Abstract
Premature birth rates and premature infant morbidity remain discouragingly high. Improving nourishment for these infants is the key for accelerating their development and decreasing disease risk. Dietary protein is essential for growth and development of infants. Studies on protein nourishment for premature infants have focused on protein requirements for catch-up growth, nitrogen balance, and digestive protease concentrations and activities. However, little is known about the processes and products of protein digestion in the premature infant. This review briefly summarizes the protein requirements of term and preterm infants, and the protein content of milk from women delivering preterm and at term. An in-depth review is presented of the current knowledge of term and preterm infant dietary protein digestion, including human milk protease and anti-protease concentrations; neonatal intestinal pH, and enzyme activities and concentrations; and protein fermentation by intestinal bacteria. The advantages and disadvantages of incomplete protein digestion as well as factors that increase resistance to proteolysis of particular proteins are discussed. In order to better understand protein digestion in preterm and term infants, future studies should examine protein and peptide fragment products of digestion in saliva, gastric, intestinal and fecal samples, as well as the effects of the gut micro biome on protein degradation. The confluence of new mass spectrometry technology and new bioinformatics programs will now allow thorough identification of the array of peptides produced in the infant as they are digested.
Keywords: Premature infant, Term infant, Human milk, Proteolysis, Protein, Digestion, Microbiota, Bacteria, Gastrointestinal tract
Introduction
Each year, more than half a million babies (about 1 in 8 deliveries) are born prematurely in the United States [1]. Survival of the smallest premature infants has increased dramatically in recent decades due to major advances in neonatal medical care [2]. This new cohort of survivors is at much higher risk for various morbidities than term infants [3]. Improving nourishment has the potential to decrease premature infant morbidity risk [4]. Protein is an essential component of infant nutrition, as it is required for growth and development of the neonate. Human milk, while ideal for the term infant, is inadequate to meet the protein requirement of premature infants and must be supplemented for adequate growth [5]. Neither milk from mothers of preterm infants nor from mothers of term infants provides enough protein for premature infant adequate growth [5].
At a minimum, protein nutrition for premature infants must provide sufficient essential and non-essential amino acids for the protein synthesis needed for growth and development. The amount of ingested protein required to meet this minimum requirement varies with an infant's ability to break down dietary protein. Nitrogen balance studies determine the amount of protein required to provide an adequate amino acid supply for the premature infant [6], but provide no information on the specific products of digestion nor their functions. Little is known about the products of protein digestion in premature or term infants. For example, lactoferrin (Lf) and secretory immunoglobulin A (sIgA) survive intact to fecal excretion in term and premature infants [7-10], but little is known about other intact proteins and digested protein products, or the implications of the passage of these intact proteins through the intestinal tract.
Studies have determined in the preterm and term infant the concentrations and activities of major gastrointestinal proteases, including pepsin, trypsin, chymotrypsin, carboxypeptidase B and enterokinase. However, digestion is more complicated than the simple sum of the cleavage patterns of well-known proteases. In addition to the effects of cleavage by major proteases, the effects of less abundant proteases, unknown proteases, the digestive environment (pH, transit time, etc.), breast milk-derived proteases and antiproteases, and microbial digestion likely affect the end result of digestion in an individual.
Incomplete breakdown of dietary protein can benefit or harm the infant, depending on the specific molecule remaining intact. Some peptides and proteins left intact may have beneficial bioactivity in the infant [11]. However, some incompletely broken down proteins can elicit an allergic response in the infant and, therefore, have detrimental effects [12,13]. Incomplete protein degradation can also limit amino acid availability for protein synthesis. Incomplete protein digestion and absorption in the upper intestinal tract also results in the availability of protein in the distal colon, where bacterial fermentation of protein— putrefaction—results in the production of potentially harmful molecules, including ammonia, amines, phenols and sulfides [14].
Through recent improvements in mass spectrometry and bioinformatic programs, it is now possible to identify the array of peptides released by digestion in the infant. Peptides can be extracted from digest using conventional solid phase extraction. Extracted peptides can then be introduced into the mass spectrometer via in-line reverse phase chromatography with electrospray ionization. Peptide ions can then be detected with high mass accuracy detectors such as time-of-fight or Orbitrap. Peptide ions can then be automatically selected for fragmentation and the fragment masses will be determined. All spectral data can be imported into proteomic analysis programs for de-novo sequencing of non-specifically cleaved peptides. These programs can then provide lists of the specific peptide sequence with protein of origin information. Now that all of these components are available, the field of nutrition and pediatrics can determine exactly how dietary protein is broken down in the digestive tract.
Premature Infant Protein Requirements
Protein and amino acid requirements for the human infant, especially the preterm infant, are high because of rapid growth. Premature infants have higher intact protein needs than term infants [15]. For term infants, protein requirements are based on the amount of protein-nitrogen in an adequate intake of breast milk [16]. Researchers have employed two approaches for determining premature infant protein requirement. The first approach sets protein requirements at amounts shown clinically to meet fetal growth rates and nitrogen accretion, while not accumulating potentially harmful protein metabolic products [17]. The second approach sets requirements based on the sum of the amount of protein incorporated into new tissues plus the obligatory nitrogen losses in urine, feces, skin, etc. [16]. Benefits of higher protein intake in premature infants include better growth and protein accretion [18]. Table 1 shows protein requirements for term and preterm infants. For infant formula, an Expert Panel from the American Society for Nutritional Sciences recommended a preterm infant protein requirement of 3.4–4.3 g/kg/d total protein without distinguishing among various stages postpartum [16] (Figure 1).
Table 1.
Protein requirements in term and preterm infants.
| Gestational age at birth | Preterm (26 weeks gestational age, 900 g average weight) | Preterm (30 weeks gestational age, 1,500 g average weight) | Term |
|---|---|---|---|
| Enteral protein requirement (g/ kg/d) | 4 [15] | 3.6 [15] | 1.98 for first month of life, 1.18 for 4–12 mo. [138] |
Figure 1.
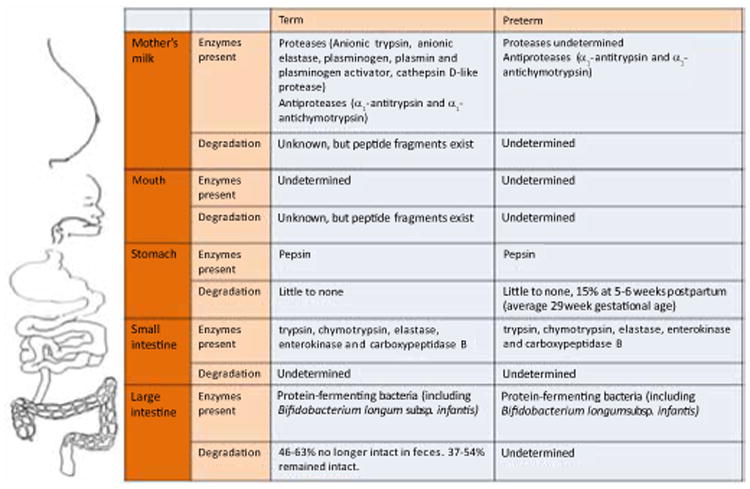
Protein degradation in term and preterm infants.
Protein Available from Premature Infants Mother's Milk
Milk from mothers delivering preterm is higher in protein than milk from mothers delivering at term (Table 2). Over the first 8 weeks of lactation, the earlier a woman delivers, the higher the protein content of her milk. With time, the milk protein content decreases in both women delivering prematurely and at term [19]. In spite of this higher protein content in preterm mothers, human milk not supplemented with protein is not adequate for the tremendous growth requirements of this unique preterm population [20]. Protein concentration is highly variable among preterm mothers and for a given woman over time [21]. This variability has prompted protocols for individualizing the protein intake of preterm infants [5]. Currently, measuring the protein content of individual samples of milk from mothers of premature infants is technically challenging [22].
Table 2.
Average protein concentration of milks from term and premature mothers over the first eight weeks of lactation (data from [19]).
| Extremely preterm human milk (<28 weeks) | Severely preterm human milk (28–31 weeks) | Moderately preterm human milk (32–33 weeks) | Term human milk | |
|---|---|---|---|---|
| Average protein concentration (g/dL) for weeks 1–8 of lactation | 2.3 ± 0.5 | 2.1 ± 0.3 | 1.9 ± 0.3 | 1.6 ± 0.4 |
Proteolysis in Milk
A variety of proteases and antiproteases exist in human milk. Ferranti et al. showed via mass spectrometry that over 100 unique protein fragments of β-, κ- and αs1-casein exist in human milk from term and premature mothers [23]. Armaforte et al. confirmed the presence of low molecular weight casein fragments with 2D-SDS-PAGE and mass spectrometry [24]. This study showed that the casein fragments were present at higher concentrations in premature infants, while the intact caseins were present at lower levels in premature infants than term mother's milk. This data suggests that premature milk undergoes more proteolysis than term milk. Christensen et al. showed that fragments of osteopontin, a common milk protein, also exist in intact term mother's milk [25].
In assessing protein digestion in infants, researchers must consider the effects of proteases and antiproteases secreted in the milk as these enzymes may affect the results of proteolytic degradation at various stages in the gastrointestinal tract.
Proteases in human milk
Proteases present in human milk include anionic trypsin [26], anionic elastase [26], plasmin (as well as its inactive zymogen precursor, plasminogen, and both tissue-type and urokinase-type plasminogen activators) [27-30], cathepsin D [31-33] and kallikrein [32]. The zymogen of thrombin—prothrombin—was identified in human colostrums, but activated thrombin has not yet been reported in milk [32]. Plasmin cleaves on the C-terminal side of lysine or arginine residues [25]. Cathepsin D, an aspartic endopeptidase, cleaves predominantly between two hydrophobic amino acids, particularly when following leucine [25]. Protease activity in term milk decreases across lactation stages [34,35]. Plasmin activity is higher in premature mother's milk than term milk [24]. Fragments of casein created by plasmin cleavage were identified by Ferranti et al. [23]. Researchers have not yet determined the concentrations and activities of proteases in preterm mother's milk. Proteases in human milk may function to initiate digestion of protein for the infant. The decrease in protease activity in human milk coincides with the increase of the infant's own degradative capacity.
Antiproteases in human milk
Antiproteases in human milk may function to protect human milk proteins from degradation. The balance of proteases and antiproteases in human milk may be important in guiding protein-specific and time-dependent digestion of proteins within the mammary gland.
Human milk from women delivering at term and preterm contains the antiproteases α1-antitrypsin and α1-antichymotrypsin from the first day of lactation [35-37]. A1-antitrypsin inhibits a wide variety of proteases, including trypsin [38]. A1-antitrypsin binds covalently to and irreversibly deactivates trypsin in vitro [39]. A1-antichymotrypsin inhibits chymotrypsin and chymotrypsin-like serine proteases such as neutrophil cathepsin G and mast cell chymases [40]. A1-antitrypsin and α1-antichymotrypsin concentrations decline in concentration across lactation from day one to 2 weeks postpartum in both term and preterm milk [35,37]. However, both α1-antitrypsin and α1- antichymotrypsin are still detectable in both term and preterm milk up to 160 d postpartum with no concentration differences noted between term and preterm samples [35].
Protease inhibitory activity was detected in both term and preterm milk samples from 4–160 d postpartum, and, in some samples, as early as the first day postpartum [35]; however, a comparison of activities in term and premature infants has not been made.
A1-antitrypsin has been identified intact in the feces of term breastfed infants. Therefore, α1-antitrypsin can potentially block trypsin and other proteases throughout the term infant digestive tract [7]. Intact survival of other antiproteases such as α1-antichymotrypsin in the feces has not been reported.
Premature Infant Protein Digestion Biology
Protein degradation in the mouth
Several antiproteases, but no proteases, have been found in adult saliva [41,42]. Whether infant saliva contains proteases or antiproteases and whether breakdown of dietary protein begins in the infant oral cavity is unknown. Small premature infants are fed through a feeding tube bypassing the oral cavity until they are old enough to suck, swallow and breathe in a coordinated fashion. Tube feeding effectively bypasses any oral protein degradation capacity except for that which might occur due to swallowed saliva.
Several studies have reported a select range of milk protein-derived peptide fragments in the saliva of human infants after milk feeding. Term and preterm infant saliva after milk feedings contained peptide fragments derived from milk histatins [43] and proline-rich proteins [44]. Term infant saliva at 3 and 6 months postpartum after a milk feed contained peptide fragments of milk-derived acidic proline-rich phosphoprotein, proline-rich protein 3 precursor and histatin 3 [45]. Problematically, however, these three studies did not determine whether these peptide fragments existed in the intact milk prior to contact with the oral cavity, and thus it remains uncertain whether protein fragment products are due to salivary degradation or due to proteases within the mammary gland.
Secretory IgA appears in term infant saliva as early as the third day of life and increases over time [46]. By 6 months of age, salivary immunoglobulin concentrations are higher in breast-fed infants than formula-fed infants, suggesting that breast feeding stimulates maturation of mucosal immunity and that these proteins are protected from oral digestion [47].
Protein degradation in the stomach
Chatterton et al. examined the in vivo gastric digestion of term infants aged 8 and 28 days at 1 and 3 h after human milk feeding with SDS-PAGE and Western blotting [48]. The study showed that many milk proteins remained intact for at least 1 hr post-ingestion. For example, α-lactablbumin, Lf and secretory component were detected intact after 1 h of digestion in both samples. B-casein was detected after 1 h in the 8 day infant but disappeared in the 1 h sample in day 28. This suggests that digestive capacity increased over time. The 1D SDS-PAGE gel images revealed hydrolysis of milk proteins at 3 h for both 8 and 28 days post-partum. Though Chatterton et al. showed that protein degradation was occurring, they did not determine the sequences of the peptides released from enzymatic cleavage.
Proteolysis in the stomach is highly influenced by pH. The generation of hydrogen ions is mediated by endocrine (via gastrin), neurocrine (via acetylcholine) and paracrine (chiefly via histamine) pathways. Typically, highly acidic pH causes protein denaturation (the loss of secondary and tertiary protein structure), which usually decreases protein resistance to protease cleavage [49,50]. By 14–15 weeks gestation, the structural development of the stomach is complete, including the components for acid production [51]. Both term and preterm infants can produce gastric acid as early as the first day of life [52,53]. Gastric acid production is influenced by parietal cell mass, which increases with growth, and by feeding regimen [54]. Premature infants produce less feeding-stimulated gastric acid than term infants; however, this difference disappears by the end of the first month postpartum [55,56]. This reported finding may not be true of extremely low birth-weight infants as they rarely survived in the cited studies. Due to the low acid production of infants in comparison with adults and because the buffering capacity of term human milk is typically pH 7.0-7.6 [57], neither term nor preterm infants can provide postprandial acid pH in the stomach in the time around birth [58,59]. Premature infants have a gastric pH of 5–7 for up to an hour after feeding, and it drops to pH 3–3.5 at three hours after a feeding [58]. Milk's buffering capacity maintains the postprandial gastric contents at near neutral pH, which is not protein-denaturing.
The extent of gastric proteolysis also depends upon the concentrations and activities of gastric proteases. Pepsin is present in the stomach of fetuses as early as 16 weeks of gestation [60], and is produced at birth by both term and preterm infants. The activity and concentration of pepsin in the gastric fluid of 5–6 week postpartum premature infants (avg. gestational age 29 weeks) prior to introduction of a milk feeding is about five-fold lower than that of adults [61]. Researchers have not yet determined pepsin concentration and activity after mother's milk feeding in term or premature infants. Henschel et al. detected a protease in the gastric aspirates of newborn infants within 6–10 h postpartum that was not pepsin [62]. The electrophoretic mobility and immunoreactivity are similar to that of calf chymosin, a protease that cleaves κ-casein and causes casein curdling. This protease is unique in that it disappears from gastric fluid at 10 days postpartum and is not found in adult gastric fluid.
Gastric proteolysis depends upon the activity of the proteases present. Activities of enzymes vary based on pH. Pepsin hydrolyzes proteins optimally at acidic pH [52,54-56,60,62-64] and is denatured when exposed to a pH greater than 7 [56,63]. As postprandial gastric pH is above 5.0 following feedings for at least the first hour [58], pepsin activity is likely to be low early in life. Gastric acid secretion is similar to that of adults by 6 months of age [64], by which time pepsin activity likely becomes significant.
As low gastric pH serves as an antibacterial barrier to the small intestine in adults, higher pH in early infancy may also facilitate bacterial colonization of the infant gut [53].
Mason assayed the gastric contents of 5–13 day old, breast-fed term infants by formol titration and qualitative biuret test methods to determine whether milk proteins were being hydrolyzed in the infant stomach. This study showed that little protein digestion occurred in the stomach of 5–13 day old term infants: no hydrolyzed protein was detected at 90 min post-feeding, and at 180 min, only 1 of 9 samples showed traces of hydrolyzed protein [59]. This may have been due in part to vigorous gastric peristalsis in the second hour post-feeding driving the majority of gastric contents into the duodenum in these infants [59]. Berfenstam et al. detected little or no proteolysis in gastric samples of breast milk-fed term or premature infants (gestational age unspecified) fed human milk over days 6–44 postpartum; however, term and premature infants fed cow's milk did show evidence of gastric proteolysis [65]. Henderson et al. using different methodology, found greater proteolytic degradation in infants fed breast milk than was found in the Berfenstam study. With this method, 5–6 week postpartum premature infants (average 29 weeks gestation) digested 15% of total human milk protein in the stomach [61].
Protein degradation in the small intestine
In adults, a large portion of dietary protein degradation occurs in the small intestine. Intestinal proteolysis occurs through the combined actions of luminal and brush border enzymes as well as enteric bacterial degradation. As is true for the products of gastric digestion, except for a few specific proteins, the overall products of intestinal digestion of milk in human infants are poorly characterized. This review provides indirect information about intestinal proteolysis via enzyme concentrations and activities in term and premature infants. Additionally, the effect of bacterial fermentation on milk proteins is considered.
Luminal proteases
Key luminal proteases in adult intestinal proteolysis include trypsin, chymotrypsin, elastase, enterokinase and carboxypeptidase B. Each of these enzymes is present in both term and premature infants, but typically at concentrations and activities lower than those in adults.
Enterokinase (also called enteropeptidase) is a protease secreted from intestinal epithelial cells in response to food stimulation [66]. Enterokinase is essential for intestinal proteolysis in both adults and infants because it is responsible for the activation of trypsinogen to trypsin [56], which leads to trypsin activation of chymotrypsinogen to chymotrypsin, proteoelastase to elastase and procarboxypeptidase to carboxypeptidase [67]. Two studies showed that enterokinase is present at birth in both term and premature infants, with detection of the enzyme in the duodenal mucosa by 24–26 weeks of gestation [68,69]. Enterokinase is active in both term and preterm infants [70]. Compared with the activity of enterokinase in older children, enterokinase activity was 6% and 20% in 26–30 week gestational age premature infants and term infants, respectively [69].
Trypsin cleaves peptides at the carboxyl side of lysine and arginine [71]. Trypsin concentrations in the duodenum in both preterm and term infants at birth are less than those of adults [72]. During the first week of life, trypsin concentration in the duodenum of premature infants was lower than in term infants, but the concentrations were similar by weeks 2-4 postpartum [72]. By one month postpartum, term and preterm infants' trypsin concentration and activity were similar to those of adults [73].
Chymotrypsin, a luminal pancreatic protease, cleaves on the carboxyl side of tyrosine, tryptophan or phenylalanine [74]. Chymotrypsin concentration in intestinal fluid is similar in term and premature infants at birth and at 30 days postpartum [75], and were 10–60% of adult concentrations [73]. In terms of activity, both at birth and at 30 days postpartum, no difference in chymotrypsin activity was detectable between term and premature infants [75]. Chymotrypsin is present in the feces of both term and preterm infants at birth and does not differ between term and premature infants [76].
Carboxypeptidase B cleaves the basic amino acids arginine and lysine from the carboxy-terminus of peptides and proteins (an excellent complement to trypsin, which produces such substrates) [77]. Carboxypeptidase B is present in similar concentrations and activities in both term and preterm infant duodenal fluids at birth and at 30 days of age. Concentrations and activities were 10–25% of those of 2-year-olds [73].
In summary, in spite of lower enterokinase activity in premature infants, the other major luminal proteases have similar concentrations and activities in term and preterm infants, particularly by 30 days post-partum; however, in the first several weeks of life—the most critical time period in terms of growth, development and survival—preterm infants are likely less capable of digesting proteins.
Brush border peptidases
Once proteins reach the brush border of the intestinal lining, a large variety of brush border peptidases such as di- and tri-peptidases begin to further degrade the peptide fragments [78]. Substantial quantities of brush border proteases, including γ-glutamyl-transpeptidase, oligoaminopeptidase, dipeptidylaminopeptidase IV and carboxypeptidase, are present by 22 weeks of gestation [79], and some brush border dipeptidases are present in the fetal gastrointestinal tract as early as 10 weeks gestation [80]. Curiously, γ-glutamyl-transpeptidase concentration was actually higher in the brush borders of 8–22 week gestation fetuses than in adults and children. Concentrations of dipeptidylaminopeptidase IV and carboxypeptidase match adult concentrations as early as 8 weeks gestation in fetuses, and oligoaminopeptidase concentrations in fetuses reach those of adults and children by 22 weeks gestation [79]. Dipeptidylaminopeptidase IV releases N-terminal dipeptides from peptides with penultimate proline, alanine or leucine residues [79]. Aminopeptidase A, however, is not as well developed in infancy—it is far lower in concentration in 8–22 week gestation fetuses than in adults and children [79]. The role of brush border peptidases in utero is unclear. Swallowed amniotic fluid has nutritional value to the growing fetus, providing about 15% of protein accretion [81,82]. These enzymes possibly contribute to maximal extraction of amino acids from amniotic fluid. These data suggest that, brush border peptidases are important in nutrition of term and premature infants; however, as no study has determined the activities of these enzymes in term and premature infants, this remains speculative.
Dipeptides and tripeptides can be transported into the intestinal enterocyte [79]. In adults, once small peptides are brought within the enterocyte, these peptides are broken down further to free amino acids [83]. The free amino acids generated are then passed by carrier-mediated mechanisms across the basolateral membrane into the portal blood [83]. Researchers have not yet studied the concentrations and activities of enterocyte internal peptidases in term or premature infants.
Bacterial proteases
In addition to the proteases produced by the host, the bacteria of the intestinal microbiota also produce proteases and contribute to the degradation of dietary proteins. A variety of human intestinal bacteria can break down protein, including Bacteroides spp., Propionibacterium spp. and some members of Streptococcus, Clostridium, Bacillus and Staphylococcus [84]. Adult intestinal bacteria degrade casein and bovine serum albumin via cellbound and extracellular proteases [85]. These proteins are first broken into peptides and then into volatile fatty acids, ammonia, dicarboxylic acids and various phenolic compounds [85]. The observation that amino acids do not accumulate when these bacteria degrade protein suggests the amino acids are quickly metabolized by the intestinal microbiota. Some bacteria can break down peptides directly, whereas others can only use amino acids that are already free [86]. A wide variety of anaerobes can ferment amino acids, including species from the genera Peptostreptococcus, Campylobacter, Acidaminococcus, Acidaminobacter, Fusobacterium and Eubacterium [87-94]. Some bacteria can utilize both carbohydrates and proteins as an energy source, whereas others are obligate amino acid fermenters [95]. Researchers have not yet determined the amount of bacterial protein degradation in the intestinal tract and colon of term and premature infants. The observation that Bifdobacterium longum subsp. infantis, a bacterial strain common in the intestinal tract of breast-fed infants, grows on culture media made of pepsin-digested human milk Lf and sIgA suggests that bacterial fermentation of dietary proteins is common in breast-fed infants [96]. A synthesized peptide called prebiotic lactoferrin-derived peptide-I (PRELP-I) that is based on these peptides stimulated growth of B. infantis at a concentration of 1–10 μM, but did not stimulate four pathogenic bacterial strains [96]. That observation that Lf and sIgA can survive intact in stools of term and preterm infants [7,10] suggests that such stimulatory peptide fragments could, indeed, survive to support growth of B. infantis in the colon, but also that even after exposure to bacteria in the infant large intestine, some milk proteins resist degradation.
Protein degradation in the colon
A comprehensive comparison of the protein content of ileostomy fluid with that of feces has not been made, so it is not possible to comment further on protein degradation that occurs in the colon. Any proteolysis in the colon would likely be primarily the result of bacterial proteases. Protein-degrading bacteria are present in the colon [14].
Resistance to Proteolysis
Studies suggest that some milk proteins are particularly resistant to proteolysis in the infant. Such resistance may reflect importance for non-nutritional function.
Resistance to in vitro degradation
In vitro, Lf is resistant to digestion, especially at pH approximating that of the infant stomach and when Lf is iron-saturated [97,98]. Likewise, sIgA resists in vitro proteolysis by trypsin and pepsin at pH 8.0 and 4.0, respectively [99,100].
Resistance to gastric degradation
Various milk proteins survive gastric digestion intact in the premature infant, including epidermal growth factor, thyrotropin-releasing hormone, sIgA, immunoglobulin G (IgG) [101-103], Lf and lactalbumin [56]. In the term infant, Lf and sIgA survive gastric digestion intact [7].
Resistance to complete gastrointestinal tract degradation
A significant fraction of dietary protein remains intact throughout digestion and transit through the gastrointestinal tract. In term infants, the amount of soluble protein excreted in the feces is highest in week 1 (∼1500 mg/24 h) and remains constant (∼700 mg/24 h fecal sample collection) for several months postpartum [7]. The predominant fecal protein is sIgA. In the first 3–4 months postpartum, 10–85% of dietary milk sIgA survived intact in the feces of term infants [7]. Lf was particularly resistant to digestion in term infants, although to a lesser degree than sIgA: 2–6% of Lf remained intact in the first week postpartum and 0.4%–1.6% remained intact for 3–4 months thereafter. Lf continued to be excreted intact in feces for long periods: >10 mg Lf was secreted in a 24-h fecal sample in this study even at 5–6 months postpartum in term infants [7]. However, Lf can be produced within adult human digestive tracts and be excreted intact in stool [104]. Therefore, in order to determine whether fecal Lf represents milk-derived or intestine-derived Lf, stable-isotope labeling of mother's milk is required. Hutchens et al. showed that for two preterm infants, nearly all the urinary Lf was of maternal origin [105]. However, no study has determined the relative amounts of fecal Lf originating from milk compared with the infant intestinal tract via isotope-labeling experiments.
In premature infants fed human milk, the predominant intact fecal proteins are sIgA, Lf and lysozyme (9%, 3% and 0.1%, respectively [10]. Haneberg and Finne detected lysozyme in premature infant fecal samples from 1 to 4 weeks postpartum, with widely variable activity [106]. Although intact lysozyme was assayed in the fecal matter of both premature [10] and term [7] infants, it was detected only in premature infants. Lack of intact lysozyme in term feces suggests either a lesser degree of proteolytic capacity in premature infants or greater production of lysozyme in the preterm mother's mammary tissue. Given the antimicrobial properties of lysozyme, this may represent a protective mechanism.
Lysozyme is produced by the Paneth cells of the small intestine and sIgA is produced by B cells in the lamina propria in the infant. Comparisons between human milk-fed and formula-fed infants suggest that most of the fecal lysozyme and sIgA come from the diet rather than the infant.
Factors that may Increase Resistance to Proteolytic Degradation of Particular Proteins
The resistance of a particular dietary protein to proteolytic degradation can be influenced by a variety of factors, including phosphorylation, size, charge, tertiary structure, amino acid content and glycosylation [107-111].
Degree of glycosylation can increase a protein's resistance to digestion with trypsin [112] and protease cocktails [113]. The glycosylated forms of human interferon-γ have higher resistance to proteolytic degradation by crude granulocyte protease, purified elastase, cathepsin G and plasmin in comparison deglycosylated forms [114].
Degradation of the glycan component of glycoproteins will affect resistance to proteolysis, and thus the peptide fragments that are produced. However, protein-linked glycan degradation is not discussed in this review.
Benefits of Incomplete Protein Digestion
Incomplete digestion could be beneficial if biologically active proteins or protein fragments are left intact as a result. Intact proteins or peptide fragments may have biological functions in the intestinal tract or be absorbed and act on other organs [115]. For example, by remaining intact, sIgA can aid in development of the infant immune system and protect against infection [116]. Likewise, Lf, by remaining intact or at least partially intact as lactoferricin, can exert protective antimicrobial actions [117,118].
Studies show a variety of functions for milk protein fragments [119-121]. Milk peptides generated from in vitro digests exert an array of biological effects, including behavioral, gastrointestinal, hormonal, immunological, neurological and nutritional responses [11]. For example, casein fragments have a wide variety of in vitro and animal model-identified effects, including aiding in calcium absorption (caseinophosphopeptides) [122] and nutrient uptake (casomorphins) [123], improving immune defense (casokinins, casomorphins) [124], acting as antimicrobials (casecidins and isracidin) [125, 126], aiding nerve transmission (casokinins) [11], modulating social behavior, causing analgesia [127], decreasing gastrointestinal transit time [128, 129], decreasing diarrheal effects [130], modulating amino acid transport [131], and stimulating hormonal secretions [132,133]. Researchers have not, however, tested the majority of these effects in term or premature infants.
Negative Consequences of Incomplete Protein Digestion
Evidence of inadequate protein accretion is common in premature infants, manifesting as poor weight gain, poor length gain, low serum albumin and low blood urea nitrogen levels. Incomplete protein digestion likely adds to inadequate protein intake, resulting in poor protein accretion. Low protein digestive capacity, and thus low capacity for extracting amino acids for protein synthesis, may be part of the reason premature infant dietary protein requirements are higher than those of term infants. Another adverse consequence of incomplete protein digestion is exposure to intact peptides, leading to allergic responses or diarrhea. For example, exposure of some infants to intact cow's milk proteins can cause allergic response that disappears when protein is first hydrolyzed before feeding [12,13]. Incomplete digestion could result in decreased release of biologically active peptide fragments. Finally, the incomplete digestion of proteins in the small intestine results in the passage of these proteins into the distal colon, where bacterial fermentation takes place [84], and this bacterial fermentation results in the formation of potentially toxic metabolites such as ammonia, amines, N-nitroso compounds, phenols , and sulfides [14,134]. However, the long-term effects of exposure to these metabolites on the gut mucosa and epithelial cells remain unclear [14].
Conclusion and Future Research Directions
Given the high number of premature births and high morbidity in a premature infant population, there is a great need for better understanding of protein digestion and improved dietary approaches. Protein supply to premature infants must not only meet overall protein requirements, but also be provided in forms that allow the release of beneficial biologically active peptides.
In order to better understand dietary protein digestion in infants, researchers now have access to remarkable new tools to carry out proteomic and peptidomic studies on the intact milk, saliva, gastric fluid, urine and feces of both term and premature infants. Proteomic studies will determine what proteins remain relatively intact at each stage of digestion as well as the concentrations of proteases, while peptidomics will reveal the peptide fragments produced from digestion at each point. Fecal transcriptomic studies can also provide information on the amounts of digestive enzymes produced in the term and premature infant gut. Use of proteomics and transcriptomics to identify enzymes may lead to discovery of enzymes important for proteolytic digestion that have not been previously considered or detected. Bacterial sequencing of fecal samples is now possible to determine which protein-degrading species exist in gastrointestinal tracts of term and premature infants. These data will provide insight to the interaction between microbial metabolism and proteolytic breakdown. The development of complex computer models [135] and in vitro models of digestion [136] will further add to a more complete understanding of human milk protein digestion in term and premature infants.
Abbreviations
- Lf
Lactoferrin
- sIgA
Secretory immunoglobulin A
- IgG
Immunoglobulin G
References
- 1.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS data brief. 2010:1–8. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, et al. Births: final data for 2006. Public Health Resources. 2009;65 [Google Scholar]
- 3.Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at< 25 weeks' gestational age. Pediatrics. 2011;127:62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–529. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arslanoglu S, Moro GE, Ziegler EE The Wapm Working Group On Nutrition. Optimization of human milk fortification for preterm infants: new concepts and recommendations. J Perinat Med. 2010;38:233–238. doi: 10.1515/jpm.2010.073. [DOI] [PubMed] [Google Scholar]
- 6.Brooke OG, Wood C, Barley J. Energy balance, nitrogen balance, and growth in preterm infants fed expressed breast milk, a premature infant formula, and two low-solute adapted formulae. Arch Dis Child. 1982;57:898–904. doi: 10.1136/adc.57.12.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson LA, Lönnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand. 1987;76:733–740. doi: 10.1111/j.1651-2227.1987.tb10557.x. [DOI] [PubMed] [Google Scholar]
- 8.Davidson LA, Donovan SM, Lönnerdal B, Atkinson SA. Excretion of human milk proteins by term and premature infants. CRC Press; 1989. [Google Scholar]
- 9.Spik G, Brunet B, Mazurier-Dehaine C, Fontaine G, Montreuil J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Paediatr Scand. 1982;71:979–985. doi: 10.1111/j.1651-2227.1982.tb09560.x. [DOI] [PubMed] [Google Scholar]
- 10.Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res. 1986;20:711–715. doi: 10.1203/00006450-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Clare DA, Swaisgood HE. Bioactive milk peptides: a prospectus. J Dairy Sci. 2000;83:1187–1195. doi: 10.3168/jds.S0022-0302(00)74983-6. [DOI] [PubMed] [Google Scholar]
- 12.Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends in Food Science & Technology. 2000;11:254–262. [Google Scholar]
- 13.Cordle CT, Mahmoud MI, Moore V. Immunogenicity evaluation of protein hydrolysates for hypoallergenic infant formulae. J Pediatr Gastroenterol Nutr. 1991;13:270–276. doi: 10.1097/00005176-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2011;56:184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler E. Nutrient requirements of premature infants. KARGER; 2007. [DOI] [PubMed] [Google Scholar]
- 16.Klein CJ. Nutrient requirements for preterm infant formulas. J Nutr. 2002;132:1395S. doi: 10.1093/jn/132.6.1395S. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler EE. Protein requirements of preterm infants 1986 [Google Scholar]
- 18.Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, et al. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatr Res. 2006;59:265–270. doi: 10.1203/01.pdr.0000196376.99101.34. [DOI] [PubMed] [Google Scholar]
- 19.Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr. 2011;30:215–220. doi: 10.1016/j.clnu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Hay WW, Thureen P. Protein for preterm infants: How much is needed? How much is enough? How much is too much? Pediatr Neonatol. 2010;51:198–207. doi: 10.1016/S1875-9572(10)60039-3. [DOI] [PubMed] [Google Scholar]
- 21.Gross SJ, Geller J, Tomarelli RM. Composition of breast milk from mothers of preterm infants. Pediatrics. 1981;68:490–493. [PubMed] [Google Scholar]
- 22.Corvaglia L, Aceti A, Paoletti V, Mariani E, Patrono D, et al. Standard fortification of preterm human milk fails to meet recommended protein intake: bedside evaluation by Near-Infrared-Reflectance-Analysis. Early Hum Dev. 2010;86:237–240. doi: 10.1016/j.earlhumdev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Ferranti P, Traisci MV, Picariello G, Nasi A, Boschi V, et al. Casein proteolysis in human milk: tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J Dairy Res. 2004;71:74–87. doi: 10.1017/s0022029903006599. [DOI] [PubMed] [Google Scholar]
- 24.Armaforte E, Curran E, Huppertz T, Ryan CA, Caboni MF, et al. Proteins and proteolysis in pre-term and term human milk and possible implications for infant formulae. Int Dairy J. 2010;20:715–723. [Google Scholar]
- 26.Christensen B, Schack L, Kläning E, Sørensen ES. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J Biol Chem. 2010;285:7929–7937. doi: 10.1074/jbc.M109.075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borulf S, Lindberg T, Månsson M. Immunoreactive anionic trypsin and anionic elastase in human-milk. Acta Paediatr Scand. 1987;76:11–15. doi: 10.1111/j.1651-2227.1987.tb10406.x. [DOI] [PubMed] [Google Scholar]
- 28.Warner RC, Polis E. On the presence of a proteolytic enzyme in casein. J Am Chem Soc. 1945;67:529–532. [Google Scholar]
- 29.Okamoto U, Horie N, Nagamatsu Y, Yamamoto JI. Plasminogen-activator in human early milk: its partial purification and characterization. Thromb Haemost. 1981;45:121–126. [PubMed] [Google Scholar]
- 30.Korycha-Dahl M, Dumas BR, Chene N, Martal J. Plasmin activity in milk. J Dairy Sci. 1983;66:704–711. [Google Scholar]
- 31.Astrup T, Sterndorff I. A Fibrinolytic System in Human Milk. Exp Biol Med. 1953;84:605–608. doi: 10.3181/00379727-84-20727. [DOI] [PubMed] [Google Scholar]
- 32.Fox P. Proteinases in dairy technology. Neth. Milk Dairy J. 1981;35:233. [Google Scholar]
- 33.Palmer DJ, Kelly VC, Smit AM, Kuy S, Knight CG, et al. Human colostrum: identification of minor proteins in the aqueous phase by proteomics. Proteomics. 2006;6:2208–2216. doi: 10.1002/pmic.200500558. [DOI] [PubMed] [Google Scholar]
- 33.Vĕtvicka V, Vágner J, Baudys M, Tang J, Foundling SI, et al. Human breast milk contains procathepsin D--detection by specific antibodies. Biochem Mol Biol Int. 1993;30:921–928. [PubMed] [Google Scholar]
- 34.Heyndrickx GV. Further investigations on the enzymes in human milk. Pediatrics. 1963;31:1019–1023. [PubMed] [Google Scholar]
- 35.Lindberg T, Ohlsson K, Weström B. Protease inhibitors and their relation to protease activity in human-milk. Pediatr Res. 1982;16:479–483. doi: 10.1203/00006450-198206000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Lindberg T. Protease inhibitors in human-milk. Pediatric Research. 1979;13:969–972. doi: 10.1203/00006450-197909000-00003. [DOI] [PubMed] [Google Scholar]
- 37.McGilligan KM, Thomas DW, Eckhert CD. Alpha-1-antitrypsin concentration in human-milk. Pediatric Research. 1987;22:268–270. doi: 10.1203/00006450-198709000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Gettins PGW. Serpin structure, mechanism, and function. ChemInform. 2003;34 [Google Scholar]
- 39.Kalsheker N. Alpha 1-antitrypsin: structure, function and molecular biology of the gene. Biosci Rep. 1989;9:129–138. doi: 10.1007/BF01115992. [DOI] [PubMed] [Google Scholar]
- 40.Kalsheker NA. [alpha] 1-antichymotrypsin. Int J Biochem Cell Biol. 1996;28:961–964. doi: 10.1016/1357-2725(96)00032-5. [DOI] [PubMed] [Google Scholar]
- 41.Ghafouri B, Tagesson C, Lindahl M. Mapping of proteins in human saliva using two dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics. 2003;3:1003–1015. doi: 10.1002/pmic.200300426. [DOI] [PubMed] [Google Scholar]
- 42.Huang CM. Comparative proteomic analysis of human whole saliva. Arch Oral Biol. 2004;49:951–962. doi: 10.1016/j.archoralbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Castagnola M, Inzitari R, Rossetti DV, Olmi C, Cabras T, et al. A cascade of 24 histatins (histatin 3 fragments) in human saliva. J Biol Chem. 2004;279:41436–41443. doi: 10.1074/jbc.M404322200. [DOI] [PubMed] [Google Scholar]
- 44.Inzitari R, Vento G, Capoluongo E, Boccacci S, Fanali C, et al. Proteomic analysis of salivary acidic proline-rich proteins in human preterm and at-term newborns. J Proteome Res. 2007;6:1371–1377. doi: 10.1021/pr060520e. [DOI] [PubMed] [Google Scholar]
- 45.Lucchi G, Chambon C, Truntzer C, Pecqueur D, Ducoroy P, et al. Mass-spectrometry based characterisation of infant whole saliva peptidome. Int J Pept Res Ther. 2009;15:177–185. [Google Scholar]
- 46.Fitzsimmons SP, Evans MK, Pearce CL, Sheridan MJ, Wientzen R, et al. Immunoglobulin A subclasses in infants' saliva and in saliva and milk from their mothers. J Pediatr. 1994;124:566–573. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]
- 47.Piirainen L, Pesola J, Pesola I, Komulainen J, Vaarala O. Breastfeeding stimulates total and cowís milk specific salivary IgA in infants. Pediatr Allergy Immunol. 2009;20:295–298. doi: 10.1111/j.1399-3038.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 48.Chatterto DEW, Rasmussen JT, Heegaard CW, Sørensen ES, Petersen TE. In vitro digestion of novel milk protein ingredients for use in infant formulas: research on biological functions. Trends in Food Science & Technology. 2004;15:373–383. [Google Scholar]
- 49.Tanford C. Protein denaturation. Advances in protein chemistry. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- 50.Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 51.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35:215–220. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 52.Euler AR, Byrne WJ, Meis PJ, Leake RD, Ament ME. Basal and pentagastrin-stimulated acid secretion in newborn human infants. Pediatr Res. 1979;13:36–37. doi: 10.1203/00006450-197901000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35:215–220. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 54.Polacek MA, Ellison EH. Gastric acid secretion and parietal cell mass in the stomach of a newborn infant. Am J Surg. 1966;111:777–781. doi: 10.1016/0002-9610(66)90171-1. [DOI] [PubMed] [Google Scholar]
- 55.Hyman PE, Clarke DD, Everett SL, Sonne B, Stewart D, et al. Gastric acid secretory function in preterm infants. J Pediatr. 1985;106:467–471. doi: 10.1016/s0022-3476(85)80682-x. [DOI] [PubMed] [Google Scholar]
- 56.Britton JR, Koldovsky O. Development of luminal protein digestion: implications for biologically active dietary polypeptides. J Pediatr Gastroenterol Nutr. 1989;9:144–162. doi: 10.1097/00005176-198908000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Hibberd CM, Brooke OG, Carter ND, Haug M, Harzer G. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch Dis Child. 1982;57:658–662. doi: 10.1136/adc.57.9.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–437. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Mason S. Some aspects of gastric function in the newborn. Arch Dis Child. 1962;37:387–391. doi: 10.1136/adc.37.194.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keene M, Hewer E. Digestive enzymes of the human foetus. Lancet Office 1929 [Google Scholar]
- 61.Henderson TR, Hamosh M, Armand M, Mehta NR, Hamosh P. Gastric proteolysis in preterm infants fed mother's milk or formula. Adv Exp Med Biol. 2001;501:403–408. doi: 10.1007/978-1-4615-1371-1_50. [DOI] [PubMed] [Google Scholar]
- 62.Henschel MJ, Newport MJ, Parmar V. Gastric proteases in the human infant. Biol Neonate. 1987;52:268–272. doi: 10.1159/000242719. [DOI] [PubMed] [Google Scholar]
- 63.Defize J, Meuwissen SG. Pepsinogens: an update of biochemical, physiological, and clinical aspects. J Pediatr Gastroenterol Nutr. 1987;6:493–508. [PubMed] [Google Scholar]
- 64.Grand RJ, Watkins JB, Torti FM. Development of the human gastrointestinal tract. A review. Gastroenterology. 1976;70:790–810. [PubMed] [Google Scholar]
- 65.Berfenstam R, Jagenburg R, Mellander O. Protein hydrolysis in the stomachs of premature and full-term infants. Acta Paediatr. 1955;44:348–354. doi: 10.1111/j.1651-2227.1955.tb04149.x. [DOI] [PubMed] [Google Scholar]
- 66.Neu J. Gastrointestinal maturation and implications for infant feeding. Early Hum Dev. 2007;83:767–775. doi: 10.1016/j.earlhumdev.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Hadorn B. Development aspects of intraluminal protein digestion 1981 [Google Scholar]
- 68.Fomina L. The activities of some enzymes in the intestine and other organs of human foetus. Vopr Med Khim. 1960;6:176–183. [PubMed] [Google Scholar]
- 69.Antonowicz I, Lebenthal E. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology. 1977;72:1299–1303. [PubMed] [Google Scholar]
- 70.Ibrahim J. Trypsinogen und Enterokinase beim menschlichen neugeborenen und Embryo. Biochem Ztschr 1909 [Google Scholar]
- 71.Leiros HK, Brandsdal BO, Andersen OA, Os V, Leiros I, et al. Trypsin specificity as elucidated by LIE calculations, X ray structures, and association constant measurements. Protein Sci. 2004;13:1056–1070. doi: 10.1110/ps.03498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borgström B, Lindquist B, Lundh G. Enzyme concentration and absorption of protein and glucose in duodenum of premature infants. AMA J Dis Child. 1960;99:338–343. [PubMed] [Google Scholar]
- 73.Lebenthal E, Lee PC. Development of functional response in human exocrine pancreas. Pediatrics. 1980;66:556–560. [PubMed] [Google Scholar]
- 74.Appel W. Chymotrypsin: molecular and catalytic properties. Clinical Biochemistry. 1986;19:317–322. doi: 10.1016/s0009-9120(86)80002-9. [DOI] [PubMed] [Google Scholar]
- 75.Kolacek S, Puntis JW, Lloyd DR, Brown GA, Booth IW. Ontogeny of pancreatic exocrine function. Arch Dis Child. 1990;65:178–181. doi: 10.1136/adc.65.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vendrell J, Aviles FX, Fricker LD. Metallocarboxypeptidases 2004 [Google Scholar]
- 77.Kim YS, Birtwhistle W, Kim YW. Peptide hydrolases in the brush border and soluble fractions of small intestinal mucosa of rat and man. J Clin Invest. 1972;51:1419. doi: 10.1172/JCI106938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Auricchio S, Stellato A, De Vizia B. Development of brush border peptidases in human and rat small intestine during fetal and neonatal life. Pediatr Res. 1981;15:991–995. doi: 10.1203/00006450-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Lebenthal E, Lee PC, Heitlinger LA. Impact of development of the gastrointestinal tract on infant feeding. J Pediatr. 1983;102:1–9. doi: 10.1016/s0022-3476(83)80276-5. [DOI] [PubMed] [Google Scholar]
- 80.Cellini C, Xu J, Buchmiller TL. Effect of esophageal ligation on small intestinal development in normal and growth-retarded fetal rabbits. J pediatr Gastroenterol Nutr. 2006;43:291–298. doi: 10.1097/01.mpg.0000231588.24491.bb. [DOI] [PubMed] [Google Scholar]
- 81.Burjonrappa SC, Crete E, Bouchard S. The role of amniotic fluid in influencing neonatal birth weight. J Perinatol. 2009;30:27–29. doi: 10.1038/jp.2009.102. [DOI] [PubMed] [Google Scholar]
- 82.Gitler C. Protein digestion and absorption in nonruminants. Mammalian protein metabolism. 1964;1:35. [Google Scholar]
- 83.Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. Microbiology. 1986;132:1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- 84.Macfarlane GT, Allison C. Utilisation of protein by human gut bacteria. Fems Microbiol Lett. 1986;38:19–24. [Google Scholar]
- 85.Smith EA, Macfarlane GT. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microbial ecology. 1997;33:180–188. doi: 10.1007/s002489900020. [DOI] [PubMed] [Google Scholar]
- 86.Whiteley H. Fermentation of amino acids by Micrococcus aerogenes. Journal of Bacteriology. 1957;74:324. doi: 10.1128/jb.74.3.324-330.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cato EP, Johnson JL, Hash DE, Holdeman LV. Synonomy of Peptococcus glycinophilus (Cardon and Barker 1946) Douglas 1957 with Peptostreptococcus micros (Prevot 1933) Smith 1957 and electrophoretic differentiation of Peptostreptococcus micros from Peptococcus magnus (Prevot 1933) Holdeman and Moore 1972. Int J Syst Evol Microbiol. 1983;33:207–210. [Google Scholar]
- 88.Dürre P, Spahr R, Andreesen RJ. Glycine fermentation via a glycine reductase in Peptococcus glycinophilus and Peptococcus magnus. Arch Microbiol. 1983;134:127–135. [Google Scholar]
- 89.Nanninga HJ, Drent WJ, Gottschal JC. Major differences between glutamate-fermenting species isolated from chemostat enrichments at different dilution rates. FEMS Microbiol Lett. 1986;38:321–329. [Google Scholar]
- 90.Buckel W. Substrate stereochemistry of the biotin dependent sodium pump glutaconyl-CoA decarboxylase from Acidaminococcus fermentans. European Journal of Biochemistry. 1986;156:259–263. doi: 10.1111/j.1432-1033.1986.tb09576.x. [DOI] [PubMed] [Google Scholar]
- 91.Stams AMJ, Hansen TA. Fermentation of glutamate and other compounds by Acidaminobacter hydrogenoformans gen. nov. sp. nov., an obligate anaerobe isolated from black mud. Studies with pure cultures and mixed cultures with sulfate-reducing and methanogenic bacteria. Arch Microbiol. 1984;137:329–337. [Google Scholar]
- 92.Rogers AH, Gully NJ, Pfennig AL, Zilm PS. The breakdown and utilization of peptides by strains of Fusobacterium nucleatum. Mol Oral Microbiol. 1992;7:299–303. doi: 10.1111/j.1399-302x.1992.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 93.Zindel U, Freudenberg W, Rieth M, Andreesen RJ, Schnell J, et al. Eubacterium acidaminophilum sp. nov., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Arch Microbiol. 1988;150:254–266. [Google Scholar]
- 94.Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria*. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- 95.Liepke C, Adermann K, Raida M, Mägert HJ, Forssmann WG, et al. Human milk provides peptides highly stimulating the growth of bifidobacteria. European Journal of Biochemistry. 2002;269:712–718. doi: 10.1046/j.0014-2956.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 96.Brock JH, Arzabe F, Lampreave F, Pineiro A. The effect of trypsin on bovine transferrin and lactoferrin. Biochim et Biophys Acta (BBA)-Protein Structure. 1976;446:214–225. doi: 10.1016/0005-2795(76)90112-4. [DOI] [PubMed] [Google Scholar]
- 97.Brines RD, Brock JH. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum: unusual resistance of human apolactoferrin to proteolytic digestion. Biochim Biophys Acta. 1983;759:229–235. doi: 10.1016/0304-4165(83)90317-3. [DOI] [PubMed] [Google Scholar]
- 98.Lindh E. Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol. 1975;114:284–286. [PubMed] [Google Scholar]
- 99.Heremans J. Immunoglobulin A. The antigens. 1974;2:365–522. [Google Scholar]
- 100.Strbak V, Alexandrova M, Macho L, Ponec J. Transport of 3H-TRH from plasma to rat milk: accumulation and slow degradation in milk and presence of unaltered hormone in gastric content of pups. Biol Neonate. 1980;37:313–321. doi: 10.1159/000241293. [DOI] [PubMed] [Google Scholar]
- 101.Britton JR, George-Nascimento C, Udall JN, Koldovsk O. Minimal hydrolysis of epidermal growth factor by gastric fluid of preterm infants. BMJ. 1989;30:327–332. doi: 10.1136/gut.30.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cathie IAB. Breast-feeding in erythroblastosis foetalis. Br Med J. 1947;2:650. doi: 10.1136/bmj.2.4529.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 104.Hutchens TW, Henry JF, Yip TT, Hachey DL, Schanler RJ, et al. Origin of intact lactoferrin and its DNA-binding fragments found in the urine of human milk-fed preterm infants. Evaluation by stable isotopic enrichment. Pediatr Res. 1991;29:243–250. doi: 10.1203/00006450-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Haneberg B, Finne P. Lysozymes in feces from infants and children. Acta Paediatr Scand. 1974;63:588–594. doi: 10.1111/j.1651-2227.1974.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 106.Bond JS, Beynon RJ. Proteolysis and physiological regulation. Mol Aspects Med. 1987;9:173–287. doi: 10.1016/0098-2997(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 107.Beynon RJ, Bond JS. Catabolism of intracellular protein: molecular aspects. Am J Physiol-Cell Physiology. 1986;251:C141–C152. doi: 10.1152/ajpcell.1986.251.2.C141. [DOI] [PubMed] [Google Scholar]
- 108.Mortimore GE, Poso AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–564. doi: 10.1146/annurev.nu.07.070187.002543. [DOI] [PubMed] [Google Scholar]
- 109.Gardner ML. Intestinal assimilation of intact peptides and proteins from the diet-a neglected field? Biol Rev Camb Philos Soc. 1984;59:289–331. doi: 10.1111/j.1469-185x.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 110.Gardner MLG. Passage of intact peptides across the intestine. Adv Biosci. 1987;65:99–106. [Google Scholar]
- 111.Olden K, Bernard BA, Humphries MJ, Yeo TK, Yeo KT, et al. Function of glycoprotein glycans. Trends in Biochemical Sciences. 1985;10:78–82. [Google Scholar]
- 112.Yu YQ, Fournier J, Gilar M, Gebler JC. Identification of N-linked glycosylation sites using glycoprotein digestion with pronase prior to MALDI tandem time-of-flight mass spectrometry. Anal Chem. 2007;79:1731–1738. doi: 10.1021/ac0616052. [DOI] [PubMed] [Google Scholar]
- 113.Sareneva T, Pirhonen J, Cantell K, Julkunen I. N-glycosylation of human interferon-gamma: glycans at Asn-25 are critical for protease resistance. Biochem J. 1995;308:9–14. doi: 10.1042/bj3080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimizu M. Food-derived peptides and intestinal functions. Biofactors. 2004;21:43–47. doi: 10.1002/biof.552210109. [DOI] [PubMed] [Google Scholar]
- 115.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatric research. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 116.Jones EM, Smart A, Bloomberg G, Burgess L, Millar MR. Lactoferricin, a new antimicrobial peptide. J Applied Microbiol. 1994;77:208–214. doi: 10.1111/j.1365-2672.1994.tb03065.x. [DOI] [PubMed] [Google Scholar]
- 117.Jenssen H, Hancock REW. Antimicrobial properties of lactoferrin. Biochimie. 2009;91:19–29. doi: 10.1016/j.biochi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Chabance B, Jollès P, Izquierdo C, Mazoyer E, Francoual C, et al. Characterization of an antithrombotic peptide from kappa-casein in newborn plasma after milk ingestion. Br J Nutr. 1995;73:583–590. doi: 10.1079/bjn19950060. [DOI] [PubMed] [Google Scholar]
- 119.Hamosh M. Bioactive factors in human milk. Pediatric Clinics of North America. 2001;48:69–86. doi: 10.1016/s0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- 120.German JB, Dillard CJ, Ward RE. Bioactive components in milk. Curr Opin Clin Nutr Metab Care. 2002;5:653–658. doi: 10.1097/00075197-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 121.Sato R, Noguchi T, Naito H. Casein phosphopeptide (CPP) enhances calcium absorption from the ligated segment of rat small intestine. J Nutr Sci Vitaminol(Tokyo) 1986;32:67–76. doi: 10.3177/jnsv.32.67. [DOI] [PubMed] [Google Scholar]
- 122.Lin L, Umahara M, York DA, Bray GA. beta-casomorphins stimulate and enterostatin inhibits the intake of dietary fat in rats. Peptides. 1998;19:325–331. doi: 10.1016/s0196-9781(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 123.Elitsur Y, Luk GD. Beta-casomorphin (BCM) and human colonic lamina propria lymphocyte proliferation. Clin Exp Immunol. 1991;85:493–497. doi: 10.1111/j.1365-2249.1991.tb05755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lahov E, Regelson W. Antibacterial and immunostimulating casein-derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol. 1996;34:131–145. doi: 10.1016/0278-6915(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 125.Zucht HD, Raida M, Adermann K, Mägert HJ, Forssmann WG. Casocidin-I: a casein-alpha s2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995;372:185–188. doi: 10.1016/0014-5793(95)00974-e. [DOI] [PubMed] [Google Scholar]
- 126.Matthies H, Stark H, Hartrodt B, Ruethrich HL, Spieler HT, et al. Derivatives of beta-casomorphins with high analgesic potency. Peptides. 1984;5:463–470. doi: 10.1016/0196-9781(84)90070-6. [DOI] [PubMed] [Google Scholar]
- 127.Daniel H, Vohwinkel M, Rehner G. Effect of casein and -casomorphins on gastrointestinal motility in rats. J Nutr. 1990;120:252–257. doi: 10.1093/jn/120.3.252. [DOI] [PubMed] [Google Scholar]
- 128.Tome D, Dumontier AM, Hautefeuille M, Desjeux JF. Opiate activity and transepithelial passage of intact beta-casomorphins in rabbit ileum. Am J Physiol Gastrointest Liver Physiol. 1987;253:G737–744. doi: 10.1152/ajpgi.1987.253.6.G737. [DOI] [PubMed] [Google Scholar]
- 129.Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. Journal of Nutrition. 1990;120:252–257. doi: 10.1093/jn/120.3.252. [DOI] [PubMed] [Google Scholar]
- 130.Brandsch M, Brust P, Neubert K, Ermisch A. Casomorphins–chemical signals of intestinal transport systems -Casomorphins and Related Peptides: Recent Developments. 1994:207–219. [Google Scholar]
- 131.Nieter M, Schatz H. Insulotropic action of endorphins and -casomorphins, an opioid-like peptide fragment of milk casein. Acta Endocrinol. 1981;97:14. [Google Scholar]
- 132.Yen SS, Quigley ME, Reid RL, Ropert JF, Cetel NS. Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. Am J Obstet Gynecol. 1985;152:485–493. doi: 10.1016/s0002-9378(85)80162-9. [DOI] [PubMed] [Google Scholar]
- 133.Mountzouris KC, McCartney AL, Gibson GR. Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr. 2002;87:405–420. doi: 10.1079/BJNBJN2002563. [DOI] [PubMed] [Google Scholar]
- 134.Arciero JC, Ermentrout GB, Upperman JS, Vodovotz Y, Rubin JE. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PloS One. 2010;5:e10066. doi: 10.1371/journal.pone.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Binsl TW, De Graaf AA, Venema K, Heringa J, Maathuis A, et al. Measuring non-steady-state metabolic fluxes in starch-converting faecal microbiota in vitro. Benef Microbes. 2010;1:391–405. doi: 10.3920/BM2010.0038. [DOI] [PubMed] [Google Scholar]
- 136.Brodsky D, Ouellette MA. Primary care of the premature infant. Elseiver; Australia: 2008. [Google Scholar]


