Summary
Benzodiazepines (BZs) allosterically modulate γ-aminobutyric acid type-A receptors (GABAARs) to increase inhibitory synaptic strength. Diazepam binding inhibitor (DBI) protein is a BZ site ligand expressed endogenously in the brain, but functional evidence for BZ-mimicking positive modulatory actions has been elusive. We demonstrate an endogenous potentiation of GABAergic synaptic transmission and responses to GABA uncaging in the thalamic reticular nucleus (nRT) that is absent in both nm1054 mice, in which the Dbi gene is deleted, and mice in which BZ binding to α3 subunit-containing GABAARs is disrupted. Viral transduction of DBI into nRT is sufficient to rescue the endogenous potentiation of GABAergic transmission in nm1054 mice. Both mutations enhance thalamocortical spike-and-wave discharges characteristic of absence epilepsy. Together these results indicate that DBI mediates endogenous nucleus-specific BZ-mimicking (“endozepine”) roles to modulate nRT function and suppress thalamocortical oscillations. Enhanced DBI signaling might serve as a novel therapy for epilepsy and other neurological disorders.
Introduction
Allosteric modulation can profoundly regulate the function of ion channels and G proteincoupled receptors in either a positive or negative direction (Conigrave and Franks, 2003; Schwartz and Holst, 2007), and is of increasing interest for both physiology and pharmacology. Benzodiazepines (BZs) act as allosteric modulators on type-A receptors for the inhibitory neurotransmitter γ-aminobutyric acid (GABA). BZs act as either positive allosteric modulators (PAMs), and prolong currents through GABAARs to increase the duration and strength of inhibitory signals, or as negative allosteric modulators (NAMs, or inverse agonists) (Sieghart, 1995). The discovery of BZ sites on GABAARs (Braestrup and Squires, 1977; Mohler and Okada, 1977; Gavish and Snyder, 1980) led to the hypothesis that the brain might synthesize its own endogenous BZ site ligands (Iversen, 1977). In the intervening years, however, functional evidence for endogenous PAM effects has been quite elusive.
The family of peptides derived from the 10 kDa protein diazepam binding inhibitor (DBI) (Guidotti et al., 1983; Alho et al., 1985), also known as acyl-CoA binding protein (ACBP) (Knudsen, 1991), has been suggested to play such roles. Most evidence, however, has indicated NAM actions, such as facilitation of anxiety behaviors (Guidotti et al., 1983; Garcia de Mateos-Verchere et al., 1998), increased aggression (Kavaliers and Hirst, 1986) and decreased sleep (Dong et al., 1999). DBI and a DBI fragment peptide, octadecaneuropeptide (ODN), also promote neurogenesis in the subventricular zone (SVZ) via negative modulation of GABA signaling (Alfonso et al., 2012). DBI is synthesized by both neurons and glia (Alho et al., 1989) and its proteolytic peptide products bind to both GABAAR and mitochondrial BZ sites (Papadopoulos et al., 1991). Functional evidence for endogenous PAM actions that would suppress neural excitability, however, has not been demonstrated.
Absence seizures, which are characterized by staring spells and brief lapses of consciousness that occur hundreds of times per day, are driven by abnormal oscillatory activity in thalamocortical (TC) networks (Crunelli and Leresche, 2002; Beenhakker and Huguenard, 2009). The thalamic reticular nucleus (nRT) is functionally and anatomically poised to play a critical gating role in this circuitry, which is normally involved in sleep rhythms and sensory processing (Steriade et al., 1993). nRT receives excitatory input from both corticothalamic and TC axons and provides GABAergic input onto TC relay cells in dorsal thalamus, such as the ventrobasal nucleus (VB), as well as intranuclear inhibition via recurrent collaterals (Cox et al., 1996; Pinault et al., 1997; Shu and McCormick, 2002). Reductions in intra-nRT inhibition result in hypersynchronous epileptiform oscillations between nRT and VB and promote absence seizures (Von Krosigk et al., 1993; Huguenard and Prince, 1994a; Huntsman et al., 1999; Sohal and Huguenard, 2003). Conversely, a gain of intra-nRT inhibition dampens oscillatory duration and power (Schofield et al., 2009). Modulation of intra-nRT inhibition can thus shape TC circuit activity, thereby influencing seizure susceptibility and duration.
In mature nRT, the predominant GABAAR α subunit is α3, whereas α1 is highly expressed in dorsal thalamus (Wisden et al., 1992; Fritschy and Mohler, 1995). Experiments utilizing mice bearing point mutations in either α3 [α3(H126R)] or α1 subunits [α1(H101R)] that selectively abolish BZ binding in GABAARs containing these subunits (Rudolph et al., 1999; Löw et al., 2000) demonstrated that BZs act via specific enhancement of intra-nRT inhibition to suppress TC oscillations (Sohal et al., 2003). A human GABAAR γ2 subunit mutation that alters BZ binding is associated with absence seizures (Wallace et al., 2001), suggesting a role for a PAM in regulating seizure activity, but this mutation also affects receptor trafficking (Kang and Macdonald, 2004) and GABA deactivation and desensitization kinetics (Bowser et al., 2002).
DBI mRNA is widely expressed throughout the brain, including the thalamus (Lein et al., 2007). Previous immunohistochemical studies have observed varying profiles of protein expression for DBI and fragment peptides in the CNS of various species (Tonon et al., 1990; Slobodyansky et al., 1992; Lihrmann et al., 1994), likely due to use of different antisera and other methodological differences, but in some cases higher expression was observed in nRT (Alho et al., 1985, 1989). We therefore hypothesized that DBI may exert endogenous effects within the thalamus, thereby modulating seizure susceptibility. Here we investigate this by comparing inhibitory transmission, effects of BZ site blockade, and seizure profiles in wild-type (WT), α3(H126R), and nm1054 (new mutation 1054) mice, which harbor a 400-kb deletion on chromosome 1 that includes the Dbi gene (Ohgami et al., 2005). Furthermore, we tested the ability of viral transduction of DBI into the thalamus to rescue the effect of the nm1054 mutation. We also examine allosteric modulation of responses to focal GABA uncaging in “sniffer” patches pulled from VB neurons and placed back in the slice in either VB or nRT. Our results provide novel functional evidence for the constitutive presence of endogenous BZ binding site ligands (“endozepines”) that mimic the PAM actions of BZs specifically within nRT.
Results
IPSC Duration in nRT is Reduced in α3(H126R) Mutant Mice
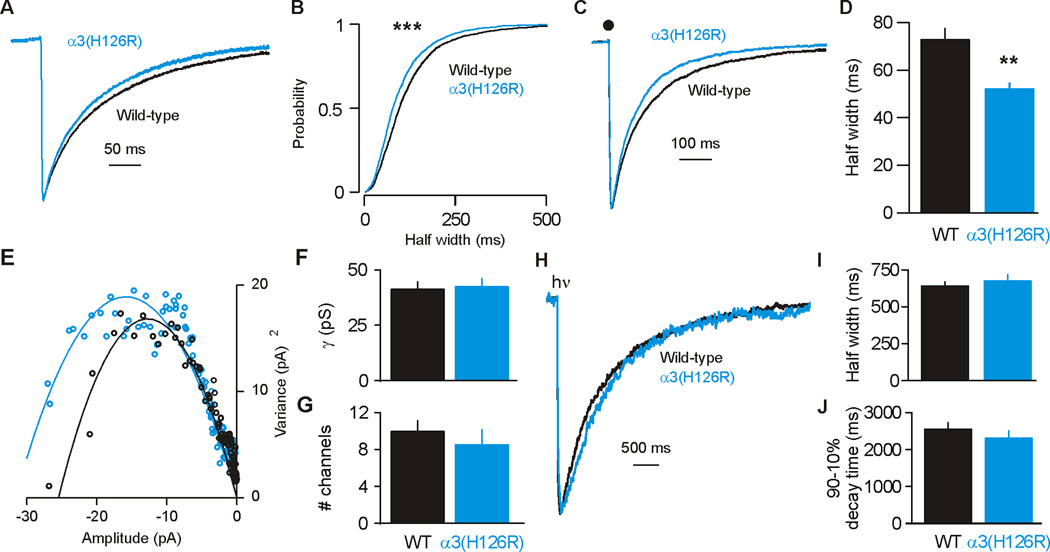
The primary PAM effect of BZs on GABAARs is to increase the duration of inhibitory postsynaptic currents (IPSCs) (Mody et al., 1994). Therefore, we focused on this parameter in assessing whether endogenous BZ site PAMs alter intrathalamic inhibition. We recorded spontaneous IPSCs (sIPSCs) and evoked monosynaptic intra-nRT IPSCs (eIPSCs) in nRT cells from C57BL/6 WT and α3(H126R) mice. As in previous reports (Huntsman and Huguenard, 2000), sIPSC duration progressively decreased through early development, reaching maturity by P20; therefore, all experiments used age-matched comparisons. α3(H126R) cells in both young and adult mice showed briefer sIPSCs (p<0.001) and eIPSCs (p<0.01) compared to WT (Figure 1A–D; Figure S1; Table S1). The decay phase of sIPSCs could be best described by a double exponential function, and both fast and slow decay time constants were shortened by the α3(H126R) mutation, while the relative contribution of fast and slow decay was unaffected (Table S1). This suggests that both components of IPSC decay are dependent on BZ-sensitive GABAARs. There was no difference in unitary conductance or numbers of channels mediating events as revealed by nonstationary variance analysis (Sigworth, 1980; De Koninck and Mody, 1994; Schofield and Huguenard, 2007) (Figure 1E–G). Outside-out membrane patches pulled from WT and α3(H126R) cells showed similar responses to laser photolysis of caged GABA (1 mM) (Figure 1H–J), indicating that differences in sIPSC duration are not due to altered GABA affinity or chloride conductance, but rather are due to the modulatory actions of a constitutively released BZ site PAM.
Figure 1. Effects of α3(H126R) Mutation on nRT IPSCs and Responses to Laser Photolysis of Caged GABA.
(A) Averaged sIPSCs from all nRT cells recorded from wild-type (n=23 cells) and α3(H126R) mice (n=20 cells) at P4–14, normalized to peak amplitude. (B) Probability distributions for >1,500 events/group comparing sIPSC half width in WT vs. α3(H126R). (C) Averaged eIPSC traces across all nRT cells recorded from WT mice (black trace) and α3(H126R) mutant mice (blue trace) at P14–21, normalized to peak amplitude. Black dot indicates time of electrical stimulation applied to nRT (stimulus artifact removed for clarity). (D) Mean + SEM for eIPSC half width in WT (black bar, n=22 cells) and α3(H126R) mutant mice (blue bar, n=10 cells). (E) Mean amplitude versus mean variance of sIPSCs from example WT (black) and α3(H126R) (blue) nRT cells. The curve fit is to the parabolic function: σ2−σ2Noise = iIm − I2m/N. (F–G) Mean + SEM for unitary conductance (γ) (F, p>0.8) and number of channels mediating sIPSC events (G, p>0.4). (H) Averaged uncaged IPSC traces for nRT patches from WT (black trace, n=8 patches) and α3(H126R) mutant mice (blue trace, n=5 patches) at P21. hν symbol indicates time of 1 ms UV laser stimulus. (I–J) Mean + SEM for uncaged IPSC half width (I; p>0.5) and 90–10% decay time (J; p>0.3). Note that in contrast to the sniffer patch experiments presented in Figure 5, here the patches were positioned in the superfusion solution >100 µm above the slice to eliminate the influence of endogenous modulators. **p<0.01, ***p<0.001 vs. WT. See also Figure S1 and Table S1.
Flumazenil Reduces IPSC Duration in nRT, but not VB
To test the hypothesis that in WT mice an endogenous BZ site PAM constitutively potentiates IPSC duration, we examined the effect of 10 min bath application of flumazenil (FLZ, 1 µM), a BZ site antagonist (Hunkeler et al., 1981). FLZ reduced sIPSC (p<0.001) and eIPSC (p<0.05) duration, along with decay rates, in WT nRT cells (Figure 2A–E; Table S1), but had no effect on sIPSC duration in α3(H126R) cells (Figure 2F–G), confirming that these effects depend on the BZ site on the α3 subunit.
Figure 2. Flumazenil Shortens IPSC Duration in nRT, but not VB, and this Effect Depends on the α3 Subunit BZ Binding Site.
(A) Representative traces recorded in individual nRT and VB cells before and during FLZ treatment. (B) Averaged sIPSCs in individual nRT and VB cells before and during FLZ treatment, normalized to peak amplitude. (C) sIPSC half width in nRT (n=13) and VB (n=17) cells before (Con) and during FLZ treatment at P4–14. Open boxes indicate mean ± SEM. (D) Averaged eIPSC traces in a representative nRT cell before (Control, black trace) and during FLZ treatment (FLZ, red trace). (E) eIPSC half width in individual WT nRT cells (n=8) before (Con), during (FLZ), and after (Wash) FLZ treatment recorded at P19–22. Open boxes indicate mean ± SEM during control, FLZ, and washout conditions. (F) Averaged traces of sIPSCs recorded in a representative α3(H126R) nRT cell before and during FLZ treatment, normalized to peak amplitude. (G) sIPSC half width in α3(H126R) nRT cells (n=10) before (Con) and during FLZ treatment at P4–14. Open boxes indicate mean ± SEM. *p<0.05, ***p<0.001 vs. Con. See also Figure S2 and Table S1.
BZs and DBI-derived peptides can increase the production of neurosteroids via upregulation of the mitochondrial BZ receptor, also known as peripheral BZ receptor (PBR) or 18-kDa translocator protein (TSPO) (Papadopoulos et al., 1991; Tokuda et al., 2010). To examine if the FLZ-sensitive potentiation of sIPSCs reflect actions of neurosteroids, we used the 5α-reductase inhibitor finasteride (1 µM) to block neurosteroidogenesis. Finasteride alone reduced nRT sIPSC duration, indicating a level of constitutive neurosteroid expression (Fig. 3A, B), but did not affect the response to FLZ, indicating that FLZ-induced reductions in sIPSC duration do not reflect blockade of neurosteroid actions (Fig. 3C, D).
Figure 3. Endozepine Effects in nRT are Distinct from Neurosteroid Actions.
(A) Averaged sIPSC traces from nRT cells in control conditions (n=14 cells) and cells preincubated and recorded in finasteride (n=13 cells) recorded at ages P17–25, normalized to peak amplitude. (B) Probability distributions for >1,300 events per group comparing sIPSC half width in control (black line) vs. finasteride conditions (purple line). (C) sIPSC half width in individual nRT cells in control (n=10) or finasteride conditions (n=9) before (Con) and during (FLZ) in vitro FLZ treatment. Open boxes indicate mean ± SEM. (D) Mean + SEM for percentage change in sIPSC half width in response to FLZ in control and finasteride conditions. ***p<0.001 vs. Control (B,C); ns, not significant.
The degree of constitutive BZ site activation in nRT was estimated at ~60%, based on the maximal modulation produced by a saturating concentration (100 nM) of clonazepam (CZP) (Gibbs III et al., 1996) (Figure 4A–D). FLZ had no effect on VB cell sIPSCs (p>0.2) (Figure 2A–C; Figure S2) but reversed the effects of CZP (Figure 4E–F), indicating that VB neurons do respond to FLZ, but only in the presence of exogenous BZs; i.e., endogenous BZ site PAMs are not functionally active in VB.
Figure 4. Constitutive Activation of BZ Sites in nRT is Approximately 60% of Maximal, and VB Neurons are BZ-Sensitive.
(A) Representative averaged traces of sIPSCs recorded in an individual WT nRT cell under control conditions (black trace), after 10 min bath application of 100 nM clonazepam (CZP, gray trace), and in CZP with subsequent 10 min bath application of FLZ (CZP+FLZ, red trace), normalized to peak amplitude. (B) sIPSC half width in individual WT nRT cells (n=8) at P20–29 in control conditions (Con), during CZP treatment (CZP), and during subsequent FLZ treatment (CZP+FLZ). Open boxes indicate mean ± SEM during control (black), CZP (gray), and CZP+FLZ conditions (red). (C) Mean + SEM for percentage change in sIPSC half width from control to CZP conditions (gray bar), CZP to CZP+FLZ conditions (red bar), and from control to CZP+FLZ conditions (black bar). (D) Mean + SEM for the within-cell ratio of the percentage change from control to CZP+FLZ conditions compared to the percentage change from CZP alone to CZP+FLZ. (E) Averaged sIPSC traces from control VB cells (black trace) and cells in slices either preincubated in 100 nM clonazepam (CZP, gray trace), or in CZP with subsequent 10 min bath application of FLZ (CZP+FLZ, red trace). Note that FLZ treatment does not reduce sIPSC duration past control values, as compared to nRT cells. (F) Probability distribution for >800 events per group comparing sIPSC half width in control (black line, n=8) and CZP-preincubated cells (gray line, n=11). **p<0.01, ***p<0.001 vs. Control (B, F), ###p<0.001 vs. CZP (B).
Dbi Gene Products Mediate Endogenous BZ-Mimicking Actions in nRT
One molecular candidate that may mediate the endogenous PAM effects in nRT is DBI. To test the role of DBI in mediating these effects, we compared nm1054 mice to WT littermates. Immunocytochemical staining confirmed that DBI protein expression in the thalamus is essentially abolished in nm1054 mice (Figure 5A–B). As with the α3(H126R) mutation, the duration, charge transfer, and fast and slow decay time constants of sIPSCs in nRT cells from nm1054 mice was reduced compared to WT (p<0.001) (Figure 5C–D; Table S2). These results suggest that loss of the Dbi gene reduces endogenous allosteric potentiation of GABAergic currents in nRT.
Figure 5. AAV-Mediated Expression of DBI Rescues Effects of nm1054 Mutation on nRT sIPSC Duration.
(A–B) Fluorescence images illustrating immunocytochemical staining for DBI and the nucleic acid marker DAPI in striatum and thalamus in WT (A) and nm1054 (B) mice. Dotted and dashed lines indicate approximate borders of striatum (Str), internal capsule (IC), and thalamic nuclei. Averaged sIPSCs from nRT cells recorded from WT (n=14 cells) and nm1054 mice (n=13 cells) at P22–29, normalized to peak amplitude. (D) Probability distributions (>1300 events/group) comparing sIPSC half width. (E) GFP fluorescence in mouse thalamus injected with AAV-DBI-GFP vector. (F–H) DBI and GFP staining in nRT of nm1054 mice injected with control AAV-GFP (F) or AAV-DBI-GFP vector (G–H). (I) Probability distributions (>800 events/group) comparing sIPSC half width. (J) sIPSC half width in nRT cells from WT mice injected with control AAV-GFP (left, n=7 cells), and nm1054 mice injected with AAV-GFP (middle, n=7) or AAV-DBI-GFP vector (right, n=11) before (Con) and during FLZ treatment. Open boxes indicate mean ± SEM. (K) Mean + SEM for percentage change in sIPSC half width in response to FLZ. Calibration: 100 µm (A–B); 200 µm (E); 25 µm (F–H). **p<0.01, ***p<0.001 vs. WT (D, I, K) or Con (J); ##p<0.01, ###p<0.001 vs. nm1054 AAV-DBI-GFP (I, K). See also Figure S3 and Table S2.
To determine whether this deficit in the nm1054 mutant was due to loss of Dbi gene products, we tested the effect of infecting nRT cells with an AAV vector expressing DBI and green fluorescent protein (GFP) (Fig. S3). Injection of AAV-DBI-GFP into nRT of nm1054 mice both increased sIPSC duration and conferred responsiveness to FLZ treatment that was not observed in nm1054 mice injected with control AAV-GFP (Figure 5E–K). Injection of AAV-GFP into WT nRT was confirmed to not affect responsiveness to FLZ (Figure 5I–K). Thus the endogenous PAM actions in nRT are mediated by products of the Dbi gene.
GABA Uncaging Responses in Sniffer Patches Pulled from VB Neurons Demonstrate nRTDependent Potentiation
The nucleus-specificity of FLZ effects may result from differential localization of PAMs and/or different GABAAR subunit composition in nRT and VB. To test these possibilities directly, we pulled outside-out membrane patches containing GABAARs from VB cells, which were then placed back into the slice to function as “sniffer patches” (Isaacson et al., 1993; Allen, 1997; Banks and Pearce, 2000). We then tested the response of these patches to laser photolysis of caged GABA (100 µM) when placed ~25–50 µm deep into the slice in either VB or nRT (Figure 6).
Figure 6. GABAARs from VB Neurons Respond to Endozepine Modulation When Placed in nRT.
(A) Uncaged GABA IPSC responses averaged across all outside-out patches pulled from VB neurons recorded when patches were placed in VB or nRT, normalized to peak amplitude. Inset, proof-of-principle experiment demonstrating potentiation of averaged responses recorded from the same patch first placed in VB (1) and then moved to nRT (2), normalized to peak amplitude. Similar results were obtained in 3 other patches. (B) Responses averaged across all WT patches in the presence of FLZ, normalized to peak amplitude. (C) Responses averaged across all patches recorded in slices from nm1054 mice, normalized to peak amplitude. Inset, schematic illustrating movement of patch pipette from VB to nRT. (D) Responses averaged across all nm1054 patches in the presence of FLZ, normalized to peak amplitude. Inset, averaged traces for responses recorded when patches were placed in nRT, re-plotted from A–D for clarity. (E) Mean + SEM for 90–10% decay time of responses. Colored bars for patches placed in nRT are all significantly different from WT control, but are not significantly different from each other (P>0.9). Each bar represents 6–8 patches. There was no difference for patches in VB (P>0.8). FIN – finasteride. hν symbol – 1 ms laser stimulus. *p<0.05, **p<0.01, ***p<0.001 vs. respective values for patches placed in VB; #p<0.05, ##p<0.01 vs. WT in nRT. See also Figure S4.
In WT slices, sniffer patches moved to nRT exhibited an increased uncaged IPSC duration compared to patches placed in VB (p<0.00001) (Figure 6A). Both FLZ treatment and the nm1054 mutation largely blocked the nRT-dependent potentiation (~25% enhancement remaining in FLZ or nm1054 vs. 72% in control, p<0.01), and FLZ had no effect on responses in nm1054 slices (p>0.9), suggesting that the nm1054 mutation removes a source of potentiating actions at BZ sites. This was confirmed further by occlusion of the nRT-dependent potentiation to a similar degree (~13% potentiation remaining) by the presence of CZP (Figure S4A–B). Combined application of GABA transporter (GAT) antagonists and FLZ in WT slices blocked all nRT-dependent potentiation (p>0.9), which was preserved in the presence of GAT antagonists alone (p<0.001) (Figure S4C–E), demonstrating that the residual potentiation in the presence of FLZ/CZP and in nm1054 mutants results from tissue-dependent differences in GABA uptake. Dbi gene products that are endogenous PAMs are thus constitutively released and bind to the extracellular BZ binding domain on GABAA receptors in nRT, but not VB. Furthermore, α1-containing GABAARs in VB are sensitive to endogenous PAMs (i.e., these actions do not depend on α3 subunits per se), but do not normally respond to FLZ treatment due to the absence of endogenous ligand in this nucleus.
Altered Spike-and-Wave Discharge Activity in α3(H126R) and nm1054 Mice
Intra-nRT inhibition plays a critical role in regulating thalamic oscillations and absence seizure activity in the thalmocortical circuit. To examine whether the endogenous PAM actions observed in nRT modulate seizure susceptibility, we performed electroencephalogram (EEG) recordings in adult mice and assessed both spontaneous and pharmacologically-induced spike-and-wave discharges (SWDs, a characteristic of absence epilepsy). SWDs in human absence epilepsy patients typically display ~3 Hz internal frequency (Steriade et al., 1993; Crunelli and Leresche, 2002), but in many rodent models the internal frequency is in the range of 4–6 Hz (Noebels and Sidman, 1979; Ryan and Sharpless, 1979; Hosford et al., 1992). Both α3(H126R) and nm1054 mice showed a higher incidence of spontaneous 4–6 Hz SWDs compared to the very rare events in WT counterparts (p<0.01, Figure 7A–D), suggesting a level of constitutive BZ site activation that is antiepileptic.
Figure 7. Endozepines Modulate Spike-and-Wave Discharge (SWD) Activity Characteristic of Absence Seizures In Vivo.
(A–B) Example traces of spontaneous SWDs recorded from α3(H126R) mice and respective WT (A) and nm1054 mice and respective WT (B). Note that SWD amplitude (power) was not significantly different between groups (p>0.2). Therefore, examples were chosen to illustrate typical SWD waveforms in each condition, which were not affected by SWD power. (C–D) Mean + SEM for spontaneous 4–6 Hz SWD rate and percentage of time spent in spontaneous SWD activity in WT (n=17) and α3(H126R) mice (n=9) (C) and WT (n=7) and nm1054 mice (n=4) (D). (E–F) Mean ± SEM for SWD period over time following PTZ injection (Time 0, 40 mg/kg) in WT (n=7) and α3(H126R) mice (n=4) (E) and WT (n=7) and nm1054 mice (n=4) (F). (G–H) Linear regression of lines of best fit for points plotted in (EF) shows positive correlations (i.e., SWD slowing) with time after PTZ injection in WT, but not in α3(H126R) nor nm1054 mice. **p<0.01, ***p<0.001 vs. WT; ##p<0.01, ###p<0.001 - repeated measures ANOVA (E–F) or Pearson correlation (G–H) across 1-min bins after PTZ injection. Gray bars in E–F indicate times at which values differed between mutants and controls (p<0.05). See also Figure S5.
Low dose pentylenetetrazol (PTZ) is a common means of chemically inducing absence seizures and SWD in rodents (Snead, 1998). In WT and α3(H126R) mice on the C57BL/6 background, however, PTZ induced generalized tonic-clonic seizures rather than absence seizures, precluding examination of strain-dependent differences in SWDs (see Supplemental Information). Therefore, we also examined α3(H126R) mutants on the 129X1/SvJ background, which we found to be less susceptible to tonic-clonic seizures. In this strain, experimental absence seizures initiated rapidly and were characterized by prominent ~4–5 Hz SWDs that peaked approximately 5 min after injection, reaching a similar peak incidence in both genotypes, but persisted at a much higher rate over time in the α3(H126R) mutants (Figure S5). SWD internal frequency showed a progressive slowing from ~4 to 3 Hz during the course of repeated seizures in WT, but remained constant at ~5 Hz in α3(H126R) mutants (p>0.8) (Figure 7E, G). Similarly, nm1054 mice failed to display the slowing of SWD internal frequency following PTZ injection (p>0.6) that was observed in WT (Figure 7F, H). These results suggest that PAM release within the TC circuit reduces seizure susceptibility and severity through a slowing of the seizure oscillations, which may destabilize them.
DISCUSSION
Following the discovery of BZ binding sites on GABAARs 35 years ago (Braestrup and Squires, 1977; Mohler and Okada, 1977; Gavish and Snyder, 1980), it was thought that the brain likely produces cognate endogenous ligands for these sites. In the intervening years, however, the search for an endogenous PAM proved elusive. The studies presented here provide novel functional evidence for endogenous BZ-mimicking (“endozepine”) PAM actions, and indicate that these effects are mediated by Dbi gene products. This was demonstrated by the deficits in IPSC potentiation exhibited in nm1054 mice, which was rescued by local viral transduction of DBI into nRT. Although the PAM effects in nRT depend on the α3 subunit BZ binding site, as demonstrated by the lack of modulation in α3(H126R) mice, GABAARs in VB neurons respond to endozepines when placed in nRT. This indicates that the nucleus-specificity of the observed effects results from nRT-specific localization of PAMs within the thalamus. Finally, we present in vivo evidence that SWD activity is altered in both α3(H126R) and nm1054 mice, suggesting that local PAM actions in nRT can exert seizure-suppressive effects on TC circuitry as a whole. Although it remains to be determined whether DBI itself or a peptide fragment act as the BZ site agonist, our data provide the first functional evidence that indeed peptides in the DBI family can act as PAMs.
Constitutive Endogenous Positive Allosteric Modulation of GABAergic Transmission by Endozepines in nRT
The principal findings of this study are the existence and functional consequences of endozepines in nRT, which act to potentiate GABAergic postsynaptic currents in this nucleus. α3(H126R) mutant mice, in which classical BZ binding to the GABAAR α3 subunit is effectively abolished (Löw et al., 2000), exhibited shorter durations of both sIPSCs and eIPSCs, indicating that in WT mice, constitutive modulation via this binding site acts to prolong inhibitory signals. Another GABAAR mutation associated with BZ binding and absence seizures (Wallace et al., 2001) has been reported to affect basic receptor properties such as receptor trafficking and expression and response to GABA (Bowser et al., 2002; Kang and Macdonald, 2004). Here, the effects of the α3(H126R) mutation on sIPSCs were confined to the duration of events, with no difference in either amplitude or frequency; thus it does not appear that this mutation leads to large differences in receptor expression, localization, or function besides the loss of BZ or endozepine binding. Both fast and slow decay time constants were reduced, suggesting that both β1 and β3 subunit-containing receptors in nRT are responsive to endozepines (Huntsman and Huguenard, 2006; Hentschke et al., 2009). Furthermore, when nRT GABAARs in excised patches were removed from the slice, and thus no longer exposed to putative endogenous modulators within the slice, the response to GABA uncaging was identical between WT and α3(H126R) mutant mice. Together, these results support the interpretation that the H126R mutation does not lead to differences in fundamental receptor properties apart from the effects on BZ/endozepine binding in α3(H126R) mutant receptors.
The ability of FLZ, a BZ binding site antagonist (Hunkeler et al., 1981), to reduce sIPSC duration also suggested an endogenous augmentation of IPSC duration in nRT. Similarly, FLZ has been observed to reduce evoked inhibitory postsynaptic potential (IPSP) amplitude in hippocampal CA1 pyramidal neurons (King et al., 1985) and eIPSC duration in dissociated cortical neurons (Vicini et al., 1986). Some reports have indicated agonist effects of FLZ (Skerritt and Macdonald, 1983; De Deyn and Macdonald, 1987; Weiss et al., 2002), including at receptors carrying α3 subunits (Ramerstorfer et al., 2010). This would not explain the reductions in duration and decay time observed here, although FLZ increased sIPSC amplitude and slightly increased charge transfer in both WT and α3(H126R) nRT cells, potentially indicating a nonspecific effect representing actions on presynaptic terminals (Table S1). Partial agonistic effects of FLZ have also been described at receptors containing α4 or α6 subunits (i.e., receptors that do not respond to classical BZs) (Hadingham et al., 1996; Knoflach et al., 1996; Whittemore et al., 1996). Here, however, α3(H126R) GABAARs did not respond to FLZ, indicating that disruption of BZ binding to these receptors does not change the direction of response to BZ binding site activation.
A recent report indicates that the CSF of hypersomnolent patients can potentiate currents evoked by GABA application in a FLZ-sensitive manner (Rye et al., 2012). This potentiation, however, is not occluded by the BZ midazolam and persists in α1(H101R) GABAARs, indicating that it does not represent a true BZ-mimicking endozepine effect.
Dbi Gene Products Mediate Endozepine Effects in nRT
The experiments in α3(H126R) mice examined the potential effects of endozepines at the level of postsynaptic GABAARs. To investigate this question at the level of the ligand, we tested the role of Dbi gene products in mediating endozepine actions by exploring intra-nRT GABAergic transmission in nm1054 mice, which lack the Dbi gene (Ohgami et al., 2005). The other known genes deleted by the mutation are: primary ciliary dyskinesia protein 1 (Pcdp1); secretin receptor (Sctr); neuronal voltage-gated calcium channel γ-like subunit (Pr1); and six-transmembrane epithelial antigen of the prostate 3 (Steap3) (Ohgami et al., 2005; Lee et al., 2007). Pr1 transcript is either absent or very low in mouse thalamus (Lein et al., 2007). The other genes are not expected to affect postsynaptic GABAA receptor function, though the secretin peptide may act on presynaptic terminals to increase GABAergic transmission in cerebellum (Yung et al., 2001). Although future work will examine the role of DBI using a specific knockout model, nm1054 mice injected with the AAV-DBI vector in nRT displayed: 1) prolonged sIPSC duration compared to nm1054 mice injected with control virus; and 2) a reduction in sIPSC duration in response to FLZ that was not observed in nm1054 mice injected with control virus, and is of the same magnitude as that observed in WT mice. DBI is thus necessary and sufficient to produce the endogenous PAM effect, and is either the endogenous modulator itself or at least a precursor.
These results stand in contrast to the majority of previous studies of DBI-related peptides, which have primarily found NAM effects. Application of DBI reduced the amplitude of GABA currents recorded in cultured spinal cord neurons (Bormann et al., 1985; Macdonald et al., 1986), as did ODN application to nucleated outside-out patches from SVZ progenitor cells (Alfonso et al., 2012). A NAM effect, however, would be disinhibitory and would not explain the endogenous potentiation observed here. Of note, these studies used high concentrations (0.5–20 µM) of applied peptide. Effects of exogenous DBI peptides on seizures exhibit dose-dependent effects, with low doses being efficacious at suppressing seizures (Garcia de Mateos-Verchere et al., 1999) and high doses promoting seizure activity (Ferrero et al., 1986). It is also possible that nRT-specific receptor-associated proteins are required to obtain a PAM effect, though this is unlikely to be solely responsible as VB receptors placed in nRT also exhibit a PAM response, as demonstrated in the sniffer patch studies (Figure 6). In addition, the dose response curve for stimulation of intracellular calcium levels in cultured astrocytes by ODN and octapeptide (OP), a smaller fragment, is bell-shaped with peak stimulation at 10 nM (Leprince et al., 1998). Therefore, it is possible that extracellular concentrations of endogenous DBI peptides in discrete areas such as nRT are at a lower level that is more likely to exert PAM rather than NAM effects.
In contrast to the stark nRT vs. VB differences in physiological PAM effects of DBI, immunoreactivity to DBI was observed in both nRT and VB (Figure 5). In this regard, it is important to note that DBI is also known as ACBP and the Dbi/Acbp gene is thought to ubiquitously serve housekeeping functions (Knudsen et al., 1993), generating an intracellular protein critically involved in facilitating intracellular transport of Acyl-CoA. We hypothesize that the nRT vs. VB differences in the electrophysiological findings likely represent nucleus-specific differences in the extracellular release and processing of DBI.
In the current studies we used a viral strategy to examine whether Dbi gene products are necessary and sufficient to produce endozepine actions in nRT. Although it is possible that viral introduction of DBI into a system in this way induces changes in its modulatory effects, it is unlikely that this explains the PAM actions observed here, as a recent report using similar viral vectors to express DBI and ODN in the SVZ exclusively observed NAM effects (Alfonso et al., 2012). The mechanism(s) underlying these differences remain to be determined, though one possible explanation is differential intra- or extracellular processing of DBI, the nature of which is specific to certain areas or cell types. It is certainly conceivable that some DBI fragments exert PAM effects, whereas others are NAMs. In addition, preliminary evidence suggests that exogenous application of DBI does not alter sIPSCs in VB (C.A.C. and J.R.H., unpublished observations), in contrast to the robust potentiation of uncaging responses when VB sniffer patches are placed in nRT. This further supports a working model in which nRT-specific processing of Dbi gene products underlies the PAM actions.
Functional Endozepines are Absent in VB
Strikingly, endogenous BZ-mimicking potentiation was observed in nRT, but not in adjacent VB thalamus. This raises the question of why such effects would be specifically localized to nRT, and not VB. Perhaps selection pressure led to evolution of an adaptive specific subcircuit modulation in nRT that reduces the possibility of seizure occurrence. Synaptic inhibition in nRT exerts a prominent desynchronizing effect to reduce the propensity for oscillatory activity (von Krosigk et al., 1993; Huntsman et al., 1999; Schofield et al., 2009), and potentiation of synaptic inhibition by BZs further suppresses oscillations (Huguenard and Prince, 1994a; Sohal and Huguenard, 2003; Sohal et al., 2003). In VB, by contrast, postsynaptic inhibition via GABAA and GABAB receptors interacts with prominent T-type calcium conductances in VB relay neurons to drive robust post-inhibitory rebound burst firing (Llinás and Jahnsen, 1982; Huguenard and Prince, 1994b; Sohal and Huguenard, 2003) and paradoxically promote oscillatory activity. Therefore, endogenous potentiation of intra-nRT inhibition is poised to exert an endogenous anti-oscillatory seizure-suppressing effect, whereas such potentiation in VB could be disadvantageous. It should be noted, however, that VB neurons are BZ-sensitive, as demonstrated here and in previous studies (Oh et al., 1995; Peden et al., 2008), although systemic treatment with BZs would influence both nRT and VB inhibition such that activity throughout the circuit would be globally suppressed. Indeed, constitutive activation in nRT was ~60% of maximal, indicating that although there is a substantial degree of endogenous modulation, there is still an extent of enhancement that can be exploited by exogenous BZs, likely explaining the therapeutic efficacy of these drugs.
Focal GABA Uncaging on Sniffer Patches Reveals Nucleus-Specific Differences in Endozepine Potentiation and GABA Uptake
Here we introduce a methodology combining the “sniffer patch” recording configuration (Isaacson et al., 1993; Allen, 1997; Banks and Pearce, 2000) with laser GABA uncaging, which is used to examine nucleus-specific differences in endogenous BZ site modulation. The main advantage of this method is that GABA exposure to the patches can be normalized independent of patch placement, so that region-specific differences in modulation of GABAergic signaling can be assessed. Combined application of GAT antagonists and FLZ was sufficient to completely block the nRT-dependent potentiation. This potentiation is thus mediated in large part by endozepines, and to a lesser extent by nucleus-specific differences in rates of GABA uptake, with more robust uptake in VB than in nRT. This is consistent with the postulated role for GATs in removing GABA from VB extracellular space in order to prevent excessive GABABR activation and oscillatory seizure activity (Beenhakker and Huguenard, 2010).
The durations of uncaging responses for both nRT and VB patches are longer than those for spontaneous or evoked IPSCs, in accordance with previous studies on patches from thalamic, hippocampal, and cortical neurons (Galarreta and Hestrin, 1997; Jones and Westbrook, 1997; Banks and Pearce, 2000; Schofield and Huguenard, 2007). Thus this appears to be an effect of the pulled patch configuration rather than the use of uncaging to apply GABA. Nevertheless, the application of GABA by laser photolysis replicates the nRT vs. VB differences in receptor affinity for GABA and kinetics of IPSC decay (Zhang et al., 1997; Mozrzymas et al., 2007; Schofield and Huguenard, 2007).
Endozepines Modulate TC Circuit Activity In Vivo
Potentiation of intra-nRT GABAergic transmission by BZs is capable of exerting powerful anti-oscillatory effects by reducing synchronization in intra-thalamic networks (Huguenard and Prince, 1994a; Huguenard, 1999; Sohal et al., 2003). Similarly, the EEG recordings here indicate that PAM potentiation of GABAergic transmission, presumably within nRT as observed in vitro, can modulate in vivo rhythmic activity in TC circuitry in a manner consistent with suppression of seizure susceptibility and severity. In particular, both α3(H126R) and nm1054 mice exhibit similar phenotypes with respect to an increased incidence of spontaneous SWDs and a lack of slowing of SWD period following PTZ treatment that is observed in WT mice. This suggests that endogenous activation of BZ binding sites on GABAARs containing the α3 subunit by DBI ligands in nRT both: 1) reduces the propensity for the circuit to generate SWDs; and 2) reduces the intensity and severity of absence seizures once initiated. Although the α3 subunit is expressed in other brain regions, including cortical layers V and VI (Pirker et al., 2000), the complementary in vitro experiments implicate actions that originate in thalamus, and specifically in nRT, in underlying these differences.
Summary
Our results describe the molecular identification of an endogenous DBI-related PAM acting via GABAAR BZ binding sites, which may represent a novel endogenous anti-seizure mechanism. The highly specific release and processing within nRT (and presumably other restricted brain regions) may explain why previous studies using α3(H126R) and similar knockin mice have not observed overt phenotypes beyond those of subtype-specific BZ actions (Rudolph and Möhler, 2004). Given the important role of nRT as a locus of control in TC circuitry, targeted release of Dbi-derived endozepine peptides in nRT may be fruitful in the development of therapies against absence seizures. Furthermore, regulation of DBI release or function may provide a safer alternative to long-term BZ administration in the treatment of epilepsy and other neurological disorders.
Experimental Procedures
Additional information on these methods can be found in Supplemental Information.
All procedures were approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
Brain Slice Preparation
Mice were anesthetized with pentobarbital sodium and killed via decapitation and the brain was quickly removed and placed in ice-cold oxygenated sucrose slicing solution. Horizontal thalamic slices (250 µm thickness) were prepared as previously described (Huguenard and Prince, 1994a). Slices were incubated and continuously oxygenated in warm (~32°C) artificial cerebrospinal fluid for 1 hour and then transferred to room temperature (~21–23 °C) for at least 15 min prior to recording.
Patch-Clamp Electrophysiology
Patch-clamp recordings were made using a MultiClamp 700A amplifier with Clampex 9.2 software. Patch pipettes were filled with a 135 mM CsCl-based solution. To isolate GABAergic IPSCs, ionotropic glutamate receptors were blocked with either kynurenic acid (1 mM) or a combination of D-(−)-2-amino-5-phosphonovaleric acid (APV, 100 µM) plus 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 µM).
IPSCs
In whole-cell recordings, membrane potential was clamped at −60 mV. Evoked IPSCs were elicited by extracellular tungsten electrode stimulation in nRT.
GABA Uncaging
CNB-caged GABA (Invitrogen) was added to a recirculating 10–20 ml bath solution containing APV and DNQX. Outside-out patches of membrane from nRT or VB cells were maintained in voltage-clamp mode at a membrane holding potential of −30 mV. Laser photolysis (DPSS Lasers) was achieved via 1-ms ultraviolet laser exposure.
EEG Recordings
EEG recordings were obtained from either metal skull screws or silver wires implanted above the left and right frontal and parietal cerebral cortices. Experimental absence seizures were induced via s.c. injection of PTZ. EEG activity and simultaneous video were recorded for up to 90 min post-PTZ injection.
Virus Injections
Bilateral stereotaxic injections of either control or DBI-expressing AAVs were performed under isoflurane anesthesia between P48–60. Brain slices were prepared for electrophysiology at 2–3 weeks post-injection. Infected cells expressing GFP were visualized using epifluorescence microscopy.
Data Analysis
sIPSCs were analyzed using the custom software programs wDetecta and WinScanSelect (J.R.H.). eIPSC and uncaging recordings were analyzed using Clampfit. EEG recordings were analyzed using a continuous wavelet transform method in MATLAB to isolate SWD events (Schofield et al., 2009). Comparisons between groups were made using two-tailed independent or paired t-tests, nonparametric Mann-Whitney Rank Sum Tests, or one-way ANOVA with Tukey’s post hoc means comparison tests. Cumulative probability distributions were constructed using up to 100 randomly selected sIPSCs (events) per cell and compared using two-sample Kolmogorov-Smirnov (KS) goodness of fit tests. Differences within each genotype for EEG parameters across different time points after PTZ injection were assessed using one-way repeated measures ANOVA. Statistical significance was set at p<0.05 for means comparisons, and p<0.001 for KS tests.
Supplementary Material
Highlights.
Nucleus-specific release of endogenous benzodiazepine site ligands in thalamus
Viral transduction of DBI into thalamus rescues allosteric potentiation
Endogenous allosteric potentiation of GABA uncaging responses in sniffer patches
Endogenous benzodiazepine site ligands modulate absence seizures in vivo
Acknowledgments
We thank Isabel Parada for expert assistance with histology experiments, Lance Lee and Mark Fleming for providing nm1054 founder mice, Richard Reimer for providing the DBI-T2AGFP plasmid and helpful discussions, Craig Garner and Michael Lochrie for helpful discussions regarding virus generation, and Istvan Mody and Stefano Vicini for useful critiques of the manuscript. This work was supported by NIH grants NS034774 (J.R.H.), NS006477 (J.R.H.), T32 NS007280 (C.A.C.), an Epilepsy Foundation Research Fellowship (C.A.C.), and a Katharine McCormick Advanced Postdoctoral Fellowship from Stanford School of Medicine (C.A.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes Extended Experimental Procedures, five figures, two tables, and Supplemental References.
Author contributions: C.A.C. and J.R.H. designed the studies, analyzed EEG data, and wrote the manuscript; C.A.C. performed and analyzed the in vitro electrophysiology and virus experiments and prepared the figures; C.A.C., A.G.H., R.L.H., K.P, and K.D.S. performed the EEG experiments; S.P.-F. performed pilot studies; U.R. provided α3(H126R) founder mice and edited the manuscript.
References
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell. 2012;10:76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Alho H, Bovolin P, Jenkins D, Guidotti A, Costa E. Cellular and subcellular localization of an octadecaneuropeptide derived from diazepam binding inhibitor: immunohistochemical studies in the rat brain. J Chem Neuroanat. 1989;2:301–318. [PubMed] [Google Scholar]
- Alho H, Costa E, Ferrero P, Fujimoto M, Cosenza-Murphy D, Guidotti A. Diazepam-binding inhibitor: a neuropeptide located in selected neuronal populations of rat brain. Science. 1985;229:179–182. doi: 10.1126/science.3892688. [DOI] [PubMed] [Google Scholar]
- Allen TGJ. The “sniffer-patch” technique for detection of neurotransmitter release. Trends Neurosci. 1997;20:192–197. doi: 10.1016/s0166-2236(96)01039-9. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci. 2010;30:15262–15276. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Ferrero P, Guidotti A, Costa E. Neuropeptide modulation of GABA receptor Cl- channels. Regul Pept Suppl. 1985;4:33–38. doi: 10.1016/0167-0115(85)90215-0. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Wagner DA, Czajkowski C, Cromer BA, Parker MW, Wallace RH, Harkin LA, Mulley JC, Marini C, Berkovic SF, et al. Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma2(R43Q)] found in human epilepsy. Proc Natl Acad Sci USA. 2002;99:15170–15175. doi: 10.1073/pnas.212320199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci USA. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave AD, Franks AH. Allosteric activation of plasma membrane receptors--physiological implications and structural origins. Prog Biophys Mol Biol. 2003;81:219–240. doi: 10.1016/s0079-6107(03)00020-8. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Heterogeneous axonal arborizations of rat thalamic reticular neurons in the ventrobasal nucleus. J Comp Neurol. 1996;366:416–430. doi: 10.1002/(SICI)1096-9861(19960311)366:3<416::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Macdonald RL. CGS 9896 and ZK 91296, but not CGS 8216 and RO 15–1788, are pure benzodiazepine receptor antagonists on mouse neurons in culture. J Pharmacol Exp Ther. 1987;242:48–55. [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Tohda M, Watanabe H. Involvement of diazepam binding inhibitor and its fragment octadecaneuropeptide in social isolation stress-induced decrease in pentobarbital sleep in mice. Life Sci. 1999;64:1779–1784. doi: 10.1016/s0024-3205(99)00116-2. [DOI] [PubMed] [Google Scholar]
- Ferrero P, Santi MR, Conti-Tronconi B, Costa E, Guidotti A. Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci USA. 1986;83:827–831. doi: 10.1073/pnas.83.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. J Neurosci. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Mateos-Verchere J, Leprince J, Tonon MC, Vaudry H, Costentin J. The octadecaneuropeptide ODN induces anxiety in rodents: possible involvement of a shorter biologically active fragment. Peptides. 1998;19:841–848. doi: 10.1016/s0196-9781(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Garcia de Mateos-Verchere J, Leprince J, Tonon MC, Vaudry H, Costentin J. Reduction of pentylenetetrazol-induced convulsions by the octadecaneuropeptide ODN. Peptides. 1999;20:1431–1436. doi: 10.1016/s0196-9781(99)00153-9. [DOI] [PubMed] [Google Scholar]
- Gavish M, Snyder SH. Benzodiazepine recognition sites on GABA receptors. Nature. 1980;287:651–652. doi: 10.1038/287651a0. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Schroder GB, Coulter DA. GABAA receptor function in developing rat thalamic reticular neurons: whole cell recordings of GABA-mediated currents and modulation by clonazepam. J Neurophysiol. 1996;76:2568–2579. doi: 10.1152/jn.1996.76.4.2568. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD, Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci USA. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human gamma-aminobutyric acid typeA receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- Hentschke H, Benkwitz C, Banks MI, Perkins MG, Homanics GE, Pearce RA. Altered GABAA, slow inhibition and network oscillations in mice lacking the GABAA receptor beta3 subunit. Journal of Neurophysiology. 2009;102:3643–3655. doi: 10.1152/jn.00651.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford DA, Clark S, Cao Z, Wilson WAJ, Lin FH, Morrisett RA, Huin A. The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science. 1992;257:398–401. doi: 10.1126/science.1321503. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Adv Neurol. 1999;79:991–999. [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Clonazepam suppresses GABAB-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994a;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994b;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkeler W, Mohler H, Pieri L, Polc P, Bonetti EP, Cumin R, Schaffner R, Haefely W. Selective antagonists of benzodiazepines. Nature. 1981;290:514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Nucleus-specific differences in GABA(A)-receptor-mediated inhibition are enhanced during thalamic development. J Neurophysiol. 2000;83:350–358. doi: 10.1152/jn.2000.83.1.350. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor beta1 subunit. J Physiol. 2006;572:459–475. doi: 10.1113/jphysiol.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Iversen L. Anti-anxiety receptors in the brain? Nature. 1977;266:678. [Google Scholar]
- Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Macdonald RL. The GABAA receptor gamma2 subunit R43Q mutation linking to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. J Neurosci. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M. An octadecaneuropeptide (ODN) derived from diazepam binding inhibitor increases aggressive interactions in mice. Brain Res. 1986;383:343–349. doi: 10.1016/0006-8993(86)90037-5. [DOI] [PubMed] [Google Scholar]
- King GL, Knox JJ, Dingledine R. Reduction of inhibition by a benzodiazepine antagonist, Ro-15–1788, in the rat hippocampal slice. Neuroscience. 1985;15:371–378. doi: 10.1016/0306-4522(85)90219-2. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Knudsen J. Acyl-CoA-binding and transport, an alternative function for diazepam binding inhibitor (DBI), which is identical with acyl-CoA-binding protein. Neuropharmacology. 1991;30:1405–1410. doi: 10.1016/s0028-3908(11)80009-2. [DOI] [PubMed] [Google Scholar]
- Knudsen J, Mandrup S, Rasmussen JT, Andreasen PH, Poulsen F, Kristiansen K. The function of acyl-CoA-binding protein (ACBP)/Diazepam binding inhibitor (DBI) Mol Cell Biochem. 1993;123:129–138. doi: 10.1007/BF01076484. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABA(A) receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Lee L, DeBono CA, Campagna DR, Young DC, Moody DB, Fleming MD. Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J Invest Dermatol. 2007;127:16–23. doi: 10.1038/sj.jid.5700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leprince J, Gandolfo P, Thoumas JL, Patte C, Fauchère JL, Vaudry H, Tonon MC. Structure-activity relationships of a series of analogues of the octadecaneuropeptide ODN on calcium mobilization in rat astrocytes. J Med Chem. 1998;41:4433–4438. doi: 10.1021/jm980275d. [DOI] [PubMed] [Google Scholar]
- Lihrmann I, Plaquevent JC, Tostivint H, Raijmakers R, Tonon MC, Conlon JM, Vaudry H. Frog diazepam-binding inhibitor: peptide sequence, cDNA cloning, and expression in the brain. Proc Natl Acad Sci USA. 1994;91:6899–6903. doi: 10.1073/pnas.91.15.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weddle MG, Gross RA. Benzodiazepine, beta-carboline, and barbiturate actions on GABA responses. Adv Biochem Psychopharmacol. 1986;41:67–78. [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mohler H, Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Vicini S. GABAergic currents in RT and VB thalamic nuclei follow kinetic pattern of alpha3- and alpha1-subunit-containing GABAA receptors. Eur J Neurosci. 2007;26:657–665. doi: 10.1111/j.1460-9568.2007.05693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels JL, Sidman RL. Inherited epilepsy: spike-wave and focal motor seizures in the mutant mouse tottering. Science. 1979;204:1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- Oh KS, Lee CJ, Gibbs JW, Coulter DA. Postnatal development of GABAA receptor function in somatosensory thalamus and cortex: whole-cell voltage-clamp recordings in acutely isolated rat neurons. J Neurosci. 1995;15:1341–1351. doi: 10.1523/JNEUROSCI.15-02-01341.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Berkovich A, Krueger KE, Costa E, Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991;129:1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Smith Y, Deschenes M. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J Neurosci. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Ramerstorfer J, Furtmüller R, Vogel E, Huck S, Sieghart W. The point mutation gamma 2F77I changes the potency and efficacy of benzodiazepine site ligands in different GABAA receptor subtypes. Eur J Pharmacol. 2010;636:18–27. doi: 10.1016/j.ejphar.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gammaaminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sharpless SK. Genetically determined spontaneous and pentylenetetrazol-induced brief spindle episodes in mice. Exp Neurol. 1979;66:493–508. doi: 10.1016/0014-4886(79)90197-3. [DOI] [PubMed] [Google Scholar]
- Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, Freeman A, Garcia PS, Owens MJ, Ritchie JC, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med. 2012;4:161ra151. doi: 10.1126/scitranslmed.3004685. [DOI] [PubMed] [Google Scholar]
- Schofield CM, Huguenard JR. GABA affinity shapes IPSCs in thalamic nuclei. J Neurosci. 2007;27:7954–7962. doi: 10.1523/JNEUROSCI.0377-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CM, Kleiman-Weiner M, Rudolph U, Huguenard JR. A gain in GABAA receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proc Natl Acad Sci USA. 2009;106:7630–7635. doi: 10.1073/pnas.0811326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci. 2007;28:366–373. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Shu Y, McCormick DA. Inhibitory interactions between ferret thalamic reticular neurons. J Neurophysiol. 2002;87:2571–2576. doi: 10.1152/jn.00850.2001. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sigworth FJ. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerritt JH, Macdonald RL. Benzodiazepine Ro 15–1788: electrophysiological evidence for partial agonist activity. Neurosci Lett. 1983;43:321–326. doi: 10.1016/0304-3940(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Slobodyansky E, Kurriger G, Kultas-Ilinsky K. Diazepam binding inhibitor processing in the rhesus monkey brain: an immunocytochemical study. J Chem Neuroanat. 1992;5:169–180. doi: 10.1016/0891-0618(92)90042-o. [DOI] [PubMed] [Google Scholar]
- Snead OC. Ganaxolone, a selective, high-affinity steroid modulator of the gamma-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence. Ann Neurol. 1998;44:688–691. doi: 10.1002/ana.410440417. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory interconnections control burst pattern and emergent network synchrony in reticular thalamus. J Neurosci. 2003;23:8978–8988. doi: 10.1523/JNEUROSCI.23-26-08978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Keist R, Rudolph U, Huguenard JR. Dynamic GABA(A) receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci. 2003;23:3649–3657. doi: 10.1523/JNEUROSCI.23-09-03649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon MC, Desy L, Nicolas P, Vaudry H, Pelletier G. Immunocytochemical localization of the endogenous benzodiazepine ligand octadecaneuropeptide (ODN) in the rat brain. Neuropeptides. 1990;15:17–24. doi: 10.1016/0143-4179(90)90155-r. [DOI] [PubMed] [Google Scholar]
- Vicini S, Alho H, Costa E, Mienville JM, Santi MR, Vaccarino FM. Modulation of gamma-aminobutyric acid-mediated inhibitory synaptic currents in dissociated cortical cell cultures. Proc Natl Acad Sci USA. 1986;83:9269–9273. doi: 10.1073/pnas.83.23.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Weiss M, Tikhonov D, Buldakova S. Effect of flumazenil on GABAA receptors in isolated rat hippocampal neurons. Neurochem Res. 2002;27:1605–1612. doi: 10.1023/a:1021674708556. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WH, Leung PS, Ng SS, Zhang J, Chan SC, Chow BK. Secretin facilitates GABA transmission in the cerebellum. J Neurosci. 2001;21:7063–7068. doi: 10.1523/JNEUROSCI.21-18-07063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl- currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78:2280–2286. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.