Abstract
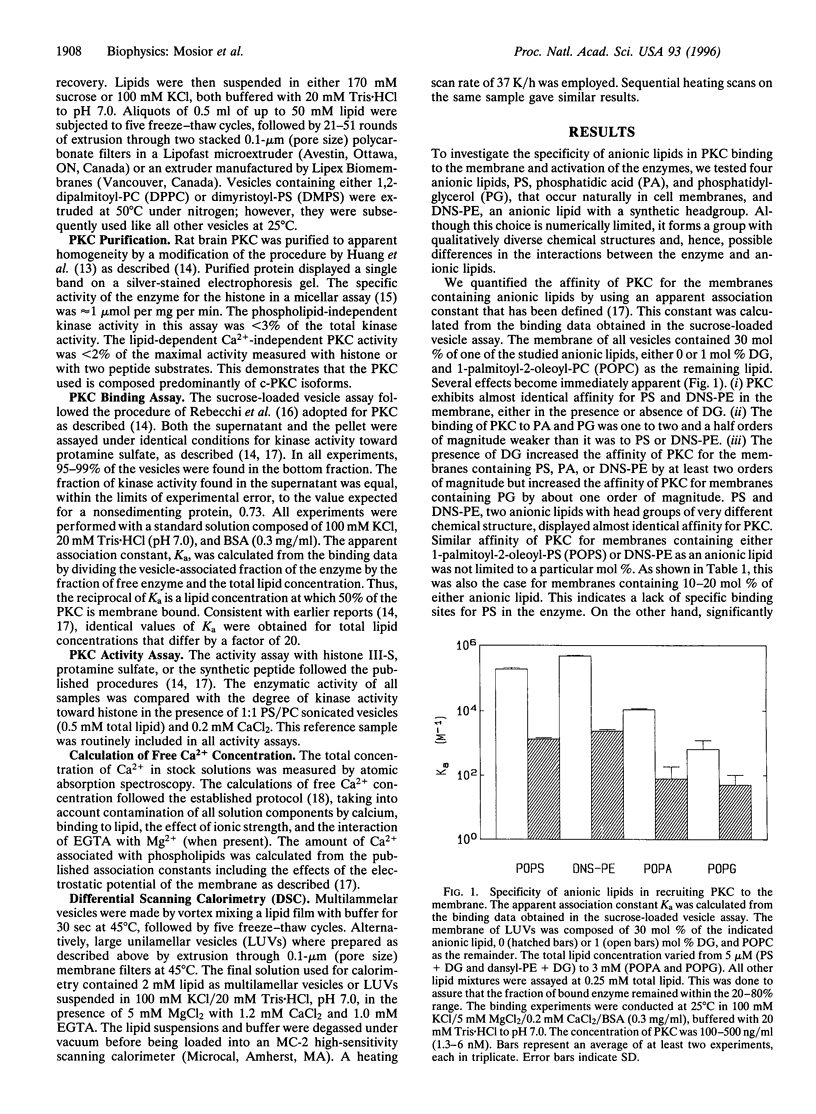
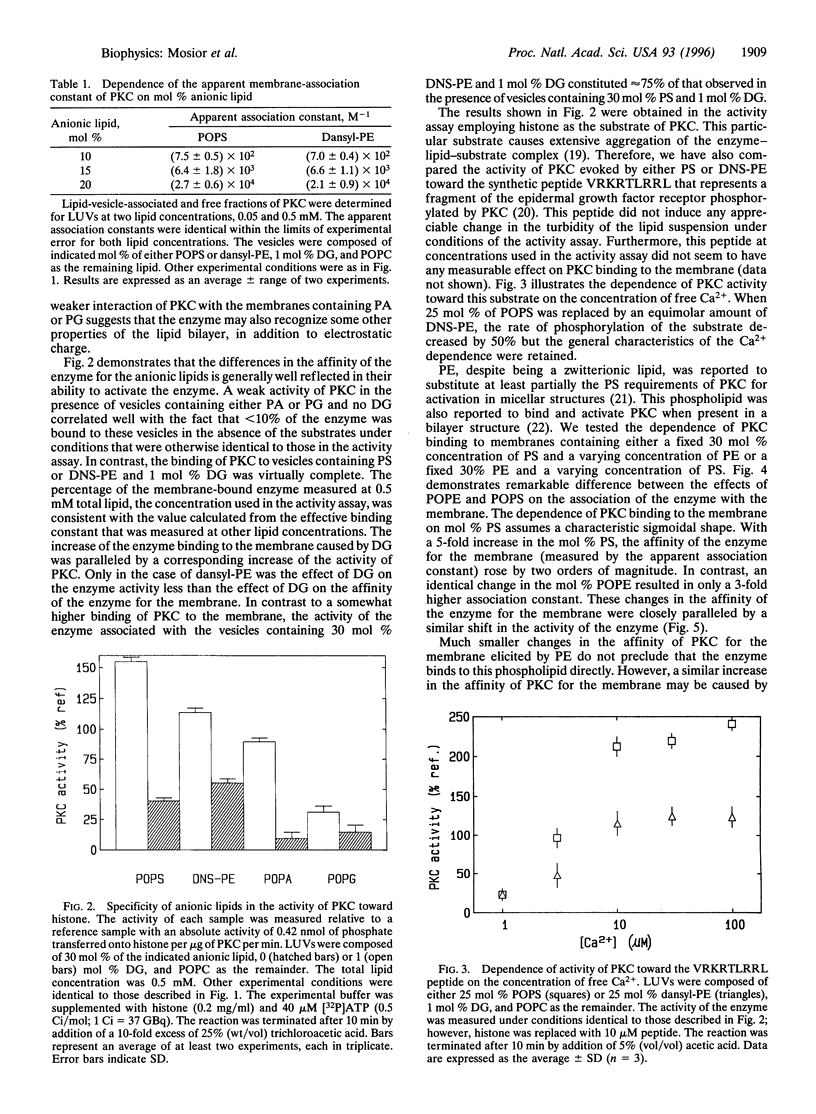
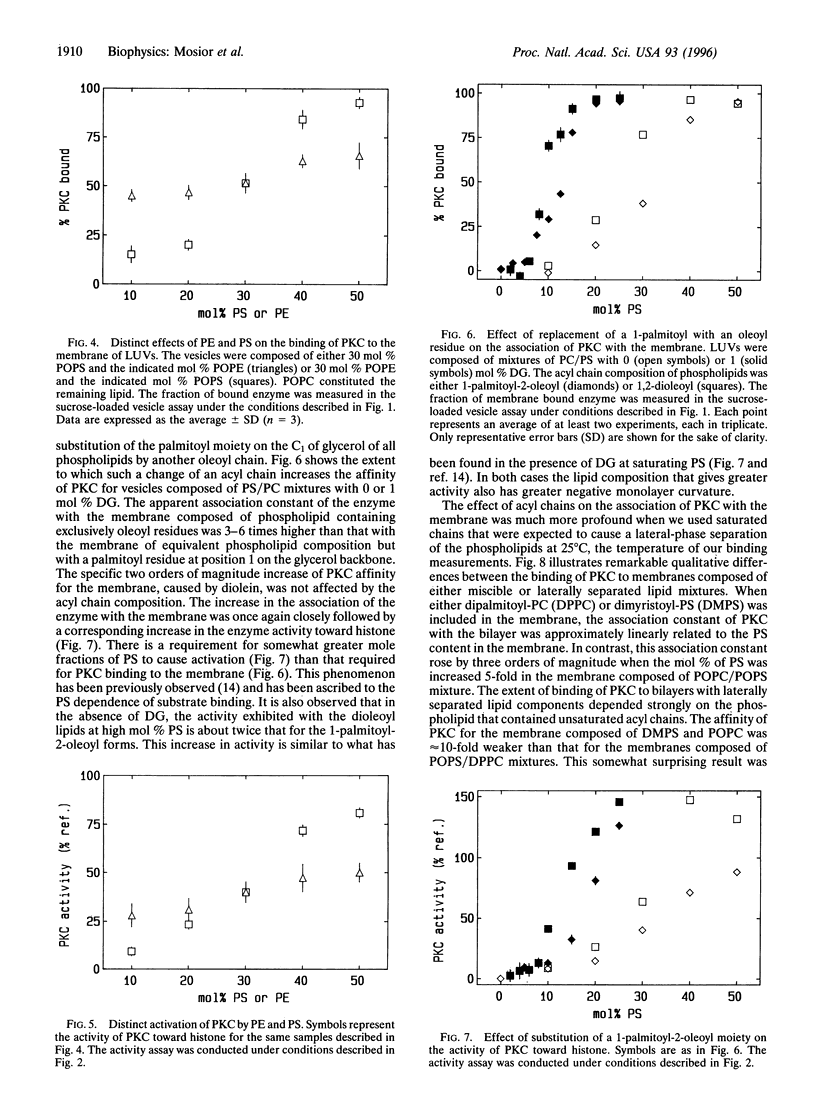
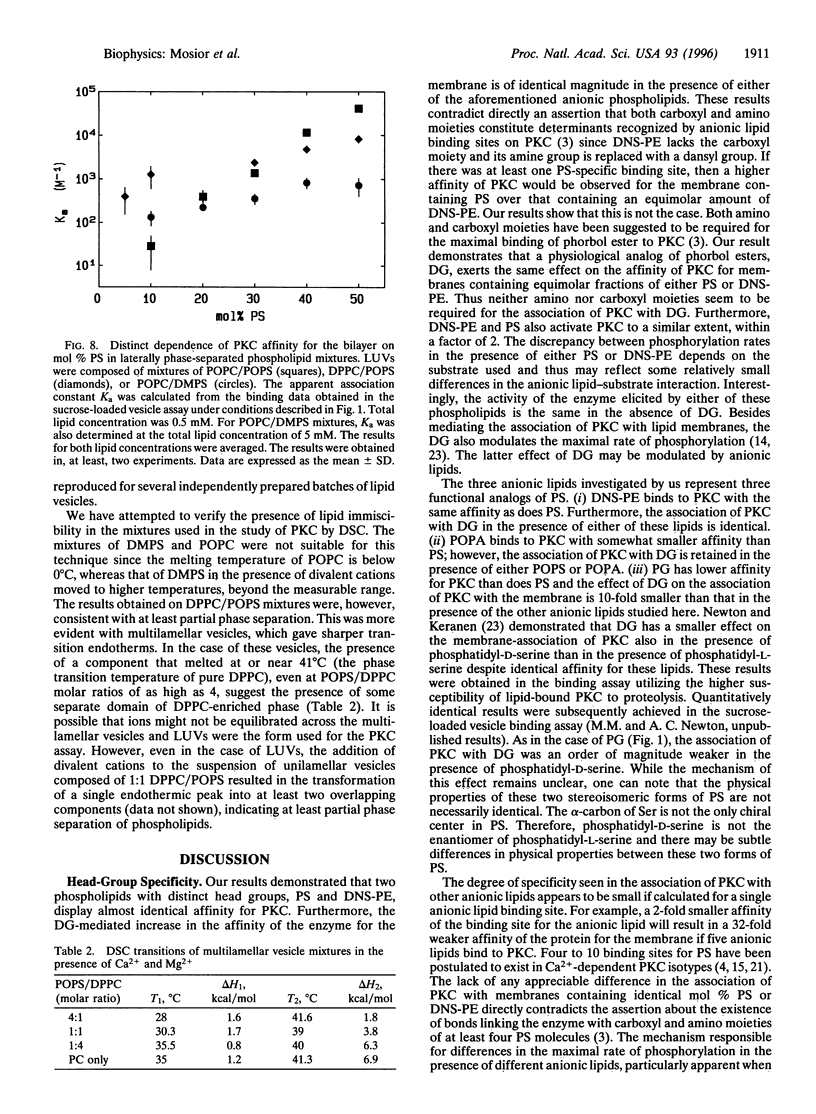
The association of protein kinase C (PKC) with membranes was found not to be specific for phosphatidyl-L-serine (PS). In particular, a synthetic phospholipid, dansyl-phosphatidylethanolamine, proved to be fully functional in the association of PKC with lipid bilayers and in mediating the interaction of this enzyme with diacylglycerol. Dansyl-phosphatidylethanolamine was also able to activate the enzyme in a Ca2+-dependent fashion. Differences in the ability to bind and activate PKC observed for an array of anionic lipids were not larger than alterations caused by changes in acyl chain composition. Thus, although different lipids interact to different extents with PKC, there are no specific binding sites for the PS headgroup on the enzyme. We found that lipids with a greater tendency to form inverted phases increased the binding of PKC to bilayers. However, these changes in lipid structure cannot be considered separately from the miscibility of lipid components in the membrane. For pairs of lipids with similar acyl chains, the dependence on PS concentration is sigmoidal, while for dissimilar acyl chains there is much less dependence of binding on PS concentration. The results can be explained in terms of differences in the lateral distribution of components in the membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Role of substrate in imparting calcium and phospholipid requirements to protein kinase C activation. Biochemistry. 1987 Apr 7;26(7):1974–1982. doi: 10.1021/bi00381a029. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Youakim M. A., Nelsestuen G. L. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry. 1992 Feb 4;31(4):1125–1134. doi: 10.1021/bi00119a022. [DOI] [PubMed] [Google Scholar]
- Bolen E. J., Sando J. J. Effect of phospholipid unsaturation on protein kinase C activation. Biochemistry. 1992 Jun 30;31(25):5945–5951. doi: 10.1021/bi00140a034. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Bloomenthal J., Lee M. H., Bell R. M. Expression of the alpha, beta II, and gamma protein kinase C isozymes in the baculovirus-insect cell expression system. Purification and characterization of the individual isoforms. J Biol Chem. 1990 Jul 15;265(20):12044–12051. [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., Leon B. T., Menger F. M., Kuo J. F. Evidence for the regulation of the activity of protein kinase C through changes in membrane properties. Biosci Rep. 1991 Feb;11(1):59–64. doi: 10.1007/BF01118606. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Detection of nerve growth factor and epidermal growth factor-regulated protein kinases in PC12 cells with synthetic peptide substrates. Mol Pharmacol. 1989 Mar;35(3):331–338. [PubMed] [Google Scholar]
- Huang J., Feigenson G. W. Monte Carlo simulation of lipid mixtures: finding phase separation. Biophys J. 1993 Nov;65(5):1788–1794. doi: 10.1016/S0006-3495(93)81234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Phospholipid functional groups involved in protein kinase C activation, phorbol ester binding, and binding to mixed micelles. J Biol Chem. 1989 Sep 5;264(25):14797–14805. [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Supplementation of the phosphatidyl-L-serine requirement of protein kinase C with nonactivating phospholipids. Biochemistry. 1992 Jun 9;31(22):5176–5182. doi: 10.1021/bi00137a013. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Mosior M., Epand R. M. Characterization of the calcium-binding site that regulates association of protein kinase C with phospholipid bilayers. J Biol Chem. 1994 May 13;269(19):13798–13805. [PubMed] [Google Scholar]
- Mosior M., Epand R. M. Mechanism of activation of protein kinase C: roles of diolein and phosphatidylserine. Biochemistry. 1993 Jan 12;32(1):66–75. doi: 10.1021/bi00052a010. [DOI] [PubMed] [Google Scholar]
- Newton A. C., Keranen L. M. Phosphatidyl-L-serine is necessary for protein kinase C's high-affinity interaction with diacylglycerol-containing membranes. Biochemistry. 1994 May 31;33(21):6651–6658. doi: 10.1021/bi00187a035. [DOI] [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr High cooperativity, specificity, and multiplicity in the protein kinase C-lipid interaction. J Biol Chem. 1989 Sep 5;264(25):14909–14915. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Orr J. W., Keranen L. M., Newton A. C. Reversible exposure of the pseudosubstrate domain of protein kinase C by phosphatidylserine and diacylglycerol. J Biol Chem. 1992 Aug 5;267(22):15263–15266. [PubMed] [Google Scholar]
- Orr J. W., Newton A. C. Interaction of protein kinase C with phosphatidylserine. 1. Cooperativity in lipid binding. Biochemistry. 1992 May 19;31(19):4661–4667. doi: 10.1021/bi00134a018. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Kour G., Marais R. M., Mitchell F., Pears C., Schaap D., Stabel S., Webster C. Protein kinase C--a family affair. Mol Cell Endocrinol. 1989 Aug;65(1-2):1–11. doi: 10.1016/0303-7207(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Rebecchi M., Peterson A., McLaughlin S. Phosphoinositide-specific phospholipase C-delta 1 binds with high affinity to phospholipid vesicles containing phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1992 Dec 29;31(51):12742–12747. doi: 10.1021/bi00166a005. [DOI] [PubMed] [Google Scholar]
- Senisterra G., Epand R. M. Role of membrane defects in the regulation of the activity of protein kinase C. Arch Biochem Biophys. 1993 Jan;300(1):378–383. doi: 10.1006/abbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- Slater S. J., Kelly M. B., Taddeo F. J., Ho C., Rubin E., Stubbs C. D. The modulation of protein kinase C activity by membrane lipid bilayer structure. J Biol Chem. 1994 Feb 18;269(7):4866–4871. [PubMed] [Google Scholar]
- Toker A., Meyer M., Reddy K. K., Falck J. R., Aneja R., Aneja S., Parra A., Burns D. J., Ballas L. M., Cantley L. C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994 Dec 23;269(51):32358–32367. [PubMed] [Google Scholar]


