Abstract
Metastatic prostate cancer is a leading cause of cancer-related death in men worldwide. We have recently discovered that IL-30 shapes the microenvironment of prostate cancer and tumor-draining lymph nodes to favor tumor progression. IL-30 supports tumor growth in vitro, and IL-30 expression in prostate cancer patients is associated with high tumor grade and metastatic stage of disease. Thus, IL-30 may constitute a valuable target for modern therapeutic approaches to hamper prostate cancer progression.
Keywords: IL-27p28, IL-30, lymph node metastasis, prostate cancer progression, tumor microenvironment, tumor-infiltrating leukocytes
IL-30 as a New Player in the Immunobiology of Prostate Cancer
As in other types of cancer, the onset and development of prostate cancer depend not only on genetic and epigenetic alterations, but also on multiple signals from the tumor microenvironment. These 2 aberrations may condition each other and synergize in favoring tumor progression.1 In an attempt to decipher microenvironmental messages released within prostate cancer lesions, we ran into the endogenous expression of a newly discovered cytokine, interleukin-30 (IL-30).
Originally identified as p28, i.e., a novel polypeptide related to interleukin-12A (IL12A, best known as IL-12p35),2 IL-30 can bind Epstein–Barr virus induced 3 (EBI3) to form IL-27, which has been shown to mediate antineoplastic effects in several tumor models, but also acts as a self-standing cytokine endowed with its own functional properties.3,4 IL-30 may thus be implicated in quite different molecular pathways depending on contextual parameters. As a matter of fact, IL-30 has been ascribed with a tumor-promoting potential as an independent cytokine, at least in the prostate cancer system.5 We have recently shown that the endogenous expression of IL-30 in the prostate and regional lymph node tissue from prostate cancer patients subjected to radical prostatectomy is associated with poorly differentiated, high tumor grade and metastatic stage of disease.5 Once again, an immunomodulatory molecule, whose functions are for the most part hitherto unknown, has gained a crucial part in the complex scenario of prostate cancer microenvironment. Therein, IL-30 is mostly produced by immune cells of myeloid origin and malignant cells themselves.
Myeloid Immune Cells and Prostate Cancer Cells are the Main Source of IL-30 in the Prostate Cancer Microenvironment
Both the epithelial and stromal components of the prostate are normally infiltrated by a variety of immune cells. Changes in the amount and functional state of prostate-infiltrating immune cells regularly occur in the course of carcinogenesis and tumor progression,6 hence allowing for the establishment of a “dangerous” crosstalk between immune cells and surrounding malignant epithelial and stromal cells. The main producers of IL-30 within prostate cancer lesions and tumor-draining lymph nodes are CD68macrophages, CD33+/CD11b+myeloid cells and CD14+monocytes, which altogether constitute major sources of several other tumor-promoting growth factors. IL-30 appears to be the last, but probably not the least, addition to this growing list.
In the prostate cancer microenvironment, IL-30, in the absence of EBI3, binds to a complex composed of the IL-6 receptor (IL6R) and a homodimer of IL-6 signal transducer IL6ST, best known as gp130.7 Thus, IL-30 may regulate the activity of tumor-infiltrating immune cells.3,4 In addition, since both epithelial and stromal prostate cancer cells express IL6R and gp130 (and their levels increase with tumor stage),8 endogenous IL-30 also appears to operate, via autocrine or paracrine circuitries, on prostate cancer cellular components. We found that IL-30 can promote the proliferation of prostate cancer cells and modulate the expression of a specific set of genes (Fig. 1). Further investigation is required to understand the regulation of IL-30 expression and functions in the (prostate) tumor microenvironment.
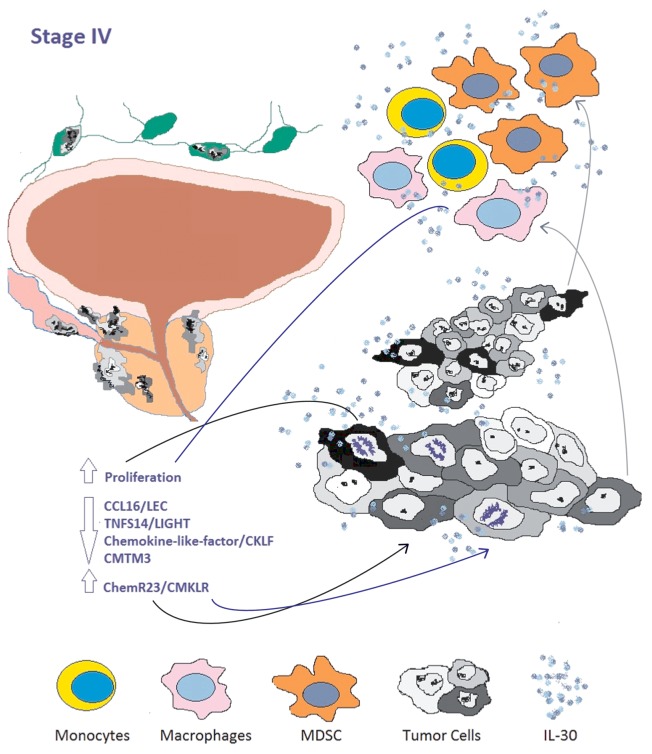
Figure 1. Role of interleukin-30 in the prostate cancer microenvironment. Interleukin-30 (IL-30) may be produced by cancer cells as well as by tumor-reactive cells of myeloid origin, such as monocytes, macrophages and myeloid-derived suppressor cells (MDSCs). These immune cells constitute the major source of IL-30 within the lymph nodes that drain metastatic prostate cancer lesions. IL-30 may act not only on cancer cells of both primary and metastatic tumor lesions, but also on local immune cells and other cell types endowed with appropriate receptors, hence displaying functions that are for the most part hitherto unknown.
Role of IL-30 Secreted within Tumor-Draining Lymph Nodes in Prostate Cancer Progression
Research designed to localize the endogenous source of IL-30 and understand some of its functional roles has yielded the following intriguing findings:
1) The levels of IL-30 in metastasis-free lymph nodes that drain metastatic prostate cancer lesions are comparable to, if not higher than, those detected in lymph nodes containing malignant cells. Thus, it appears that the pre-metastatic lymph node niche is fostered by myeloid-derived cells establishing a cross-talk with components of the primary tumor, which may have triggered their recruitment and activation.
2) The direct effects of IL-30 on human prostate cells in vitro do not involve the activation of canonical metastasis-related genes, but the regulation of genes coding for specific chemokines and chemokine receptors. In particular, IL-30 suppresses the expression of selective leukocyte chemoattractants such as chemokine (C-C motif) ligand 16 (CCL16, best known as LEC), tumor necrosis factor (ligand) superfamily, member 14 (TNFSF14, also known as LIGHT) and chemokine-like factor (CKLF), which recruit immune cells at the tumor site, and consistently downregulates the tumor suppressor and androgen co-repressor CKLF-like MARVEL transmembrane domain containing 3 (CMTM3).9 Human recombinant IL-30 also robustly upregulates the multifunctional receptor chemokine-like receptor 1 (CMKLR1), which is usually expressed by human immature dendritic cells, macrophages and natural killer cells. CMKLR1 binds to retinoic acid receptor responder (tazarotene induced) 2 (RARRES2, best known as chemerin) and is involved in cell migration and inflammation.10 It may therefore be hypothesized that the expression of CMKLR1 by prostate cancer cells may drive their migration toward a chemerin-rich lymph node or more distant sites. This possibility must be precisely addressed to understand the actual significance of IL-30 in the biology of prostate cancer.
A Personalized IL-30-Targeting Treatment to Inhibit the Metastatic Dissemination of Prostate Cancer
Since prostate cancer is a typical age-related malignancy, its incidence is expected to augment in the foreseeable future as a consequence of the increased longevity of the Western populations. Prostate cancer is a heterogeneous tumor, manifesting in variants that can range from a slow-growing to a rapidly fatal systemic disease with overt metastatic dissemination at presentation. The clinical management of fragile elderly patients bearing locally invasive or metastatic prostate cancer will thus become a substantial public health issue. The elaboration of non-invasive, well-tolerated therapeutic strategies aimed at extending patient survival and at preserving quality of life has a strong rationale in this context. The assessment of both genetic aberrations and specific microenvironmental signals in prostate cancer biopsies is the mandatory first step toward the design of patient-tailored and effective treatment protocols. Our study reveals that only prostate cancer cells forming moderately-to-poorly differentiated tumors, may effectively produce IL-30. Indeed, the production of IL-30 was documented in only about 21% of localized, organ-confined (Stage I-III) tumors, and in about 41% of tumors with lymph node involvement (Stage IV). Altogether, our findings further highlight the heterogeneity of prostate cancer, which depends not only on histological and genetic features, but also on a variety of microenvironmental clues. These characteristics may be harnessed jointly to distinguish patients diagnosed with the same disease but a having different prognosis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the Associazione Italiana Ricerca Cancro (AIRC, Investigator Grant n. 13134), Milano, Italy, and the “Umberto Veronesi” Foundation for the Progress of Sciences, Milano, Italy to E. Di Carlo.
Citation: Di Carlo E. Interleukin-30: a novel microenvironmental hallmark of prostate cancer progression. OncoImmunology 2013; 2:e27618; 10.4161/onci.27618
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27618
References
- 1.Prendergast GC, Jaffee EM. Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res. 2007;67:3500–4. doi: 10.1158/0008-5472.CAN-06-4626. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–90. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 3.Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Wang Z, Ye N, Chen Z, Zhou X, Teng X, Huang N, Liu N, Zhang N, Guan T, et al. A protective role of IL-30 via STAT and ERK signaling pathways in macrophage-mediated inflammation. Biochem Biophys Res Commun. 2013;435:306–12. doi: 10.1016/j.bbrc.2013.03.136. [DOI] [PubMed] [Google Scholar]
- 5.Di Meo S, Airoldi I, Sorrentino C, Zorzoli A, Esposito S, Di Carlo E. Interleukin-30 expression in prostate cancer and its draining lymph nodes correlates with advanced grade and stage. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-2240. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 6.Di Carlo E, D’Antuono T, Pompa P, Giuliani R, Rosini S, Stuppia L, Musiani P, Sorrentino C. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clin Cancer Res. 2009;15:2979–87. doi: 10.1158/1078-0432.CCR-08-1951. [DOI] [PubMed] [Google Scholar]
- 7.Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Hölscher C, Rose-John S, Grötzinger J, Lorenzen I, Scheller J. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem. 2013;288:4346–54. doi: 10.1074/jbc.M112.432955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobisch A, Rogatsch H, Hittmair A, Fuchs D, Bartsch G, Jr., Klocker H, Bartsch G, Culig Z. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–44. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H, Li H, Shu XS, Li H, Liu W, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res. 2009;69:5194–201. doi: 10.1158/0008-5472.CAN-08-3694. [DOI] [PubMed] [Google Scholar]
- 10.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–8. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]