric Association . Diagnostic and Statistical manual of mental disorders, Fourth Edition (DSM-IV) The American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 49.American Academy of Sleep Medicine . The International Classification of Sleep Disorders: diagnostic and coding manual. 2nd ed. American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- 50.Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest. 1991;99:600–608. doi: 10.1378/chest.99.3.600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




