SUMMARY
Stratification of retinal neuronal cell bodies and lamination of their processes provide a scaffold upon which neural circuits can be built. However, the molecular mechanisms that direct retinal ganglion cells (RGCs) to resolve into a single-cell retinal ganglion cell layer (GCL) are not well understood. The extracellular matrix protein laminin conveys spatial information that instructs the migration, process outgrowth, and reorganization of GCL cells. Here, we show that the β1-Integrin laminin receptor is required for RGC positioning and reorganization into a single-cell GCL layer. β1-Integrin signaling within migrating GCL cells requires Cas signaling-adaptor proteins, and in the absence of β1-Integrin or Cas function retinal neurons form ectopic cell clusters beyond the inner limiting membrane (ILM), phenocopying laminin mutants. These data reveal a novel and essential role for Cas adaptor proteins in β1-Integrin-mediated signaling events critical for the formation of the single-cell GCL in the mammalian retina.
INTRODUCTION
An early step in the development of laminar organization within the nervous system is the stratification of neuronal cell bodies, providing a foundation upon which lamination of neuronal processes and precisely ordered neural circuits can be assembled. The mature murine retina is organized into three distinct cell body layers: the multilayered outer and inner nuclear layers (ONL and INL, respectively), and the single cell-layered retinal GCL (Sanes and Zipursky, 2010). The GCL includes retinal ganglion cells (RGCs) and displaced amacrine cells (dACs) that temporarily reside in the inner neuroblastic layer (INbL) but then migrate early postnatally through the INbL to form a single-cell GCL (Reese, 2011; Zolessi et al., 2006). While progress has been made toward understanding how processes emanating from retinal neurons become stratified, the molecular mechanisms that direct RGC and dAC migration to form the single-cell GCL remain to be elucidated.
Secreted extracellular matrix laminin proteins guide GCL progenitors as they migrate to their final position in the retina (Randlett et al., 2011). Laminins are highly enriched in the basal lamina of the inner limiting membrane (ILM) and serve as cell non-autonomous instructive signals that orient RGC axon emergence and initial guidance during GCL cell migration (Libby et al., 2000; Libby et al., 1997; Randlett et al., 2011). Laminins are required for the establishment of the single-cell GCL; null mutations in the genes encoding β2 or γ3 laminin chains produce ectopic multilayered GCL cell aggregates that form beyond the ILM (Edwards et al., 2010; Pinzon-Duarte et al., 2010). Although accumulating evidence supports an instructive role for laminins in GCL establishment, how differential laminin distribution is sensed and interpreted by migrating neurons is not known.
Integrins are laminin receptors essential for neural migration and stratification in regions of the nervous system other than the retina, acting both neuron-autonomously and non-autonomously (Anton et al., 1999; Graus-Porta et al., 2001; Niewmierzycka et al., 2005; Schmid et al., 2004; Stanco et al., 2009). Integrins can activate a myriad of signaling pathways to instruct cell migration. One of these pathways involves phosphorylation of Crk-associated substrate (Cas) cytosolic adaptor proteins by activated focal adhesion kinase (FAK) and Src tyrosine phosphorylation; this results in the recruitment of Crk, activation of Rac and actin cytoskeleton remodeling (Bouton et al., 2001; Defilippi et al., 2006). Cas signaling adaptor proteins are thought to mediate integrin signal transduction during neural development (Bargon et al., 2005; Bourgin et al., 2007; Huang et al., 2006; Huang et al., 2007). In Drosophila, dCas is essential for integrin-mediated motor axon guidance and fasciculation (Huang et al., 2007). However, evidence supporting a role for Cas proteins downstream of integrins during vertebrate neural development comes from in vitro tissue culture studies (Bargon et al., 2005; Bourgin et al., 2007; Bouton et al., 2001).
Here, we show that β1-Integrin and Cas adaptor proteins are highly expressed in the inner neuroblastic layer (INbL) of the mouse retina, and that β1-Integrin is required for proper GCL organization. We also provide the first in vivo evidence that Cas signaling adaptor proteins are essential for β1-Integrin function during vertebrate neural development by investigating their role in the establishment of the GCL. These results provide insight into how migrating retinal neurons respond to the laminin-rich ILM, resulting in the characteristic single-cell layer organization of the GCL.
RESULTS
To explore the instructive role played by laminin in establishing the GCL, we first asked whether the laminin receptor β1-Integrin is expressed during retinal development. We performed β1-Integrin immunohistological analyses and found that during embryonic development expression of β1-Integrin is enriched in the INbL, with higher levels of expression close to the ILM (Figures 1A and 1B). This expression continues during the first week after birth (P4; Figure 1C), however at P14 β1-Integrin expression in the retina is down regulated and becomes restricted to vascular tissue (Figure 1D). This expression pattern suggests that β1-Integrin participates in GCL progenitor migration and organization.
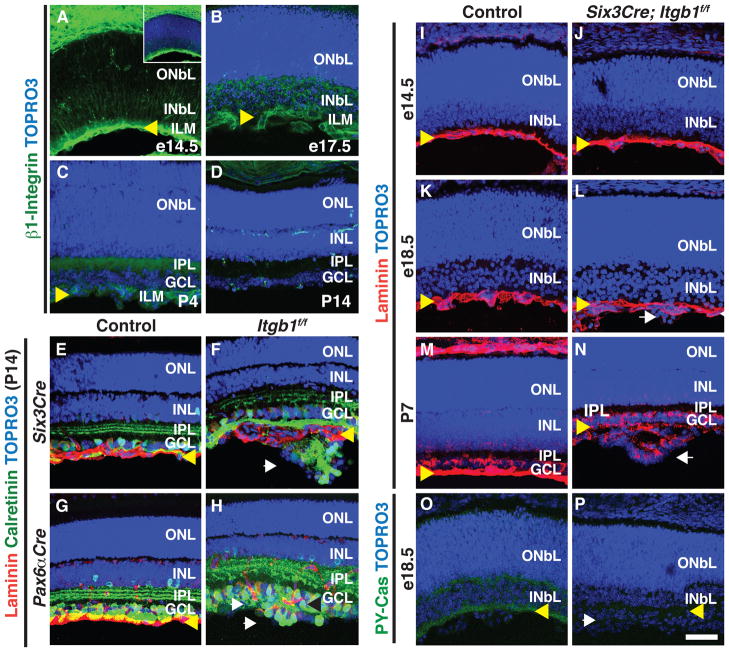
Figure 1. β1-Integrin is Required for GCL Formation and p130Cas Phosphorylation.
(A–D) Immunostaining of mouse retinas at e14.5 (A), e17.5 (B), P4 (C) and P14 (D) using an antibody against β1-Integrin (green), and TOPRO3 (blue). During embryonic and early postnatal development (A–C), β1-Integrin is enriched in the INbL but later becomes restricted to capillaries (D). Inset (A) shows co-staining with TOPRO3. (E–H) Conditional removal of β1-Integrin in the neural retina results in severe GCL defects. Control (E and G), Six3Cre; Itgb1f/f (F) and Pax6αCre; Itgb1f/f (H) adult retinas were sectioned and stained with antibodies against laminin (red) and calretinin (green). Ectopic cell aggregates form beyond the laminin+ ILM in Six3Cre; Itgb1f/f (F) and Pax6αCre; Itgb1f/f (H) retinas (n=5 independent animals for all genotypes). (I–N) Histological assessment of ILM integrity during retinal developmental in Control (I, K and M) and Six3Cre; Itgb1f/f (J, L and N) retinas stained with anti-laminin (red) and TOPRO3 (blue). At e18.5 ectopic aggregates have already formed in Six3Cre; Itgb1f/f mice but the ILM is still intact (L). (O–P) Histological assessment of p130Cas phosphorylation in Control (O) and Six3Cre; Itgb1f/f (P) retinas using a phospho-Tyrosine165-p130Cas antibody (PY-Cas, green) and TOPRO3 (blue); n=3. INbL: Inner Neuroblastic Layer; ONbL: Outer Neuroblastic Layer; INL: Inner Nuclear Layer; ONL: Outer Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer. Yellow arrowheads:ILM; white arrows: ectopic aggregates. Scale bar, 50 μm.
To investigate the requirement for β1-Integrin during retinal development, we used mouse lines that express pan-retinal Cre recombinase expression (Six3Cre) (Furuta et al., 2000) or neural retina-specific Cre (Pax6αCre) (Marquardt et al., 2001) together with a conditional β1-Integrin allele (Itgb1f) (Raghavan et al., 2000), to selectively remove β1-Integrin (Itgb1) in the retina. In postnatal day 14 (P14) control animals, GCL cells are organized into a single-cell layer adjacent to the ILM (Figures 1E and 1G). In Six3Cre;Itgb1f/f or Pax6αCre;Itgb1f/f retinas, the GCL fails to resolve into a single-cell layer (Figures 1F and 1H). In these mutant retinas ectopic aggregates that include GCL cells form multilayered “rosette” structures beyond the ILM, which is disrupted at the sites where these aggregates form (Figures 1F and 1H). These rosette structures include displaced ACs (anti-Chat+: starburst ACs; Figures S1A and S1B), and RGCs (anti-Brn3b+; Figures S1C and S1D). Ectopically localized ACs and RGCs in these mutant retinas recruit at least some of their presynaptic partners since rod bipolar neurites (PKCα+; Figures S1E and S1F) invade the rosettes. These results show that β1-Integrin is required for the formation of the single-cell GCL during retina development.
During cortical development, β1-Integrin regulates Cajal-Retzius cell organization and maintains basal lamina integrity (Graus-Porta et al., 2001). To determine whether β1-Integrin is required for formation of the ILM basal lamina, we analyzed ILM integrity at different developmental stages in Six3Cre;Itgb1f/f animals. At embryonic day 14.5 (e14.5) formation of the ILM appears normal, as revealed by anti-laminin immunohistochemistry in both control and Six3Cre;Itgb1f/f retinas (Figures 1I and 1J). Furthermore, the ILM is mostly intact in Six3Cre;Itgb1f/f retinas at e18.5, even at sites where aggregates have started to form (Figures 1K, 1L, S1G and S1H). Later in retinal development, ectopic aggregates continue to grow and the ILM in Six3Cre;Itgb1f/f mutant retinas becomes highly disorganized (P7, Figures 1M and 1N), suggesting that ILM disruption arises as a secondary consequence of RGC positioning defects.
Phosphorylation of Cas adaptor proteins is thought to mediate integrin signaling during neural development (Bourgin et al., 2007; Huang et al., 2007). Does β1-Integrin signaling during RGC and AC positioning proceed through Cas, and is Cas phosphorylation relevant for GCL elaboration in the vertebrate retina? To address these issues we first asked if Cas phosphorylation is affected by loss of β1-Integrin. In control animals at e18.5, phosphotyrosine-p130Cas (PY-Cas) is highly enriched in the INbL, particularly in close proximity to the ILM (Figure 1O). In contrast, PY-Cas is almost completely absent in Six3Cre;Itgb1f/f retinas (Figure 1P), although total p130Cas protein expression is not apparently affected (Figures S1G and S1H). The antibody we used to detect PY-Cas is specific for p130Cas since the protein band it detects in Western analysis of embryo lystates is dramatically reduced when exon 2 of p130Cas is removed in our p130Cas targeted mutant (Figure S2). These results show that Itgb1 is required for p130Cas phosphorylation during GCL formation.
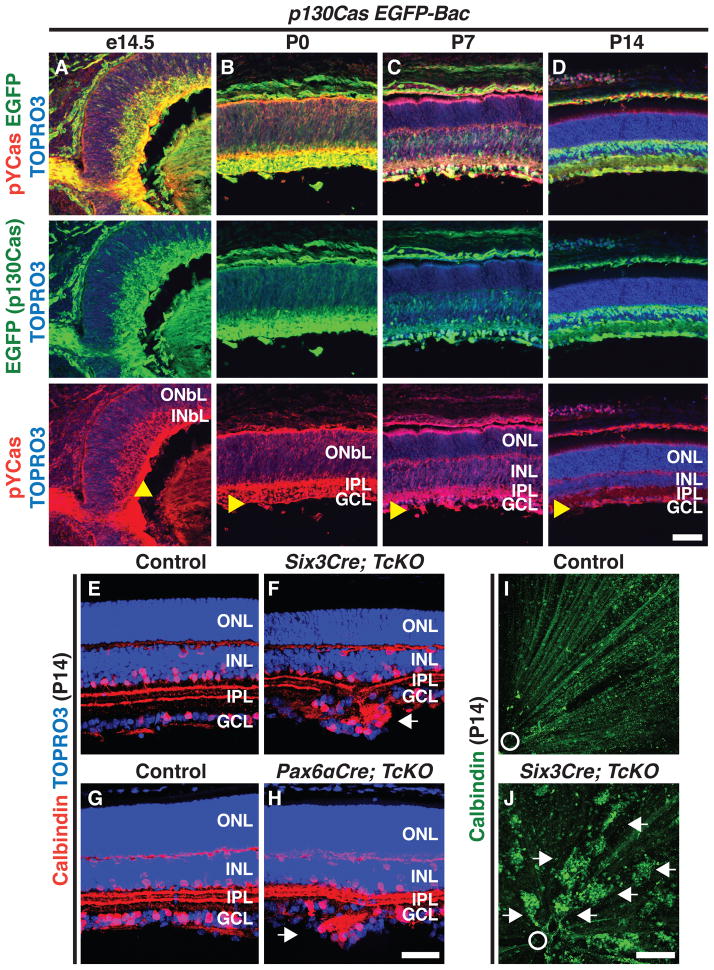
We next undertook detailed histological analyses of p130Cas expression in the developing retina, utilizing a GENSAT BAC transgenic mouse line that expresses EGFP under the control of p130Cas regulatory sequences (Gong et al., 2003; Heintz, 2004). This line allows detection of cells expressing p130Cas and phosphorylated p130Cas in the same section. The p130Cas-EGFP-Bac retinal EGFP expression pattern is consistent with that of endogenous p130Cas in WT animals (Figures 2A–2D and Figures S2A–S2D; data not shown). Although p130Cas is expressed broadly in the INbL during embryonic development, phosphorylated p130Cas is highly enriched in the cells that are in close proximity to the ILM (Figures 2A and Figure S2E). Soon after birth (P0 and P7), high levels of phosphorylated Cas are mainly present in close proximity to the ILM and also in the incipient IPL (Figures 2B and 2C). As development progresses, p130Cas phosphorylation becomes restricted to the GCL and a small subset of cells in the INL (P14 and adult, Figure 2D and Figure S2F). Therefore, p130Cas and phospho-tyrosine-p130Cas expression patterns are consistent with Cas involvement in GCL organization.
Figure 2. Cas Signaling Adaptor Proteins are Required for the GCL to Resolve into a Single Cell Layer.
(A–D) Expression of a p130Cas EGFP reporter (EGFP, left green) and phosophorylated-p130Cas (PY-Cas, red) in a p130Cas EGFP Bac transgenic mouse (p130Cas EGFP-Bac) throughout retina development. Retinas were counterstained with TOPRO3 (blue). From e14.5 to P0 (A and B) p130Cas phosphorylation is mainly found in the INbL and is enriched in close proximity to the ILM. (E–H) Cryo-sectioned Control (E, G), Six3Cre; TcKO (F) and Pax6αCre; TcKO (H) retinas immunostained with anti-calbindin (red) and TOPRO3 (blue). Retina-specific removal of Cas (F and H) results in the formation of ectopic cell aggregates in the GCL beyond the ILM (n=5 independent animals for each genotype). (I and J) Whole-mount calbindin immunostaining (green) confirms the presence of ectopic GCL cell aggregates throughout the retina in Six3Cre; TcKO (J) mice, as compared to Control (I). Arrowheads: ILM; white arrows: ectopic aggregates; white circle: optic nerve head. Scale bars, 50 μm for A–D and E–H, and 200 μm for I and J.
Since p130Cas is expressed during retinal development and Cas phosphorylation is regulated by β1-Integrin, we next asked whether p130Cas is required during RGC and AC migration. Since p130Cas−/− mice die early in embryogenesis (Honda et al., 1998), we generated a conditional p130Cas allele (p130Casf; Figure S2). p130Cas alone is not required for GCL development since Six3Cre; p130Casf/Δ and Pax6αCre; p130Casf/Δ retinas develop normally and are indistinguishable from WT (Figures S3A–S3C; data not shown). The expression patterns during retina development of the two other mouse Cas family members, CasL and Sin, highly overlap with p130Cas (Figures S3D–S3L), suggesting that CasL and Sin compensate for the lack of p130Cas in Six3Cre; p130Casf/Δ and Pax6αCre; p130Casf/Δ mice, and that Cas adaptor proteins might act redundantly. Therefore, we performed retina-specific p130Cas ablation in a CasL−/−;Sin−/− double null mutant genetic background (we refer to p130Casf/Δ;CasL−/−;Sin−/− mice as triple conditional knock-outs: “TcKO”) (Donlin et al., 2005; Seo et al., 2005). When examined at P14, both Six3Cre;TcKO and Pax6αCre;TcKO retinas exhibit, with full penetrance and expressivity, severe disorganization of the GCL and formation of ectopic rosette structures beyond the INbL, phenotypes not observed in control retinas (Figures 2E–2H; n=5 for each genotype; 3±1.3 aggregates/mm of sectioned retina). Similar phenotypes are also observed in Six3Cre; p130Casf/Δ;CasL−/−;Sin+/− animals, but not in any other combination of Cas family alleles (Figures S3M–O). Ectopic cell clusters are found throughout the Six3Cre;TcKO retina, and they can be visualized using retina whole mount staining with anti-calbindin, a marker for subsets of RGCs and ACs. This is in sharp contrast to the even distribution of unclustered calbindin+ cells in control animals (Figures 2I and 2J).
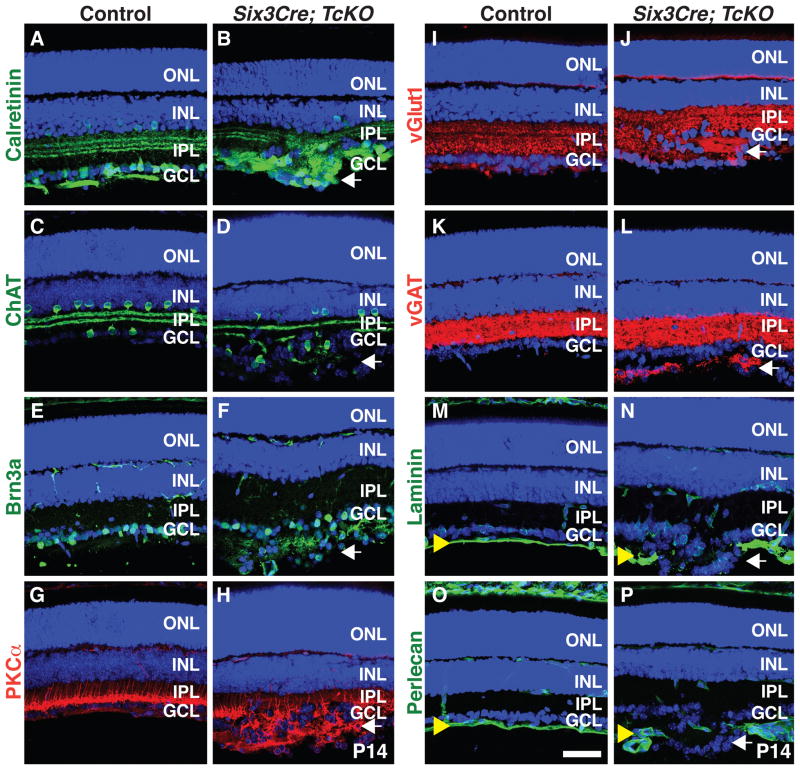
What are the identities of cells in these ectopic rosette structures? Using a battery of RGC and AC markers we found that many cells present in the ectopic aggregates express calretinin (subsets of ACs and RGCs; Figures 3A and 3B), ChAT (starburst ACs; Figures 3C and 3D), and Brn3a (RGCs; Figure 3E and 3F), showing that ectopic aggregates include both RGCs and ACs. Bipolar cells send projections into these aggregates since we observe neurites expressing the rod bipolar cell marker PKCα in the rosettes (Figures 3G and 3H). Further, small ectopic presynaptic terminals appear in and around the ectopic aggregates, indicated by the presence of neurites labeled with excitatory (vGlut1; Figures 3I and 3J) and inhibitory (vGAT; Figures 3K and 3L) presynaptic markers. No obvious phenotypes were observed in RGC axon projection orientation when Six3Cre;TcKO embryonic retinas were stained using neurofilament antibodies (data not shown). We next assessed the integrity of the ILM in Six3Cre;TcKO retinas using antibodies raised against laminin and the proteoglycan perlecan (Figures 3M–3P). Although the ILM is well organized in control animals at P14 (Figures 3M and 3O), it is completely disrupted in Six3Cre;TcKO animals at sites where aggregates form (Figures 3N and 3P). These phenotypes closely resemble Six3Cre;Itgb1f/f mutant retinas (Figure 1) and demonstrate a requirement for Cas adaptor proteins during RGC and AC positioning and formation of the single-cell GCL.
Figure 3. Ectopic GCL Aggregates in Cas TcKO Mutant Retinas are Formed by Multiple Cell-types.
(A–P) Control (A, C, E, G, I, K, M and O) and Six3Cre; TcKO (B, D, F, H, J, L, N and P) P14 retina sections were immunostained with antibodies against the AC and RGC marker calretinin (A and B), the starburst AC marker ChAT (C and D), the RGC marker Brn3a (E and F), the rod BP cell marker PKCα (G and H), the presynaptic terminal markers vGlut1 (I and J) and vGAT (K and L), and the ILM markers laminin (M and N) and perlecan (O and P). Six3Cre; TcKO ectopic aggregates contain both RGCs and displaced ACs (B, D and F). Note that the ILM is completely disrupted at sites where aggregates form in the Six3Cre; TcKO (N and P) retinas compared to control (M and O). All sections were counterstained with TOPRO3 (Blue). White arrows: ectopic aggregates; yellow arrowheads: ILM. Scale bar, 50 μm.
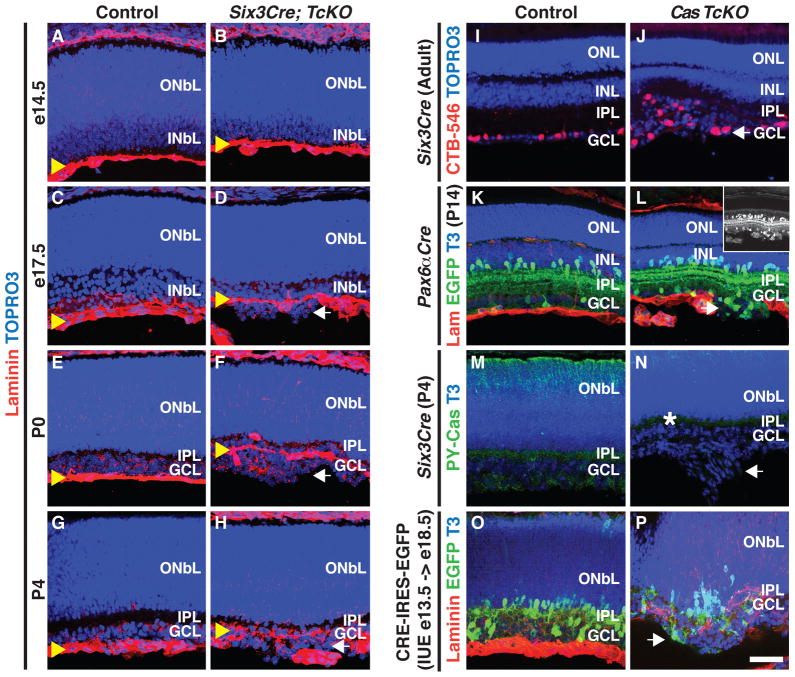
Though severe ILM defects are observed in Six3Cre;TcKO retinas, is ILM integrity compromised throughout retinal development in these mutant retinas? The ILM forms normally in Six3Cre;TcKO retinas and is indistinguishable in mutants and controls at e14.5 (Figures 4A and 4B). In e17.5 Six3Cre;TcKO retinas, ectopic cell aggregates form prior to the complete disruption of the ILM (Figures 4C and 4D), similar to Six3Cre;Itgb1f/f mutants (Figure 1L). Thus, retinal neuron positioning defects in these mutants arise in the presence of a nearly intact ILM. By P0, the ILM becomes more disorganized in Six3Cre;TcKO retinas relative to controls, and this occurs only at sites where aggregates have formed (Figures 4E and 4F, and Figures S4F and S4G); no obvert disruption of the ILM is observed where aggregates do not form (Figure S4G). The severity of ILM disruption in mutant retinas increases as development progresses and as ectopic aggregates increase in size (P4; Figures 4G and 4H). Aggregate formation and ILM disruption result in the secondary recruitment of vascular tissue into these rosettes (Figures S4H–S4K), and also mild to moderate defects in Müller Glia organization (Figures S4L and S4M). These data strongly suggest that ILM defects at sites where ectopic aggregates form are a secondary consequence of initial migration defects and ectopic rosette growth.
Figure 4. Cas Adaptor Proteins are Required Rosette-autonomously for GCL Cell Positioning and Organization.
(A–H) Developmental progression of ILM formation and maintenance in Control (A, C, E, G) and Six3Cre; TcKO (B, D, F, H) retinas, here immunolabeled with anti-laminin (Red), and TOPRO3 (Blue). (A and B) The ILM forms normally in Control and Six3Cre; TcKO retinas at e14.5. Ectopic cell aggregates begin to appear in Six3Cre; TcKO retinas before any disruption of the ILM is evident (e17.5, D; n=3 for each stage and genotype). (I and J) Retrograde labeling of WT (I) and Six3Cre; TcKO (J) RGCs by injection of CTB-Alexa546 into the LGN. Ganglion cells in ectopic rosettes that form in Six3Cre; TcKO retinas (J) are labeled by CTB. (K and L) Histological analysis of Control (K) and Pax6αCre;TcKO (L) P14 retinas stained with Topro3 (T3, Blue) and antibodies against EGFP (green) and laminin (Lam, red). Cells that form ectopic aggregates in Pax6αCre;TcKO (L, inset) animals are Cre/EGFP+.
(M and N) Phospho-Cas (PY-Cas, green) imunohistochemistry in P4 Control (M) and Six3Cre; TcKO (N) retinas. Though some PY-Cas staining remains in the Six3Cre; TcKO retinas (white asterisk, N), cells that form aggregates show no PY-Cas expression compared to Control (M). (O and P) Cryo-sections of WT (O) and Cas TcKO (P) e18.5 retinas electroporated in utero with pCAGGs-Cre-IRES-EGFP at e13.5. Sections were immunolabelled with anti-GFP (Green), TOPRO3 (T3, Blue) and anti-Laminin (Red). In utero electroporation of Cre-EGFP into Cas TcKO retinas results in formation of ectopic aggregates that contain EGFP+ cells (5/5 aggregates, n=3 independent animals); no aggregates form in WT retinas (n=3). White arrows: ectopic aggregates; yellow arrowheads: ILM. Scale bar, 50 μm.
A large number of RGCs are mislocalized in Six3Cre;TcKO retinas. Does the final RGC location affect central projection targeting by Six3Cre;TcKO RGC axons? We performed injections of cholera toxin subunit B (CTB) conjugated to fluorescent alexa dyes into the retinas of control and Six3Cre;TcKO animals and found no obvious differences in the central axonal projections of control or Six3Cre;TcKO animals (Figure S4A–4D′). This suggests that retino-recipient targeting of RGC axons is not affected even though many RGCs end up in an ectopic location. To ask whether RGCs in ectopic rosette structures project to retinorecipient areas, we performed retrograde labeling by injecting CTB-Alexa546 into the LGN in Six3Cre;TcKO and control mice (Figures 4I and J, and Figures S4E and S4E′). All ectopic aggregates we observed in Six3Cre;TcKO retinas included CTB+ cells (Figure 4J; n=3 animals, 6 retinas, and 5–10 aggregates per retina). Therefore, RGCs in Six3Cre;TcKO retina rosettes are indeed able to project to retinorecipient areas in the thalamus.
Although Six3Cre;TcKO and Pax6αCre;TcKO retinas exhibit similar phenotypes (Figures 2E–2H, data not shown), the Pax6αCre transgene has the added advantage of driving co-expression of Cre recombinase and EGFP (Marquardt et al., 2001). The Pax6αCre transgene is expressed broadly in neural progenitors for only a limited developmental period, following which expression becomes restricted to subpopulations of ACs and RGCs (Marquardt et al., 2001). We took advantage of these Pax6αCre transgene properties to assess whether cells that form ectopic rosettes are ACs and RGCs in which Cre recombinase expression was maintained during development. Most of the cells that compose ectopic aggregates in Pax6αCre;TcKO P4 retinas are EGFP+ (Figures 4K–4L), and all rosettes co-segregate with EGFP+ cells, suggesting that cells in which efficient recombination of the conditional allele occurs contribute to the rosettes. To test whether inefficient recombination of the conditional p130Casf allele might account for the formation of rosettes, we stained control and Six3Cre;TcKO retinas with the phosphotyrosine-Cas antibody (PY-Cas, Figures 4M and 4N). Though reduced, some PY-Cas staining remains in the Six3Cre;TcKO retinas compared to control retinas, suggesting that disruption of Cas expression does not occur in some retinal cells. Importantly, all of the cells that form ectopic aggregates show strong abrogation of PY-Cas expression (Figures 4M and 4N). Since Six3Cre and Pax6αCre transgenes do not drive expression of Cre recombinase in the neuroepithelial cells that form the ILM (Figures S4N and S4N′; Marquardt et al., 2001), our findings suggest that Integrin-Cas signaling plays a neuron-autonomous role in the INbL during the positioning of RGCs and ACs required for organizing the GCL.
To investigate whether Integrin-Cas signaling functions cell autonomously during GCL organization, we used in utero electroporation to introduce a plasmid expressing both Cre recombinase and EGFP under the strong CAGGs promoter (CRE-IRES-EGFP) into WT or TcKO retinas at e13.5 (Figures 4O and 4P). At this stage of retina development GCL progenitors are already born and migrating through the INbL (Garcia-Frigola et al., 2007; Petros et al., 2009). In WT retinas RGC and AC migration proceeds normally, and no electroporated (EGFP+) cells are detected beyond the laminin-rich ILM (Figure 4O; n=3 animals). However, Cre/EGFP-electroporated TcKO retinas exhibit ectopic cell aggregates beyond the ILM (Figure 4P). Some of the cells in every aggregate we observe are EGFP+ (n=5 aggregates from 3 independent animals). These observations show a rosette-autonomous requirement for Cas proteins during GCL organization. Taken together with the phenotypes we observe in the neural retina-specific conditional knock-outs (Pax6αCre; TcKO and Pax6αCre; Itgb1f/f; Figures 1H, 2H, 4L, data not shown), our results strongly suggest a role for Integrin-Cas signaling in regulating GCL progenitor positioning and organization into a single cell layer. Importantly, although all ectopic rosettes contain Cre-EGFP+ cells, we find that the average rosette is composed of 51±21% GFP+ cells (mean±SD), and some cells in every rosette in mutant retinas are EGFP− (Figure 4P). This is consistent with secondary, cell non-autonomous, defects caused by the initial disruption of the ILM by aberrant migration of EGFP+/Cas− cells.
DISCUSSION
The stratification of cell bodies within developing laminar structures is a progressive event that occurs throughout the nervous system and is critical for the assembly of neural circuits (Franco and Müller, 2011). The process by which the single-cell retina GCL is generated provides a relatively simple example of how laminar organization within the nervous system arises. We describe here a molecular mechanism by which the retina GCL is resolved into a single-cell layer through signaling interactions with ECM components. We identify a signaling pathway that acts in a neuron-autonomous fashion to sense and interpret the differential distribution of laminin during GCL progenitor positioning. We find that Cas signaling-adaptor proteins are critical for resolving the GCL into a single cell layer in vivo, functioning redundantly downstream of the laminin receptor β1-integrin in a neuron-autonomous manner.
During the process of cortical lamination, integrin signaling acts neuron-nonautonomously, functioning in glia and Cajal-Retzius cells to preserve basal lamina and pial surface integrity (Graus-Porta et al., 2001). Similarly, Abl and Arg tyrosine kinases are required in radial glia for basement membrane integrity and cortical lamination in the cerebellum (Qiu et al., 2010). In contrast, our data suggest that integrin-Cas signaling acts primarily in a neuron-autonomous manner to resolve the GCL into a single cell layer. The initial assembly of the ILM basal lamina in retina-specific Cas TcKO and β1-Integrin mutants apparently proceeds normally. The ILM is completely intact in these conditional mutants during early embryogenesis; ectopic rosette structures begin forming beyond the ILM well before we detect gross disruption of the basal lamina. This is consistent with the observation that the Six3Cre and Pax6αCre transgenes do not drive expression of Cre recombinase in the neuroepithelial cells that form the ILM (Figures S4N and S4N′; Marquardt et al., 2001). Further, when β1-Integrin or Cas are removed early in retinal development through the protracted expression of Cre/EGFP, mutant cells constitute the majority of cells observed in ectopic rosettes. As cell migration past the ILM in Six3Cre;Itgb1f/f and Six3Cre;Cas TcKO mutant retinas progresses, the ILM breaks down and ectopias become larger, likely a secondary effect of ILM disruption. These data suggest an initial cell-autonomous role for integrin-Cas signaling in migrating retinal neurons during the establishment and organization of the GCL. Interestingly, although there is a severe disruption of retina morphology, with multiple GCL cell bodies located in ectopic positions in Six3Cre;Itgb1f/f and Six3Cre;Cas TcKO mutant retinas, RGC central projections reach their retinorecipient targets. RGCs that form rosettes in Six3Cre;Cas TcKO mutants can project to the LGN, a retinorecipient area in the thalamus, and this is consistent with the developmental timing of RGC axon targeting during late embryonic development (Badea et al., 2009; Marcus and Mason, 1995; Simon and O’Leary, 1992).
At postnatal stages we observe a modest, but significant, defect in Müller Glia (MG) endfeet organization. Since MG differentiate postanatally (Jadhav, et al. 2009), the initial disruption in GCL organization we observe in Six3Cre;Cas TcKO mutant retinas arises independently of neuron-MG interactions. These defects in MG cell end-feet organization could be due to a primary deficiency in MG attachment or, alternatively, they could arise as a secondary consequence of ILM disruption by mislocalized neurons. Later in retina postnatal development, disorganization of MG end-feet likely contributes to the severity of the phenotype, since MG cells are known to maintain the integrity of the ILM postnatally (Jadhav, et al. 2009).
Migrating cells that settle in precise locations must interpret a variety of cues to find their final position, and so it is interesting to consider how these cells detect the end of their migration trajectories. In the cortex, apart from non-autonomous β1-Integrin functions that are important for preserving the basal lamina, β1-Integrin signaling is essential in migrating neurons for cell-cell recognition of radial glia and for reelin-induced repulsion at the pial surface (Anton et al., 1999; Dulabon et al., 2000; Schmid et al., 2004). Reelin in a more superficial cortical location provides a repulsive signal that promotes the switch from gliophilic to neurophilic cell-cell adhesion (Dulabon et al., 2000). In contrast, retinal neurons initiate migration without the aid of radial glia, and the laminin-rich ILM provides an instructive cue that initially regulates the orientation of migrating cells but ultimately defines the position where the single-cell GCL will form (Edwards et al., 2010; Pinzon-Duarte et al., 2010; Randlett et al., 2011; Sanes and Zipursky, 2010; Zolessi et al., 2006). Integrin-Cas signaling acts in migrating neurons to sense the ILM and is necessary for the reorganization of the GCL. This signaling pathway allows migrating cells to interpret the ILM as a “stop, adhere and spread” signal, thereby promoting the alignment of the GCL into a single-cell layer. In the absence of integrin-Cas signaling, cells fail to detect the ILM, poke through and migrate past it, ultimately disrupting the basal lamina and initiating subsequent formation of ectopias. Since stratification and laminar organization of neuronal processes is observed throughout the nervous system, these molecular mechanisms may be relevant for the assembly of a wide range of neural circuits.
We show here that Cas phosphorylation in the developing retina is dependent upon β1-Integrin function, providing a direct in vivo link between integrin signaling and Cas phosphorylation during GCL formation. Furthermore, Six3Cre;Cas TcKO mutant retinas phenocopy Six3Cre;Itgb1f/f (this study) and β2/ γ3 laminin−/− mutants (Pinzon-Duarte et al., 2010). These results provide the first in vivo evidence that Cas proteins act redundantly in mammals to transduce integrin signaling during neuronal development. Since Cas phosphorylation and function can be modulated by integrin-independent signaling pathways in vitro (Bourgin et al., 2007; Defilippi et al., 2006; Liu et al., 2007), it will be important to determine how these key signaling adaptor proteins integrate multiple signaling pathways during neuronal circuit assembly and refinement.
EXPERIMENTAL PROCEDURES
Animals
The day of vaginal plug observation was designated as embryonic day 0.5 (e0.5) and the day of birth postnatal day 0 (P0). Generation of the β1-integrinf/f (Itgb1f/f), Six3Cre, Pax6αCre, CasL−/− and Sin−/− mouse lines has been described previously (Donlin et al., 2005; Furuta et al., 2000; Marquardt et al., 2001; Raghavan et al., 2000; Seo et al., 2005). The mouse line carrying a targeted allele for the Bcar1 (p130Cas) locus was generated using the vector PL253, modified by Bac recombineering (Liu et al., 2003; Figure S2).
Immunohistochemistry and In situ hybridization
Eyes were fixed and processed essentially as previously described (Matsuoka et al., 2011).
Cholera toxin subunit B (CTB) injection
Bilateral CTB injection was performed as previously described (Matsuoka et al., 2011). Briefly, the animals were anesthetized using isoflurane, and then injected with 2 μL CTB-Alexa-555 or -488 (Life Technologies, 1mg/mL), bilaterally into the vitreous of each eye.
In utero retina electroporation
In utero retina electroporation was performed as previously described with a few modifications (Garcia-Frigola et al., 2007; Petros et al., 2009). Briefly, e13.5 Control or p130CasF/F; CasL−/−; Sin−/− retinas were injected intravitreally with pCAGGS-Cre-IRES-EGFP plasmid (4 μg/μL) and electroporated with five 40 volts, 50 ms square pulses at a frequency of 60 Hz.
Supplementary Material
HIGHLIGHTS.
Cas proteins and β1-Integrin are expressed in the INbL during retina development
β1-Integrin is required for RGC organization into a single cell layer
Phosphorylation of Cas adaptor proteins in the INbL is β1-Integrin dependent
Cas adaptor proteins act redundantly and cell-autonomously during GCL organization
Acknowledgments
We thank Dr. Jeremy Nathans for Pax6αCre and SixCre mice, and for the Brn3b antibody. We thank Drs. Seth Blackshaw and Rebecca James for helpful comments on the manuscript. We also thank Joshua G. J. Macopson and members of Kolodkin laboratory for assistance and discussions. This work was supported by a postdoctoral fellowship from the National Ataxia Foundation (M.M.R) and NIH RO1 NS35165 (A.L.K.). A.L.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Additional information about methods used in this study is found in Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargon SD, Gunning PW, O’Neill GM. The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochim Biophys Acta. 2005;1746:143–154. doi: 10.1016/j.bbamcr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Donlin LT, Danzl NM, Wanjalla C, Alexandropoulos K. Deficiency in expression of the signaling protein Sin/Efs leads to T-lymphocyte activation and mucosal inflammation. Mol Cell Biol. 2005;25:11035–11046. doi: 10.1128/MCB.25.24.11035-11046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Edwards MM, Mammadova-Bach E, Alpy F, Klein A, Hicks WL, Roux M, Simon-Assmann P, Smith RS, Orend G, Wu J, et al. Mutations in Lama1 disrupt retinal vascular development and inner limiting membrane formation. J Biol Chem. 2010;285:7697–7711. doi: 10.1074/jbc.M109.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Müller U. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Dev Neurobiol. 2011;71:889–900. doi: 10.1002/dneu.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- Garcia-Frigola C, Carreres MI, Vegar C, Herrera E. Gene delivery into mouse retinal ganglion cells by in utero electroporation. BMC Dev Biol. 2007;7:103. doi: 10.1186/1471-213X-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Müller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Huang J, Sakai R, Furuichi T. The docking protein Cas links tyrosine phosphorylation signaling to elongation of cerebellar granule cell axons. Mol Biol Cell. 2006;17:3187–3196. doi: 10.1091/mbc.E05-12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Xu Y, Selfors LM, Brunken WJ, Hunter DD. Identification of the cellular source of laminin beta2 in adult and developing vertebrate retinae. J Comp Neurol. 1997;389:655–667. doi: 10.1002/(sici)1096-9861(19971229)389:4<655::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, et al. p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RC, Mason CA. The first retinal axon growth in the mouse optic chiasm: axon patterning and the cellular environment. J Neurosci. 1995;15:6389–6402. doi: 10.1523/JNEUROSCI.15-10-06389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, et al. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci. 2005;25:7022–7031. doi: 10.1523/JNEUROSCI.1695-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA. In utero and ex vivo electroporation for gene expression in mouse retinal ganglion cells. J Vis Exp. 2009 doi: 10.3791/1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Duarte G, Daly G, Li YN, Koch M, Brunken WJ. Defective formation of the inner limiting membrane in laminin beta2- and gamma3-null mice produces retinal dysplasia. Invest Ophthalmol Vis Sci. 2010;51:1773–1782. doi: 10.1167/iovs.09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Cang Y, Goff SP. Abl family tyrosine kinases are essential for basement membrane integrity and cortical lamination in the cerebellum. J Neurosci. 2010;30:14430–14439. doi: 10.1523/JNEUROSCI.2861-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70:266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. Development of the retina and optic pathway. Vision Res. 2011;51:613–632. doi: 10.1016/j.visres.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Seo S, Asai T, Saito T, Suzuki T, Morishita Y, Nakamoto T, Ichikawa M, Yamamoto G, Kawazu M, Yamagata T, et al. Crk-associated substrate lymphocyte type is required for lymphocyte trafficking and marginal zone B cell maintenance. J Immunol. 2005;175:3492–3501. doi: 10.4049/jimmunol.175.6.3492. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DD. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.