Abstract
Background
Human genomes harbor copy number variants (CNVs), regions of DNA gains or losses. While pathogenic CNVs are associated with congenital heart disease (CHD), their impact on clinical outcomes is unknown. This study sought to determine whether pathogenic CNVs among infants with single ventricle (SV) physiology were associated with inferior neurocognitive and somatic growth outcomes.
Methods and Results
Genomic DNAs from 223 subjects of two National Heart, Lung, and Blood Institute-sponsored randomized clinical trials with infants with SV CHD and 270 controls from The Cancer Genome Atlas project were analyzed for rare CNVs >300 kb using array comparative genomic hybridization. Neurocognitive and growth outcomes at 14 months from the CHD trials were compared among subjects with and without pathogenic CNVs. Putatively pathogenic CNVs, comprising 25 duplications and 6 deletions, had a prevalence of 13.9%, significantly greater than the 4.4% rate of such CNVs among controls. CNVs associated with genomic disorders were found in 13 cases but no control. Several CNVs likely to be causative of SV CHD were observed, including aberrations altering the dosage of GATA4, MYH11, and GJA5. Subjects with pathogenic CNVs had worse linear growth, and those with CNVs associated with known genomic disorders had the poorest neurocognitive and growth outcomes. A minority of children with pathogenic CNVs were noted to be dysmorphic on clinical genetics examination.
Conclusions
Pathogenic CNVs appear to contribute to the etiology of SV forms of CHD in at least 10% of cases, are clinically subtle but adversely affect outcomes in children harboring them.
Keywords: copy number variant, congenital cardiac defect, outcome, hypoplastic left heart syndrome
Congenital heart disease (CHD) care has advanced remarkably over the past 40 years.1 This has shifted focus to decreasing morbidity and improving neurologic and developmental outcomes, which are affected by genetic factors. For example, aneuploidies and genomic lesions such as 22q11.2 deletions, are associated with poorer neurocognitive outcomes. For most cases of CHD, however, the underlying genetic basis remains unknown. Identification of causative genetic factors could help to explain a larger percent of the variance in CHD outcomes, and might be useful for patient management as well as clinical trial design.
Copy number variants (CNVs), DNA gains or losses greater than 1000 base pairs,2 are detectable due to recent advances in molecular cytogenetics, particularly in microarray-based methods. Using high-resolution, whole-genome scanning methods, it has become evident that a significant proportion of the normal healthy human genome harbors benign CNVs.3, 4 A smaller subset of CNVs, more often large and de novo, are considered pathogenic and are increasingly associated with disease such as schizophrenia and intellectual and developmental disabilities (IDD).5–8
Pathogenic CNV prevalence in CHD has been estimated at 5–15%.9–17 Two small-to-modest-sized studies of patients with hypoplastic left heart syndrome (HLHS), a single ventricle (SV) form of CHD, have suggested that CNV frequency is not increased for that heart lesion.12, 17 CNVs appear to be present at higher rates among patients with CHD plus extracardiac or developmental abnormalities,9–12 although roles for de novo CNVs causing isolated CHD have also been observed.13, 14 No prior study of CNVs has included careful follow-up of outcomes, particularly growth and neurocognitive development, in children with CHD.
Methods
Study Cohort
Study participants from the Pediatric Heart Network’s Infants with Single Ventricle (ISV) and Single Ventricle Reconstruction (SVR) trials were combined into a single cohort based on the similarity of their demographics, assessment tools and CHD lesions as previously described.18, 19 Additional subject demographics are provided in the Supplement. Genomic DNAs (gDNAs) and outcome data were provided anonymously through the New England Research Institute. The protocol was deemed exempt by the Mount Sinai Institutional Review Board.
Genomic DNA Samples
The quality and quantity of gDNA for each sample was examined via NanoDrop™ (Thermo Fisher Scientific, Waltham, MA, USA). For partially degraded or insufficient (<500 ng) samples as determined by agarose gel electrophoresis, whole genome amplification (WGA) was performed using the GenomePlex Complete WGA Kit (Sigma-Aldrich, St. Louis, MO, USA) as described by the manufacturer.
Array Comparative Genomic Hybridization (aCGH)
aCGH was performed on microarrays according to the manufacturer's instructions (Agilent Human CGH 1 × 244A; Agilent Technologies, Santa Clara, CA, USA). The data were analyzed with DNA Analytics 5.0.14 software (Agilent Technologies) via the Aberration Detection Method-1 algorithm with a sensitivity threshold of 6.0 and a data filter rejecting aberrations with less than five probes with a log2 ratio ± 0.25.
CNV Characterization
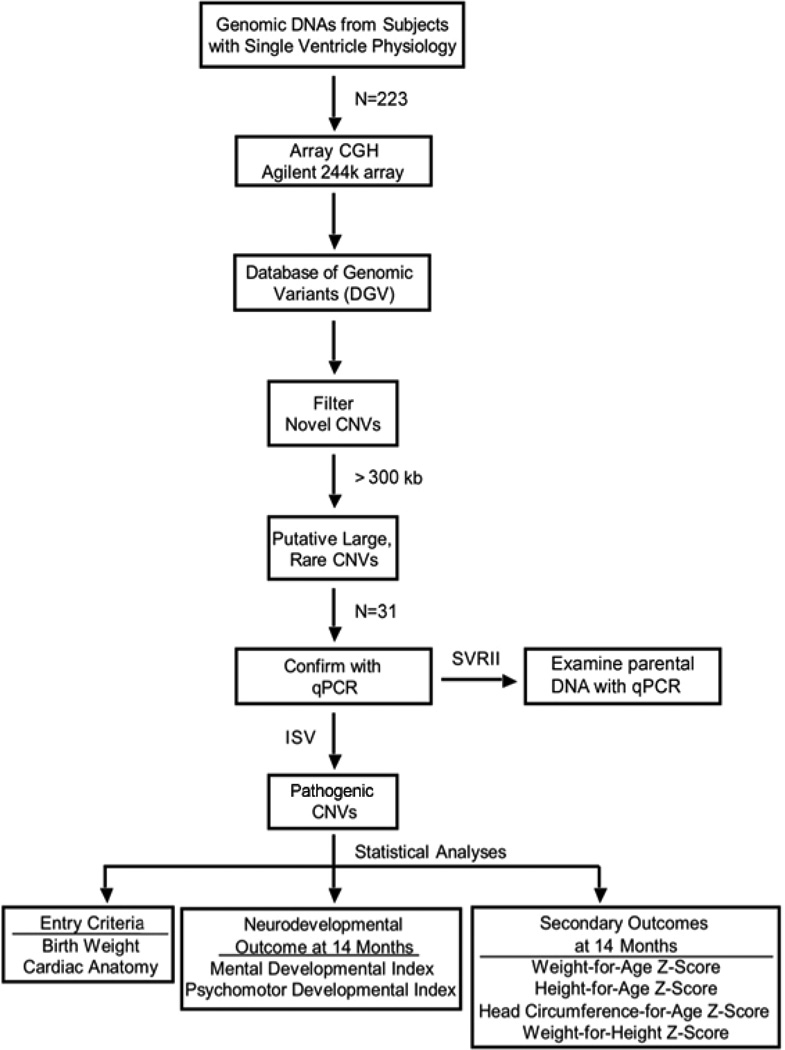
CNVs were considered pathogenic if they were >300 kb in size, contained genes20 and were either novel or well-established as abnormal21, 22 (Figure 1). Novelty was determined by comparing CNVs to the Database of Genomic Variants (DGV) (http://projects.tcag.ca/variation/). CNVs were deemed polymorphisms if there was >50% overlap with CNVs already catalogued in the DGV, unless they were well established in the literature as associated with a genomic disorder. Putatively pathogenic CNVs were also checked against CNV data from 2500 controls released by the Eichler group23 and against a set of pathogenic CNVs identified from a proprietary database of >40,000 individuals, most of whom had IDD, at Signature Genomic Laboratories (SGL). CNVs that were observed infrequently or deemed pathogenic in the SGL system24 were designated as pathogenic for our study.
Figure 1.
Workflow for aCGH studies. Starting with the available cohorts from the Infants with Single Ventricle and the Single Ventricle Reconstruction-Extension trials of the Pediatric Heart Network, the flow of statistical and genetic analyses is outlined. All pathogenic CNVs identified were successfully confirmed via secondary methods.
CNV Confirmation
Putatively pathogenic CNVs were confirmed via quantitative qPCR using the Universal Probe Library (UPL; Roche, Indianapolis, IN) system (details in the Supplement).
Pathogenic CNV Inheritance
For 12 SVRII subjects harboring pathogenic CNVs, one or both parental gDNAs were available (n=19) and analyzed for those CNVs with qPCR.
Normal Controls
Two-hundred-seventy controls were obtained from The Cancer Genome Atlas Project (TCGA). We used aCGH data from peripheral blood gDNAs from subjects with solid tumors, either glioblastoma multiforme (GBM) or ovarian cancer (OV). Of note, an aged-matched control group would have been inferior as some neonates are destined to have IDD, often related to pathogenic CNVs. Adults enrolling in cancer clinical trials provide a better “healthy” comparison for the outcomes of interest. We eliminated the possibility of missing CNVs associated with early lethality by interrogating DGV and the SGL database.
The TCGA blood gDNAs were analyzed at the Dana Farber Cancer Institute using a custom 415K array (Agilent Technologies), which included 230,000 of the 244,000 probes used for the CHD cases. Reference DNA was from Promega, as for the CHD cases. The raw aCGH data were re-analyzed identically to the cases. All pathogenic CNVs (>300 kb) identified in CHD cases and TCGA controls would have been called using the other array.
Neurocognitive and Somatic Growth Outcomes
Subjects were dichotomized into subgroups of those with pathogenic CNVs (referred to as genotype+) and those without pathogenic CNVs (referred to as genotype−), and compared according to entry characteristics (sex, race/ethnicity, birth weight, and cardiac diagnoses). Subgroups were also assessed with respect to neurocognitive function using the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) measured with the Bayley Scales of Infant Development II at 14 months of age. Growth outcomes at 14 months of age were compared (weight-, height-, and head circumference-for-age Z-scores and weight-for-height Z-scores). For SVRII subjects, clinical or research genetic evaluations at 14 months of age were used for subgroup analysis.25
Statistical Analysis
Comparisons of demographic characteristics between the subgroup of 223 subjects included in this study and the larger cohort from which they were derived, and of those with and without CNV were based on Fisher’s exact tests for categorical measures and t-tests for continuous measures. Normality was tested using the Shapiro-Wilk test and confirmed by the observed distribution. Secondary analyses with other subgroups were not adjusted for the multiplicity of comparisons. Z scores for height, weight and head circumference for age were calculated from the measured values using the National Health and Nutrition Examination Survey (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). T-tests were used to compare neurocognitive and somatic growth measurements among those with CNV gains and losses and those without CNVs. All tests were performed at the 0.05 level.
Results
We studied 82 ISV-only subjects, 113 SVRII-only subjects, and 28 subjects enrolled in both trials. Among these 223 subjects, 147 were male (66%). Racial composition was 85% Caucasian, 11% African-American, 4% other or unknown. All enrolled subjects had SV forms of CHD, 76% being HLHS. No statistically significant difference in demographic characteristics was noted between our subjects derived from the ISV and SVRII cohorts and the larger cohorts from which they were obtained.
Pathogenic CNVs were detected in 31 subjects (13.9%, Table 1). The median sizes of the twenty-five duplications and six deletions were 674 kb and 1.5 Mb, respectively. Twenty-nine CNVs were successfully confirmed by qPCR. Two other CNVs, for which there was insufficient DNA, were confirmed after whole-genome amplification using aCGH with a 105K Agilent array. The demographic characteristics, including birth weight, gestational age, sex, race, and ethnicity, were not significantly different among these 31 subjects with pathogenic CNVs compared to the 192 subject without such CNVs (Supplementary Table 1). Cardiac anatomy also did not differ with genotype (HLHS: genotype+ 84% vs. genotype− 75%, not significant).
Table 1.
Pathogenic CNVs detected among 223 subjects with isolated single ventricle heart defects
| Subject | Location | Start | Stop | Size (kb) | CN | Status | Inheritance |
|---|---|---|---|---|---|---|---|
| 92022989 | 1p31.1 | 76420271 | 76809986 | 390 | Loss | Novel | Unknown |
| GT04009571 | 1q21.1 | 145009267 | 146318068 | 1309 | Gain | Known | Unknown |
| 92027535/GT04007822 | 1q21.1 | 145009267 | 147203477 | 2194 | Gain | Known | De novo |
| 92026280/GT04009083 | 2q13 | 111108466 | 112819206 | 1711 | Gain | Novel | Unknown |
| GT04013352 | 2q14.1 | 114390707 | 114975263 | 585 | Gain | Novel | Not maternal |
| GT04008671 | 3p14.1 | 65445598 | 66643726 | 1198 | Gain | Novel | Unknown |
| 92026850/GT04007792 | 4q35.1-q35.2 | 186295054 | 188154168 | 1859 | Loss | Novel | Maternal |
| 92022561 | 5q12.3 | 6435972 | 65164354 | 328 | Gain | Novel | Unknown |
| 92124531 | 8p23.1 | 8131939 | 11898119 | 3766 | Loss | Known | Unknown |
| 92022496 | 8p23.1 | 10791639 | 11898119 | 1106 | Gain | Known | Unknown |
| 92120189 | 9q21.31 | 81376647 | 83149610 | 1773 | Gain | Novel | Unknown |
| GT04006712 | 9q21.32 | 83460453 | 83972537 | 512 | Loss | Novel | Unknown |
| 92024262 | 10q25.3 | 116304612 | 116870123 | 566 | Gain | Novel | Unknown |
| 92021139 | 12p13.33 | 551096 | 2543903 | 1993 | Loss | Known | Unknown |
| 92024760/GT04010341 | 16p13.12-p13.11 | 14669540 | 16190029 | 1520 | Gain | Known | Maternal |
| GT04011611 | 16p13.11 | 14817506 | 16515223 | 1698 | Gain | Known | Paternal |
| GT04012411 | 16p13.11 | 14955977 | 16199882 | 1244 | Loss | Known | Paternal |
| GT04011421 | 16p13.11 | 15398828 | 16166985 | 768 | Gain | Known | De novo |
| 92020315 | 16p12.3 | 16701973 | 17654093 | 952 | Gain | Novel | Unknown |
| 92124101 | 16p11.2 | 29500084 | 30107008 | 607 | Gain | Known | Unknown |
| 92020074 | 16p11.2 | 29500084 | 30107008 | 607 | Gain | Known | Unknown |
| GT04011942 | 16p11.2 | 29560300 | 30265521 | 705 | Gain | Known | De novo |
| 92023830 | 16q23.1 | 74558771 | 75090033 | 531 | Gain | Novel | Unknown |
| GT04010673 | 17p13.1 | 9922263 | 10359076 | 437 | Gain | Novel | Unknown |
| 92021026 | 17p12 | 13709239 | 14218834 | 510 | Gain | Novel | Unknown |
| 92121321/GT04008312 | 18p11.31-p11.23 | 6837549 | 8088466 | 1251 | Gain | Novel | Not paternal |
| 92029147 | 20q11.21 | 29297070 | 29971177 | 674 | Gain | Novel | Unknown |
| GT04010851 | 22q11.21-q11.22 | 20294861 | 20753261 | 458 | Gain | Known | De novo |
| GT04009061 | Xp22.2 | 10709385 | 11255827 | 546 | Gain | Novel | Maternal |
| GT04013021 | Xq24 | 117055728 | 117415164 | 359 | Gain | Novel | Paternal |
| 92021913 | Xq28 | 148573118 | 149245260 | 672 | Gain | Novel | Unknown |
Human Genome Build 36.1 (hg18) used for genomic coordinates. CN, copy number. Novel, aberration has not been previously reported in CHD patients. Known, aberration reported in genomic disorders with CHD.
Among the TCGA controls, 164 had glioblastoma multiforme and 106 had ovarian cancer, resulting in a female predominance (64.5%). The racial composition was 87% Caucasian, 6.6% African-American, 6.4% other or unknown. Pathogenic CNVs were observed in 12 individuals (4.4%, Supplementary Table 2). The median sizes of the nine duplications and three deletions were 675 kb and 326.6 kb, respectively.
A statistically significant increase in pathogenic CNVs was observed in the CHD population relative to the TCGA cohort (13.9% vs. 4.4%, p=0.0003 from the Fisher’s exact test). The ratios of pathogenic gain and loss CNVs were similar between the CHD and TCGA cohorts (CHD, 25:6; TCGA 9:3, p = 0.69).
Analysis of parental gDNAs of 12 SVRII subjects harboring pathogenic CNVs revealed inherited lesions in 3/7 for whom both parents were assessed and 3/5 for whom one parent was analyzed (Table 1).
To examine possible effects of pathogenic CNVs on health outcomes, neurocognitive and somatic growth measurements were compared between the genotype+ and genotype− cohorts (Table 2; details by subject with pathogenic CNV, Supplementary Table 3). Genotype+ subjects were significantly shorter by an average of 0.65 z-score (p=0.031). No other significant difference was observed. For the genotype+ subgroups with gain or loss CNVs (Table 2), the PDI scores were significantly lower among children who harbored loss CNVs than those in the genotype− cohort (p=0.032). The MDI scores also trended lower among those with deletions but did not achieve statistical significance in this small cohort (p = 0.29).
Table 2.
Fourteen-month outcomes for subgroups by genotype
| N‡ | MDI | PDI | Weight Z | Length Z | HC Z | |
|---|---|---|---|---|---|---|
| Genotype− | 192 | 91.4 (17.0) | 77.6 (19.1) | −0.61 (1.15) | −1.00 (1.37) | −0.18 (1.32) |
| Genotype+ | 31 | 90.6 (17.8) | 71.4 (20.4) | −0.61 (1.37) | −1.65* (1.82) | −0.16 (1.66) |
| Gain CNV | 25 | 92.5 (16.3) | 74.7 (20.9) | −0.80 (1.24) | −1.81* (1.87) | −0.30 (1.66) |
| Loss CNV | 6 | 83.2 (23.1) | 59.1* (13.5) | 0.16 (1.77) | −0.94 (1.55) | 0.46 (1.70) |
| Known CNV | 11 | 79.8* (18.3) | 56.8† (7.7) | −0.87 (1.57) | −1.89* (2.16) | −0.43 (1.69) |
p < 0.05 compared to Genotype−;
p < 0.005 compared to Genotype−;
Abbreviations: MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index; Weight Z, Weight-for-Age Z Score at 14 months; Length Z, Length-for-Age Z score at 14 months; HC Z, Head Circumference Z Score at 14 months. All data shown as mean (standard deviation).
Size of each cohort. Incomplete data for outcomes resulted in lower N’s.
Among the 31 pathogenic CNVs found among the CHD subjects, 13 (42%) have previously been associated with genomic disorders (Table 1). The demographics of this group did not differ from the genotype− cohort (Supplementary Table 1) and 77% had HLHS. As compared to the genotype− cohort, the 11 children with known CNVs for whom 14-month outcomes were available had the worst outcomes with globally reduced neurocognitive development (MDI and PDI) as well as the slowest growth.
To determine the sensitivity of clinical examination in detecting children with CHD who harbored pathogenic CNVs, we reviewed the data from 116 subjects from the SVR study genotyped in our study who had been evaluated by a clinical geneticist. Four children (3.4%) were diagnosed with known genetic syndromes and an additional 29 (25%) were observed to have one or more dysmorphic features and/or extra-cardiac malformations. Of interest, none of the 14 subjects with a putatively pathogenic CNV who had a clinical genetic evaluation was diagnosed with a syndrome and only three (21%) had dysmorphic features or extra-cardiac malformations. Seven of those 14 subjects harbored CNVs previously associated with genomic disorders; two of those had dysmorphic features or extra-cardiac malformations.
The outcomes for the 18 subjects with a genetic syndrome or a putatively pathogenic CNV were worse than those for the 69 children without genetic abnormalities, dysmorphic features, or extra-cardiac malformations with lower PDI scores, weights and lengths (Table 3). The outcomes for the 29 individuals with dysmorphic features and/or extra-cardiac malformations but without a genetic diagnosis or pathogenic CNV did not differ from the 69 subjects without genetic abnormalities, dysmorphic features or extra-cardiac malformations. Inclusion of the subjects with only dysmorphic features or extra-cardiac malformations with those without genetic abnormality provide the same conclusions about the inferior outcomes for the children with a genetic syndrome or pathogenic CNV.
Table 3.
Fourteen-month outcomes for subgroups based on genetic examination
| N‡ | MDI | PDI | Weight Z | Length Z | HC Z | |
|---|---|---|---|---|---|---|
| CNV− Syndrome− Dysmorphic− Extracardiac− | 69 | 89.1 (18.0) | 77.5 (20.2) | −0.71 (1.07) | −1.13 (1.32) | −0.34 (1.24) |
| CNV+ | 14 | 85.4 (20.1) | 65.1* (17.6) | −0.94 (0.88) | −1.61 (1.08) | −0.04 (1.38) |
| CNV+ or Syndrome | 18 | 83.2 (18.7) | 67.9* (19.4) | −1.19* (1.11) | −1.99* (1.73) | −0.16 (1.27) |
| Dysmorphic/Extracardiac | 29 | 89.3 (18.1) | 78.1 (20.0) | −0.63 (1.25) | −1.18 (1.30) | −0.10 (1.28) |
| CNV− Syndrome− | 98 | 89.4 (17.5) | 77.8 (19.8) | −0.73 (1.10) | −1.18 (1.43) | −0.25 (1.32) |
p < 0.05 compared to CNV−/Syndrome−/Dysmorphic−/Extracardiac−;
Abbreviations: MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index; Weight Z, Weight-for-Age Z Score at 14 months; Length Z, Length-for-Age Z score at 14 months; HC Z, Head Circumference Z Score at 14 months. All data shown as mean (standard deviation).
Size of each cohort. Incomplete data for outcomes resulted in lower N’s.
Discussion
Here, we provide a case-control study of the role of pathogenic CNVs in the etiology of SV heart defects, particularly HLHS, and the first study to relate pathogenic CNVs to formal outcome assessments for children with CHD but without a known syndrome. Based on our results, SV forms of CHD are associated with a 10% excess of pathogenic CNVs, which may underestimate their importance. For the ISV and SVR trials, gDNAs were procured after the Stage II operations. Since the 12-month mortality in the SVR trial was nearly 30%, we were unable to assess CNV frequency in most subjects who died during these trials. A birth cohort study is needed to determine pathogenic CNV prevalence among those not surviving. We also suspect that some CNVs <300 kb, ignored in the present study, may be pathogenic. They were not included because public databases are far less populated for smaller CNVs, making separation of benign polymorphic CNVs from pathogenic ones less accurate.
Although we were only able to analyze a limited number of parents, it appears that roughly 50% of the pathogenic CNVs are inherited. Three of the six inherited pathogenic CNVs altered a region on chromosome 16p13.1, an established genomic disorder with variable expressivity and incomplete penetrance.26 Since parents were not examined, we cannot exclude that those harboring these pathogenic CNVs had subtle phenotypes.
The 4.4% rate of pathogenic CNVs among the controls has plausible explanations. As noted above, CNVs associated with genomic disorders are incompletely penetrant. An excess of second-site CNVs has been found among individuals with CNVs associated with variable phenotypes.27 While no large second-site CNV was observed in our study, other mutations were not excluded. Lastly, CNVs labeled as pathogenic could also be false positives.
With our secondary analyses, we examined the outcomes among several subgroups such as subjects with pathogenic gain or loss CNVs, known genomic lesions, etc. A limitation of this study is that these subgroups were small, underpowering those analyses. Specifically, secondary comparisons were not adjusted for multiple hypothesis testing.
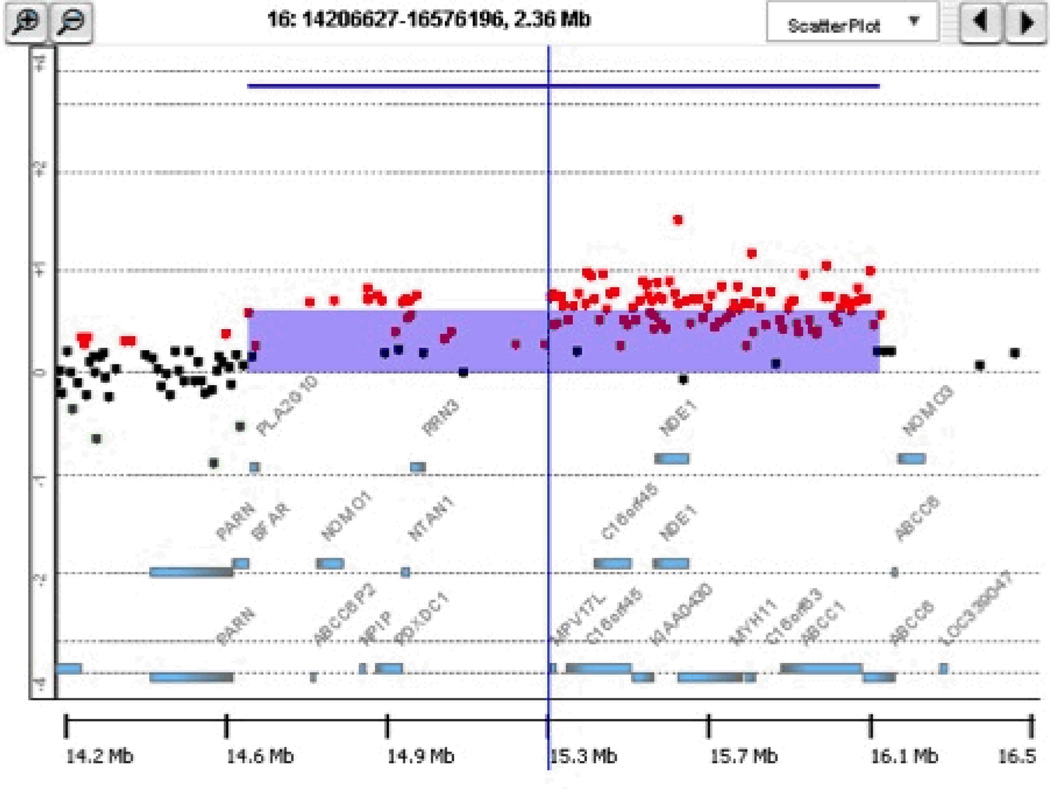
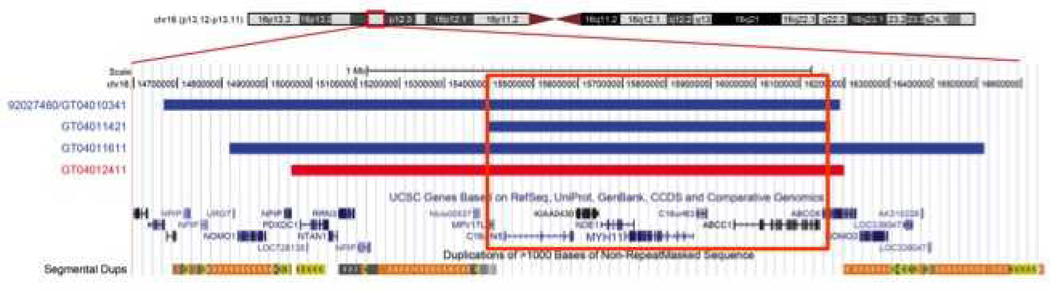
The most commonly identified pathogenic CNVs in our CHD cohort were three overlapping duplications and a deletion at chromosome 16p13.1 (Figures 2 and 3), CNVs previously associated with IDD, neuropsychiatric disorders, aortic dissection and other forms of CHD.28–31 Compared to 8329 controls from a recent study,5 16p13.1 duplications were significantly enriched in our CHD cohort (p=0.004). Among the eight genes in the region, MYH11 and ABCC6 are of interest because MYH11 mutations are associated with aortic aneurysm and bicuspid aortic valve and ABCC6 mutations cause pseudoxanthoma elasticum.29, 32, 33 In addition to possible etiologic relevance for CHD, parents carrying loss CNVs may be at risk for aortic aneurysm.
Figure 2.
Pathogenic deletion and duplication CNVs likely causative for CHD defects. (A) 1.5-Mb deletion CNV at 12p13.33 affecting 223 consecutive probes. (B) 1.6-Mb duplication CNV at 16p13.12-p13.11 affecting 124 consecutive probes and altering MYH11. For each 244K array clone, the Cy3/Cy5 signal intensity ratios are plotted. The green and red dots correspond to log2 ratios of ≤ −0.25 (loss) and ≥ 0.25 (gain), respectively. All chromosomes are depicted according to hg18.
Figure 3.
Schematic illustration of the 16p13.1 region. The smallest region of overlap (SRO) between the three duplications (shown in blue) and the deletion (shown in red) at 16p13.1 is 758 kb. This region includes eight genes, including MYH11 and ABCC6. MYH11 encodes the smooth muscle myosin, heavy chain.
We identified gain and loss CNVs altering GATA4 dosage. GATA4 point mutations cause CHD,34 and deletions also altering neighboring genes underlie CHD with IDD.35, 36 Our subject with the GATA4 deletion had a PDI score of 53, >1 standard deviation below the mean for our CHD cohort. Gain CNVs altering GATA4 gains have also been associated with CHD, including HLHS, and may be associated with IDD37, 38
We observed a subtelomeric loss CNV at 12p13.33 (Figure 2), which has been associated with IDD with or without CHD.39 The subject harboring this CNV had a PDI score of 50.
We identified three nearly identical duplications at 16p11.2, which have been implicated in IDD and neuropsychiatric disease.40–42 While the PDI score in one subject harboring this CNV was 50, outcomes were not inferior in the other two. Interestingly, deletions at 16p11.2 have also been associated with aortic valvular defects.43
We detected two distal 1q21.1 duplication CNVs, which have previously been associated with CHD, particularly non-syndromic tetralogy of Fallot,13, 44 and poor neurocognitive and growth outcomes.45 For the one child with this CNV with outcome results, there was marked global neurocognitive delay (MDI and PDI scores of 77 and 54, respectively) and poor growth (weight- and height-for-age Z scores of −3.1 and −7.4, respectively).
Lastly, one subject with double-inlet left ventricle harbored a de novo duplication altering 22q11.2, which has been linked to cardiac and extra-cardiac abnormalities46, 47 and partially overlapped with the proximal region of the 22q11 distal deletion syndrome.48, 49 This individual had a PDI score of 56.
The most interesting novel CNV was the 18p11.31-p11.2 duplication, which overlapped with a duplication in a patient with HLHS from the SGL database. Among the 40,000 individuals in that database, only 53 are known to have HLHS. PTPRM, the sole gene residing within the overlapping region for these two CNVs, appears to be relevant for cardiac development based on its expression pattern. At mouse embryonic day (E) 9.5, Ptprm is expressed in the endocardium, dorsal aortic and branchial arch endothelia as well as portions of the brain;50 at E14.5, it is expressed most highly in the ascending aorta and at lower levels in the developing brain and lung.51 Thus, PTPRM gains appear to be a new genomic lesion underlying HLHS.
With regard to the types of pathogenic CNVs detected, disproportionately more gains were observed than losses. In the general population, deletion CNVs exceed duplications by a 2:1 ratio.2 Among individuals with neurocognitive disorders, de novo losses outnumber duplications by 3:1.52 Similarly, children with CHD plus extra-cardiac abnormalities have an excess of deletions over duplications. Of interest, an excess of gain CNVs over losses was observed in children with isolated tetralogy of Fallot.13 In our study, the most striking adverse effects on neurocognitive development and growth were observed among the children with CNVs previously associated with genomic disorders, which included gains and losses with a predominance of the former.
The subgroup of genotyped SVR subjects phenotyped by clinical geneticists provided interesting insights. Children harboring putatively pathogenic CNVs were not clinically obvious as none was diagnosed with a syndrome and dysmorphic features or extra-cardiac malformations were not enriched. Most strikingly, more than 70% of the children with CNVs previously associated with genomic disorders had no dysmorphic feature or extra-cardiac anomaly. Of note, these CNVs would have been declared as abnormalities if clinical testing had been performed.
The findings from this study support the routine use of CNV testing in newborns with SV forms of CHD to enable better prognostication and early intervention. Similarly, the poorer linear growth associated with all pathogenic CNVs, the worse neurocognitive outcomes with deletions, and particularly the globally poor outcomes with CNVs associated with known genomic disorders could impact clinical trial outcomes depending on the designated endpoints. Thus, future CHD clinical trials might benefit from an incorporation of CNV status when determining entry criteria or randomization strategies.
Supplementary Material
Acknowledgments
We thank the ISV and SVRII study families for their participation and the National Heart, Lung, and Blood Institute (NHLBI)-Pediatric Heart Network for sponsorship of the ISV, SVR and SVRII studies and enabling this ancillary study.
Funding Sources: This project was funded by R21 HL104243 from NIH to L.E. and B.D.G. A.S. was funded by the Doris Duke Clinical Research Fellowship at the Icahn School of Medicine at Mount Sinai. J.E. was funded by a fellowship from the Sarnoff Cardiovascular Research Foundation. The project was supported in part by the NIH-funded Pediatric Heart Network.
Footnotes
Conflict of Interest Disclosure: JAR is an employee of Signature Genomic Laboratories, a subsidiary of PerkinElmer, Inc.
References
- 1.Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 2.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H, Kim JI, Ju YS, Gokcumen O, Mills RE, Kim S, et al. Discovery of common Asian copy number variants using integrated high-resolution array CGH and massively parallel DNA sequencing. Nat Genet. 2010;42:400–405. doi: 10.1038/ng.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld JA, Ballif BC, Torchia BS, Sahoo T, Ravnan JB, Schultz R, et al. Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med. 2010;12:694–702. doi: 10.1097/GIM.0b013e3181f0c5f3. [DOI] [PubMed] [Google Scholar]
- 7.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 9.Thienpont B, Mertens L, de Ravel T, Eyskens B, Boshoff D, Maas N, et al. Submicroscopic chromosomal imbalances detected by array-CGH are a frequent cause of congenital heart defects in selected patients. Eur Heart J. 2007;28:2778–2784. doi: 10.1093/eurheartj/ehl560. [DOI] [PubMed] [Google Scholar]
- 10.Breckpot J, Thienpont B, Peeters H, de Ravel T, Singer A, Rayyan M, et al. Array comparative genomic hybridization as a diagnostic tool for syndromic heart defects. J Pediatr. 2010;156:810–817. 817 e811–817 e814. doi: 10.1016/j.jpeds.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Richards AA, Santos LJ, Nichols HA, Crider BP, Elder FF, Hauser NS, et al. Cryptic chromosomal abnormalities identified in children with congenital heart disease. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31818095d0. [DOI] [PubMed] [Google Scholar]
- 12.Payne AR, Chang SW, Koenig SN, Zinn AR, Garg V. Submicroscopic chromosomal copy number variations identified in children with hypoplastic left heart syndrome. Pediatr Cardiol. 2012;33:757–763. doi: 10.1007/s00246-012-0208-9. [DOI] [PubMed] [Google Scholar]
- 13.Greenway SC, Pereira AC, Lin JC, DePalma SR, Israel SJ, Mesquita SM, et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat Genet. 2009;41:931–935. doi: 10.1038/ng.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdogan F, Larsen LA, Zhang L, Tumer Z, Tommerup N, Chen W, et al. High frequency of submicroscopic genomic aberrations detected by tiling path array comparative genome hybridisation in patients with isolated congenital heart disease. J Med Genet. 2008;45:704–709. doi: 10.1136/jmg.2008.058776. [DOI] [PubMed] [Google Scholar]
- 15.Hitz MP, Lemieux-Perreault LP, Marshall C, Feroz-Zada Y, Davies R, Yang SW, et al. Rare copy number variants contribute to congenital left-sided heart disease. PLoS Genet. 2012;8:e1002903. doi: 10.1371/journal.pgen.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soemedi R, Wilson IJ, Bentham J, Darlay R, Topf A, Zelenika D, et al. Contribution of global rare copy-number variants to the risk of sporadic congenital heart disease. Am J Hum Genet. 2012;91:489–501. doi: 10.1016/j.ajhg.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita-Mitchell A, Mahnke DK, Struble CA, Tuffnell ME, Stamm KD, Hidestrand M, et al. Human gene copy number spectra analysis in congenital heart malformations. Physiol Genomics. 2012;44:518–541. doi: 10.1152/physiolgenomics.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz GBGoUS. Human (Homo sapiens) Genome Browser Gateway. http://genome.ucsc.edu/cgi-bin/hgGateway?hgsid=331446057&clade=mammal&org=Human&db=hg18. [Google Scholar]
- 21.Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med. 2011;13:777–784. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, et al. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet. 2009;84:148–161. doi: 10.1016/j.ajhg.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer LG, Dabell MP, Rosenfeld JA, Neill NJ, Ballif BC, Coppinger J, et al. Referral patterns for microarray testing in prenatal diagnosis. Prenat Diagn. 2012;32:611. doi: 10.1002/pd.3909. [DOI] [PubMed] [Google Scholar]
- 25.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grayton HM, Fernandes C, Rujescu D, Collier DA. Copy number variations in neurodevelopmental disorders. Prog Neurobiol. 2012;99:81–91. doi: 10.1016/j.pneurobio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367:1321–1331. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingason A, Rujescu D, Cichon S, Sigurdsson E, Sigmundsson T, Pietilainen OP, et al. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry. 2011;16:17–25. doi: 10.1038/mp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang SQ, Guo DC, Prakash SK, McDonald ML, Johnson RJ, Wang M, et al. Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genet. 2011;7:e1002118. doi: 10.1371/journal.pgen.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagamani SC, Erez A, Bader P, Lalani SR, Scott DA, Scaglia F, et al. Phenotypic manifestations of copy number variation in chromosome 16p13.11. Eur J Hum Genet. 2011;19:280–286. doi: 10.1038/ejhg.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullmann R, Turner G, Kirchhoff M, Chen W, Tonge B, Rosenberg C, et al. Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28:674–682. doi: 10.1002/humu.20546. [DOI] [PubMed] [Google Scholar]
- 32.Varadi A, Szabo Z, Pomozi V, de Boussac H, Fulop K, Aranyi T. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671–682. doi: 10.2174/138945011795378612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 34.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 35.Devriendt K, Matthijs G, Van Dael R, Gewillig M, Eyskens B, Hjalgrim H, et al. Delineation of the critical deletion region for congenital heart defects, on chromosome 8p23.1. Am J Hum Genet. 1999;64:1119–1126. doi: 10.1086/302330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wat MJ, Shchelochkov OA, Holder AM, Breman AM, Dagli A, Bacino C, et al. Chromosome 8p23.1 deletions as a cause of complex congenital heart defects and diaphragmatic hernia. Am J Med Genet A. 2009;149A:1661–1677. doi: 10.1002/ajmg.a.32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu S, Zhou XG, Fiedler SD, Brawner SJ, Joyce JM, Liu HY. Cardiac defects are infrequent findings in individuals with 8p23.1 genomic duplications containing GATA4. Circ Cardiovasc Genet. 2011;4:620–625. doi: 10.1161/CIRCGENETICS.111.960302. [DOI] [PubMed] [Google Scholar]
- 38.Barber JC, Bunyan D, Curtis M, Robinson D, Morlot S, Dermitzel A, et al. 8p23.1 duplication syndrome differentiated from copy number variation of the defensin cluster at prenatal diagnosis in four new families. Mol Cytogenet. 2010;3:3. doi: 10.1186/1755-8166-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macdonald AH, Rodriguez L, Acena I, Martinez-Fernandez ML, Sanchez-Izquierdo D, Zuazo E, et al. Subtelomeric deletion of 12p: Description of a third case and review. Am J Med Genet A. 2010;152A:1561–1566. doi: 10.1002/ajmg.a.33401. [DOI] [PubMed] [Google Scholar]
- 40.Degenhardt F, Priebe L, Herms S, Mattheisen M, Muhleisen TW, Meier S, et al. Association between copy number variants in 16p11.2 and major depressive disorder in a German case-control sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:263–273. doi: 10.1002/ajmg.b.32034. [DOI] [PubMed] [Google Scholar]
- 41.Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci U S A. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenfeld JA, Coppinger J, Bejjani BA, Girirajan S, Eichler EE, Shaffer LG, et al. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J Neurodev Disord. 2010;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. Am J Med Genet A. 2007;143A:1462–1471. doi: 10.1002/ajmg.a.31837. [DOI] [PubMed] [Google Scholar]
- 44.Soemedi R, Topf A, Wilson IJ, Darlay R, Rahman T, Glen E, et al. Phenotype-specific effect of chromosome 1q21.1 rearrangements and GJA5 duplications in 2436 congenital heart disease patients and 6760 controls. Hum Mol Genet. 2012;21:1513–1520. doi: 10.1093/hmg/ddr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenfeld JA, Traylor RN, Schaefer GB, McPherson EW, Ballif BC, Klopocki E, et al. Proximal microdeletions and microduplications of 1q21.1 contribute to variable abnormal phenotypes. Eur J Hum Genet. 2012;20:754–761. doi: 10.1038/ejhg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunet A, Gabau E, Perich RM, Valdesoiro L, Brun C, Caballin MR, et al. Microdeletion and microduplication 22q11.2 screening in 295 patients with clinical features of DiGeorge/Velocardiofacial syndrome. Am J Med Genet A. 2006;140:2426–2432. doi: 10.1002/ajmg.a.31499. [DOI] [PubMed] [Google Scholar]
- 47.Yang YH, Hu YL, Zhu XY, Mo XM, Wang DJ, Yao JC, et al. Diagnosis of 22q11 deletion and duplication in congenital heart disease by multiplex ligation dependent probe amplification. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:892–896. [PubMed] [Google Scholar]
- 48.Ben-Shachar S, Ou Z, Shaw CA, Belmont JW, Patel MS, Hummel M, et al. 22q11.2 distal deletion: a recurrent genomic disorder distinct from DiGeorge syndrome and velocardiofacial syndrome. Am J Hum Genet. 2008;82:214–221. doi: 10.1016/j.ajhg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coppinger J, McDonald-McGinn D, Zackai E, Shane K, Atkin JF, Asamoah A, et al. Identification of familial and de novo microduplications of 22q11.21-q11.23 distal to the 22q11.21 microdeletion syndrome region. Hum Mol Genet. 2009;18:1377–1383. doi: 10.1093/hmg/ddp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koop EA, Lopes SM, Feiken E, Bluyssen HA, van der Valk M, Voest EE, et al. Receptor protein tyrosine phosphatase mu expression as a marker for endothelial cell heterogeneity; analysis of RPTPmu gene expression using LacZ knock-in mice. Int J Dev Biol. 2003;47:345–354. [PubMed] [Google Scholar]
- 51.Mouse atlas project. www.emouseatlas.org.
- 52.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.