Abstract
Patterns of biomass and carbon (C) storage distribution across Chinese pine (Pinus tabulaeformis) natural secondary forests are poorly documented. The objectives of this study were to examine the biomass and C pools of the major ecosystem components in a replicated age sequence of P. tabulaeformis secondary forest stands in Northern China. Within each stand, biomass of above- and belowground tree, understory (shrub and herb), and forest floor were determined from plot-level investigation and destructive sampling. Allometric equations using the diameter at breast height (DBH) were developed to quantify plant biomass. C stocks in the tree and understory biomass, forest floor, and mineral soil (0–100 cm) were estimated by analyzing the C concentration of each component. The results showed that the tree biomass of P. tabulaeformis stands was ranged from 123.8 Mg·ha–1 for the young stand to 344.8 Mg·ha–1 for the mature stand. The understory biomass ranged from 1.8 Mg·ha–1 in the middle-aged stand to 3.5 Mg·ha–1 in the young stand. Forest floor biomass increased steady with stand age, ranging from 14.9 to 23.0 Mg·ha–1. The highest mean C concentration across the chronosequence was found in tree branch while the lowest mean C concentration was found in forest floor. The observed C stock of the aboveground tree, shrub, forest floor, and mineral soil increased with increasing stand age, whereas the herb C stock showed a decreasing trend with a sigmoid pattern. The C stock of forest ecosystem in young, middle-aged, immature, and mature stands were 178.1, 236.3, 297.7, and 359.8 Mg C ha–1, respectively, greater than those under similar aged P. tabulaeformis forests in China. These results are likely to be integrated into further forest management plans and generalized in other contexts to evaluate C stocks at the regional scale.
Introduction
Biomass and carbon (C) storage in forest ecosystems play dominant roles in global C cycle [1], [2], [3], [4], and serve as the most significant C sinks to reduce global warming [5]. This is largely due to their huge potential for sequestering carbon in vegetation and soil [6], [7], [8], [9], and interact with atmospheric processes through the absorption and respiration of CO2 [2], [10], [11]. As an important C pool in the terrestrial ecosystems, forests store more C than any other terrestrial ecosystems [8] and contain about 80% of all aboveground terrestrial C [12] and 40% of soil C [2], [13]. Therefore, a slight change of the C pool could have an important impact on global C balance. In the past few decades, many studies have estimated biomass or C storage in ecosystem components such as vegetation, forest floor and mineral soil [14], [15], [16], [17], [18]. However, considerable problems and uncertainties existed in these studies due to site-specific characteristics, and inconsistent methodologies and definitions [19]. Thus, there is a need for accurate information concerning the biomass and C storage in forest ecosystems to improve our understanding in processes and mechanisms of the global C cycle.
Generally speaking, stand development is bound up with C storage over the entire life cycle of forest ecosystems because tree growth rates vary greatly with stand age [20], [21]. Furthermore, stand age is one of the crucial factors affecting changes in C allocation among different forest ecosystem components such as the forest floor, soil, and coarse woody debris [15], [22], [23], [24], [25]. Moreover, it affects allometry, wood density and structure [26], [27], [28], which has a considerable impact on the quantitative analysis of forest biomass. To date, many studies based on age have reported that the biomass and C storage patterns in forest ecosystems [3], [4], [29]; however, few studies have examined biomass and C storage of P. tabulaeformis forests across a chronosequence, particularly in Northern China.
P. tabulaeformis is a geographically widely distributed native tree species that spans in Northern China from latitude 31°13′ N to 43°33′ N and from longitude 103°20′ E to 124°45′E. Its ability to grow in poor site conditions as well as regenerate naturally as a secondary succession pioneer species following disturbances has led to the tree species covering a total of 228.10×104 ha [30] of forestland in China. Although biomass or C storage of this tree species in China have been quantified [31], [32], studies on the forest C stock and C allocation patterns along stand development are scarce [29]. Due to rapid land use changes, P. tabulaeformis and other secondary forests have been continuously increasing in their coverage in China. Secondary forests provide important ecosystem services, including erosion prevention, biodiversity maintenance, water conservation and watershed protection [33]. In recent years, many efforts have been made to understand the ecological processes of secondary forests [34], [35], [36]. However, there is still a lack of information on the biomass and C storage in P. tabulaeformis natural secondary forests in an age sequence,which is crucial for us to predict the responses of regional and global carbon balance to future climate change.
The objectives of this study were (1) to estimate the biomass of the ecosystem components across an age sequence of P. tabulaeformis stands, and (2) to assess the changes in the size and contribution of these C pools to total ecosystem C stock with increasing stand age.
Materials and Methods
Site description
The study was conducted in Liaoheyuan (41°01′∼41°21′N, 118°22′∼118°37′E), located in Pingquan County, Hebei Province (Fig. 1) (Pingquan forestry bureau issued the permission to conduct this study for each location). Altitude ranges from 625 to 1738 m above sea level. This area is warm temperate to cold temperate transition zone, a semi-humid and semi-arid continental monsoon mountain climate with a humid and rainy summer and a cold and snowy winter. The mean annual temperature is 7.3°C (−10.8°C in January and 22.9°C in July, respectively), and the mean annual precipitation is 540 mm (most rainfall occurs from May to August). The annual frost-free period is 110–125 days, and annual mean total sunshine time is 2,000–2,900 h. The soil is a typical brown forest soil with a thickness of approximately 100 cm.
Figure 1. The location of Liaoheyuan, Hebei Province, China.
Experimental design
Based on the “National Forest Resource Continuous Investigation Technical Regulations”[37], our P. tabulaeformis chronosequence (four age groups) includes twelve stands (three stands in each age group, and they were natural regeneration) with pure overstories, ≤30, 31–50, 51–60, and 61–80-year-old, representing the young, middle-aged, immature, and mature stages, respectively. Understory of all stands is dominated by Quercus liaotungensis, Quercus mongolica, Lespedeza bicolor, Spiraea trilobata, Rhamnus parvifolia, Corylus mandshurica, Rhododendron micranthum, Deutzia grandiflora, Prunus armeniaca, and herbs such as Carex rigescens, Saussurea nivea, Dianthus chinensis, Polygonatum odoratum, Dontostemon dentatus, Vicia unijuga,chinensis, and Goodyera schlechtendaliana.
Within each stand, three 20 m×30 m permanent plots (50 m apart each plot) were randomly set up in July 2012. Species identities, diameter at breast height (DBH), height and crown dimensions were documented for all trees within each plot. Detailed information for these forest stands is shown in Table 1.
Table 1. Characteristics of stands of P. tabulaeformis natural secondary forests.
| Age groups | Stand age (years) | Location (lat, long) | Elevation (m) | Slope degree (°) | Stand density (stems·ha–1) | Bulk density* (g·cm–3) | DBH * (cm) | Height* (m) | Basal area of DBH (m2·ha–1) | Stand volume** (m3·ha–1) |
| Young | <30 | 41°18′N, 118°30′E | 1097±6 | 27±2 | 2567±112 | 1.5±0.1 | 11.0±5.1 | 9.2±2.0 | 29.5 | 270.2 |
| 41°19′N, 118°33′E | 1094±5 | 29±2 | 2334±35 | 1.7±0.2 | 11.8±5.5 | 10.2±2.1 | 31.0 | 283.4 | ||
| 41°18′N, 118°31′E | 1058±10 | 30±1 | 1900±93 | 1.4±0.1 | 11.0±6.1 | 9.6±2.4 | 23.5 | 215.3 | ||
| Middle-aged | 31–50 | 41°17′N, 118°31′E | 1008±8 | 30±2 | 1034±9 | 1.6±0.1 | 18.4±7.5 | 14.9±4.2 | 32.1 | 307.2 |
| 41°18′N, 118°32′E | 982±3 | 23±1 | 1050±28 | 1.6±0.1 | 18.5±5.2 | 15.8±2.0 | 30.4 | 291.5 | ||
| 41°20′N, 118°34′E | 985±2 | 25±1 | 1034±41 | 1.5±0.1 | 17.2±4.6 | 13.2±2.6 | 25.8 | 247.6 | ||
| Immature | 51–60 | 41°15′N, 118°31′E | 1011±14 | 31±2 | 884±17 | 1.6±0.1 | 18.9±7.7 | 15.9±4.2 | 28.9 | 276.7 |
| 41°17′N, 118°30′E | 993±15 | 30±3 | 850±53 | 1.5±0.1 | 20.4±8.4 | 17.5±4.4 | 32.5 | 263.9 | ||
| 41°19′N, 118°31′E | 1018±9 | 31±1 | 867±22 | 1.4±0.1 | 20.0±5.3 | 17.0±2.1 | 29.2 | 237.3 | ||
| Mature | 61–80 | 41°18′N, 118°28′E | 1066±18 | 29±2 | 917±80 | 1.6±0.1 | 23.3±7.1 | 20.9±3.7 | 42.5 | 419.3 |
| 41°16′N, 118°31′E | 1080±15 | 31±2 | 934±106 | 1.5±0.2 | 22.9±10.2 | 20.1±4.3 | 45.8 | 452.4 | ||
| 41°20′N, 118°30′E | 1089±10 | 23±1 | 717±37 | 1.5±0.2 | 23.1±12.5 | 19.8±4.9 | 38.5 | 379.9 |
*: stand mean ± within-stand deviation (SD).
**: Stand volume (M) and sample tree volume (V) was calculated by the following formula (1) and (2), respectively.
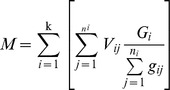 (1);
(1); 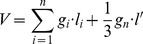 (2)Where M is the stand volume (m3·ha–1), ni is the number of i–th class of the sample tree, k is graded series (i = 1, 2,…, k), Gi is the i–th class of basal area of DBH (m2·ha–1), Vij and gij are the volume (m3) and basal area of DBH (m2) of j–th sample tree in i–th class, respectively. V is the sample tree volume (m3), gi is the central basal area (m2) of the i–th log, li is the length (m) of the i–th log, gn is the last basal area (m2) at the top of tree, l′is the length (m) between the last basal area and the top of tree, n is the total number of logs.
(2)Where M is the stand volume (m3·ha–1), ni is the number of i–th class of the sample tree, k is graded series (i = 1, 2,…, k), Gi is the i–th class of basal area of DBH (m2·ha–1), Vij and gij are the volume (m3) and basal area of DBH (m2) of j–th sample tree in i–th class, respectively. V is the sample tree volume (m3), gi is the central basal area (m2) of the i–th log, li is the length (m) of the i–th log, gn is the last basal area (m2) at the top of tree, l′is the length (m) between the last basal area and the top of tree, n is the total number of logs.
Field sampling and measurements
Tree and understory biomass estimation
This work was conducted based on Forestry Standards“Observation Methodology for Long-term Forest Ecosystem Research” of People's Republic of China [38].
In early August 2012, five P. tabulaeformis trees within representative stand-specific DBH range were selected and harvested destructively in each stand (The Pingquan forestry bureau issued the permission to conduct this study for each location). Trees were cut at the buttress near the ground surface. After the measurements of the diameters at DBH and at ground level (D0), and the total height (H), the tree (including the tree stem, branch, needle, and pine cone) was first cut open at 1.3 m. The top part (from 1.3 m to the tips) was then sectioned into 1 m (if H<15 m) or 2 m (if H>15 m) intervals using “stratified cutting method” [38]. All branches, needles and pine cones of each stem section were clipped from the tree stems and branches, respectively. The fresh mass of logs (sectioned tree stems) and canopy (branches, needles and pine cones separately) were measured in situ. Stem disks of approximately 5 cm thickness from the stump were cut out of each section, and separated into two subsamples: stem wood and stem bark. In each stand, roots of each sampled tree were excavated manually in 1 m depth within the radius of 1 m from the tree center, and sorted into five size classes: piles, coarse roots (with a diameter >5 cm), big roots (with a diameter between 2 and 5 cm), taproots (with a diameter between 1 and 2 cm) and fine roots (with a diameter <1 cm). Then the entire root system was washed lightly to remove soil particles, air dried, and weighed. Subsamples (stems, barks, branches, needles, pine cones, and roots) of each tree were brought back to the laboratory and oven dried at 80°C to constant weight for dry biomass determination.
Previous study demonstrated that DBH-based allometric equations [25] could be used to estimate tree biomass in an age sequence of pine forests. Cao et al. [29] also reported that tree components biomass of P. tabulaeformis forests in northern China were highly correlated with DBH (R2 values were all over 96%). Using the same DBH-based allometric equations, we found they almost explained more than 90% of the variability in all components between the young and mature P. tabulaeformis stands in our study area (Table 2). Thus, these equations were used to estimating tree components biomass of the P. tabulaeformis forests.
Table 2. Parameters and statistics of biomass equations for different tree components in P. tabulaeformis forests.
| Age groups | Components | a | b | R | F value | S.E.E a | MSRb | P |
| Young | Total tree | 4.401 | 1.031 | 0.965 | 83.156 | 0.046 | 0.002 | <0.01 |
| Aboveground | 3.135 | 1.085 | 0.965 | 82.283 | 0.049 | 0.002 | <0.01 | |
| Tree stem | 1.281 | 1.220 | 0.966 | 84.735 | 0.054 | 0.003 | <0.01 | |
| Stem wood | 1.477 | 1.078 | 0.947 | 53.952 | 0.060 | 0.004 | <0.01 | |
| Stem bark | 0.048 | 1.874 | 0.972 | 105.233 | 0.075 | 0.006 | <0.01 | |
| Branch | 1.045 | 0.919 | 0.958 | 68.570 | 0.045 | 0.002 | <0.01 | |
| Needle | 1.243 | 0.802 | 0.946 | 52.324 | 0.045 | 0.002 | <0.01 | |
| Pine cone | 6E-05 | 3.587 | 0.942 | 48.760 | 0.210 | 0.044 | <0.01 | |
| Roots | 1.450 | 0.800 | 0.926 | 37.480 | 0.053 | 0.003 | <0.01 | |
| Middle-aged | Total tree | 9.799 | 1.009 | 0.949 | 55.928 | 0.025 | 0.001 | <0.01 |
| Aboveground | 10.057 | 0.965 | 0.918 | 33.467 | 0.031 | 0.001 | <0.05 | |
| Tree stem | 8.440 | 0.950 | 0.859 | 18.336 | 0.041 | 0.002 | <0.05 | |
| Stem wood | 6.597 | 0.997 | 0.845 | 16.400 | 0.045 | 0.002 | <0.05 | |
| Stem bark | 2.765 | 0.551 | 0.933 | 42.096 | 0.016 | 0.000 | <0.01 | |
| Branch | 0.303 | 1.386 | 0.932 | 40.826 | 0.040 | 0.002 | <0.01 | |
| Needle | 2.653 | 0.589 | 0.910 | 30.200 | 0.020 | 0.000 | <0.05 | |
| Pine cone | 0.010 | 1.554 | 0.927 | 38.054 | 0.046 | 0.002 | <0.01 | |
| Roots | 0.281 | 1.427 | 0.929 | 38.974 | 0.042 | 0.002 | <0.01 | |
| Immature | Total tree | 0.036 | 2.914 | 0.944 | 50.821 | 0.042 | 0.002 | <0.01 |
| Aboveground | 0.041 | 2.835 | 0.948 | 54.753 | 0.040 | 0.002 | <0.01 | |
| Tree stem | 0.021 | 2.981 | 0.935 | 43.438 | 0.047 | 0.002 | <0.01 | |
| Stem wood | 0.021 | 2.945 | 0.938 | 45.006 | 0.046 | 0.002 | <0.01 | |
| Stem bark | 0.001 | 3.285 | 0.910 | 30.366 | 0.062 | 0.004 | <0.05 | |
| Branch | 0.960 | 1.003 | 0.936 | 44.046 | 0.016 | 0.000 | <0.01 | |
| Needle | 0.003 | 2.945 | 0.978 | 135.618 | 0.026 | 0.001 | <0.01 | |
| Pine cone | 2E-11 | 8.395 | 0.901 | 27.297 | 0.167 | 0.028 | <0.05 | |
| Roots | 0.001 | 3.577 | 0.913 | 31.448 | 0.066 | 0.004 | <0.05 | |
| Mature | Total tree | 0.785 | 1.932 | 0.949 | 55.366 | 0.036 | 0.001 | <0.01 |
| Aboveground | 0.465 | 2.063 | 0.950 | 57.171 | 0.037 | 0.001 | <0.01 | |
| Tree stem | 0.493 | 1.974 | 0.941 | 48.147 | 0.039 | 0.002 | <0.01 | |
| Stem wood | 0.536 | 1.911 | 0.934 | 42.307 | 0.040 | 0.002 | <0.01 | |
| Stem bark | 0.010 | 2.510 | 0.967 | 86.847 | 0.037 | 0.001 | <0.01 | |
| Branch | 0.023 | 2.253 | 0.970 | 97.205 | 0.031 | 0.001 | <0.01 | |
| Needle | 0.052 | 1.988 | 0.942 | 48.299 | 0.039 | 0.002 | <0.01 | |
| Pine cone | 4E-05 | 6.228 | 0.979 | 139.558 | 0.072 | 0.005 | <0.01 | |
| Roots | 2.010 | 0.918 | 0.906 | 28.889 | 0.023 | 0.001 | <0.05 |
Equations follow the form Y = axb + ε, where a and b are the equation parameters, Y is the biomass of the respective tree component (kg), x is the diameter at breast height (cm), ε is the error term.
S.E.E is the standard error of estimation, bMSR is the mean square residuals.
Understory biomass was determined using destructive sampling techniques (total harvesting); sampling for shrub layer and herb layer conducted in five 2 m×2 m subplots and 1 m×1 m subplots, respectively. These subplots were randomly selected within each 20 m×30 m plot. Shrubs and herbs were both separated into aboveground and belowground components, and the aboveground components of shrubs were further divided into leaves and branches. Biomass of each component was air dried and subsamples were oven dried at 80°C to constant weight for dry biomass determination.
Subsamples (tissue sample) of P. tabulaeformis and understory were collected from each chronosequence stand. Total C stock was obtained by multiplying tissue C concentration by the total dry weight of each component. Biomass of tree and understory were separately calculated and summed to estimated vegetation C density of each stand.
Forest floor biomass estimation
Forest floor components were sampled through collecting the entire organic material within five 1 m×1 m subplot randomly chosen in each 20 m×30 m plot. All plant materials collected within each subplot were sorted into three components: undecomposed layer, semi-decomposed layer and full decomposed layer. After the measurements of thickness, subsamples of different components were collected separately, transported to the lab, and oven-dried at 80°C to constant weight. Subsamples were ground and used for C concentration analysis.
The C concentrations of all samples (components of tree, understory, and forest floor) were analyzed by vario Macro Elemental Analyzer (Elementar Analysensysteme GmbH, Germany).
Mineral soil sampling and measurement
Soil samples were taken to a depth up to 100 cm with three replicates in each chronosequence stand. At each sampling point, soil samples were extracted from six depths (0–10, 10–20, 20–40, 40–60, 60–80, and 80–100 cm) using a 100 cm3 stainless cylinder with. Prior to the measurement of bulk density, samples for each soil layer were sieved through a 2 mm mesh. Bulk density for each soil depth was measured by weighing the whole sample and drying subsamples at 105°C. Soil organic carbon was established by the oil-bath K2Cr2O7 titration method.
Calculation of C stocks
Forest ecosystem C stocks were calculated as follows: 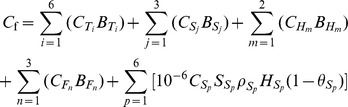
where Cf is the forest ecosystem C stock (Mg C ha–1), i is the tree tissue type (i.e., stem wood, stem bark, branch, needle, pine cone, and roots), CTi is the C concentration of the tree tissue (%), BTi is the biomass of the tree tissue (Mg·ha–1), j is the shrub tissue type (i.e., branch, leaf, and root), CSj is the C concentration of the shrub tissue (%), BSj is the shrub tissue biomass (Mg·ha–1), m is the component (i.e., the aboveground and belowground) of herb, CHm is the C concentration of the component (%), BHm is the component biomass (Mg·ha–1), n is the forest floor component (i.e., undecomposed layer, semi-decomposed layer and full decomposed layer), CFn is the C concentration of the component (%), BFn is the biomass of the forest floor component (Mg·ha–1), p is the layer of mineral soil, CSp is the C concentration of the mineral soil (%), SSp is the area of the calculated soil (ha), ρSp is the bulk density of the measured soil layer (g·cm–3), HSp is the depth of the measured soil layer (10 or 20 cm), and  Sp is the volumetric percentage of fragments >2 mm.
Sp is the volumetric percentage of fragments >2 mm.
Statistical analysis
Statistical analysis of data and regression analysis for developing allometric equations were performed using the SPSS software package (ver.17.0; SPSS, Chicago, IL). The difference between the stand mean and variation within that stand was examined by one-way analysis of variance (ANOVA) test.
Results
Biomass of ecosystem components
Based upon the power regression equations of tree components (stem wood, stem bark, branch, needle, pine cone, and roots) (Table 2), the biomass were estimated for all P. tabulaeformis forests (Table 3). The biomass of each tree component showed that an increasing trend with increasing stand age, with the exception of branch, needle, pine cone and roots. For young and middle-aged stands, the biomass distribution among different tree components was in an order of stem wood >roots >branch >needle >stem bark >pine cone. For the immature stand, however, the average needle biomass was slightly greater than the average branch biomass. In the mature stand, the average stem bark biomass was higher than the average branch biomass. Total tree biomass was 123.8, 189.3, 259.8, and 344.8 Mg·ha–1 for the young, middle-aged, immature, and mature stands, respectively, demonstrating a rapid increase from young to mature stands. The stem made the largest contribution to the total biomass regardless of stand age, accounting for 46.9%, 72.2%, 70.6% and 70.7% of total tree biomass for young, middle-aged, immature, and mature stands, respectively. The root to shoot ratios of four stands ranged from 0.1 for the mature stand to 0.2 for the young stand, with an average value of 0.1 across the entire age sequence.
Table 3. Biomass of different ecosystem components in P. tabulaeformis forests.
| Components | Biomass (Mg·ha–1) | |||
| Young | Middle-aged | Immature | Mature | |
| Total tree | 123.8±19.2d | 189.3±8.6c | 259.8±32.1b | 344.8±26.2a |
| Aboveground | 101.3±15.7d | 170. 5±7.4c | 228.8±27.0b | 314.4±21.0a |
| Tree stem | 58.0±9.0d | 136.7±5.8c | 183. 3±24.2b | 243.8±21.5a |
| Stem wood | 45.9±7.3d | 122.8±5.5c | 163.1±21.1b | 213.6±19.7a |
| Stem bark | 12.1±1.8c | 13.9±0.4c | 20.3±3.1b | 30.1±2.2a |
| Branch | 21.8±3.4b | 17.8±1. 2bc | 16.6±0.4c | 29. 6±2.2a |
| Needle | 19.3±3.1bc | 15.0±0.4c | 20.6±2.7b | 26.9±2.4a |
| Pine cone | 2.2±0.2b | 1.02±0.1b | 8.2±4.4b | 14.2±1.7a |
| Roots | 22.4±3.5b | 18.7±1.3b | 31.0±5.4a | 30.5±4.4a |
| Total understory | 3.5±0.9a | 1.8±1.1b | 2.8±0.5ab | 2.0±0.6ab |
| Total shrub | 0.4±0.4a | 0.6±0.5a | 0.8±0.7a | 0.9±0.5a |
| Shrub foliage | 0.0±0.0b | 0.1±0.1ab | 0.0±0.0b | 0.1±0.1a |
| Shrub branch | 0.1±0.1a | 0.3±0.3a | 0.2±0.2a | 0.3±0.3a |
| Shrub root | 0.2±0.2a | 0.2±0.2a | 0.6±0.6a | 0.4±0.2a |
| Total herb | 3.1±1.1a | 1.2±0.6b | 2.0±0.3ab | 1.2±1.2b |
| Aboveground herb | 1.0±0.5a | 0.3±0.2a | 0.4±0.1a | 0. 5±0.4a |
| Belowground herb | 2.1±0.6a | 0.9±0.4b | 1.6±0.2ab | 0.7±0.7b |
| Forest floor | 14.9±2.5b | 17.5±4.0b | 20.0±2.7ab | 23.0±0.9a |
| Undecomposed | 6.6±1.0b | 8.7±2.2ab | 9.3±1.2a | 9.7±0.4a |
| Semi-decomposed | 4.9±1.0b | 5.2±1.0b | 6.5±1.3ab | 8.2±0.7a |
| Full decomposed | 3.4±1.1a | 3.6±1.2a | 4.1±2.2a | 5.1±1.6a |
Data are presented as the mean value ± the standard deviation (SD). Mean values of biomass within a row followed by different lowercase letters are significantly different at p<0.05.
The total understory biomass ranged from 1.8 Mg ha–1 in the middle-aged stand to 3.5 Mg·ha–1 in the young stand. Forest floor biomass increased steady with stand age, ranging from 14.9 to 23.0 Mg·ha–1.
C concentrations and C pools of ecosystem components
Tree, understory, and forest floor
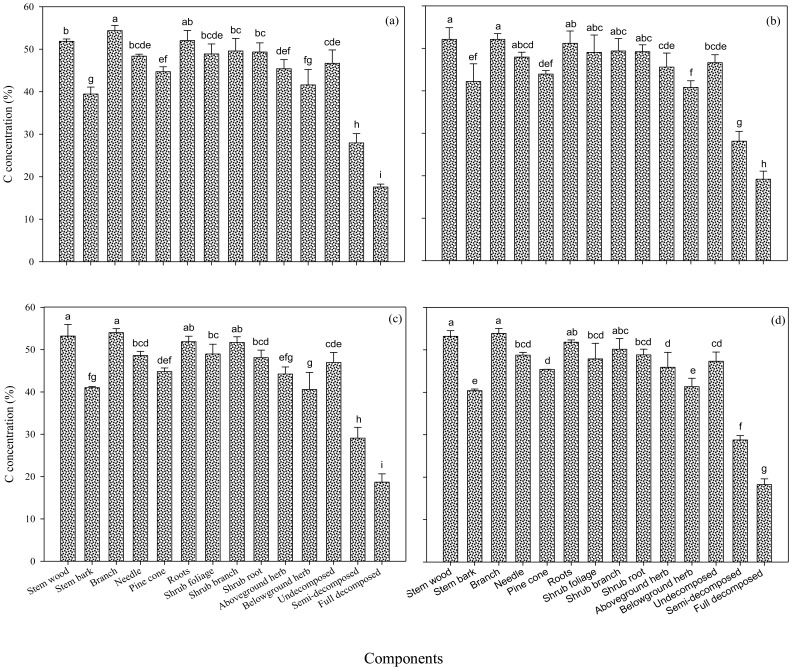
The C concentrations of different components of tree, understory, and forest floor in four age groups were shown in Fig. 2. In general, tree had the highest C concentration while the forest floor had the lowest. The C concentration varied from 39.5% to 54.4% among components within individual trees. The highest and lowest C concentrations in each stand was found to be the branch and stem bark, respectively. The mean C concentration of total tree ranged from 48.2% in the middle-aged stand to 48.9% in the immature stand. Shrub and herb had smaller variation in C concentrations among different components, ranging from 47.9% to 51.7%, and 40.6% to 45.9% of dry biomass, respectively. Within shrub components, higher C concentration was found in the branch than in the other components, with a mean value of 50.2% across all stands. On average, the C concentrations in the shrub foliage had the lowest values compared to the other components. For the immature stand, however, the shrub foliage C concentration was slightly greater than the shrub root C concentration. For all stands, the pattern of C concentrations distribution for the different components of the herb was in an order of aboveground > belowground. Within forest floor components, C concentrations ranged from 17.6% to 47.3% among the P. tabulaeformis stands. The C concentrations of forest floor in each stand showed a decreasing trend with the increase in the decomposition level.
Figure 2. Biomass C concentrations of different components in the young (a), middle-aged (b), immature (c), and mature (d) P. tabulaeformis forests.
Different lowercase letters indicate a significant difference within-stand (p<0.05).
The C stock of trees across the age sequence followed a similar pattern as tree biomass due to the steady C concentration for the same component of the differently aged stands (Fig. 2). The aboveground and total tree biomass C increased significantly from 50.8 and 62.4 Mg C ha−1 in the young stand to 161.2 and 177.0 Mg C ha−1 in the mature stand (Table 4). The contribution of tree stem C to total biomass C of vegetation and forest floor ranged from 41.4% for the young stand to 67.6% for the middle-aged stand. The contribution of pine cone C to the total biomass C was the smallest in each stand.
Table 4. C pools of different biomass components in P. tabulaeformis forests.
| Components | Young | Middle-aged | Immature | Mature | ||||
| (Mg C ha–1)(%) | (Mg C ha–1)(%) | (Mg C ha–1)(%) | (Mg C ha–1)(%) | |||||
| Total tree | 62.4±9.6d | 90.3±2.4a | 96.4±4.5c | 93.3±0.6a | 133.8±18.7b | 94.1±0.9a | 177.0±11.8a | 95.2±0.5a |
| Aboveground | 50.8±7.8d | 73.4±1.9a | 86.8±3.8c | 84.0±0.8b | 117.8±15.9b | 82.8±0.5b | 161.2±10.8a | 86.8±0.5b |
| Tree stem | 28.6±4.4d | 41.4±1.1b | 69.9±3.0c | 67.6±0.8c | 95.1±12.5b | 66.9±0.2c | 125.8±11.1a | 64.5±3.3c |
| Stem wood | 23.8±3.8d | 34.4±1.1c | 64.0±2.9c | 62.0±0.6d | 86.7±11.2b | 61.0±0.2d | 113.6±10.5a | 58.3±3.3d |
| Stem bark | 4.8±0.7c | 6.9±0.2f | 5.9±0.2c | 5.7±0.2g | 8.3±1.3b | 5.9±0.1g | 12.2±0.9a | 6.2±0.1f |
| Branch | 11.8±1.9b | 17.1±0.6d | 9.3±0.6c | 9.0±0.2e | 9.0±0. 2c | 6.4±0.8fg | 15.9±1.2a | 8.2±0.2f |
| Needle | 9.4±1.5b | 13.5±0.5e | 7.2±0.2c | 6.9±0.2f | 10.0±1.3b | 7.1±0.0f | 13. 1±1.1a | 6.7±0.3ef |
| Pine cone | 1.0±0.1b | 1.4±0.2hi | 0.4±0.0b | 0.4±0.0j | 3.7±2.0b | 2.5±1.0i | 6.440±0.8a | 3.5±0.6g |
| Roots | 11.7±1.8b | 16.9±0.6d | 9.6±0.7b | 9.3±0.2e | 16.1±2.8a | 11.3±0.5e | 15.8±2.3a | 8.1±1.0e |
| Total understory | 1.5±0.4a | 2.2±0.5h | 0.8±0.5b | 0.8±0.6ij | 1.2±0.3ab | 0.9±0.1jk | 0.9±0.2ab | 0.5±0.1i |
| Total shrub | 0.2±0.2a | 0.3±0.2i | 0.3±0.3a | 0.3±0.3j | 0.4±0.3a | 0.3±0.2kl | 0.4±0.3a | 0.2±0.1i |
| Shrub foliage | 0.0±0.0b | 0.0±0.0j | 0.0±0.0ab | 0.0±0.0j | 0.0±0.0b | 0.0±0.0l | 0.1±0.0a | 0.0±0.0i |
| Shrub branch | 0.1±0.1a | 0.1±0.1j | 0.1±0.2a | 0.2±0.2j | 0.1±0.1a | 0.1±0.1l | 0.2±0.1a | 0.1±0.1i |
| Shrub root | 0.1±0.1a | 0.2±0.2j | 0.1±0.1a | 0.1±0.1j | 0.3±0.3a | 0.2±0.2kl | 0.2±0.1a | 0.1±0.1i |
| Total herb | 1.3±0.5a | 1.9±0.7hi | 0.5±0.3b | 0.5±0.3j | 0.8±0.1ab | 0.6±0.1jkl | 0.5±0.5b | 0.3±0.3i |
| Aboveground herb | 0.4±0.2a | 0.6±0.3hi | 0.1±0.1a | 0.2±0.1j | 0.2±0.1a | 0.1±0.0kl | 0.2±0. 2a | 0.1±0.1i |
| Belowground herb | 0.9±0.2a | 1.3±0.4hi | 0.4±0.2b | 0.4±0.2j | 0.6±0.1ab | 0.5±0.1kl | 0.3±0.3b | 0.2±0.2i |
| Forest floor | 5.1±0.8c | 7.5±2.2f | 6.2±1.4bc | 6.0±1.1g | 7.1±0.5ab | 5.1±0.9h | 7.9±0.2a | 4.1±0.3g |
| Undecomposed | 3.1±0.4b | 4.6±1.3g | 4.0±1.0ab | 3.9±0.8h | 4.4±0.6a | 3.1±0.7i | 4.6±0.2a | 2.4±0.2gh |
| Semi-decomposed | 1.4±0.3b | 2.0±0.6h | 1.5±0.3b | 1.4±0.2i | 1.9±0.4ab | 1.4±0.4j | 2.3±0.2a | 1.2±0.1h |
| Full decomposed | 0.6±0.2a | 0.9±0.4hi | 0.7±0.2a | 0.7±0.2ij | 0.8±0.4a | 0.6±0.3kl | 0.9±0.3a | 0.5±0.2i |
Data are presented as the mean value ± the standard deviation (SD). Mean values of C stocks within a row and mean percentages of C stocks of biomass components within a column followed by different lowercase letters are both significantly different at p<0.05.
The predicted C stocks stored in different components of total understory (shrub and herb) were generally higher than the observed C stocks if using the C conversion factor (0.5). The observed C stocks of total understory in the P. tabulaeformis stands varied from 0.8 Mg C ha–1 in the middle-aged stand to 1.5 Mg C ha–1 in the young stand, most of which came from herb layer (Table 4). The C stored in the total herb for the young, middle-aged, immature, and mature stands accounted for 1.9%, 0.5%, 0.6%, and 0.3% of the total biomass C pools, respectively.
The observed C stocks in forest floor increased steadily with stand age, from 5.1 Mg C ha–1 in the young stand to 7.9 Mg C ha–1 in the mature stand (Table 4). The C stored in the forest floor for the young, middle-aged, immature, and mature stands accounted for 7.5%, 6.0%, 5.1%, and 4.1% of the total biomass C pools, respectively.
Mineral soil
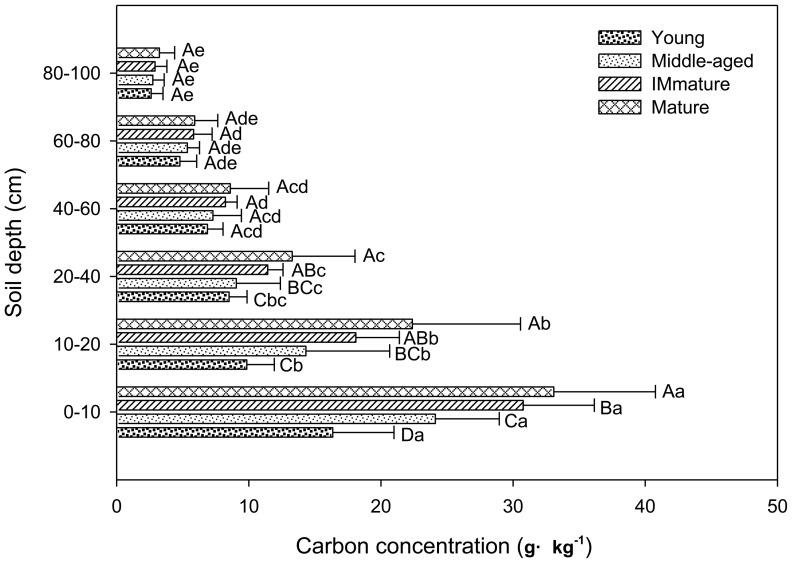
Mineral soil organic carbon (MSOC) concentration at all soil depths increased with stand age. At depth of 0–10, 10–20, 20–40, 40–60, 60–80, and 80–100 cm, MSOC concentrations of the young stand were 16.4, 9.9, 8.5, 6.9, 4.8, and 2.6 g·kg−1, respectively, and MSOC concentrations of the mature stand were 33.1, 22.4, 13.3, 8.6, 5.9, and 3.2 g·kg−1, respectively. The MSOC concentration decreased significantly with increasing soil depth in all stands (Fig.3). However, it increased significantly in the same depth cross the chronosequence. The observed C stocks in total mineral soil continuously increased with stand age, from 109.1 Mg C ha–1 in the young stand to 174.0 Mg C ha–1 in the mature stand. The MSOC stock between the 0–40 cm was greater than that in deeper soil (Fig.4), accounting for 58.7%, 63.3%, 66.4%, and 66.6% of entire soil profile (0–100 cm) in the young, middle-aged, immature, and mature stands, respectively.
Figure 3. MSOC concentrations at different soil depths in the P. tabulaeformis forests.
Different uppercase letters indicate a significant difference between age groups in the same depth (p<0.05), different lowercase letters indicate a significant difference between different soil depths within-stand (p<0.05). Error bars standard deviation (SD).
Figure 4. MSOC stocks at different soil depths in the P. tabulaeformis forests.
Different uppercase letters indicate a significant difference between age groups at the same horizon (p<0.05), different lowercase letters indicate a significant difference between different soil depths within-stand (p<0.05). Error bars standard deviation (SD).
Forest ecosystem
The C stocks of the tree, shrub, forest floor, mineral soil, and total ecosystem increased with increasing stand age, but for herb, C stock was the highest in the young stand (Table 5).
Table 5. C pools of ecosystem components in P. tabulaeformis forests.
| Components | C stock (Mg C ha–1) | |||
| Young | Middle-aged | Immature | Mature | |
| Tree | 62.4 ±5.6 | 96.4±2.6 | 133.8 ±10.8 | 177.0±6.8 |
| Shrub | 0.2±0.1 | 0.3±0.2 | 0.4±0.2 | 0.4±0.1 |
| Herb | 1.3±0.3 | 0.5±0.1 | 0.8±0.1 | 0.5±0.3 |
| Forest floor | 5.1±0.5 | 6.2±0.8 | 7.1±0.3 | 7.9±0.1 |
| Mineral soil | 109.1±5.8 | 133.0±10.6 | 155.6±4.2 | 174.0±11.7 |
| Ecosystem | 178.1±7.7 | 236.3±18.4 | 297.7±11.0 | 359.8±21.0 |
Data are presented as the mean value ± the standard error (SE).
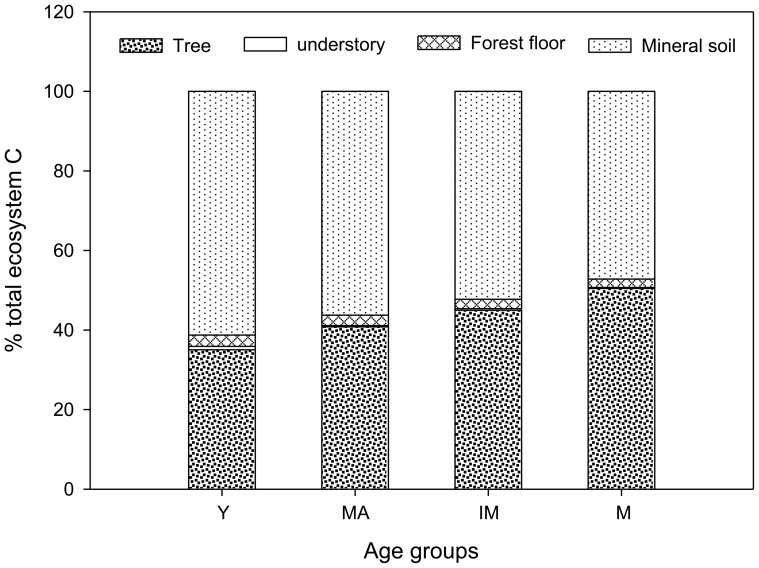
Tree and mineral soil were the two largest contributors to the total ecosystem C pool in our age sequence stands (Fig.5). The contribution of tree biomass C ranged from 35.1% for the young stand to 49.2% for the mature stand, with an average value of 42.5% in this chronosequence study. Mineral soil was the dominant C pool, representing 61.3% of the total ecosystem in the young stand, whereas the contribution of MSOC decreased only to 48.4% in the mature stand. Forest floor C represented 2.2% to 2.8% of the total ecosystem C pool. The total understory contributed little to the forest ecosystem C pool.
Figure 5. Percentage contribution of C pool in individual components of ecosystem in the young (Y), middle-aged (MA), immature (IM), and mature (M) P. tabulaeformis forests.
Discussion
Biomass
Tree
Our estimates of P. tabulaeformis biomass across the chronosequence demonstrated that tree biomass increased with the age of stands. This pattern was in agreement with many previous reports on other pine forests [16], [27], [29], [39], confirming that stand age is an important variable for accurately estimating biomass of different components in pine forests. Meanwhile, our results revealed P. tabulaeformis biomass was greater than other pine species across the same chronosequence studied in China (Table 6). For example, Gower et al. [40] reported that the average value of total tree biomass of temperate pines is 120.5 Mg·ha–1, which is smaller than the average value we found. Cao et al. [29] and Li et al. [41] studied the biomass accumulation across different stages of stand development of pine forests, and the result is also smaller than what we reported here. The greater biomass revealed in our study indicates that P. tabulaeformis forests in Liaoheyuan have a greater potential to accumulate biomass. These differences may be affected by the stand age and origin.
Table 6. Comparison of biomass between P. tabulaeformis and published results (FRSC, 1994) of pine species.
| Pine species | Biomass (Mg·ha–1) | ||||
| Young | Middle-aged | Immature | Mature | Fang's data* | |
| P.sylvestris var. mongolica | 40.42 | 93.01 | 113.37 | 122.9 | 49.3 |
| P.tabulaefomis | 62.71 | 81.92 | 82.05 | 93.76 | 88.7 |
| P.armandii, P. densata | 76.71 | 134.22 | 169.25 | 191.87 | 71.8 |
| P.koraiensis | 74.91 | 171.89 | 214.46 | 245.52 | 65.4 |
| P.massoniana | 70.53 | 126.31 | 322.20 | 319.49 | 81.1 |
| P.yunnaensis, P. khasya | 58.79 | 108.87 | 197.11 | 219.99 | nd* |
*nd indicates that the results were not determined at these sites.
The patterns of biomass distribution among various components of the tree were the same for both young and middle-aged stands, average biomass decreasing in an order of stem wood > roots > branch > needle > stem bark > pine cone (Table 3). Similar patterns were observed in an age sequence of P. densiflora stands [19] and lacebark pine plantation forests [41]. However, inconsistent with other reports, we found that branch biomass was lower than needle biomass and stem bark biomass in the immature and mature stands, which is likely due to the large within-stand variability.
Previous studies have reported that the proportion of stem to total tree biomass varied significantly among stands of different ages [39]. The ratios in our study were 46.9%, 72.2%, 70.6% and 70.7% for young, middle-aged, immature, and mature stands, respectively. Therefore, total tree biomass may be considerably underestimated by forest inventories that are normally limited to stem biomass. At the same time, root biomass accounted for a large proportion of the total tree biomass, highlighting the importance of roots in the total biomass estimation of P. tabulaeformis forests.
Understory and forest floor
Only a few studies have measured understory and forest floor biomass in P. tabulaeformis natural forests in Northern China. In our study, the understory biomass ranged from 1.8 Mg·ha–1 in the middle-aged stand to 3.5 Mg·ha–1 in the young stand. These numbers were in agreement with other previously reported values of 1.61–3.76 Mg·ha–1 [39] and 0.87–3.55 Mg·ha–1 [29]. However, our results were lower than Li et al. [41] whose estimates of understory biomass were 6.17 and 20.25 Mg·ha–1 in 16- and 35-year old lacebark pine stands, respectively. We also found a sigmoid pattern of changes in understory biomass across the chronosequence. Understory biomass might not be responsive to stand age. It may depend more on forest management, stand-specific canopy, and soil conditions, which affect light, water, and nutrient availability for the development of understory.
Forest floor biomass, however, did increase steadily from young to mature stands along the chronosequence, ranging from 14.9 to 23.0 Mg·ha–1. This pattern was in accordance with the results from Li et al. [41], who reported that the forest floor biomass of secondary lacebark pine increases with stand age.
C concentration
A factor of 0.5 of the C concentration has been commonly used for C conversion from biomass [42]. Recent studies showed that the C concentration of tree components or tree species might be either above or below 0.5 [29], [39], [41], [43]. In our study, C concentrations fell within a range of 39.5% to 54.4% of dry biomass, and the mean C concentration of total tree ranged from 48.2% in the middle-aged stand to 48.9% in the immature stand. Overall, the C concentration of shrub layer (47.9–51.7%) was higher than herb layer (40.6–45.9%), similar to previous results reported for various pine species [29], [39], [41]. Within forest floor, higher C concentration was found in the undecomposed layer than that in other components, with a mean value of 46.9% across the chronosequence stands. We found that the C concentrations of ecosystem components changed significantly in each chronosequence stand (Fig. 2). This indicates that the use of a component-specific C concentration to estimate the forest C pool is more appropriate than a fixed conversion factor (0.5). We also found that the C concentrations of ecosystem components in P. tabulaeformis forests did not change significantly with stand age (p>0.05), which is similar to the results reported by Cao et al. [29] and Uri et al. [44]. We suggest that a component-specific C concentration value is suitable for estimating forest C pool among all stands.
Carbon storage
The estimated total tree biomass increase steadily with stand age, from 62.4 Mg C ha–1 in the young stand to 177.0 Mg C ha–1 in the mature stand. The pattern of changes in total tree biomass among stand ages is the same between our study and previous studies. For example, Li et al. [41] reported the total tree C stock of lacebark pine increased from 23.6 to 148.8 Mg C ha–1 when stand ages increased from 16 to 68 years old. Total tree biomass estimates often vary among pine forests [19], [29], [45], [46], which is likely due to the differences in the stand density and nutrients of forests.
The pattern of understory C stock changes with stand ages was different in our study than in other coniferous chronosequence studies (Table 7). Previous research has shown that the C stock of the understory decreased with the increasing stand age [21], [29], but the C stock of the understory in our study was not significantly related to stand age. This may be explained by the increasing competition for light and nutrients with increasing stand age. Interestingly, we found total shrub C increased with stand age, similar to previous result reported for conifer [43]. However, herbs dominated understory C pool in our chronosequence of P. tabulaeformis stands, and the contribution of herb C to the total understory C varied from 55.3% to 87.6%, resulting in a sigmoid pattern of changes in total understory C pool along our chronosequence.
Table 7. Comparison of C storage between P. tabulaeformis and published results of pine species.
| Pine species | Age (years) | C stock (Mg C ha–1) | |||||
| Tree | Understory | Forest floor | Mineral soil | Ecosystem | Reference | ||
| P. strobus | 16–114 | 24.5–174.4 | nd* | nd* | 2.4–17.1 | 33.6–239.4 | [45] |
| P. strobus | 2–65 | 0.2–103.4 | 2.1–3.6 | 0.8–12.1 | 33.9–39.1 | 40.2–156.1 | [16] |
| P. elliottii | >14 | 31.1 | 8.1 | 2.2 | 65.4 | 104.07 | [43] |
| P. massoniana | >14 | 35.2 | 19.5 | 3.0 | 84.3 | 142.0 | [43] |
| P. densiflora | 10–71 | 9.3–104.9 | nd* | 7.0–11.2 | 2.5–84.7 | 18.8–201.4 | [19] |
| P. koraiensis | 8–51 | 0.8–122.3 | 0.6–1.1 | 3.14–6.1 | 32.9–37.6 | 42.2–162.7 | [39] |
| P. kesiya | 65–80 | 222.9 | 0.4 | 2.2 | 58.7 | 283.1 | [46] |
| P. tabulaeformis | 25–105 | 20.7–103.8 | 1.6–3.7 | 5.9–16.6 | 53.7–85.6 | 84.1–207.5 | [29] |
| P. bungeana | 16–68 | 23.6–148.8 | 2.6–13.6 | 1.1–3.5 | 66.1–74.4 | 93.4–240.3 | [41] |
| P. tabulaeformis | < 80 | 62.4–177.0 | 0.8–1.5 | 5.1–7.9 | 109.1–174.0 | 178.1–359.8 | Our results |
*nd indicates that the results were not determined at these sites.
We found that the total C stock of forest floor increased with stand age. This pattern is consistent with some of the coniferous chronosequence studies [15], [47], but not with others [16], [39], suggesting a large within-stand variability. The previous studies has been reported to be highly impressionable to disturbances and variations in stand treatment, litter input, and decomposition rate [15], [16], [21]. By comparing the trends, it was clear that each component of forest floor C stock increases as the stand age increases and this was due to the increase of litter input and slow decomposition. We also found that the C stock of the forest floor in each stand is greater than that of the total understory, indicating that forest floor should be considered as an important component in total C stock calculation.
The effect of stand age on MSOC is debatable. Among those chronosequence-based studies listed in Table 7, there exist great deals of controversies regarding whether or not MSOC stock could respond to stand ages [48], [49]. Some studies reported no significant increase in mineral soil C stocks with stand age [16], [48], [50], whereas other studies indicate an increasing soil C stocks in the early decades after afforestation [15], [45], [49]. This discrepancy may be due in part to how much other influencing factors, such as climate, soil properties, and forest type, could overshadow the effect of stand age [16]. In our study, we observed soil C stocks increased with stand age, joining with some of the most recent reports to support that conifer forest MSOC does increase with stand age, possibly due to a larger accumulation of organic matter in older stands [29], [41]. Meanwhile, the C stocks of mineral soil in our study were much higher than most others reported (Table 7). These differences may result from the different depths of soil sampling in the profile.
In terms of vertical distribution of MSOC, our data revealed that a large quantity of the MSOC was sequestrated in upper 20 cm of the mineral soil horizon in all stands, indicating that higher amounts of MSOC were stored in the surface layer. Approximately 58.7% to 66.6% of MSOC in the 0–100 cm range of soil was stored in the 0–40 cm range, where soils can be disturbed by human disturbances. Hence, research the spatial variability of MSOC [51], and protection of the topsoil from disturbances is important for C sequestration.
Another important finding through our study was that although the aboveground (sum of vegetation, and forest floor) and belowground (mineral soil) C stocks both increased with stand ages, the contribution of the MSOC stock to total ecosystem C stock decreased gradually from 61.3% in the young stand to 48.4% in the mature stand. This pattern did not occur in all forests as the rates of aboveground and belowground C accumulation differed among tree species during stand development [16], [29], [39]. Considering that the aboveground C accumulation would ultimately exceed the mineral soil C accumulation in mature stand, we suggest that aboveground tree biomass may make major contribution to total ecosystem C over time.
Conclusions
The biomass of each tree component could be predicted from an allometric equation using DBH as the independent variable. Biomass of tree, shrub, and forest floor increased with stand age, particularly the biomass within tree stem which comprised the main proportion of the aboveground and total tree biomass increased with increasing stand age. The highest C concentration was found in tree branch while the lowest C concentration was observed in the forest floor. The use of component-specific C concentration values other than a fixed factor of 0.5 to convert biomass to C stock is needed in order to more accurately estimate the forest C pool. Belowground components contribute a great deal to the total C accumulation, but aboveground tree biomass becomes increasingly important as stands age, indicating the necessity of considering the entire development stages of a forest in estimating its ecosystem-level C stock. The C sequestration potential of P. tabulaeformis forests in northern China is greater than previously estimated, so its importance in combating global warming deserves more attention and further studies.
These findings given by our study will improve our understanding of C stocks and dynamics in P. tabulaeformis forests and can be used in forest management activities to enhance C sequestration. Due to the lack of over-mature stands in our study, the results do not represent the whole growth pattern of a stand development. Thus, further evidence of the effect of stand age on ecosystem C pool development will be needed from a whole chronosequence studies.
Acknowledgments
This research is part of the forest ecological service function distributed localization observation and model simulation. We gratefully acknowledge the support from the Pingquan forestry bureau and Liaoheyuan Forest Ecological Research Station for field monitoring and sampling, and they are Weimin Hu, Shuwen Han, Tianyu Li, Chuan Chen, Zhiyuan Ma, Hongyang Wang, Qiqi Zhang, and Chenyi Zhu.
Funding Statement
This research was jointly supported by the Special Fund for Forestry Scientific Research in the Public Interest (No. 201204101) and CFERN&GENE Award Funds on Ecological Paper. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Choi S, Lee K, Chang YS (2002) Large rate of uptake of atmospheric carbon dioxide by planted forest biomass in Korea. Global Biogeochemical Cycles 16(4): 1089. [Google Scholar]
- 2. Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, et al. (2002) Forest carbon sinks in the Northern Hemisphere. Ecological Applications 12(3): 891–899. [Google Scholar]
- 3. Houghton RA (2005) Aboveground forest biomass and the global carbon balance. Global Change Biology 11(5): 945–958. [Google Scholar]
- 4. Somogyi Z, Cienciala E, Makipaa R, Muukkonen P, Lehtonen A, et al. (2007) Indirect methods of large-scale forest biomass estimation. European Journal of Forest Research 126 (2): 197–207. [Google Scholar]
- 5. Schimel DS, House JI, Hibbard KA, Bousquet P, Ciais P, et al. (2001) Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 414(6860): 169–172. [DOI] [PubMed] [Google Scholar]
- 6.Heath LS, Smith JE, Birdsey RA (2003) Carbon Trends in U.S. forestlands: a context for the role of soils in forest carbon sequestration. The potential of US forest soils to sequester carbon and mitigate the greenhouse effect, CRC Press, Boca Raton, FL. p: 35–45.
- 7. Zhou GY, Liu SG, Li ZA, Zhang DQ, Tang XL, et al. (2006) Old-growth forests can accumulate carbon in soils. science 314(5805): 1417. [DOI] [PubMed] [Google Scholar]
- 8. Houghton RA (2007) Balancing the global carbon budget. Annual review of earth planetary Science 35: 313–347. [Google Scholar]
- 9. Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451(Suppl (1)) 289–292. [DOI] [PubMed] [Google Scholar]
- 10. Houghton RA, Hackler JL, Lawrence KT (1999) The US carbon budget: contributions from land-use change. Science 285: 574–578. [DOI] [PubMed] [Google Scholar]
- 11. Brown SL, Schroeder PE (1999) Spatial patterns of aboveground production and mortality of wood biomass for eastern US Forests Ecological Applications, 9 (3): 968-980. DOI 10: 1051–1761. [Google Scholar]
- 12.Waring RH, Running SW (1998) Forest ecosystems: analysis at multiple scales Academic Press. San Diego, California: Academic Press.
- 13. Dixon RK, Brown S, Houghton REA, Solomon AM, Trexler MC, et al. (1994) Carbon pools and flux of global forest ecosystems. Science (Washington) 263(5144): 185–189. [DOI] [PubMed] [Google Scholar]
- 14. Joosten R, Schumacher RJ, Wirth C, Schulte A (2004) Evaluating tree carbon predictions for beech (Fagus sylvatica L.) in western Germany. Forest Ecology and Management 189(1): 87–96. [Google Scholar]
- 15. Pregitzer KS, Euskirchen ES (2004) Carbon cycling and storage in world forests: biome patterns related to forest age. Global Change Biology 10(12): 2052–2077. [Google Scholar]
- 16. Peichl M, Arain MA (2006) Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agricultural and Forest Meteorology 140(1): 51–63. [Google Scholar]
- 17. Chmura DJ, Rahman MS, Tjoelker MG (2007) Crown structure and biomass allocation patterns modulate aboveground productivity in young loblolly pine and slash pine. Forest ecology and management 243(2): 219–230. [Google Scholar]
- 18. Vesterdal L, Schmidt IK, Callesen I, Nilsson LO, Gundersen P (2008) Carbon and nitrogen in forest floor and mineral soil under six common European tree species. Forest Ecology and Management 255(1): 35–48. [Google Scholar]
- 19. Noh NJ, Son Y, Lee SK, Seo KW, Heo SJ, et al. (2010) Carbon and nitrogen storage in an age-sequence of Pinus densiflora stands in Korea. Science China Life Sciences 53(7): 822–830. [DOI] [PubMed] [Google Scholar]
- 20. Law BE, Sun OJ, Campbell J, Van TS, Thornton PE (2003) Changes in carbon storage and fluxes in a chronosequence of ponderosa pine. Global Change Biology 9(4): 510–524. [Google Scholar]
- 21. Taylor AR, Wang JR, Chen HY (2007) Carbon storage in a chronosequence of red spruce (Picea rubens) forests in central Nova Scotia, Canada. Canadian Journal of Forest Research 37(11): 2260–2269. [Google Scholar]
- 22.Covington WW (1981) Changes in forest floor organic matter and nutrient content following clear cutting in northern hardwoods. Ecology: 41–48.
- 23. Martin JL, Gower ST, Plaut J, Holmes B (2005) Carbon pools in a boreal mixedwood logging chronosequence. Global Change Biology 11(11): 1883–1894. [Google Scholar]
- 24. Zerva A, Ball T, Smith KA, Mencuccini M (2005) Soil carbon dynamics in a Sitka spruce (Picea sitchensis Carr.) chronosequence on a peaty gley. Forest Ecology and Management 205(1): 227–240. [Google Scholar]
- 25. Peichl M, Arain MA (2007) Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. Forest Ecology and Management 253(1): 68–80. [Google Scholar]
- 26. Wang JR, Letchford T, Comeau P, Kimmins JP (2000) Above- and below-ground biomass and nutrient distribution of a paper birch and subalpine fir mixed-species stand in the Sub-Boreal Spruce zone of British Columbia. Forest Ecology and Management 130(1): 17–26. [Google Scholar]
- 27. Litton CM, Ryan MG, Knight DH (2004) Effects of tree density and stand age on carbon allocation patterns in postfire lodgepole pine. Ecological Applications 14(2): 460–475. [Google Scholar]
- 28. Lehtonen A, Makipaa R, Heikkinen J, Sievanen R, Liski J (2004) Biomass expansion factors (BEFs) for Scots pine, Norway spruce and birch according to stand age for boreal forests. Forest Ecology and Management 188(1): 211–224. [Google Scholar]
- 29. Cao J, Wang X, Tian Y, Wen Z, Zha T (2012) Pattern of carbon allocation across three different stages of stand development of a Chinese pine (Pinus tabulaeformis) forest. Ecological Research 27(5): 883–892. [Google Scholar]
- 30. Guo H, Wang B, Ma X, Zhao G, Li S (2008) Evaluation of ecosystem services of Chinese pine forests in China. Science in China Series C: Life Sciences 51(7): 662–670. [DOI] [PubMed] [Google Scholar]
- 31. Zhao M, Zhou G (2005) Estimation of biomass and net primary productivity of major planted forests in China based on forest inventory data. Forest Ecology and Management 207(3): 295–313. [Google Scholar]
- 32. Tan K, Piao S, Peng C, Fang J (2007) Satellite-based estimation of biomass carbon stocks for northeast China's forests between 1982 and 1999. Forest Ecology and Management 240(1): 114–121. [Google Scholar]
- 33. Feldpausch TR, Rondon MA, Fernandes EC, Riha SJ, Wandelli E (2004) Carbon and nutrient accumulation in secondary forests regenerating on pastures in central Amazonia. Ecological Applications 14: 164–176. [Google Scholar]
- 34. Hashimoto T, Tange T, Masumori M, Yagi H, Sasaki S, et al. (2004) Allometric equations for pioneer tree species and estimation of the aboveground biomass of a tropical secondary forest in East Kalimantan. Tropics 14(1): 123–130. [Google Scholar]
- 35. Li Y, Xu M, Zou X, Shi P, Zhang Y (2005) Comparing soil organic carbon dynamics in plantation and secondary forest in wet tropics in Puerto Rico. Global Change Biology 11(2): 239–248. [Google Scholar]
- 36. Kenzo T, Ichie T, Hattori D, Itioka T, Handa C, et al. (2009) Development of allometric relationships for accurate estimation of above-and below-ground biomass in tropical secondary forests in Sarawak, Malaysia. Journal of Tropical Ecology 25(4): 371–386. [Google Scholar]
- 37.State Forest Administration (2003) Technical regulations of national forest continuous inventory. Internal Document of Chinese Government, Beijing (in Chinese).
- 38.Wang B, Lu SW, Li HJ (2011) Observation methodology for long-term forest ecosystem research (LY/T 1952—2011).: Fenghuang Press. Beijing, China, p: 1–128 (in Chinese).
- 39. Li X, Yi MJ, Son Y, Park PS, Lee KH, et al. (2011) Biomass and carbon storage in an age-sequence of Korean pine (Pinus koraiensis) plantation forests in central Korea. Journal of Plant Biology 54(1): 33–42. [Google Scholar]
- 40.Gower ST, Gholz HL, Nakane K, Baldwin VC (1994) Production and carbon allocation patterns of pine forests. Ecological Bulletins: 115–135.
- 41. Li C, Zha T, Liu J, Jia X (2013) Carbon and nitrogen distribution across a chronosequence of secondary lacebark pine in China. The Forestry Chronicle 89(2): 192–198. [Google Scholar]
- 42. Fang J, Chen A, Peng C, Zhao S, Ci L (2001) Changes in forest biomass carbon storage in China between 1949 and 1998. Science 292(5525): 2320–2322. [DOI] [PubMed] [Google Scholar]
- 43. Zheng H, Ouyang Z, Xu W, Wang X, Miao H, et al. (2008) Variation of carbon storage by different reforestation types in the hilly red soil region of southern China. Forest Ecology and Management 255(3): 1113–1121. [Google Scholar]
- 44. Uri V, Varik M, Aosaar J, Kanal A, Kukumagi M, et al. (2012) Biomass production and carbon sequestration in a fertile silver birch (Betula pendula) forest chronosequence. Forest Ecology and Management 267: 117–126. [Google Scholar]
- 45. Hooker TD, Compton JE (2003) Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonment. Ecological Applications 13(2): 299–313. [Google Scholar]
- 46. Baishya R, Barik SK (2011) Estimation of tree biomass, carbon pool and net primary production of an old-growth Pinus kesiya Royle ex. Gordon forest in north-eastern India. Annals of forest science 68(4): 727–736. [Google Scholar]
- 47. Bradford JB, Birdsey RA, Joyce LA, Ryan MG (2008) Tree age, disturbance history, and carbon stocks and fluxes in subalpine Rocky Mountain forests. Global change biology 14(12): 2882–2897. [Google Scholar]
- 48. Farley KA, Kelly EF, Hofstede RG (2004) Soil organic carbon and water retention after conversion of grasslands to pine plantations in the Ecuadorian Andes. Ecosystems 7(7): 729–739. [Google Scholar]
- 49. Lemma B, Kleja DB, Nilsson I, Olsson M (2006) Soil carbon sequestration under different exotic tree species in the southwestern highlands of Ethiopia. Geoderma 136(3): 886–898. [Google Scholar]
- 50.Cheng XQ, Han HR, Kang FF, Song YL, Liu K (2013) Variation in biomass and carbon storage by stand age in pine (Pinus tabulaeformis) planted ecosystem in Mt. Taiyue, Shanxi, China. Journal of Plant Interactions, DOI:10.1080/17429145.2013.862360
- 51. Peng G, Bing W, Guangpo G, Guangcan Z (2013) Spatial Distribution of Soil Organic Carbon and Total Nitrogen Based on GIS and Geostatistics in a Small Watershed in a Hilly Area of Northern China. PLoS ONE 8(12): e83592 doi:10.1371/journal.pone.0083592 [DOI] [PMC free article] [PubMed] [Google Scholar]