Abstract
Cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP), two intracellular Ca2+ mobilizing second messengers, have been recognized as a fundamental signaling mechanism regulating a variety of cell or organ functions in different biological systems. Here we reviewed the literature regarding these ADP-ribosylcyclase products in vascular cells with a major focus on their production, physiological roles, and related underlying mechanisms mediating their actions. In particular, several hot topics in this area of research are comprehensively discussed, which may help understand some of the controversial evidence provided by different studies. For example, some new models are emerging for the agonist receptor coupling of CD38 or ADP-ribosylcyclase and for the formation of an acidic microenvironment to facilitate the production of NAADP in vascular cells. We also summarized the evidence regarding the NAADP-mediated two-phase Ca2+ release with a slow Ca2+-induced Ca2+ release (CICR) and corresponding physiological relevance. The possibility of a permanent structural space between lysosomes and sarcoplasmic reticulum (SR), as well as the critical role of lysosome trafficking in phase 2 Ca2+ release in response to some agonists are also explored. With respect to the molecular targets of NAADP within cells, several possible candidates including SR ryanodine receptors (RyRs), lysosomal transient receptor potential-mucolipin 1 (TRP-ML1) and two pore channels (TPCs) are presented with supporting and opposing evidence. Finally, the possible role of NAADP-mediated regulation of lysosome function in autophagy and atherogenesis is discussed, which may indicate a new direction for further studies on the pathological roles of cADPR and NAADP in the vascular system.
Keywords: Calcium Mobilization, Lysosomal Channels, Signal Transduction, ADP-Ribose, Vasoconstriction, Autophagic Flux, Vesicle Trafficking, Intracellular Ca2+ Stores
INTRODUCTION
It is widely accepted that in vascular cells, which mainly include vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), Ca2+ signaling is one of the most important signaling mechanisms leading to vascular cell activation, producing vascular tone and vasomotor responses to various agonists and stimuli (Berridge, 1994; Berridge, 1997; Himpens et al., 1995; Nelson et al., 1990). Over the last 30 years, numerous studies have shown that intracellular Ca2+ concentration ([Ca2+]i) in vascular cells, particularly in VSMCs, is determined by both the influx of extracellular Ca2+ and the mobilization of Ca2+ from intracellular stores. In the search for intracellular Ca2+ mobilizing second messengers, the discovery both of inositol 1,4,5-tris-phosphate (IP3) in the early 1980s (Streb et al., 1983) and of cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) in the late 1980s (Lee et al., 1989) made a paradigm shift in how we understand intracellular Ca2+ mobilization in vascular cells and its associated physiological and pathological relevance. In this review we briefly summarized the current progress of knowledge on cADPR and NAADP in vascular cells and provided an overview regarding the role of both second messengers in the regulation of vascular function and development of related vascular diseases.
PRODUCTION OF cADPR AND NAADP IN VASCULAR CELLS
cADPR was first discovered by Lee and associates in sea urchin eggs (Lee et al., 1989) and then detected in a variety of mammalian tissues or cells such as heart, liver, spleen, brain and red blood cells, lymphocytes, pituitary cells and cultured renal epithelial cells (Beers et al., 1995; Galione et al., 1991; Koshiyama et al., 1991; Lee and Aarhus, 1993; Takasawa et al., 1993). Basal concentrations of cADPR in cardiac muscle, liver and brain are estimated at 100–200 nM (Ladd et al., 1996). Homogenates prepared from dissected small bovine coronary arteries, cultured arterial ECs and smooth muscle cells produced cADPR and its metabolite ADPR, when incubated with NAD (Li et al., 2000a; Li et al., 2000b; Li et al., 1997; Li et al., 1998). Tissue cADPR concentration in the coronary smooth muscle is about 150 nM (Li et al., 1998). The cADPR extracted from the reaction mixture of NAD with cultured smooth muscle cells or arterial homogenates is capable of stimulating Ca2+ release in vitro using single cell Ca2+ fluorospectrometry. Recently, we detected cADPR in coronary arterial ECs, also in the nM range (Zhang et al., 2006b).
Similarly, homogenates or microsomes from VSMCs converted NADP+ along with nicotinic acid into NAADP in a concentration-dependent manner at pH of 4.5, which had similar efficiency to that observed for cADPR production under pH 7.4, indicating that NAADP is an enzymatic product of NADP+ in these vascular cells. In VSMCs from other vascular beds such as renal, cerebral and pulmonary vasculatures, NAADP was also detected with a range of 4–16 nM (Churamani et al., 2004; Kinnear et al., 2004). More recently, intracellular NAADP levels were also detected in ECs (1.774 88 ±0.65 pmol/mg protein), which could be produced by selective histamine 1 receptor (H1R) stimulation (Esposito et al., 2011). It is clear that both VSMCs and ECs are capable of producing NAADP as a specific second messenger to mobilize Ca2+ from intracellular stores.
Enzymatic Products of ADP-Ribosylcyclase
cADPR
cADPR can be synthesized from NAD via the action of ADP-ribosylcyclase. Once formed, cADPR can be further hydrolyzed by cADPR hydrolase to ADPR. Therefore, the cellular cADPR level is determined by the expression and activity of these enzymes. Both ADP-ribosylcyclase and cADPR hydrolase are membrane-bound enzymes in a wide range of mammalian tissues including arterial smooth muscle (Franco et al., 1994; Zocchi et al., 1993). It has been reported that the human lymphocyte differentiate antigens CD38 and CD157 are highly homologous with Aplysia ADP-ribosylcyclase, which possesses multiple forms of enzymatic activity including NAD glycohydrolase, ADP-ribosylcyclase and cADPR hydrolase activity (Adebanjo et al., 2000; Franco et al., 1994; Zocchi et al., 1993). These CD proteins are considered to be a molecular switch in regulating the cellular levels of cADPR by balancing its synthesis and hydrolysis. In response to stimuli, this multi-functional enzyme can be aggregated and internalized into the cytoplasm where it can more efficiently produce or metabolize cADPR. By Western blot analysis and RT-PCR, we demonstrated that CD38 was detectable in coronary arterial smooth muscle. In these experiments, two immunoreactive bands with molecular sizes of 42 and 90 kDa were recognized by a monoclonal antibody against CD38 in coronary arterial homogenates and microsomes (Li et al., 1997). Removal of CD38 by immunoprecipitation significantly decreased the production and catabolism of cADPR in these arterial homogenates. In CD38−/− mice, very low cADPR levels and no detectable ADP-ribosylcyclase activity were observed in lung, kidney, coronary arterial tissue, and renal afferent arterioles dissected from these mice (Boini et al., 2011; Deshpande et al., 2005; Li et al., 2000a; Teggatz et al., 2005a; Zhang et al., 2010). Interestingly, this CD38-associated enzyme in coronary VSMCs not only produces cADPR, but also metabolizes cADPR into ADPR through its bifunctional domain. Therefore, intracellular cADPR levels could be dynamically maintained by switching of different functional CD38 domains (Li et al., 1998). It is now well accepted that an enzymatic pathway responsible for the formation and metabolism of cADPR is present in vascular cells. cADPR is formed from NAD+ by ADP-ribosylcyclase and metabolized into ADPR by the hydrolase activity of the same enzyme, which could be associated with the multifunctional activity of CD38 (Li et al., 1997; Li et al., 2002; Li et al., 1998; Zhang et al., 2001).
NAADP
Although the metabolites of NADP+ was reported to induce Ca2+ release from sea urchin egg microsomes by Lee and his associates in 1987 (Clapper et al., 1987), this NADP+ metabolite was only identified as NAADP nearly a decade later (Chini et al., 1995; Lee and Aarhus, 1995). Since then, the enzymatic pathways for NAADP production and metabolism have been characterized. Similar to cADPR production, soluble protein Aplysia ADP-ribosylcyclase and its membrane-bound homologs, CD38 and CD157, have also been reported to be involved in the production of NAADP (Aarhus et al., 1995; Galione et al., 1993; Lee, 1997; Lee, 2005). These enzymes can exchange the terminal nicotinamide group of the NADP+ with nicotinic acid to produce NAADP through a base—exchange reaction, which has been shown in a variety of cells and tissues such as sea urchin eggs, pancreatic acinar cells, human T lymphocytes, rat brain, and smooth muscle cells (Ge et al., 2002; Ge et al., 2003; Lee and Aarhus, 2000; Li et al., 2001). In addition to membrane-bound CD38 and CD157, a cytosolic soluble ADP-ribosylcyclase isoform or CD38 are also interestingly found in VSMCs (Lee and Aarhus, 1991; Rusinko and Lee, 1989). Our previous studies demonstrated that cytosolic ADP-ribosylcyclase activity may be primarily derived from internalized CD38 in coronary arterial smooth muscle, which may be associated with lipid raft clustering and endocytosis in seconds (Jia et al., 2008). Other studies also demonstrated that CD38 internalization is important in mediating cADPR production in cell cytosol (Chidambaram and Chang, 1999; Han et al., 2002; Zocchi et al., 1999). Therefore, based on the current understanding, conversion of NAAP+ to NAADP due to high levels of ADP-ribosylcyclase activity may be associated with the internalization of a membrane-bound enzyme, although there remains to be some unidentified pathways. This internalized cytosolic ADP-ribosylcyclase or CD38 is responsible for catalyzing the exchange of the nicotinamide group of NADP+ with nicotinic acid to produce NAADP under acidic conditions in VSMCs (Zhang et al., 2006a). Our recent studies in mouse coronary artery indeed demonstrated that CD38 and its cytosolic isoforms are responsible for NAADP production in response to death receptor activation, endothelin and oxidant stimulation (Xu et al., 2012b; Zhang et al., 2010).
More recently, CD38 has also been found to hydrolyze NAADP to ADP-ribose 2′-phosphate. This activity of CD38 in the degradation of NAADP was greatly increased at acidic pH, which is determined by acidic residues at the active site of this enzyme. X-ray crystallography of the CD38 complex or purified ADP-ribosylcyclase with substrates demonstrated that acidic residues at the active sites of both enzymes determine NAADP synthesis or hydrolysis and that these residues are Glu-146 and Asp-155. Changing Glu-146 or Asp-155 by site-directed mutagenesis could eliminate their strong pH dependence (Graeff et al., 2006). However, in myometrial cells, NAADP could not be produced by either CD38 or a base-exchange reaction (Soares et al., 2007), but by some unidentified pathway. It seems that different cell types use different enzymatic pathways to produce NAADP.
Although NAADP production has been reported in various tissues and cells, it remains a mystery how an acidic reaction of ADP-ribosylcyclase or its human homologue CD38 produces NAADP either at the cell membrane or in the cytosol, where the pH is around 7.4. Furthermore, it is unknown why some agonists or stimuli can selectively elicit such acidic reaction of ADP-ribosylcyclase. Based on our current studies on redox signaling and lipid raft-associated transmembrane signaling mechanisms (Jia et al., 2008; Xu et al., 2012a; Xu et al., 2012b), we believe that an acid microenvironment may be generated locally in response to some agonists or stimuli in vascular cells. For example, we found that both ET-1 and FasL preferably stimulate NAADP production in VSMCs (Zhang et al., 2010; Zhang et al., 2006a), which may be associated with their ability to generate a local acidic microenvironment at the cell membrane, facilitating a base exchange reaction via ADP-ribosylcyclase or CD38. It is assumed that lipid raft clustering may be involved in the formation of this local acidic environment if agonist receptors are linked to lipid rafts. As shown in our previous studies and by others, CD38 or ADP-ribosylcyclase activity may be activated through membrane lipid raft clustering, (Deaglio et al., 2007; Jia et al., 2008; Munoz et al., 2003; Zilber et al., 2005). Upon agonist stimulation, membrane raft clustering is usually induced by lysosome fusion to the cell membrane, which translocates not only critical molecules for raft clustering such as acid sphingomyelinase (ASM) (only present in lysosomes), but also lysosomal vacuolar H+-ATPase. The latter provides a local acidic environment which maintains the activity of translocated ASM to amplify the production of ceramide and form lipid raft platforms (Xu et al., 2012a). Such membrane microenvironment of acidic pH generated and maintained by vacuolar H+-ATPase may be critical for ADP-ribosylcyclase or CD38 to produce NAADP within lipid raft platforms.
The formation of this acidic microenvironment may be an important step for the enzymatic activity of CD38, which is actively regulated by agonists or other stimuli outside the cells. Since such acidic environment is formed through rapid lysosome trafficking and fusion and subsequent lipid rafts clustering, the substrates in CD38-mediated enzymatic response such as nicotinic acid or other related substrates may be transported and maintained enough concentrations in the local area of lipid raft platforms. It has been well known that lysosome membrane has numerous transporter systems including nucleosides and nucleotides (Pisoni and Thoene, 1991). However, so far there is no study done to elucidate how these transporters work to provide nicotinic acid for NAADP production, which will be another interesting topic for future studies.
Activation of ADP-Ribosylcyclase in Vascular Cells
VSMCs
Many studies have been done in VSMCs to test whether ADP-ribosylcyclase can be activated in response to different agonists or stimuli (Bai et al., 2005; Evans et al., 2005; Zhang and Li, 2006). In this regard, we have reported that incubation of coronary VSMCs with oxotremorine, a specific M1 mAChR agonist, produced time- and concentration-dependent activation of ADP-ribosylcyclase, which was blocked by both ADP-ribosylcyclase inhibitor nicotinamide and by specific M1 mAChR antagonist, pirenzepine (PIR). The activation of ADP-ribosylcyclase occurred rapidly within the first minute of oxotremorine incubation with coronary arterial smooth muscle cells (CASMCs) (Ge et al., 2003). In pulmonary circulation, activation of ADP-ribosylcyclase has been reported as a primary trigger of hypoxia-induced contraction of small arteries or arterioles (Dipp and Evans, 2001; Dipp et al., 2001). In addition, Ang II has been demonstrated to activate ADP-ribosylcyclase in neonatal rat cardiac myocytes (Higashida et al., 2000), and ET-1 may increase cADPR production via activation of ADP-ribosylcyclase in rat mesenteric small arteries (Giulumian et al., 2000) or shark anterior mesenteric arteries (Fellner and Parker, 2004). However, recent studies have demonstrated that ET-1 may also stimulate the production of NAADP (Kinnear et al., 2004; Lee, 2005; Zhang et al., 2006a). More recently, we reported that death receptor ligand FasL markedly increased NAADP production in CASMCs from wild-type mice (CD38+/+), but not in CASMCs from CD38 knockout (CD38−/−) mice (Zhang, et al., 2010). Other vascular agonists that increase ADP-ribosylcyclase activity and thereby enhance cADPR production include β-adrenergic agonists (Boittin et al., 2003) and urocortin (Sanz et al., 2003).
On the other hand, NO donor sodium nitroprusside (SNP) was reported to decrease ADP-ribosylcyclase activity, inhibiting the production of cADPR in VSMCs, either from coronary arteries (Yu et al., 2000) or from airway (White et al., 2002). However, these results are not in concordance with the findings of previous studies in nonvascular cells, where NO increases the production of cADPR in nonvascular cells such as rat parotid acinar cells (Looms et al., 2001) and urchin eggs (Willmott et al., 1996). It remains unknown why NO decreases ADP-ribosylcyclase activity in VSMCs, but increases it other cells.
ECs
It has been reported that in non-vascular tissues CD38 or ADP-ribosylcyclase may be a link from bradykinin receptors to its effector enzymes such as NOS (Deshpande et al., 2003; Higashida et al., 1996; Higashida et al., 2001). Given that bradykinin is a typical stimulator of endothelium-dependent vasodilator, it is possible that cADPR production via CD38-ADP-ribosylcyclase may be a critical mechanism regulating endothelial Ca2+ and corresponding function. By direct measurement of cADPR production in ECs, we indeed demonstrated that bradykinin induced cADPR production and thereby evoked a Ca2+ release from RyR-sensitive stores, which was accompanied by an increase in NO production (Yu et al., 2000; Zhang et al., 2005; Zhang et al., 2006b) Interestingly, a recent study reported that in rat ECs, NAADP production may be activated by endothelium-dependent vasodilator acetylcholine (Brailoiu et al., 2010c). In addition, human ECs were also shown to produce NAADP in response to the specific histamine H1 receptor agonist, 2-[(3-Trifluoromethyl)phenyl] histamine dimaleate (TMPH), which mediates H1R-induced Ca2+ release from acidic organelles and the endoplasmic reticulum (Naylor et al., 2009). It has been suggested that NAADP production and subsequent Ca2+ release may underlie histamine-mediated endothelial activation and the exocytosis of VWF (Esposito et al., 2011).
Receptor-Effector Coupling
As discussed above, many agonists can activate or inhibit ADP-ribosylcyclase to produce cADPR and NAADP, which mediate Ca2+ release from intracellular stores. It has been shown that these agonists exert action through their receptors in different cells, such as muscarinic acetylcholine receptors in NG108–15 neuronal cells (Higashida et al., 2007), Ang II or β-adrenergic receptors in ventricular myocytes (Gul et al., 2008), and M1 muscarinic receptors, ET-1 receptors and Fas in VSMCs (Ge et al., 2003; Zhang et al., 2010; Zhang et al., 2006a). However, little is known regarding the link between activation of these agonist receptors and ADP-ribosylcyclase. The major mammalian analogues of ADP-ribosylcyclase, namely CD38 and CD157, are pleiotropic ectoenzymes, which act independently as both receptors and enzymes. However, it still remains unknown how CD38 or CD157 is able to produce intracellular signaling molecules. Although there is evidence that internalization and aggregation of CD38 are importantly involved in signaling of this cADPR-producing enzyme (Franco et al., 1994; Howard et al., 1993; Koguma et al., 1994; Lacapere et al., 2003; Lee et al., 1993; Zocchi et al., 1995; Zocchi et al., 1999), how CD38 internalization and aggregation is linked to agonist receptors is still an unresolved issue.
In some studies, various receptors have been proposed to be directly coupled with ADP-ribosylcyclase, where their activation may result in increased production of cADPR (Higashida et al., 1999; Higashida et al., 1997). With respect to bradykinin, there is evidence that B2 receptors are directly coupled to the membrane-bound form of ADP-ribosylcyclase in human airway smooth muscle cells (Deshpande et al., 2003) and NG108–15 neuronal cells (Higashida et al., 1996), suggesting that bradykinin could stimulate cADPR production via this direct coupling mechanism. G-proteins may contribute to this linking of agonist receptor to ADP-ribosylcyclase since it is well known that G-proteins play an important role in linking cell surface receptors to intracellular second messengers. In this context, G-proteins may link Ach receptors to ADP-ribosylcyclase and therefore Ach stimulates the production of cADPR through G-protein activation. This view has been supported by several reports from vascular and non-vascular tissues or cells (Higashida et al., 1999; White et al., 2003; Wu et al., 1997). Given the ectoenzyme nature of CD38, how such G-protein-mediated receptor-effector coupling occurs is still speculative and further studies are needed to address the direct structural or functional connection between G-proteins and any enzyme determining intracellular cADPR levels.
Rather than focusing on this classical receptor-effector coupling model of transmembrane signaling, we recently developed a new model for temporospatial activation of ADP-ribosylcyclase in VSMCs in response to different agonists or stimuli. In this model, selected agonists or stimuli will concurrently activate membrane NADPH oxidase to convert NAD(P)H to NAD(P)+ and . This membrane NADPH oxidase-derived is released out of VSMCs and nearby activates ADP-ribosylcyclase (CD38) through its rapid internalization and dimerization. Activated CD38 uses increased NAD+ as the substrate to produce cADPR (or NADP+ to produce NAADP). The coupling of agonist receptors to ADP-ribosylcyclase is via membrane NADPH oxidase rather than G-proteins. This cross talk between NADPH oxidase and ADP-ribosylcyclase may explain why some agonists link to ADP-ribosylcyclase, but others do not (Fig. 1). There are several lines of evidence supporting this working model for agonist receptor-ADP-ribosylcyclase coupling. First, NADPH oxidase can be activated by many agonists such as Ang-II, Oxo, ET-1, and FasL (Bao et al., 2010; Zhang et al., 2008; Zhang et al., 2007). NADPH oxidase is also reported to produce and release outside VSMCs (Zhang et al., 2006b). Importantly, one of the intracellular products from NADPH oxidase, NAD+ or NADP+ can provide substrate for ADP-ribosylcyclase. Second, it has been reported that ADP-ribosylcyclase or CD38 dimerizes in response to increases in intracellular oxidants, which enhances the catalytic activity of ADP-ribosylcyclase (Chidambaram et al., 1998; Guida et al., 1995). Several studies have demonstrated that cysteine residues in CD38 or ADP-ribosylcyclase determine the enzyme function as ADP-ribosylcyclase or cADPR hydrolase (Tohgo et al., 1994). The oxidation of cysteine molecules may lead to the formation of one or several disulfide bonds, which induces dimerization of the enzyme protein. In other studies, the activity of ADP-ribosylcyclase was found to be inhibited by disulfide bond reducing reagents (Berruet et al., 1998; Galione et al., 1993). Lastly, some agonists such as oxtremorine, ET-1 and FasL have been reported to stimulate membrane lipid raft clustering and internalization of CD38 (Gambara et al., 2008; Jia et al., 2008; Trubiani et al., 2004). Internalized CD38 or ADP-ribosylcyclase can form dimers to produce maximal activity. As mentioned above, lipid raft clustering of NADPH oxidase and CD38 with or without vacuolar H+-ATPase may determine the production of cADPR or NAADP since vacuolar H+-ATPase is important for the generation and maintenance of local acidic microenvironment (Xu et al., 2012a). If the local pH is around 4 in those raft platforms, NAADP may be produced; otherwise, cADPR may be synthesized and released.
Figure 1. Cross talk between NADPH oxidase and CD38/ADP-ribosylcyclase in cell plasma membrane.
NOX-C: NADPH oxidase complex. VP: vasopressin. 5-HT: serotonin. Em: membrane potential.
Ca2+ MOBILIZING ACTION OF cADPR AND NAADP IN VASCULAR CELLS
Independent of IP3, cADPR stimulates Ca2+ release from intracellular Ca2+ stores when given directly in VSMCs or in response to different agonists or stimuli. Kannan et al., reported that cADPR induces SR Ca2+ release in β-escin-permeabilized smooth muscle cells freshly isolated from porcine coronary arteries. In α-toxin permeabilized cells, we found that cADPR produces SR Ca2+ release in both cultured and freshly dissociated bovine coronary and rat renal VSMCs (Li et al., 2000a; Yu et al., 1996). This cADPR-induced Ca2+ release from the SR can be completely blocked by cADPR antagonist, 8-Br-cADPR, but not by IP3R blockers. It is concluded that cADPR mobilizes intracellular Ca2+ through a mechanism independent of IP3 in VSMCs. Recently, we also determined whether bradykinin-induced vasodilator response is directly linked to cADPR-mediated Ca2+ release from the endoplasmic reticulum (ER) in bovine coronary arterial ECs (Zhang et al., 2006b). Using a newly developed fluorescence imaging system to simultaneously measure Ca2+ transient and NO production in the intact arterial endothelium, we showed that bradykinin produced a rapid and transient increase in [Ca2+]i that was accompanied by enhanced NO production (Zhang et al., 2006b). However, bradykinin-induced Ca2+ release and NO production were significantly attenuated by pretreatment of the arteries with cADPR-RyRs signaling inhibitors such as nicotinamide, 8-Br-cADPR or ryanodine. This supports the view that bradykinin-induced Ca2+ increase in arterial ECs is through cADPR-mediated Ca2+ release from the ER and via RyR activation.
NAADP has been proposed to act as a ubiquitous Ca2+ messenger, and this nucleotide is one of the most potent intracellular Ca2+ mobilizing molecules (Evans et al., 2005; Galione, 2006; Lee, 2005; Yamasaki, et al., 2005a). The Ca2+ mobilizing action of NAADP is even stronger than that induced by commonly known Ca2+ mobilizing second messengers IP3 and cADPR (Clapper et al., 1987; Lee and Aarhus, 1995). Two working models have been proposed to interpret the different actions of NAADP in mobilizing intracellular Ca2+ (Yamasaki et al., 2005a). In the first model, the ER or SR that expresses IP3Rs and RyRs is responsible for NAADP-induced Ca2+ release, where NAADP may interact either directly with RyRs or via a separate protein that may indirectly activate RyRs (Dammermann and Guse, 2005; Gerasimenko et al., 2003b). This model may work in several cell types such as T-lymphocytes (Dammermann and Guse, 2005), cardiac cells (Mojzisova et al., 2001) and skeletal muscle (Hohenegger et al., 2002). In these cells, the target for the actions of NAADP is the RyR on the ER or SR (Dammermann and Guse, 2005). The second model relates to a two-pool mechanism, which is based on the assumption that an NAADP-sensitive Ca2+ store exists in arterial myocytes, which is possibly a thapsigargin-insensitive acidic store (Churchill et al., 2002). This NAADP-sensitive Ca2+ store is responsible for a localized signal, which triggers CICR to cause global Ca2+ increases through IP3Rs and RyRs on the SR (Cancela et al., 1999; Cancela et al., 2000; Churchill and Galione, 2000; Churchill and Galione, 2001). Considering a wide variety of cellular processes regulated by changes in intracellular Ca2+ concentrations including fertilization to cell death, it is possible that this temporospatial Ca2+ signaling pattern related to NAADP importantly contributes to the regulation of different cell functions, either through its action as second messenger or via its activity to synchronize the actions of other second messengers (Albrieux et al., 1998; Brailoiu et al., 2005; Brailoiu et al., 2001; Chameau et al., 2001; Johnson and Misler, 2002; Kinnear et al., 2004; Masgrau et al., 2003; Yamasaki et al., 2004; Yamasaki et al., 2005b).
Over the last two decades, numerous studies have been conducted to explore the possible molecular mechanisms mediating the action of cADPR and NAADP to release Ca2+ from the intracellular stores. It is clear that several molecules centered on RyRs may be the targets of cADPR to mobilize Ca2+ from the SR in VSMCs. For NAADP, in addition to targeting RyRs on the SR, lysosomal channels are reported to be involved in a lysosomal burst of Ca2+ that triggers CICR, leading to global Ca2+ mobilization. Below is an overview of these intracellular targets for the action of cADPR and NAADP.
Intracellular Targets of cADPR Action
RyR in SR
There is considerable electrophysiological evidence showing that the RyR/Ca2+ release channels reconstituted into a planar lipid bilayer are activated by cADPR in a variety of tissues or cells. In coronary arterial smooth muscle, a calcium channel with 245 pS conductance is present on the SR membrane and cADPR was found to increase the NPO of these RyR/Ca2+ release channels in a concentration-dependent manner (Li et al., 2001). In the presence of ryanodine (50 μM), cADPR-induced activation of these channels was completely abolished. These results provided direct evidence that cADPR activates RyRs and therefore may serve as an endogenous activator or modulator of RyRs in these VSMCs. However, this view has been challenged by studies using other tissues such as neurons, myocardium and other smooth muscle, where cADPR was found to release Ca2+ independently of RyR (Kannan et al., 1996; Lahouratate et al., 1997; Sitsapesan et al., 1994). It seems that there exists tissue specific effects of cADPR on RyRs, which may be associated with the intermediate proteins or accessory proteins that regulate RyR activity.
It has been reported that cADPR participates in KCl, CaCl2, Bay K 8644 (Ca2+ channel activator) and caffeine-induced Ca2+ release response in coronary and renal VSMCs, suggesting that cADPR contributes to Ca2+-induced Ca2+ release (CICR) (Teggatz et al., 2005b). In these smooth muscle cells, high extracellular Ca2+ (5 mM CaCl2) and agonist Ach produced 1–1.5 Hz oscillations, which were blocked by CICR inhibitor tetracaine and cADPR antagonist 8-Br-cADPR (Li et al., 2000a). Kannan et al., have also reported that cADPR increased Ach-induced Ca2+ oscillations which was blocked by cADPR antagonist 8-amino-cADPR in porcine tracheal smooth muscle cells (Kannan et al., 1997). Taken together, these results demonstrated that cADPR is necessary for CICR and intracellular Ca2+ oscillation and that RyRs are the mechanistic link between cADPR and CICR or Ca2+ oscillations.
There are also two mechanistic models proposed to elucidate the role of endogenous cADPR in mediating vascular Ca2+ mobilization through RyRs. First, cADPR acts as a mediator to activate RyRs. In this regard, various agonists or stimuli activate ADP-ribosylcyclase to produce cADPR, which induces Ca2+ release from the SR by its direct action on the RyRs. Second, cADPR serves as a modulator of CICR or RyR reactivity. In this way, cytosolic cADPR sensitizes the RyRs, enhancing CICR activated by agonists or Ca2+ influx.
FKBP 12.6 Proteins
FKBP12.6 is a ubiquitous 12.6-kDa cytosolic protein that binds to one RyR monomer and its activity is inhibited by the immunosuppressant drug FK506 and rapamycin. In nonvascular cells, Ca2+ release from the SR is inhibited when FKBP12.6 is bound to the RyR, and dissociation of FKBP 12.6 from the RyR releases Ca2+. This 12.6 kDa protein is also expressed in coronary arterial smooth muscle (Tang et al., 2002). Blockade, dissociation or removal of FKBP12.6 protein from the RyR substantially abolished cADPR-induced activation of RyR/Ca2+ release channels on lipid bilayer membrane. Ligand binding experiments have demonstrated that cADPR can directly bind to FKBP12.6 in islet microsomes (Tang et al., 2002). Using confocal fluorescence imaging, we have demonstrated that FKBP12.6 colocalizes with RyRs in renal arterial myocytes (Teggatz et al., 2005b). Ca2+ influx by CaCl2 significantly decreased this colocalization, and 8-Br-cADPR reversed CaCl2 effects suggesting that cADPR is involved in the dissociation of FKBP 12.6 protein from RyRs under this condition. These results indicate that cADPR exerts its action by dissociating FKBP12.6, resulting in Ca2+ release from the SR in VSMCs. Recently, studies from other groups also demonstrated such contribution of FKBP to the action of cADPR in the mobilization of Ca2+ (Morita et al., 2006; Noguchi et al., 1997; Wang et al., 2004). However, some studies could not obtain similar results (Bradley et al., 2003; Copello et al., 2001; Zhang et al., 2009b). Therefore, further investigations are needed to solve such controversy by demonstrating the possible tissue specific action of this intracellular regulatory mechanism.
Intracellular Targets of NAADP Action
RyRs in SR
Depending on the tissues or cells studied, RyRs were reported to be a possible target for the action of NAADP in mobilizing Ca2+ from intracellular stores. There is evidence that reconstituted RyR1 and RyR2 channels from cardiac cells (Mojzisova et al., 2001) and skeletal muscle (Hohenegger et al., 2002) are sensitive to NAADP to increase Ca2+ channel activity. In some other tissues or cell preparations, in particular T-lymphocytes, NAADP was found to target RyRs localized to the ER (Dammermann and Guse, 2005; Gerasimenko et al., 2003a; Gerasimenko et al., 2003b; Langhorst et al., 2004). However, other studies agreed more with the findings that in sea urchin eggs, there is an acidic compartment related to lysosomes (Churchill et al., 2002). It has been proposed that an NAADP-sensitive acidic Ca2+ store exists in some type of cells including arterial myocytes, which is a thapsigargin-insensitive acidic store mainly shown in lysosomes or lysoendosomal compartments. NAADP is demonstrated to first activate Ca2+ bursts as a triggering mechanism and then lead to global Ca2+ mobilization through IP3Rs and RyRs in the SR, a so-called two-pool mechanism. It is assumed that the NAADP-sensitive Ca2+ store or acidic Ca2+ store is responsible for a localized signal, where latter triggers CICR to cause global Ca2+ increases through RyRs or IP3Rs on the SR (Kinnear et al., 2004; Kinnear et al., 2008; Zhang et al., 2010). In VSMCs, this two-pool mechanism has been demonstrated to function in response to different agonists such as ET-1 and FasL or by delivery of NAADP into the cells (Zhang et al., 2010; Zhang et al., 2006a). Lipid bilayer reconstitution and Ca2+ imaging in intact CASMCs did not confirm that NAADP directly activates RyRs on the SR from VSMCs (Zhang et al., 2009a; Zhang and Li, 2007; Zhang et al., 2006a). However, RyR is an important SR receptor mediating CICR upon NAADP stimulation, and therefore blockade of RyRs still abolishes global Ca2+ increases, which may be of physiological significance in many biological processes.
Lysosome (Lyso)-SR Junction
Although the two-pool mechanism is attractive in its interpretation of a two phase Ca2+ release induced by NAADP, some issues remain to be addressed. For example, NAADP-induced CICR has a long delayed second phase Ca2+ release (seconds to minutes) (Evans and Cannell, 1997; Zhang et al., 2010; Zhu et al., 2010a), which is very different from the classical CICR reported previously (Fleischer and Inui, 1989; Franco et al., 1994; Galione 1993; Galione et al., 1991; Hirst et al., 1994). It remains unknown why such a long delay occurs and what physiological relevance this delayed second phase Ca2+ in CICR may have. Given the principle of economic design in the biological system, the significance of such unconventional CICR through lysosomal triggering is imperative to define.
The Lyso-SR junction between lysosomal clusters and a subpopulation of SR was proposed to form a trigger zone, where Ca2+ released from lysosomes activates a global Ca2+ response via CICR in pulmonary VSMCs and some other cells (Kinnear et al., 2004; Zhang et al., 2010; Zhang et al., 2006a). However, the structural and functional characteristics of this Lyso-SR junction have yet to be completely revealed. In collaboration with Dr. van Breemen, who has extensive experience in characterizing plasma membrane-SR (PM-SR) and mitochondria-SR (Mito-SR) junctions in smooth muscle cells under resting and contracting condition (Dai et al., 2005; Poburko et al., 2004), we performed electronic microscopy and found that there are Lyso-SR junctions around 30–80 nm in VSMCs, which are relatively large compared to PM-SR and Mito-SR junctions. However, these Lyso-SR junctions are rather heterogeneous within arterial myocytes (unpublished data). Although it seems that some Lyso-SR junctions are present within VSMCs, there may not be a permanent structural space between lysosomes and SRs. Given the great mobility of lysosomes within cells, it is possible that Lyso-SR junctions depend upon lysosome trafficking and aggregation toward the SR as we hypothesized in CASMCs (Zhang et al., 2009a). It has been proposed that the global Ca2+ release following small Ca2+ bursts from lysosomes may be associated with lysosomal trafficking to the SR. Although small amounts of Ca2+ released from lysosomes may not be enough to activate global Ca2+ release from the SR, it may be enough to drive lysosome movement or aggregation. When these clustered or aggregated lysosomes work together, global Ca2+ release from the SR is activated (Zhang et al., 2009a). This interaction of lysosome and SR was also proposed later by Zhu et al., in pulmonary VSMCs (Zhu et al., 2010a). In some preliminary studies, we detected colocalization of lysosomal marker (GFP-Lamp1) with RyR3 or SR tracker (red fluorescent labeled) suggesting that there is lysosomal trafficking and aggregation toward the SR in coronary VSMCs when they were stimulated by death factor, FasL (Xu et al., 2011). In another study, we indeed demonstrated that NAADP stimulates lysosome trafficking through its Ca2+ mobilizing action (Zhang et al., 2011).
TRP-ML Channels in Lysosomes
The transient receptor potential-mucolipin (TRP-ML) subfamily of TRP channels consists of three mammalian members (TRP-ML1–3), which are relatively small proteins consisting of <600 amino acid residues with an expected molecular mass of approximately 56–65 kDa. TRP-ML1 is widely expressed and mainly resides in late endosomes/lysosomes (Bach 2005; Laplante et al., 2002; Laplante et al., 2004). It has been demonstrated that the protein encoded by the TRP-ML1 gene, MCOLN1, has six predicted transmembrane domains, a putative channel pore, and is predominantly expressed in endosomes or lysosomes. Due to cleavage and other modifications, TRP-ML1 may be detected in different sizes from 36–75 kDa in lysosome membranes of native cells (Kiselyov et al., 2005; Yamaguchi et al., 2011). Within TRP-ML1, there is a TRP channel-homologous region located within amino acids 331–521 and an internal Ca2+ and Na+ channel pore region between amino acids 496 and 521. Mutations of MCOLN1 are implicated in the pathogenesis of a neurological disease, namely, mucolipidosis Type IV (MLIV). This disease is a lysosomal storage disorder that is characterized by severe neurologic and ophthalmologic abnormalities (Bach 2001; Kiselyov et al., 2005; Laplante et al., 2004; Raychowdhury et al., 2004; Slaugenhaupt, 2002; Sun et al., 2000). Compared to TRP-ML1, the functions of TRP-ML2 and TRP-ML3 are as extensively characterized until recently (Nilius et al., 2007).
In the search for the molecular target of NAADP action in lysosomes from native cells or tissues, reconstitution of liver lysosome preparations to characterize the possible Ca2+ channels was first done in our laboratory using lipid bilayer. Liver lysosomes were used since lysosome preparations were well established with using large volumes of tissues. Our work, published in 2007, demonstrated a 174 pS of a reconstituted Ca2+ channel with all the biophysical and pharmacological features of TRP-ML in liver lysosomes (Zhang and Li, 2007). We further characterized this TRP-ML in bovine coronary arterial muscle cells using lysosome preparations by constitution in lipid bilayer. It was demonstrated that the reconstituted lysosomal channel is also a voltage-dependent Ca2+ channel with a conductance of 145 pS in arterial muscle preparation (Zhang et al., 2009a). At the same time, Xu and his associates developed an elegant approach using patch clamp techniques and recorded Fe2+ and Ca2+ channel activity directly froms lysosome membrane in different cell types with TRP-ML transgenes, discovering that TRP-ML1 is an inwardly rectifying, proton-impermeable, Ca2+ and Fe2+/Mn2+ dually permeable cation channel (Dong et al., 2008; Dong et al., 2010; Dong et al., 2009). More recently, the same group used a genetically encoded Ca2+ indicator (GCaMP3) attached directly to TRP-ML1 to directly measure Ca2+ release from lysosomes via TRP-ML1 (Martelli et al., 2012), which further confirm the nature of TRP-ML1 as a lysosomal Ca2+ release channel.
NAADP was found to activate TRP-ML channels reconstituted from liver and coronary arterial muscle lysosome preparations in a concentration-dependent manner, which was featured by a self-desensitization at high concentrations of NAADP. In particular, when the bilayer preparations containing coronary arterial muscle lysosomal channels were pretreated with a subthreshold concentration of NAADP, the activity of these channels in response to higher concentrations of NAADP was substantially attenuated, suggesting that these lysosomal channels can be desensitized. This self-desensitization property of lysosomal ion channels has also been shown in the actions of NAADP as a Ca2+ releasing second messenger in other cells such as sea urchin egg fractions or intact egg cells (Aarhus et al., 1996; Bach, 2005; Genazzani et al., 1996). Although the mechanism mediating this self-desensitization is not yet clear, it is assumed that the NAADP receptor allosteric site transformation between high- and low- binding affinity to NAADP may play an important role, which is very similar to the regulatory machinery observed in well-studied IP3 receptors (Hirata et al., 1990). However, more studies will be needed to further elucidate the underlying mechanism responsible for such lysosomal channel desensitization and to address the possible physiological significance of this phenomenon, in particular, in the regulation of NAADP-mediated Ca2+ signaling and related function in the vasculature.
Pharmacologically, these reconstituted lysosomal channels in both liver and coronary arterial lysosomes were blocked by commonly used antagonists of TRP-ML1 channels such as dihydropyridine derivatives nifedipine and verapamil, sodium channel antagonist amiloride and an NAADP receptor antagonist PPADS (Yusufi et al., 2002; Zhang and Li, 2007). Furthermore, silencing the expression of TRP-ML1 gene in CASMCs with its specific siRNA substantially attenuated reconstituted lysosomal NAADP-sensitive Ca2+ release channel activity. Similarly, immunoprecipitation of TRP-ML1 from lysosome preparations of CASMCs with a specific antibody that was raised against its 101–150th amino acids, an epitope antigen sequence located on the lysosomal lumen-oriented TRP-ML1 loop between segment one and two, almost completely removed the channel activity and related response to NAADP. The use of an anti-TRP-ML1 antibody that was raised against a peptide mapping at the C terminus of TRP-ML1, a channel pore forming region, also attenuated NAADP-induced activation of reconstituted lysosomal Ca2+ channels. It is known that TRP-ML1 is a protein with full length of 580 amino acid containing six transmembrane segments and cytoplasm-resided C- and N- termini. The 101–150 antigen epitope is located on the lysosomal lumen-oriented loop between segment 1 and 2. The TRP cation channel domain of amino acids 331 to 521 spans transmembrane segments 3 to 6 (Cheng et al., 2010; Sun et al., 2000). All the results presented above that were obtained by gene silencing, deprivation of TRP-ML1 protein or interference of its channel pore formation strongly suggest that the activity of this reconstituted lysosomal NAADP-sensitive Ca2+ release channel represents a function of TRP-ML1 in CASMCs (Zhang et al., 2009a).
More recently, TRP-ML channel activity was reconstituted by using lysosomal preparation from wild-type (TRP-ML1+/+) human fibroblasts, but not from TRP-ML1−/− cells. Reconstituted TRP-ML1 channels in wild-type cells were stimulated by NAADP (0.01–1.0 μM) in a concentration-dependent manner. However, when a TRP-ML transgene was expressed in TRP-ML1−/− cells, the channel activity can be observed and NAADP enhanced this channel activity. In intact cell experiments, microscopic Ca2+ imaging showed that NAADP significantly increased intracellular [Ca2+] in TRP-ML1+/+ cells, but had no effect in TRP-ML1−/− cells. If a TRP-ML1 transgene was expressed in TRP-ML1−/− cells, the Ca2+ response to NAADP was restored to the level comparable to TRP-ML1+/+ cells. This further support the view that NAADP increases lysosomal TRP-ML1 channel activity to release Ca2+ and TRP-ML channels in lysosomes may regulate local compartmental Ca2+ which may be important for the control of lysosome function such as trafficking or intracellular signaling (Zhang et al., 2011).
Since the report of TRP-ML1 as an NAADP-sensitive lysosomal Ca2+ channel, some studies doubt that TRP-ML1 serves as a target of NAADP because different ligand binding experiments did not demonstrate TRP-ML1 binding to NAADP agonistic or antagonistic probes (Lin-Moshier et al., 2012; Pryor et al., 2006; Walseth et al., 2012). Given the multiple sizes of TRP-ML1 channel from 36–75 kDa in the lysosome membrane of native cells and its possible cleavage after function (Kiselyov et al., 2005; Yamaguchi et al., 2011), the results with negative binding of intracellular cytosol, particles or cell membrane with overexpressed TRP-ML1 gene may not rule out the role of TRP-ML1 as a target of NAADP or NAADP-sensitive lysosomal channels. It should be noted that in our original report (Zhang and Li, 2007), the action of NAADP through TRP-ML1 channels does not necessarily indicate that it must use this channel as its receptor. Since there are evidence that mucolipin-1 is able to oligomerize and/or form complexes with other proteins (Manzoni et al., 2004; Miedel et al., 2006) and that this heteromeric formation to constitute cation-permeable pores is common in TRP channels (Clapham, 2003), it is possible that stimulation of some regulatory protein or some common accessory NAADP binding protein may facilitate NAADP-associated Ca2+ release from lysosomes (Zhang and Li, 2007; Zhang et al., 2011). Most recently, Dr. Guse further proposed NAADP binding proteins as a unifying hypothesis for the action of NAADP to activate multiple channels in different Ca2+ stores (Guse, 2012).
Interestingly, a recent study reported that TRP-ML1 and two-pool channels (TPCs) are present in the same complex, yet function as two independent organellar ion channels (Yamaguchi et al., 2011). Although TRP-ML1 channels were not confirmed to be the target for NAADP, it seems that such conclusion is only for TRP-ML1 channels in the plasma membrane rather than lysosomes, because the channel activity in those studies were only recorded in the plasma membrane of cells with overly expressed TRP-ML1 gene. However, these studies using patch clamp recordings of calcium activated ionic currents in acinar cells indeed showed that membrane channel activity is extensively correlated with both local (short-lived) and global (longer-lived) calcium increases. In these experiments, the lack of difference in Ca2+-activated Cl− current oscillation upon NAADP stimulation between wild-type and knockout cells may not be specific enough to define the role of TRP-ML1, because this Ca2+-activated Cl− current oscillation depends upon global Ca2+ increase, which may be influenced by NAADP action on cell depolarization (Brailoiu et al., 2009b; Moccia et al., 2006a; Moccia et al., 2006b). The cell depolarization will activate Ca2+-activated Cl− current. Furthermore, when cells are clamped at certain membrane potentials, NAADP may amplify the globalization of Ca2+ signals (Cancela et al., 2002). Therefore, direct recording of lysosomal TRP-ML1 currents or more direct measurements of Ca2+ release from lysosomes, rather than global Ca2+ levels are needed for a solid conclusion to specify the action of TRP-ML1 channels as an NAADP-sensitive lysosome responder.
Another concern relates to the use of cell lines or transgenic cells in many studies for testing the target of NAADP action (Kiselyov et al., 2005; Yamaguchi et al., 2011), where TRP-ML1 was very overly expressed and spread to other compartments of cells in addition to lysosomes. It has been reported that although the majority of TRP-ML1 is expressed in intracellular compartments when a transgene was introduced into cell lines, some of the overexpressed TRP-ML1 can be targeted to the plasma membrane, while under a moderate overexpression condition TRP-ML1 variants were not found at the plasma membrane. This is because saturation of the protein trafficking pathway by marked overexpression forces expression of significant amounts of TRP-ML1 on the plasma membrane. Such plasma membrane mistargeting provides opportunity for studies on TRP-ML1 channel properties using the whole cell patch clamp configuration of the cell plasma membrane (Kiselyov et al., 2005; Soyombo et al., 2006). Given that TRP-ML1 on the plasma membrane may not occur in native organs or cells, whether the results obtained from the plasma membrane regarding TRP-ML1 channels indicate its channel property in lysosomes may be questionable. In some studies, the results that TRP-ML1 failed to act as an NAADP sensitive channel were obtained by manipulating TRP-ML1 expression or modifying TRP-ML integrability in SKBR3 cell lines (Yamaguchi et al., 2011). However, as mentioned above, overexpressed TRP-ML1 by these approaches may not reflect the real circumstances of TRP-ML1 in the native cells in terms of location and configuration. In addition, recent studies (Lin-Moshier et al., 2012; Walseth et al., 2012) have demonstrated that there may be some specific NAADP binding proteins, which may function as a cofactor to activate a Ca2+ channel such as TRP-ML1 or TPCs in response to NAADP. It is possible that the overexpressed TRP-ML1 has differences from the native channel protein in terms of the access of the NAADP binding proteins.
TPC Channels in Lysosome-Like Acidic Organelles
In 2009, Zhu, Evans, Galione and their associates in collaboration with other groups published a work indicating that TPCs may be an NAADP-targeted Ca2+ channel in lysosome-like acidic organelles mediating lysosomal Ca2+ bursts and consequent global Ca2+ release in HEK293 cells transfected with human TPC2 channels (Calcraft et al., 2009). Since then, TPC1 and TPC2 were reported to serve as major NAADP-sensitive Ca2+-permeable channels in different cells with overly expressed transgenes of these TPCs (Brailoiu et al. 2009a; Brailoiu et al., 2010a; Brailoiu et al., 2010b; Bright et al., 2005; Bund and Lee, 2003; Churchill and Galione 2001; Yamaguchi et al., 2011; Zinchuk et al., 2007). With some positive results in ligand binding experiments (Calcraft et al., 2009), these investigators proposed that like IP3 receptors in the SR, TPCs in lysosome-like acidic organelles serve as an NAADP receptor with Ca2+ release channel activity, whereby NAADP binds and elicits Ca2+ release from these acidic organelles. In addition, blockade of endogenous NAADP responses by siRNA, gene knockout and/or use of dominant negative constructs also demonstrated that TPCs may mediate NAADP-induced Ca2+ response (Brailoiu et al., 2009a; Calcraft et al., 2009; Pereira et al., 2011; Rybalchenko et al., 2012). Some pharmacological and functional characteristics of this NAADP-TPCs working model (including binding and activation) have been described in several reviews by the investigators who first reported the role of TPCs as NAADP-sensitive channels (Galione, 2011; Galione et al., 2009; Zhu et al., 2010b), and the readers are directed to these reviews for details.
Unfortunately, this NAADP-TPC working model for NAADP-mediated Ca2+ response together with its hypothetic basis, namely, the two-pool Ca2+ release mechanism, has not yet widely been accepted as an general pathway of Ca2+ mobilization, at least in the area of vascular biology. Several lines of evidence have challenged this NAADP-TPC working model. First, photoaffinity binding analysis using more potent and specific probes has recently shown that NAADP did not bind to TPCs in different cells. Walsethand colleagues developed a photoaffinity probe for the NAADP receptor, 5-N3-NAADP (Lin-Moshier et al., 2012; Walseth et al., 2012) and confirmed that its binding proteins are 30, 40, and 45 kD in sea urchin egg homogenates and 22- and 23-kD doublet proteins in mammalian SKBR3 cells, HEK 293 cells, and mouse pancreas, which were much smaller than TPC channel proteins. In particular, such binding patterns were not changed by overexpression or knocking out of TPC genes in different cells (Lin-Moshier et al., 2012). These results suggest that TPCs may not be NAADP binding proteins and challenge the view of TPCs as an NAADP receptor. The NAADP-TPC working model for NAADP-mediated Ca2+ response requires further evidence for an NAADP-binding domain of TPCs.
Second, previous studies have reported that TPCs can be detected in the plasma membrane (Ishibashi et al., 2000) and that TPCs probably assemble as dimers in cell membrane through differential interactions between transmembrane regions (Churamani et al., 2012). These results suggest that TPCs may be a channel in the plasma membrane, which is able to mediate the action of NAADP to regulate Ca2+ influx and membrane depolarization (Moccia et al., 2006a; Moccia et al., 2004). Although the localization of TPCs in the cell plasma membrane may be due to overexpression of TPC genes during experiments, a majority of evidence supporting TPCs as an NAADP receptor or target is also from experiments using TPC transgenes in different cell lines (Calcraft et al., 2009; Zong et al., 2009). Until more evidence shows the similar role of TPCs in mediating NAADP mobilization of Ca2+ from lysosome-like acidic organelles or vesicles of native cells or cells isolated from animal tissues or organs without exogenously introduced TPC genes, a cautious conclusion should be made regarding the role of TPCs in the action of NAADP intracellular Ca2+ mobilization and its related physiological relevance. In this regard, we recently demonstrated that TPC2 was ubiquitously expressed in various compartment of arterial smooth muscle cells isolated from bovine hearts, which was colocalized with plasma membrane marker caveolin-1, endoplasmic reticulum marker protein-disulfide isomerase, lysosome marker LAMP-1, as well as mitochondrial marker Mito-tracker. While mitochondrial expression of TPCs was much higher compared to other organelles (unpublished data), it is interesting to note that the SR and lysosomes had almost equal abundance of TPC proteins suggesting that NAADP may also act on the SR TPCs to release Ca2+. Lysosomal Ca2+ release is perhaps coinciding event with SR Ca2+ release in VSMCs where NAADP may simultaneously act on the TPCs of lysosomes and SR to mediate different cellular regulation. However, more studies are certainly needed to confirm this hypothesis.
Finally, in a recent study large efforts were made to distinguish the trigger event of TPCs mediated by NAADP from its amplification via ER Ca2+ stores by targeting TPCs on the cell membrane (Brailoiu et al., 2010b). This is because resolving triggering events of TPCs in lysosomes is relatively difficult. It is true that many studies aiming to define the role of TPCs in the Ca2+ response to NAADP used relatively indirect methods of measuring Ca2+ release from lysosome-like acidic organelles (Brailoiu et al., 2010c; Calcraft et al., 2009; Morgan and Galione, 2007; Yamasaki et al., 2004) by testing bafilomycin-sensitive or -dependent Ca2+ release. Although bafilomycin is considered to be a selective vacuolar H+-ATPase inhibitor, its wide spectrum for inhibition of ATPase (Bowman et al., 1988) has also been reported. In addition, the findings that the vacuolar H+-ATPase is also expressed in the plasma membrane and other cellular compartments or organelles (Forgac, 2007; Gluck, 1992; Rojas, et al., 2006; Tapper and Sundler, 1995) further raise a concern about the reliability of bafilomycin-sensitive lysosomal Ca2+ release as a parameter to define the role of TPCs in lysosomes or acidic organelles. In many studies, global Ca2+ transient response and plasma membrane Ca2+-sensitive Cl− channel or K+ channel activity were used to study the role of TPC as a lysosomal Ca2+ release channel to trigger intracellular global Ca2+ increase (Arredouani et al., 2010; Calcraft et al., 2009; Zong et al., 2009). Findings from these studies that measure global Ca2+ response may not differentiate the triggering action of TPCs in lysosomes since no lysosomal Ca2+ burst could be detected in those experiments. Therefore, it is imperative to develop useful measurements that can be used to directly monitor lysosomal Ca2+ release such as Ca2+ indicator (GCaMP3) attached directly to lysosome proteins, as was done for TRP-ML1 (Shen et al., 2012), and localization of Ca2+ release signals with lysosome markers (Zhang et al. 2010). Such direct measurements may provide reliable evidence for the Ca2+ channel nature of TPC as a trigger of NAADP-induced global Ca2+ release. Unfortunately, a most recent study by direct patch clamp recording of ion channels in endosome/lysosomes demonstrated that TPCs are not activated by NAADP and that TPC currents are absent in pancreatic β-cell lines that exhibit NAADP-induced lysosomal Ca2+ release. In addition, both TPC1 and TPC2 are not required for NAADP-or glucose-induced Ca2+ responses in pancreatic islets. It is concluded that TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes (Wang et al., 2012).
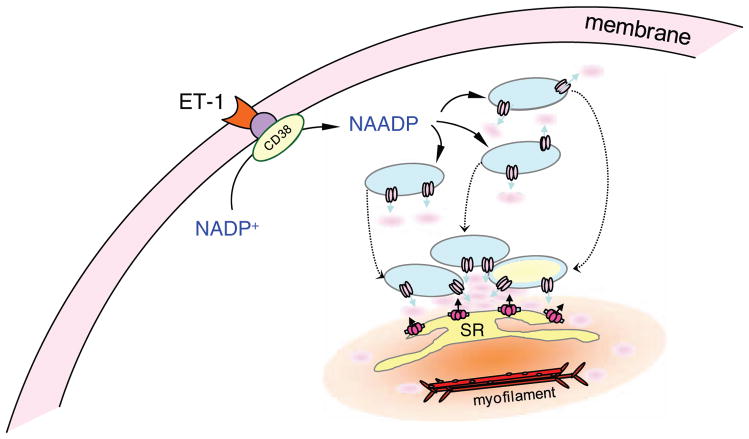
Based on the current knowledge, NAADP can be produced in response to ET-1, Ang II, norepinephrine, FasL, hypoxia and other agonists in VSMCs (Thai and Arendshorst, 2009; Zhang et al., 2006a). NAADP may induce lysosomal Ca2+ release through TRP-ML channels or TPCs either by direct binding or through a cytosolic binding protein. Such local Ca2+ bursts promote lysosome trafficking toward the SR, where aggregated lysosomes further release Ca2+ to activate RyRs or IP3Rs to produce large Ca2+ release from the SR, thereby increasing global Ca2+ concentrations within cells (Fig. 2).
Figure 2. NAADP-induced lysosomal Ca2+ release, lysosome trafficking and CICR in VSMCs.
SR: sarcoplasmic reticulum. ET-1: endothelin-1.
FUNCTIONAL RELEVANCE OF cADPR OR NAADP-MEDIATED SIGNALING
Vascular Regulation
Vascular Tone
Vascular smooth muscle (VSM) usually operates in a contracted state, which is referred to as vascular tone. It has been proposed that [Ca2+]i importantly contributes to the production of this “resting” vascular tone. Under resting conditions, [Ca2+]i in VSMCs is dependent upon Ca2+ influx, spontaneous brief releasing bursts of Ca2+ from the SR into the cytoplasm and CICR (Berridge, 1997). cADPR participates in the control of resting Ca2+ levels in smooth muscle cells through RyRs and CICR. Therefore, cADPR plays an important role in forming basic vascular tone. In isolated, perfused and pressurized small coronary arteries, basic vascular tone or spontaneous tension can be developed during a 1.5-hour equilibration period. Under this condition, SR Ca2+-ATPase inhibitor thapsigargin decreased the arterial diameter, and CICR blocker tetracaine and cADPR antagonist 8-Br-cADPR slightly dilated these arteries, suggesting that [Ca2+]i associated with the cADPR-RyR signaling pathway and CICR is one determinant of basic vascular tone (Boini et al., 2011; Deshpande et al., 2005; Li et al., 2000a; Li et al., 1997; Li et al., 2002; Li et al., 1998; Teggatz et al., 2005b; Zhang et al., 2001; Zhang et al., 2010).
Little is known so far whether NAADP-mediated two-phase Ca2+ release is implicated in the control of basic vascular tone. Recently, we reported that a death receptor agonist, FasL, can induce a two-phase Ca2+ release and enhance U46619-induced vasoconstriction in mouse coronary arteries. Such enhanced vasoconstrictor response was consistent with the time frame of the FasL-induced two-phase Ca2+ response (Zhang et al., 2010). However, FasL itself did not alter vascular tension. It seems that the slow development of FasL-induced slow development of vascular tension is different from that of the instant vasoconstriction provoked by some classical vasoactive agonists (e.g., norepinephrine, Ang II, or ATP), which is dependent on PLC-mediated direct Ca2+ release from the SR. Thus, FasL-induced two-phase Ca2+ release may be an important mechanism maintaining vascular tone where the NAADP signaling pathway in VSM is critical for the development of sustained vascular tone. In some other experiments using perfused small renal artery, we indeed found that blockade of the CD38-NAADP pathway by nicotinamide or PPADS had no effect on activation of arterial contraction in response to phenylnephrine at the beginning of tension increase. However, it significantly attenuated maintenance of vascular tension when it reached a plateau (unpublished data). This suggests that NAADP-induced two-phase or multiple phase Ca2+ release is an important source of intracellular Ca2+ to regulate the related molecular mechanisms for the maintenance of vascular tone such as protein kinase C and myosin light-chain kinase (Gao et al., 2001; Rasmussen et al., 1987).
Another important mechanism regulating vascular tone is the production of endothelium-derived relaxing factors (EDRFs), where blood vessels, particularly arteries, can produce relaxation in response to blood flow, shear stress and circulatory vasodilators, which may importantly contribute to the control of vascular tone under physiological or pathological conditions (Edwards et al., 2010; Qi et al., 2011; Vanhoutte et al., 2009). It is well known that Ca2+ activation of ECs is critically implicated in the production of EDRFs such as nitric oxide (NO), epoxyeicosatrienoic acids (EETs) and prostacyclins (Yi et al., 2002; Zhang et al., 2005; Zhang et al., 2004). Upon stimulation with different factors such as bradykinin, thrombin, histamine, bradykinin and oxidants, [Ca2+]i could increase 5–10 fold compared with the basal level. Increased Ca2+ stimulates the binding of Ca2+/CaM to endothelial NO synthase (eNOS), resulting in rapid conversion of L-arginine into L-citrulline, producing NO (Freichel et al., 2001; Putney, 1999; Tiruppathi et al., 2002). Interestingly, ADP-ribosylcyclase gives rise to bradykinin signal transduction from receptors to its effector enzymes (Deshpande et al., 2003; Higashida et al., 1996; Higashida et al., 2001), suggesting that cADPR/RyR signaling may be present in ECs to modulate endothelial function by regulating EDRF production. In this regard, we indeed demonstrated that inhibition of cADPR production or antagonism of its action significantly attenuated bradykinin-induced concentration-dependant coronary arterial vasodilation, endothelial Ca2+ release from RyR-sensitive stores and increases in NO production. Measurement of endothelial ADP-ribosylcyclase activity and intracellular cADPR concentrations confirmed that bradykinin induced cADPR production via enhanced ADP-ribosylcyclase activity (Zhang et al., 2006b). It is concluded that such bradykinin-induced intracellular Ca2+ increase and NO response are not mainly associated with IP3 signaling, but with cADPR levels in coronary ECs cells that participates in endothelium-dependent vasodilation. More recently, NAADP acetoxymethyl ester (NAADP-AM), a cell-permeant NAADP analog, was also demonstrated to increase cytosolic Ca2+ concentration in aortic ECs. This increase in intracellular Ca2+ and those evoked by acetylcholine were accompanied by hyperpolarization of ECs and NO production. Correspondingly, NAADP-AM was found to dilate aortic rings in an endothelium- and NO-dependent manner. In anesthetized rats, intravenous administration of NAADP-AM markedly decreased mean arterial pressure. Taken together, this study suggest that NAADP may regulate endothelial function by participating in the control of vascular tone and arterial blood pressure (Brailoiu et al., 2010c).
Vasomotor Response
As discussed above, acetylcholine (Ach) M-type receptor (mAChR) agonist, oxotremorine was demonstrated to markedly enhance the activity of ADP-ribosylcyclase and to increase the production of cADPR in cultured CASMCs, which was blocked by M1 mAChR blocker pirenzepine and by ADP-ribosylcyclase inhibitor nicotinamide (Ge et al., 2003). It seems that ADP-ribosylcylcase is directly coupled to M1 mAChRs through G-proteins. In isolated, perfused and pressurized small coronary arteries, vasoconstriction induced by Ach or oxotremorine was also attenuated by the inhibition of ADP-ribosylcyclase and blockade of cADPR action. These results confirmed that cADPR is linked to M1 mAChRs and mediates the vasoconstrictor response through activation of this subtype of mAChRs in CASMCs (Ge et al., 2003). This oxotremorine-induced vasoconstriction is also directly associated with cADPR-mediated Ca2+ release from the SR in these cells because oxotremorine-induced Ca2+ release with Ca2+-free extracellular solution was significantly attenuated by inhibition of cADPR production by nicotinamide and by blockade of cADPR action by 8-Br-cADPR (Ge et al., 2003). In isolated and perfused small coronary septal arteries from CD38−/− mice, oxotremorine produced much smaller vasoconstrictor response than in the same arteries from wild-type mice, and oxotremorine-induced intracellular Ca2+ increase was significantly lowered in freshly isolated septal arterial VSMCs from CD38−/− mice than in VSMCs isolated from wild-type mice (Teggatz et al., 2005a). Taken together, these results provide direct evidence that endogenous cADPR contributes to oxotremorine-induced Ca2+ mobilization in VSMCs and that cADPR serves as a second messenger to activate M1 receptors and mediate the vasoconstrictor response (Prakash et al., 1998). In renal circulation, Arendshorst and his associates demonstrated that basal ADP-ribosylcyclase activity is important in the control of renal blood flow since its inhibition resulted in increased renal blood flow in anesthetized rats (Thai et al., 2007). They also showed that inhibitors of ADP-ribosylcyclase attenuated renal vascular responses to Ang II and norepinephrine by approximately 60% when injected into the renal artery (Thai and Arendshorst 2008; Thai et al., 2007). In these experiments, it was also found that more pronounced renal ADP-ribosylcyclase inhibition produced local vasodilation without altering arterial pressure, indicating that the basal levels of ADP-ribosylcyclase activity contribute to resting renal vascular resistance. This role of ADP-ribosylcyclase activity in mediating the vasoconstrictor response was further confirmed by using CD38−/− mice. It was shown that acute renal vasoconstrictor responses to Ang II and norepinephrine were reduced by approximately 50% in CD38−/− mice compared with wild-type mice. All these studies suggest that CD38/ADP-ribosylcyclase is importantly involved in mediating renal vasoconstrictor responses to stimulation of G-protein coupled receptors in mice and rats (Thai and Arendshorst, 2008; Thai et al., 2007).
ET-1 is a potent endothelium-derived vasoconstrictor peptide that increases intracellular Ca2+ via activation of ETA and/or ETB receptors in different vascular beds. Recent studies have shown that ET-1-induced Ca2+ response is associated with cADPR/RyR signaling. In rat mesenteric arteries, Giulumian et al., reported that ET-1-induced Ca2+ increase and vasoconstriction were significantly attenuated by ADP-ribosylcylcase inhibitor nicotinamide and RyR Ca2+ release channel inhibitor dantrolene (Giulumian et al., 2000). In isolated pulmonary arteries, membrane-permeant cADPR antagonist 8-Br-cADPR was demonstrated to block sustained hypoxic pulmonary vasoconstriction (Dipp and Evans, 2001). In rat peritubular VSMCs, Barone et al., observed that both ETA- and ETB-mediated Ca2+ signaling were completely abolished by cADPR antagonist 8-NH2-cADPR (Barone et al., 2002). In porcine airway smooth muscle, White et al., showed that ET-1-induced Ca2+ response was inhibited by cADPR antagonist 8-Br-cADPR (White et al., 2003). Although there was direct detection of cADPR production in response to ET-1 in some vascular beds in these studies, these functional data have suggested that cADPR/RyR Ca2+ signaling may importantly participate in ET-1-induced vasoconstrictor response.
In addition to cADPR, NAADP-mediated Ca2+ signaling was also found to regulate agonist-induced vasoconstrictor response. In this regard, Evans and associates first reported that intracellular dialysis of NAADP induced spatially restricted “bursts” of Ca2+ release that initiated a global Ca2+ wave and contraction in pulmonary artery smooth muscle cells. Depletion of SR Ca2+ stores with thapsigargin and inhibition of RyRs with ryanodine both blocked the global Ca2+ waves by NAADP (Boittin et al. 2002). They suggest that NAADP may act in concert with cADPR to promote hypoxic pulmonary vasoconstriction (Evans 2010). Consistent with a previous study by Kinnear et al., in coronary arteries, we also found that ET-1 induced NAADP production, which mobilized intracellular Ca2+ in a manner dependent of normal lysosome function. ET-1-induced maximal coronary arterial constriction was substantially blocked by lysosome function inhibitor bafilomycin A1 and NAADP antagonist PPADS. It is obvious that a lysosome-mediated Ca2+ regulatory mechanism via NAADP contributes to ET-1-induced Ca2+ mobilization in CASMCs and consequent vasoconstriction of coronary arteries (Zhang et al., 2006a). More recently, we further demonstrated that FasL also increased NAADP production, but FasL itself did not produce vasoconstriction in coronary arterial preparation. However, FasL significantly enhanced IP3-producing agonist U46619-induced coronary arterial contraction, suggesting that NAADP may also sensitize arterial contraction when its production is increased (Zhang et al., 2010). Despite these reports, more studies are needed to differentiate the role of intracellular Ca2+ stores or signaling pathways in the mediation or modulation of vasomotor response. In particular, definition of the temporospatial action of NAADP, cADPR and IP3 in VSMCs is imperative.
Vascular Diseases
Although cADPR and NAADP have been extensively investigated as Ca2+ signaling second messengers with their related molecular signaling mechanisms, the pathological role of both signaling molecules is still under studied. Based on acute experiments in cells or isolated vessels, cADPR or NAADP are indicated to be involved in the development of hypertension and pulmonary hypertension (Evans et al., 2005). However, so far there is no direct evidence showing that both ADP-ribosylcyclase-derived second messengers are implicated in any vascular diseases. More recently, our laboratory is working on the potential contribution of NAADP-mediated regulation of lysosome function in the development of atherosclerosis. The general hypothesis is that NAADP regulates lysosome function, which plays an essential role in the control of lysosome trafficking or fusion to autophagosomes (APs), and regulates autophagy via its effect on autophagic flux (Fasano et al., 2012; Ryter et al., 2010; Seedorf et al., 1995; Xu et al., 2011).
Given the different cell types in the artery wall, the role of autophagy in the development of atherosclerosis is complex due to its action on different vascular cell functions. It has been recently assumed that autophagy may have both protective and detrimental roles during atherosclerosis, depending upon the status of autophagy or stages of atherosclerosis (Bampton et al., 2005; Martinet and De Meyer, 2009). Since autophagy is important in the degradation of damaged materials, it is possible that autophagy in the arterial wall helps clean up damaged components and recover cells from the damage upon atherosclerotic stimuli. In addition, autophagy activation interferes with cell apoptosis due to engulfment of defective or damaged mitochondria by APs, which limits the release of proapoptotic proteins (Gutierrez et al., 2004; Kim et al., 2008; Zhu et al., 2007). This autophagic process may protect arterial cells from atherogenic injury. However, if acute or persistent oxidative stress occurs during atherosclerosis, lysosomes may be damaged to release hydrolases, engage as part of oxidative stress, and enhance cellular damages (Jia et al. 2006; Martinet and De Meyer 2008; Martinet and De Meyer 2009; Xu et al., 2010). Enhanced or reduced autophagy plays different roles in the development of atherosclerosis depending on the different cells involved. In macrophages, autophagy increasescholesterol transport out of these cells, which may prevent lipid droplet formation reducing foam cell formation. Similarly, enhanced autophagic death of macrophages also possibly attenuates foam cell formation, reducing atherosclerotic injury. However, excessive activation of autophagy in ECs may lead to damage of the endothelium enhancing atherogenic injury. In arterial SMCs, enhanced autophagy induces their modulation to a differentiated, quiescent, and contractile phenotype, decreasing cell proliferation and preventing fibrosis. Nevertheless, excessive autophagy in arterial SMCs may result in their death, increasing the instability of atherosclerotic plaques (Jia et al., 2007; Jia et al., 2006; Schrijvers et al., 2007; Verheye et al. 2007; Xu et al., 2010). Although the role of augmented autophagy in atherosclerosis has been extensively studied, there is no evidence that a defective or reduced autophagy is involved in the pathogenesis of atherosclerosis.
In some of our preliminary studies, we demonstrated that defective autophagy is also importantly involved in atherogenesis, which is associated with molecular dysregulation of lysosome function. As shown in Figure 3, proatherogenic stimuli such as 7-keto-cholesterol (7-keto) or ox-LDL activates autophagy in arterial SMCs, leading to the formation of APs. Under normal condition, lysosome trafficking and fusion to APs are controlled by CD38-ADP-ribosylcyclase-mediated regulation, particularly by NAADP production, which leads to the formation of autophagolysosomes (APLs) and subsequent breakdown of the autophagic vesicles within the cells. This regulated autophagic process via NAADP signaling pathway protects SMCs from atherosclerotic injury upon atherogenic stimulations. When the controlling mechanism of lysosome function is insufficient, such as impaired CD38-ADP-ribosylcyclase activity or reduced NAADP production, the formation of APLs and breakdown of autophagic vesicles also become impaired, which activates cell dedifferentiation, proliferation and growth, thereby stimulating production of extracellular matrix and ultimately inducing or accelerating atherosclerosis. Indeed, CD38−/− mice developed atherosclerosis when they were exposed to the atherogenic diet (Xu et al., 2011). Under such condition, dysregulation of lysosome function due to the lack of CD38 product, mainly NAADP, caused deficient autophagy by failed formation of APLs and impaired degradation of autophagic content, which resulted in arterial smooth muscle remodeling by enhanced cell proliferation and extracellular matrix production.
Figure 3. Lysosome trafficking and fusion to autophagosomes controlled by NAADP via CD38- ADP-ribosylcyclase.
FasL: Fas ligand. LDL: Low density lipoprotein.
CONCLUDING REMARKS
Since the discovery of cADPR and NAADP as Ca2+ signaling second messengers, a large body of evidence shows that they are importantly involved in the regulation of intracellular Ca2+ concentrations in vascular cells including ECs and VSMCs (Bai et al., 2005; Evans, 2010; Zhang and Li, 2006). In ECs, cADPR mediates agonist (such as bradykinin)-induced Ca2+ mobilization from the ER, resulting in production of NO or other EDRFs, participating in the EDVD response. In VSMCs, cADPR serves as a second messenger to stimulate Ca2+ release from the SR via RyRs and is involved in the regulation of CICR and consequent Ca2+ waves, producing global Ca2+ increase within these cells. cADPR activates RyRs by binding to FKBP12.6 and results in dissociation of this accessory protein from RyRs, whereby Ca2+ release from the SR is enhanced. There is considerable evidence showing that this cADPR-mediated Ca2+ signaling plays a critical role in Ca2+ release and vasoconstrictor response to different agonists, oxidative stress, cell membrane depolarization, and Ca2+ influx. Similarly, NAADP has been found to play similar roles in the regulation of intracellular Ca2+ mobilization, vascular tone and vasomotor responses (Zhang et al., 2010; Zhang et al., 2006a). It has been demonstrated that NAADP is a more potent Ca2+ mobilizer compared with other Ca2+ signaling second messengers. It first produces small Ca2+ bursts via lysosomes and then leads to a global Ca2+ increases in VSMCs due to CICR-mediated large Ca2+ release from the SR, known as a two-phase Ca2+ release. Functionally, NAADP-mediated Ca2+ release has been reported to also participate in the control of vascular tone and vasomotor response to different agonists, which may occur in a temporospatial way in concert with other pathways such as cADPR and IP3. In addition, NAADP-mediated Ca2+ regulation in vascular cells may be relevant to vascular function such as EDVD or cell apoptosis. Although many studies assume that cADPR and NAADP-mediated Ca2+ signaling may be implicated in the pathogenesis of some vascular diseases such as hypertension, pulmonary hypertension and atherosclerosis, there is not much direct evidence that both Ca2+ second messengers indeed contribute to the development of these diseases. This is an area necessary to be studied and addressed in the future.
Acknowledgments
Most of the studies from our laboratory cited in this report were supported by NIH Grants HL057244, HL075316 and DK054927.
Biographies

Pin-Lan Li is a Professor and Vice Chair in the Department of Pharmacology and Toxicology of the Virginia Commonwealth University. She was trained as M.D. in Yi-Chang Medical College and Tongji Medical University, China in 1973–1979 and then received her Ph.D. from the Heidelberg University, Germany in 1992. She joined the Medical College of Wisconsin as an Assistant Professor in1994 and then promoted to full professor in 2004. She moved to the Virginia Commonwealth University as a Professor with tenure in 2005. Her research is mainly focused on the vascular signaling mechanisms including cADPR, NAADP, ceramide, NO and redox-mediated signal transduction and their relevance to vascular diseases such as vascular inflammation, atherosclerosis and hypertension.

Yang Zhang is an Assistant Professor in the Department of Pharmacology & Toxicology, Virginia Commonwealth University (VCU). He obtained his Ph.D. in Pharmacology from the Medical College of Wisconsin in 2006. After two years postdoctoral training at the Department of Molecular Biology, University of Duisburg-Essen, Germany, he was promoted to a junior professor at the same department. He joined VCU as an Assistant Professor in 2011. His research interests are in studying the roles of ADP ribosylcyclase/CD38, NADPH oxidase, and acid sphingomyelinase in the control of vascular autophagy and inflammasome activity as well as their pathogenic roles in atherosclerosis, pulmonary fibrosis and chronic obstructive pulmonary disease.

Justine M. Abais is a Ph.D. student in the Department of Pharmacology and Toxicology at the Virginia Commonwealth University (VCU), located in Richmond, Virginia. Justine received her Bachelor’s degree in Chemistry from VCU in 2009. Her current research interest is in understanding the molecular mechanisms of hyperhomocysteinemia-associated vascular and renal degenerative diseases such as atherosclerosis and end-stage renal disease. She is currently a recipient of Ruth L. Kirschstein National Research Service Award for individual predoctoral fellows from the National Institute of Aging.

Joseph K. Ritter is an Associate Professor of Pharmacology and Toxicology at the Virginia Commonwealth University School of Medicine. He received his Ph.D. degree at the University of Utah in 1987 and was a postdoctoral fellow at the National Institute of Child Health and Human Development in Bethesda, Maryland. His research interests are in the role of metabolism as a modulator of pharmacologic and toxic effects of xenobiotic and endogenous substances. Recent research projects in his laboratory focus on the functional relevance of endocannabinoids and associated signaling mechanisms to hypertension and kidney diseases.

Fan Zhang is an Assistant Professor of Pharmacology at the Virginia Commonwealth University (VCU). He obtained his Ph.D. in Pharmacology at the Tongji Medical College of Huazhong University of Science and Technology, China in 2002. After postdoctoral training in the Department of Pharmacology, VCU, he was promoted to Assistant Professor in 2009 at the same department. His research focus is on the vascular regulation by ADP ribosylcyclase/CD38 and its products, cADPR and NAADP. Recent research projects in his laboratory deal with lysosomal TRP-ML1 channels, regulation of lysosomal lipid metabolism in macrophages, and their roles in coronary arterial atherosclerosis.
References
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+ J Biol Chem. 1996;271:8513–8516. doi: 10.1074/jbc.271.15.8513. [DOI] [PubMed] [Google Scholar]
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- Adebanjo OA, Koval A, Moonga BS, Wu XB, Yao S, Bevis PJ, Kumegawa M, Zaidi M, Sun L. Molecular cloning, expression, and functional characterization of a novel member of the CD38 family of ADP-ribosyl cyclases. Biochem Biophys Res Commun. 2000;273:884–889. doi: 10.1006/bbrc.2000.3041. [DOI] [PubMed] [Google Scholar]
- Albrieux M, Lee HC, Villaz M. Calcium signaling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J Biol Chem. 1998;273:14566–14574. doi: 10.1074/jbc.273.23.14566. [DOI] [PubMed] [Google Scholar]
- Arredouani A, Evans AM, Ma J, Parrington J, Zhu MX, Galione A. An emerging role for NAADP-mediated Ca2+ signaling in the pancreatic beta-cell. Islets. 2010;2:323–330. doi: 10.4161/isl.2.5.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach G. Mucolipidosis type IV. Mol Genet Metab. 2001;73:197–203. doi: 10.1006/mgme.2001.3195. [DOI] [PubMed] [Google Scholar]
- Bach G. Mucolipin 1: Endocytosis and cation channel—A review. Pflugers Arch. 2005;451:313–317. doi: 10.1007/s00424-004-1361-7. [DOI] [PubMed] [Google Scholar]
- Bai N, Lee HC, Laher I. Emerging role of cyclic ADP-ribose (cADPR) in smooth muscle. Pharmacol Ther. 2005;105:189–207. doi: 10.1016/j.pharmthera.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM. The dynamics of autophagy visualized in live cells: From autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. doi: 10.4161/auto.1.1.1495. [DOI] [PubMed] [Google Scholar]
- Bao JX, Jin S, Zhang F, Wang ZC, Li N, Li PL. Activation of membrane NADPH oxidase associated with lysosome-targeted acid sphingomyelinase in coronary endothelial cells. Antioxid Redox Signal. 2010;12:703–712. doi: 10.1089/ars.2009.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F, Genazzani AA, Conti A, Churchill GC, Palombi F, Ziparo E, Sorrentino V, Galione A, Filippini A. A pivotal role for cADPR-mediated Ca2+ signaling: Regulation of endothelin-induced contraction in peritubular smooth muscle cells. Faseb J. 2002;16:697–705. doi: 10.1096/fj.01-0749com. [DOI] [PubMed] [Google Scholar]
- Beers KW, Chini EN, Lee HC, Dousa TP. Metabolism of cyclic ADP-ribose in opossum kidney renal epithelial cells. Am J Physiol. 1995;268:C741–746. doi: 10.1152/ajpcell.1995.268.3.C741. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The biology and medicine of calcium signalling. Mol Cell Endocrinol. 1994;98:119–124. doi: 10.1016/0303-7207(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berruet L, Muller-Steffner H, Schuber F. Occurrence of bovine spleen CD38/NAD+glycohydrolase disulfide-linked dimers. Biochem Mol Biol Int. 1998;46:847–855. [PubMed] [Google Scholar]
- Boini KM, Xia M, Xiong J, Li C, Payne LP, Li PL. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Dipp M, Kinnear NP, Galione A, Evans AM. Vasodilation by the calcium-mobilizing messenger cyclic ADP-ribose. J Biol Chem. 2003;278:9602–9608. doi: 10.1074/jbc.M204891200. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Galione A, Evans AM. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ Res. 2002;91:1168–1175. doi: 10.1161/01.res.0000047507.22487.85. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KN, Currie S, Macmillan D, Muir TC, Mccarron JG. Cyclic ADP-ribose increases Ca2+ removal in smooth muscle. J Cell Sci. 2003;116:4291–4306. doi: 10.1242/jcs.00713. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009a;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S, Dun NJ. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem. 2010a;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Miyamoto MD, Dun NJ. Nicotinic acid adenine dinucleotide phosphate enhances quantal neurosecretion at the frog neuromuscular junction: Possible action on synaptic vesicles in the releasable pool. Mol Pharmacol. 2001;60:718–724. [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010b;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Brailoiu E, Parkesh R, Galione A, Churchill GC, Patel S, Dun NJ. NAADP-mediated channel ‘chatter’ in neurons of the rat medulla oblongata. Biochem J. 2009b;419:91–97. doi: 10.1042/BJ20081138. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Gurzu B, Gao X, Parkesh R, Aley PK, Trifa DI, Galione A, Dun NJ, Madesh M, Patel S, Churchill GC, Brailoiu E. Acidic NAADP-sensitive calcium stores in the endothelium: Agonist-specific recruitment and role in regulating blood pressure. J Biol Chem. 2010c;285:37133–37137. doi: 10.1074/jbc.C110.169763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Bund SJ, Lee RM. Arterial structural changes in hypertension: A consideration of methodology, terminology and functional consequence. J Vasc Res. 2003;40:547–557. doi: 10.1159/000075678. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Cancela JM, Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. Two different but converging messenger pathways to intracellular Ca(2+) release: The roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J. 2000;19:2549–2557. doi: 10.1093/emboj/19.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Van Coppenolle F, Galione A, Tepikin AV, Petersen OH. Transformation of local Ca2+ spikes to global Ca2+ transients: The combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 2002;21:909–919. doi: 10.1093/emboj/21.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Van De Vrede Y, Fossier P, Baux G. Ryanodine-, IP3- and NAADP-dependent calcium stores control acetylcholine release. Pflugers Arch. 2001;443:289–296. doi: 10.1007/s004240100691. [DOI] [PubMed] [Google Scholar]
- Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett. 2010;584:2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram N, Chang CF. NADP+-Dependent internalization of recombinant CD38 in CHO cells. Arch Biochem Biophys. 1999;363:267–272. doi: 10.1006/abbi.1999.1103. [DOI] [PubMed] [Google Scholar]
- Chidambaram N, Wong ET, Chang CF. Differential oligomerization of membrane-bound CD38/ADP-ribosyl cyclase in porcine heart microsomes. Biochem Mol Biol Int. 1998;44:1225–1233. doi: 10.1080/15216549800202322. [DOI] [PubMed] [Google Scholar]
- Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J Biol Chem. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- Churamani D, Carrey EA, Dickinson GD, Patel S. Determination of cellular nicotinic acid-adenine dinucleotide phosphate (NAADP) levels. Biochem J. 2004;380:449–454. doi: 10.1042/BJ20031754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churamani D, Hooper R, Brailoiu E, Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Galione A. Spatial control of Ca2+ signaling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J Biol Chem. 2000;275:38687–38692. doi: 10.1074/jbc.M005827200. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 2001;20:2666–2671. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- Copello JA, Qi Y, Jeyakumar LH, Ogunbunmi E, Fleischer S. Lack of effect of cADP-ribose and NAADP on the activity of skeletal muscle and heart ryanodine receptors. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- Dai J, Kuo KH, Leo JM, Van Breemen C, Lee CH. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium. 2005;37:333–340. doi: 10.1016/j.ceca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Dammermann W, Guse AH. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J Biol Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Vaisitti T, Billington R, Bergui L, Omede P, Genazzani AA, Malavasi F. CD38/CD19: A lipid raft-dependent signaling complex in human B cells. Blood. 2007;109:5390–5398. doi: 10.1182/blood-2006-12-061812. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. Faseb J. 2003;17:452–454. doi: 10.1096/fj.02-0450fje. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in CD38-deficient mice. Am J Respir Cell Mol Biol. 2005;32:149–156. doi: 10.1165/rcmb.2004-0243OC. [DOI] [PubMed] [Google Scholar]
- Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res. 2001;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Shen D, Chen S, Liu M, Wang Y, Mills E, Cheng X, Delling M, Xu H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Esposito B, Gambara G, Lewis AM, Palombi F, D’alessio A, Taylor LX, Genazzani AA, Ziparo E, Galione A, Churchill GC, Filippini A. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood. 2011;117:4968–4977. doi: 10.1182/blood-2010-02-266338. [DOI] [PubMed] [Google Scholar]
- Evans AM. The role of intracellular ion channels in regulating cytoplasmic calciumin pulmonary arterial mmooth muscle: Which store and where? Adv Exp Med Biol. 2010;661:57–76. doi: 10.1007/978-1-60761-500-2_4. [DOI] [PubMed] [Google Scholar]
- Evans AM, Cannell MB. The role of L-type Ca2+ current and Na+ current-stimulated Na/Ca exchange in triggering SR calcium release in guinea-pig cardiac ventricular myocytes. Cardiovasc Res. 1997;35:294–302. doi: 10.1016/s0008-6363(97)00117-x. [DOI] [PubMed] [Google Scholar]
- Evans AM, Wyatt CN, Kinnear NP, Clark JH, Blanco EA. Pyridine nucleotides and calcium signalling in arterial smooth muscle: From cell physiology to pharmacology. Pharmacol Ther. 2005;107:286–313. doi: 10.1016/j.pharmthera.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Fasano T, Pisciotta L, Bocchi L, Guardamagna O, Assandro P, Rabacchi C, Zanoni P, Filocamo M, Bertolini S, Calandra S. Lysosomal lipase deficiency: Molecular characterization of eleven patients with Wolman or cholesteryl ester storage disease. Mol Genet Metab. 2012;105:450–456. doi: 10.1016/j.ymgme.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Parker LA. Endothelin B receptor Ca2+ signaling in shark vascular smooth muscle: Participation of inositol trisphosphate and ryanodine receptors. J Exp Biol. 2004;207:3411–3417. doi: 10.1242/jeb.01134. [DOI] [PubMed] [Google Scholar]
- Fleischer S, Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Franco L, Zocchi E, Calder L, Guida L, Benatti U, De Flora A. Self-aggregation of the transmembrane glycoprotein CD38 purified from human erythrocytes. Biochem Biophys Res Commun. 1994;202:1710–1715. doi: 10.1006/bbrc.1994.2132. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose: A new way to control calcium. Science. 1993;259:325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- Galione A. NAADP, a new intracellular messenger that mobilizes Ca2+ from acidic stores. Biochem Soc Trans. 2006;34:922–926. doi: 10.1042/BST0340922. [DOI] [PubMed] [Google Scholar]
- Galione A. NAADP receptors. Cold Spring Harb Perspect Biol. 2011;3:a004036. doi: 10.1101/cshperspect.a004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. The acid test: The discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca(2+) release channels. Pflugers Arch. 2009;458:869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: Modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Galione A, White A, Willmott N, Turner M, Potter BV, Watson SP. cGMP mobilizes intracellular Ca2+ in sea urchin eggs by stimulating cyclic ADP-ribose synthesis. Nature. 1993;365:456–459. doi: 10.1038/365456a0. [DOI] [PubMed] [Google Scholar]
- Gambara G, Billington RA, Debidda M, D’alessio A, Palombi F, Ziparo E, Genazzani AA, Filippini A. NAADP-induced Ca(2+ signaling in response to endothelin is via the receptor subtype B and requires the integrity of lipid rafts/caveolae. J Cell Physiol. 2008;216:396–404. doi: 10.1002/jcp.21407. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ye LH, Kishi H, Okagaki T, Samizo K, Nakamura A, Kohama K. Myosin light chain kinase as a multifunctional regulatory protein of smooth muscle contraction. IUBMB Life. 2001;51:337–344. doi: 10.1080/152165401753366087. [DOI] [PubMed] [Google Scholar]
- Ge ZD, Li PL, Chen YF, Gross GJ, Zou AP. Myocardial ischemia and reperfusion reduce the levels of cyclic ADP-ribose in rat myocardium. Basic Res Cardiol. 2002;97:312–319. doi: 10.1007/s00395-002-0348-9. [DOI] [PubMed] [Google Scholar]
- Ge ZD, Zhang DX, Chen YF, Yi FX, Zou AP, Campbell WB, Li PL. Cyclic ADP-ribose contributes to contraction and Ca2+ release by M1 muscarinic receptor activation in coronary arterial smooth muscle. J Vasc Res. 2003;40:28–36. doi: 10.1159/000068936. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J Biol Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Mezna M, Summerhill RJ, Galione A, Michelangeli F. Kinetic properties of nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. J Biol Chem. 1997;272:7669–7675. doi: 10.1074/jbc.272.12.7669. [DOI] [PubMed] [Google Scholar]
- Gerasimenko J, Maruyama Y, Tepikin A, Petersen OH, Gerasimenko O. Calcium signalling in and around the nuclear envelope. Biochem Soc Trans. 2003a;31:76–78. doi: 10.1042/bst0310076. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003b;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulumian AD, Meszaros LG, Fuchs LC. Endothelin-1-induced contraction of mesenteric small arteries is mediated by ryanodine receptor Ca2+ channels and cyclic ADP-ribose. J Cardiovasc Pharmacol. 2000;36:758–763. doi: 10.1097/00005344-200012000-00011. [DOI] [PubMed] [Google Scholar]
- Gluck S. V-ATPases of the plasma membrane. J Exp Biol. 1992;172:29–37. doi: 10.1242/jeb.172.1.29. [DOI] [PubMed] [Google Scholar]
- Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem. 2006;281:28951–28957. doi: 10.1074/jbc.M604370200. [DOI] [PubMed] [Google Scholar]
- Guida L, Franco L, Zocchi E, De Flora A. Structural role of disulfide bridges in the cyclic ADP-ribose related bifunctional ectoenzyme CD38. FEBS Lett. 1995;368:481–484. doi: 10.1016/0014-5793(95)00715-l. [DOI] [PubMed] [Google Scholar]
- Gul R, Kim SY, Park KH, Kim BJ, Kim SJ, Im MJ, Kim UH. A novel signaling pathway of ADP-ribosyl cyclase activation by angiotensin II in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H77–88. doi: 10.1152/ajpheart.01355.2007. [DOI] [PubMed] [Google Scholar]
- Guse AH. Linking NAADP to ion channel activity: A unifying hypothesis. Sci Signal. 2012;5:18. doi: 10.1126/scisignal.2002890. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Han MK, Kim SJ, Park YR, Shin YM, Park HJ, Park KJ, Park KH, Kim HK, Jang SI, An NH, Kim UH. Antidiabetic effect of a prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid, through CD38 dimerization and internalization. J Biol Chem. 2002;277:5315–5321. doi: 10.1074/jbc.M106439200. [DOI] [PubMed] [Google Scholar]
- Higashida H, Bowden SE, Yokoyama S, Salmina A, Hashii M, Hoshi N, Zhang JS, Knijnik R, Noda M, Zhong ZG, Jin D, Higashida K, Takeda H, Akita T, Kuba K, Yamagishi S, Shimizu N, Takasawa S, Okamoto H, Robbins J. Overexpression of human CD38/ADP-ribosyl cyclase enhances acetylcholine-induced Ca2+ signalling in rodent NG108-15 neuroblastoma cells. Neurosci Res. 2007;57:339–346. doi: 10.1016/j.neures.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Higashida H, Egorova A, Higashida C, Zhong ZG, Yokoyama S, Noda M, Zhang JS. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem. 1999;274:33348–33354. doi: 10.1074/jbc.274.47.33348. [DOI] [PubMed] [Google Scholar]
- Higashida H, Egorova A, Hoshi N, Noda M. Streptozotocin, an inducer of NAD+ decrease, attenuates M-potassium current inhibition by ATP, bradykinin, angiotensin II, endothelin 1 and acetylcholine in NG108-15 cells. FEBS Lett. 1996;379:236–238. doi: 10.1016/0014-5793(95)01516-7. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Hashii M, Taketo M, Higashida M, Takayasu T, Ohshima T, Takasawa S, Okamoto H, Noda M. Muscarinic receptor-mediated dual regulation of ADP-ribosyl cyclase in NG108-15 neuronal cell membranes. J Biol Chem. 1997;272:31272–31277. doi: 10.1074/jbc.272.50.31272. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Hoshi N, Hashii M, Egorova A, Zhong ZG, Noda M, Shahidullah M, Taketo M, Knijnik R, Kimura Y, Takahashi H, Chen XL, Shin Y, Zhang JS. Signal transduction from bradykinin, angiotensin, adrenergic and muscarinic receptors to effector enzymes, including ADP-ribosyl cyclase. Biol Chem. 2001;382:23–30. doi: 10.1515/BC.2001.004. [DOI] [PubMed] [Google Scholar]
- Higashida H, Zhang J, Hashii M, Shintaku M, Higashida C, Takeda Y. Angiotensin II stimulates cyclic ADP-ribose formation in neonatal rat cardiac myocytes. Biochem J. 2000;352:197–202. [PMC free article] [PubMed] [Google Scholar]
- Himpens B, Missiaen L, Casteels R. Ca2+ homeostasis in vascular smooth muscle. J Vasc Res. 1995;32:207–219. doi: 10.1159/000159095. [DOI] [PubMed] [Google Scholar]
- Hirata M, Yanaga F, Koga T, Ogasawara T, Watanabe Y, Ozaki S. Stereospecific recognition of inositol 1,4,5-trisphosphate analogs by the phosphatase, kinase, and binding proteins. J Biol Chem. 1990;265:8404–8407. [PubMed] [Google Scholar]
- Hirst DG, Kennovin GD, Flitney FW. The radiosensitizer nicotinamide inhibits arterial vasoconstriction. Br J Radiol. 1994;67:795–799. doi: 10.1259/0007-1285-67-800-795. [DOI] [PubMed] [Google Scholar]
- Hohenegger M, Suko J, Gscheidlinger R, Drobny H, Zidar A. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem J. 2002;367:423–431. doi: 10.1042/BJ20020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Agrawal DK. Autophagy of vascular smooth muscle cells in atherosclerotic lesions. Autophagy. 2007;3:63–64. doi: 10.4161/auto.3427. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Jia SJ, Jin S, Zhang F, Yi F, Dewey WL, Li PL. Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1743–1752. doi: 10.1152/ajpheart.00617.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proc Natl Acad Sci USA. 2002;99:14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan MS, Fenton AM, Prakash YS, Sieck GC. Cyclic ADP-ribose stimulates sarcoplasmic reticulum calcium release in porcine coronary artery smooth muscle. Am J Physiol. 1996;270:H801–806. doi: 10.1152/ajpheart.1996.270.2.H801. [DOI] [PubMed] [Google Scholar]
- Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol. 1997;272:L659–664. doi: 10.1152/ajplung.1997.272.4.L659. [DOI] [PubMed] [Google Scholar]
- Kim JS, Nitta T, Mohuczy D, O’malley KA, Moldawer LL, Dunn WA, Jr, Behrns KE. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium. 2008;44:190–201. doi: 10.1016/j.ceca.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem. 2005;280:43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- Koguma T, Takasawa S, Tohgo A, Karasawa T, Furuya Y, Yonekura H, Okamoto H. Cloning and characterization of cDNA encoding rat ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (homologue to human CD38) from islets of Langerhans. Biochim Biophys Acta. 1994;1223:160–162. doi: 10.1016/0167-4889(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Koshiyama H, Lee HC, Tashjian AH., Jr Novel mechanism of intracellular calcium release in pituitary cells. J Biol Chem. 1991;266:16985–16988. [PubMed] [Google Scholar]
- Lacapere JJ, Boulla G, Lund FE, Primack J, Oppenheimer N, Schuber F, Deterre P. Fluorometric studies of ligand-induced conformational changes of CD38. Biochim Biophys Acta. 2003;1652:17–26. doi: 10.1016/j.bbapap.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Ladd C, Bourke MT, Scherczinger CA, Pagliaro EM, Gaensslen RE, Lee HC. A PCR-based strategy for ABO genotype determination. J Forensic Sci. 1996;41:134–137. [PubMed] [Google Scholar]
- Lahouratate P, Guibert J, Faivre JF. cADP-ribose releases Ca2+ from cardiac sarcoplasmic reticulum independently of ryanodine receptor. Am J Physiol. 1997;273:H1082–1089. doi: 10.1152/ajpheart.1997.273.3.H1082. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Schwarzmann N, Guse AH. Ca2+ release via ryanodine receptors and Ca2+ entry: Major mechanisms in NAADP-mediated Ca2+ signaling in T-lymphocytes. Cell Signal. 2004;16:1283–1289. doi: 10.1016/j.cellsig.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Laplante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett. 2002;532:183–187. doi: 10.1016/s0014-5793(02)03670-0. [DOI] [PubMed] [Google Scholar]
- Laplante JM, Ye CP, Quinn SJ, Goldin E, Brown EM, Slaugenhaupt SA, Vassilev PM. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun. 2004;322:1384–1391. doi: 10.1016/j.bbrc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- Lee HC. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem. 2005;280:33693–33696. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. ADP-ribosyl cyclase: An enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Wide distribution of an enzyme that catalyzes the hydrolysis of cyclic ADP-ribose. Biochim Biophys Acta. 1993;1164:68–74. doi: 10.1016/0167-4838(93)90113-6. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Functional visualization of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. J Cell Sci. 2000;113:4413–4420. doi: 10.1242/jcs.113.24.4413. [DOI] [PubMed] [Google Scholar]
- Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DsL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- Lee HC, Zocchi E, Guida L, Franco L, Benatti U, De sFlora A. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;191:639–645. doi: 10.1006/bbrc.1993.1265. [DOI] [PubMed] [Google Scholar]
- Li N, Teggatz EG, Li PL, Allaire R, Zou AP. Formation and actions of cyclic ADP-ribose in renal microvessels. Microvasc Res. 2000a;60:149–159. doi: 10.1006/mvre.2000.2255. [DOI] [PubMed] [Google Scholar]
- Li N, Zou AP, Ge ZD, Campbell WB, Li PL. Effect of nitric oxide on calcium-induced calcium release in coronary arterial smooth muscle. Gen Pharmacol. 2000b;35:37–45. doi: 10.1016/s0306-3623(01)00089-1. [DOI] [PubMed] [Google Scholar]
- Li P, Zou AP, Campbell WB. Metabolism and actions of ADP-riboses in coronary arterial smooth muscle. Adv Exp Med Biol. 1997;419:437–441. doi: 10.1007/978-1-4419-8632-0_56. [DOI] [PubMed] [Google Scholar]
- Li PL, Tang WX, Valdivia HH, Zou AP, Campbell WB. cADP-ribose activates reconstituted ryanodine receptors from coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;280:H208–215. doi: 10.1152/ajpheart.2001.280.1.H208. [DOI] [PubMed] [Google Scholar]
- Li PL, Zhang DX, Ge ZD, Campbell WB. Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H1229–1236. doi: 10.1152/ajpheart.00736.2001. [DOI] [PubMed] [Google Scholar]
- Li PL, Zou AP, Campbell WB. Regulation of KCa-channel activity by cyclic ADP-ribose and ADP-ribose in coronary arterial smooth muscle. Am J Physiol. 1998;275:H1002–1010. doi: 10.1152/ajpheart.1998.275.3.H1002. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looms DK, Tritsaris K, Nauntofte B, Dissing S. Nitric oxide and cGMP activate Ca2+-release processes in rat parotid acinar cells. Biochem J. 2001;355:87–95. doi: 10.1042/0264-6021:3550087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, Borsani G. Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: Effects on the late endocytic compartment organization. FEBS Lett. 2004;567:219–224. doi: 10.1016/j.febslet.2004.04.080. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, Fini M, Mccubrey JA. Two hits are better than one: Targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 2012;3:371–394. doi: 10.18632/oncotarget.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR. Autophagy in atherosclerosis. Curr Atheroscler Rep. 2008;10:216–223. doi: 10.1007/s11883-008-0034-y. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR. Autophagy in atherosclerosis: A cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, Galione A. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr Biol. 2003;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Miedel MT, Weixel KM, Bruns JR, Traub LM, Weisz OA. Posttranslational cleavage and adaptor protein complex-dependent trafficking of mucolipin-1. J Biol Chem. 2006;281:12751–12759. doi: 10.1074/jbc.M511104200. [DOI] [PubMed] [Google Scholar]
- Moccia F, Billington RA, Santella L. Pharmacological characterization of NAADP-induced Ca2+ signals in starfish oocytes. Biochem Biophys Res Commun. 2006a;348:329–336. doi: 10.1016/j.bbrc.2006.05.157. [DOI] [PubMed] [Google Scholar]
- Moccia F, Lim D, Kyozuka K, Santella L. NAADP triggers the fertilization potential in starfish oocytes. Cell Calcium. 2004;36:515–524. doi: 10.1016/j.ceca.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Moccia F, Nusco GA, Lim D, Kyozuka K, Santella L. NAADP and InsP3 play distinct roles at fertilization in starfish oocytes. Dev Biol. 2006b;294:24–38. doi: 10.1016/j.ydbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Mojzisova A, Krizanova O, Zacikova L, Kominkova V, Ondrias K. Effect of nicotinic acid adenine dinucleotide phosphate on ryanodine calcium release channel in heart. Pflugers Arch. 2001;441:674–677. doi: 10.1007/s004240000465. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007;402:301–310. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kitayama T, Kitayama S, Dohi T. Cyclic ADP-ribose requires FK506-binding protein to regulate intracellular Ca2+ dynamics and catecholamine release in acetylcholine-stimulated bovine adrenal chromaffin cells. J Pharmacol Sci. 2006;101:40–51. doi: 10.1254/jphs.fp0050991. [DOI] [PubMed] [Google Scholar]
- Munoz P, Navarro MD, Pavon EJ, Salmeron J, Malavasi F, Sancho J, Zubiaur M. CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and the CD3-zeta subunit of the T cell antigen receptor. J Biol Chem. 2003;278:50791–50802. doi: 10.1074/jbc.M308034200. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- Pereira GJ, Hirata H, Fimia GM, Do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, Piacentini M, Patel S, Smaili SS. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni RL, Thoene JG. The transport systems of mammalian lysosomes. Biochim Biophys Acta. 1991;1071:351–373. doi: 10.1016/0304-4157(91)90002-e. [DOI] [PubMed] [Google Scholar]
- Poburko D, Kuo KH, Dai J, Lee CH, Van Breemen C. Organellar junctions promote targeted Ca2+ signaling in smooth muscle: Why two membranes are better than one. Trends Pharmacol Sci. 2004;25:8–15. doi: 10.1016/j.tips.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274:C1653–1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Hang C, Zhu L, Shi J. Involvement of endothelial-derived relaxing factors in the regulation of cerebral blood flow. Neurol Sci. 2011;32:551–557. doi: 10.1007/s10072-011-0622-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Takuwa Y, Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987;1:177–185. [PubMed] [Google Scholar]
- Raychowdhury MK, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF. Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum Mol Genet. 2004;13:617–627. doi: 10.1093/hmg/ddh067. [DOI] [PubMed] [Google Scholar]
- Rojas JD, Sennoune SR, Maiti D, Bakunts K, Reuveni M, Sanka SC, Martinez GM, Seftor EA, Meininger CJ, Wu G, Wesson DE, Hendrix MJ, Martinez-Zaguilan R. Vacuolar-type H+-ATPases at the plasma membrane regulate pH and cell migration in microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H1147–1157. doi: 10.1152/ajpheart.00166.2006. [DOI] [PubMed] [Google Scholar]
- Rusinko N, Lee HC. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:11725–11731. [PubMed] [Google Scholar]
- Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J Biol Chem. 2012;287:20407–20416. doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Lee SJ, Smith A, Choi AM. Autophagy in vascular disease. Proc Am Thorac Soc. 2010;7:40–47. doi: 10.1513/pats.200909-100JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Monge L, Fernandez N, Climent B, Dieguez G, Garcia-Villalon AL. Mechanisms of relaxation by urocortin in renal arteries from male and female rats. Br J Pharmacol. 2003;140:1003–1007. doi: 10.1038/sj.bjp.0705516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Seedorf U, Wiebusch H, Muntoni S, Christensen NC, Skovby F, Nickel V, Roskos M, Funke H, Ose L, Assmann G. A novel variant of lysosomal acid lipase (Leu336–>Pro) associated with acid lipase deficiency and cholesterol ester storage disease. Arterioscler Thromb Vasc Biol. 1995;15:773–778. doi: 10.1161/01.atv.15.6.773. [DOI] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Mcgarry SJ, Williams AJ. Cyclic ADP-ribose competes with ATP for the adenine nucleotide binding site on the cardiac ryanodine receptor Ca(2+)-release channel. Circ Res. 1994;75:596–600. doi: 10.1161/01.res.75.3.596. [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt SA. The molecular basis of mucolipidosis type IV. Curr Mol Med. 2002;2:445–450. doi: 10.2174/1566524023362276. [DOI] [PubMed] [Google Scholar]
- Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN. NAADP as a second messenger: Neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol. 2007;292:C227–239. doi: 10.1152/ajpcell.00638.2005. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Takasawa S, Nata K, Yonekura H, Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science. 1993;259:370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1304–1310. doi: 10.1152/ajpheart.00843.2001. [DOI] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Bafilomycin A1 inhibits lysosomal, phagosomal, and plasma membrane H(+)-ATPase and induces lysosomal enzyme secretion in macrophages. J Cell Physiol. 1995;163:137–144. doi: 10.1002/jcp.1041630116. [DOI] [PubMed] [Google Scholar]
- Teggatz EG, Zhang G, Yi F, Zou AP, Li PL. Vasoconstrictor responses of coronary arteries in CD38 gene knockout mice: Role of cyclic ADP-ribose. Faseb J. 2005a;19:1088. [Google Scholar]
- Teggatz EG, Zhang G, Zhang AY, Yi F, Li N, Zou AP, Li PL. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc Res. 2005b;70:65–75. doi: 10.1016/j.mvr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Thai TL, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol. 2008;295:F360–368. doi: 10.1152/ajprenal.00512.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TL, Arendshorst WJ. Mice lacking the ADP ribosyl cyclase CD38 exhibit attenuated renal vasoconstriction to angiotensin II, endothelin-1, and norepinephrine. Am J Physiol Renal Physiol. 2009;297:F169–176. doi: 10.1152/ajprenal.00079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TL, Fellner SK, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol. 2007;293:F1107–1114. doi: 10.1152/ajprenal.00483.2006. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Takasawa S, Noguchi N, Koguma T, Nata K, Sugimoto T, Furuya Y, Yonekura H, Okamoto H. Essential cysteine residues for cyclic ADP-ribose synthesis and hydrolysis by CD38. J Biol Chem. 1994;269:28555–28557. [PubMed] [Google Scholar]
- Trubiani O, Guarnieri S, Orciani M, Salvolini E, Di Primio R. Sphingolipid microdomains mediate CD38 internalization: Topography of the endocytosis. Int J Immunopathol Pharmacol. 2004;17:293–300. doi: 10.1177/039463200401700309. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP, Ellis JT, Kilpatrick DL, De Meyer GR. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol. 2007;49:706–715. doi: 10.1016/j.jacc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol. 2004;286:C538–546. doi: 10.1152/ajpcell.00106.2003. [DOI] [PubMed] [Google Scholar]
- White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. Faseb J. 2003;17:482–484. doi: 10.1096/fj.02-0622fje. [DOI] [PubMed] [Google Scholar]
- White TA, Walseth TF, Kannan MS. Nitric oxide inhibits ADP-ribosyl cyclase through a cGMP-independent pathway in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1065–1071. doi: 10.1152/ajplung.00064.2002. [DOI] [PubMed] [Google Scholar]
- Willmott N, Sethi JK, Walseth TF, Lee HC, White AM, Galione A. Nitric oxide-induced mobilization of intracellular calcium via the cyclic ADP-ribose signaling pathway. J Biol Chem. 1996;271:3699–3705. doi: 10.1074/jbc.271.7.3699. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua NH. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- Xu K, Yang Y, Yan M, Zhan J, Fu X, Zheng X. Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res. 2010;51:2581–2590. doi: 10.1194/jlr.M005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xia M, Li XX, Han WQ, Boini KM, Zhang F, Zhang Y, Ritter JK, Li PL. Requirement of translocated lysosomal V1H(+)-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells. Mol Biol Cell. 2012a;23:1546–1557. doi: 10.1091/mbc.E11-09-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang F, Xia M, Li XX, Abais JM, Boini KM, Li PL. Lysosomal regulation of autophagy efflux via CD38-mediated signaling in mouse coronary arterial myocytes. Hypertension. 2011;58:E178–E178. [Google Scholar]
- Xu M, Zhang Y, Xia M, Li XX, Ritter JK, Zhang F, Li PL. NAD(P)H oxidase-dependent intracellular and extracellular O2*-production in coronary arterial myocytes from CD38 knockout mice. Free Radic Biol Med. 2012b;52:357–365. doi: 10.1016/j.freeradbiomed.2011.10.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Jha A, Li Q, Soyombo AA, Dickinson GD, Churamani D, Brailoiu E, Patel S, Muallem S. Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J Biol Chem. 2011;286:22934–22942. doi: 10.1074/jbc.M110.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Churchill GC, Galione A. Calcium signalling by nicotinic acid adenine dinucleotide phosphate (NAADP) Febs J. 2005a;272:4598–4606. doi: 10.1111/j.1742-4658.2005.04860.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Thomas JM, Churchill GC, Garnham C, Lewis AM, Cancela JM, Patel S, Galione A. Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr Biol. 2005b;15:874–878. doi: 10.1016/j.cub.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Yi FX, Zhang AY, Campbell WB, Zou AP, Van Breemen C, Li PL. Simultaneous in situ monitoring of intracellular Ca2+ and NO in endothelium of coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2725–2732. doi: 10.1152/ajpheart.00428.2002. [DOI] [PubMed] [Google Scholar]
- Yu J, Chait BT, Toll L, Kreek MJ. Nociceptin in vitro biotransformation in human blood. Peptides. 1996;17:873–876. doi: 10.1016/0196-9781(96)00079-4. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca(2+) mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H873–881. doi: 10.1152/ajpheart.2000.279.3.H873. [DOI] [PubMed] [Google Scholar]
- Yusufi AN, Cheng J, Thompson MA, Burnett JC, Grande JP. Differential mechanisms of Ca(2+) release from vascular smooth muscle cell microsomes. Exp Biol Med (Maywood) 2002;227:36–44. doi: 10.1177/153537020222700107. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger—Cyclic ADP-ribose. J Cell Mol Med. 2006;10:407–422. doi: 10.1111/j.1582-4934.2006.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AY, Teggatz EG, Zou AP, Campbell WB, Li PL. Endostatin uncouples NO and Ca2+ response to bradykinin through enhanced O2*-production in the intact coronary endothelium. Am J Physiol Heart Circ Physiol. 2005;288:H686–694. doi: 10.1152/ajpheart.00174.2004. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Yi F, Teggatz EG, Zou AP, Li PL. Enhanced production and action of cyclic ADP-ribose during oxidative stress in small bovine coronary arterial smooth muscle. Microvasc Res. 2004;67:159–167. doi: 10.1016/j.mvr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Zou AP, Li PL. Adenosine diphosphate ribose dilates bovine coronary small arteries through apyrase- and 5′-nucleotidase-mediated metabolism. J Vasc Res. 2001;38:64–72. doi: 10.1159/000051031. [DOI] [PubMed] [Google Scholar]
- Zhang F, Jin S, Yi F, Li PL. TRP-ML1 functions as a lysosomal NAADP-sensitive Ca2+ release channel in coronary arterial myocytes. J Cell Mol Med. 2009a;13:3174–3185. doi: 10.1111/j.1582-4934.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Jin S, Yi F, Xia M, Dewey WL, Li PL. Local production of O2- by NAD(P)H oxidase in the sarcoplasmic reticulum of coronary arterial myocytes: cADPR-mediated Ca2+ regulation. Cell Signal. 2008;20:637–644. doi: 10.1016/j.cellsig.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Li PL. Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J Biol Chem. 2007;282:25259–25269. doi: 10.1074/jbc.M701614200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xia M, Li PL. Lysosome-dependent Ca(2+) release response to Fas activation in coronary arterial myocytes through NAADP: Evidence from CD38 gene knockouts. Am J Physiol Cell Physiol. 2010;298:C1209–1216. doi: 10.1152/ajpcell.00533.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu M, Han WQ, Li PL. Reconstitution of lysosomal NAADP-TRP-ML1 signaling pathway and its function in TRP-ML1(−/−) cells. Am J Physiol Cell Physiol. 2011;301:C421–430. doi: 10.1152/ajpcell.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhang G, Zhang AY, Koeberl MJ, Wallander E, Li PL. Production of NAADP and its role in Ca2+ mobilization associated with lysosomes in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2006a;291:H274–282. doi: 10.1152/ajpheart.01064.2005. [DOI] [PubMed] [Google Scholar]
- Zhang G, Teggatz EG, Zhang AY, Koeberl MJ, Yi F, Chen L, Li LP. Cyclic ADP ribose-mediated Ca2+ signaling in mediating endothelial nitric oxide production in bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2006b;290:H1172–1181. doi: 10.1152/ajpheart.00441.2005. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H483–495. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, Miao L, Xin HB, Kotlikoff MI, Ji G. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not beta-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res. 2009b;84:253–262. doi: 10.1093/cvr/cvp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. Calcium signaling via two-pore channels: Local or global, that is the question. Am J Physiol Cell Physiol. 2010a;298:C430–441. doi: 10.1152/ajpcell.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Galione A, Evans AM. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010b;584:1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber MT, Setterblad N, Vasselon T, Doliger C, Charron D, Mooney N, Gelin C. MHC class II/CD38/CD9: A lipid-raft-dependent signaling complex in human monocytes. Blood. 2005;106:3074–3081. doi: 10.1182/blood-2004-10-4094. [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: Pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101–111. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;196:1459–1465. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- Zocchi E, Franco L, Guida L, Calder L, De Flora A. Self-aggregation of purified and membrane-bound erythrocyte CD38 induces extensive decrease of its ADP-ribosyl cyclase activity. FEBS Lett. 1995;359:35–40. doi: 10.1016/0014-5793(95)00005-t. [DOI] [PubMed] [Google Scholar]
- Zocchi E, Usai C, Guida L, Franco L, Bruzzone S, Passalacqua M, De Flora A. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: Role of NAD+ transport across cell membranes. FASEB J. 1999;13:273–283. doi: 10.1096/fasebj.13.2.273. [DOI] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]