Abstract
Background
We aimed to investigate prevalence and prognostic role of SOX2, PIK3CA, FGFR1 and BRF2 gene gain in patients with surgically resected non-small cell lung cancer (NSCLC).
Methods
SOX2, PIK3CA, FGFR1 and BRF2 gene copy number was assessed by fluorescence in situ hybridization (FISH) in arrayed tissue cores from 447 resected NSCLCs.
Results
Increased gene copy number (FISH+) for SOX2, PIK3CA, FGFR1 and BRF2 was observed in 23.6%, 29.2%, 16.6% and 14.9% of cases, respectively. FISH+ status for each gene was significantly associated with smoking history, squamous cell carcinoma (SCC) histology, and increased copy number of the other studied genes. Multivariate analysis of overall survival indicated increased SOX2 gene copy number (P = 0.008), stage I-II (P<0.001), and adenocarcinoma or SCC histology (P = 0.016) as independent, favorable prognostic factors. A statistically significant interaction was observed between stage and SOX2 gene status (P = 0.021), indicating that the prognostic impact of SOX2 gene gain differs across stages and is limited to patients with stage I-II disease (HR 0.44, 95% CI: 0.25–0.77; P = 0.004, adjusted for histology).
Conclusions
Increased SOX2 gene copy number is an independent and favorable prognostic factor in surgically resected, early stage NSCLC, regardless of histology. SOX2, PIK3CA, FGFR1 and BRF2 gene gains are likely to occur concurrently, with potentially relevant implications for the development of new therapeutic strategies.
Introduction
Lung cancer is the leading cause of cancer death worldwide and survival rates are poor. In the last few years, progress in the knowledge of lung cancer biology led to the identification of activated oncogenes that can be therapeutically targeted by novel agents. Among those are the tyrosine kinase inhibitors, such as gefitinib and erlotinib for patients with Epidermal Growth Factor Receptor (EGFR) mutations [1]–[2] and, more recently, crizotinib for subjects with Anaplastic Lymphoma Kinase (ALK) rearrangements [3]. These agents have significantly improved the outcome of biologically selected non-small cell lung cancer (NSCLC) patients with advanced disease, encouraging the identification of novel therapeutic targets.
The SRY (sex determining region Y)-box 2 (SOX2) gene, located on chromosome 3q26.33, encodes for a member of the SRY-related HMG-box family of transcription factors and has been implicated in pluripotency regulation in embryonic stem cells [4]. Upregulation of SOX2 induces cell proliferation and anchorage-independent growth in lung squamous cell carcinoma (SCC) cell lines and drives lung tumorigenesis in mice [5]-[6]. Amplification of SOX2 has been associated with lung SCC [5]–[8], with retrospective data suggesting a trend for improved survival in favor of SOX2 gene gain in surgically resected patients [7]–[8].
The PIK3CA gene encodes for the catalytic subunit p110α of class IA phosphatidylinositol 3-kinases (PI3K) and has been identified to function as an oncogene in human malignancies activated by gene amplification or mutations [9]. Importantly, preclinical models showed that inhibition of the PI3K pathway impairs survival of NSCLC cells harboring PIK3CA gene alterations [10]–[11], and numerous novel PI3K, Akt and mTOR inhibitors have entered clinical trial testing for the treatment of PI3K-addicted cancers. PIK3CA is located on chromosome 3q26.32, close to the SOX2 locus. Comparative genomic hybridization studies have shown distinct amplification levels for SOX2 and PIK3CA in lung SCC with 3q26 gain [5], suggesting that they might play independent oncogenic roles.
The fibroblast growth factor receptor 1 (FGFR1), which is encoded by a gene located on chromosome 8p12.1, is a member of a four tyrosine kinase receptor family (FGFR1–4) and has been widely studied as a key receptor involved in embryonic development [12]. Amplification of FGFR1 has been recently reported in up to 22% of lung SCC [13] and has been associated with sensitivity to FGFR1 tyrosine kinase inhibitors in multiple preclinical models, including lung SCC cell lines [13]–[15], leading to clinical trials of these agents in FGFR1-amplified tumors.
The B-related factor 2 (BRF2) gene, located on chromosome 8p11.23 very close to FGFR1, encodes a subunit of RNA polymerase III (Pol III) transcription initiation complex, which is responsible for the transcription of a number of noncoding RNA genes whose products are involved in protein synthesis, RNA processing and transcription [16]. The RNA pol III is often deregulated in cancer, and BRF2 was recently identified as a novel oncogene in lung SCC activated by increased copy number [17]. In fact, BRF2 amplification determines an increased RNA Pol III activity and sustained cell proliferation and survival in vitro [17].
Alterations in copy number of the SOX2, PIK3CA, FGFR1 and BRF2 genes have been individually studied in a number of NSCLC populations, particularly in lung SCC, with limited and sometimes conflicting data regarding their prognostic impact. Moreover, the prevalence of copy number gains of more than one gene and its effect on patient survival is still largely unexplored. The present study was conducted to investigate the prevalence of SOX2, PIK3CA, FGFR1 and BRF2 gene copy number changes in a large, unselected cohort of surgically resected NSCLC patients, to verify concurrent genomic gains, and to determine whether copy number alterations in these genes affect patient outcome.
Patients and Methods
Ethics Statement
Institutional Review Board (IRB) approval (#304/12) was obtained from the Istituto Clinico Humanitas ethical committee. Written informed consent was obtained from patients undergoing active follow up at our institution. IRB waived the requirements for written informed consent from patients that could not be reached. The study was conducted in accordance with ethical principles stated in the most recent version of the Declaration of Helsinki.
Patients
This retrospective study was conducted in a previously reported cohort of 447 Caucasian NSCLC patients that received a radical resection of a primary NSCLC between 2000 and 2004 at Istituto Clinico Humanitas (Rozzano, Italy) [18]. A tissue microarray (TMA) was constructed using 0.6 mm diameter cores.
Fluorescence In Situ Hybridization (FISH) Analysis
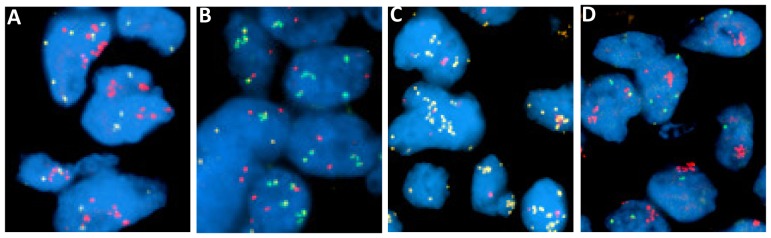
The Bacterial Artificial Chromosome (BAC) clones RP11-459K06, RP11-245C23, RP11-168H08 and RP11-350N15, respectively containing human DNA inserts from regions homologous to SOX2, PIK3CA, BFR2 and FGFR1, were used for the enumeration FISH probes, according to previously described protocols [19]–[20]. Copy number per cell for each gene was enumerated on at least 50 tumor cells from 2 tissue cores per patient by an expert cytogeneticist blinded to patient data. In absence of validated FISH scoring criteria for these genes, a pre-specified cut-off of mean ≥4 gene copies/cell, previously used to define gene gain with other techniques [13], or presence of gene clusters indicating true gene amplification, was set to identify cases with increased gene copy number (FISH+). Examples of FISH patterns are shown in figure 1.
Figure 1. Specimen B5b showing gene amplification for FGFR1 (A, red) and BRF2 (B, green) and low copy number for SOX2 (A, gold), and PIK3CA (B, red); specimen C4d showing gene amplification for SOX2 (C, gold) and PIK3CA (D, red) and low copy number for FGFR1 (C, red) and BRF2 (D, green).
Statistical Analysis
Associations between demographical and clinical characteristics and FISH status were estimated using the continuity adjusted Chi-square or the Fisher exact test, when appropriate. Pearson correlation coefficient was used to test the correlation between FISH status of each gene.
Overall survival (OS), calculated from the time of diagnosis to patient death or last contact, was evaluated using the Kaplan–Meier method. Differences between groups were evaluated by using log rank test. Hazard ratios with their corresponding 95% confidence intervals (95% CI) were calculated using the Cox proportional hazards regression model. Statistical significance was set at <0.05 for each analysis. All statistical analyses were carried out using R package.
Results
Patient Characteristics
The study included a cohort of 447 patients with surgically resected NSCLC. Details of the cohort have been previously described [18] and patient characteristics are summarized in table 1. Briefly, the majority of patients were male (82.8%), former (53.2%) or current smokers (36.0%), with histological grade 1 or 2 tumors (64.2%). All patients underwent radical surgery and tumors were staged according to the TNM classification with evidence of pathological stage I in 37.1% of cases, stage II in 22.1%, stage III in 32.7% and stage IV in 8.1%. The latter group included patients with a solitary metastatic lesion, mostly in the brain or adrenal glands, amenable of surgery or stereotactic radiosurgery. Patients with stage III pN2 disease (n = 101) received mediastinal post-operative radiotherapy. Overall, given the surgical timeframe (2000–2004), no patients with stage I-II tumors received post-operative systemic treatment, while only 18 subjects with stage III disease were treated with adjuvant platinum-based chemotherapy. The majority of patients were diagnosed with adenocarcinoma (54.6%), while 138 (30.9%) and 65 (14.5%) patients had SCC or other histology, respectively. After a median follow up of 60.4 months, 244 deaths occurred and median survival was 42.5 months. Significantly longer survival was observed for patients with stage I–II (herein referred to as “early stage”) tumors when compared with those with stage III–IV disease (P<0.001). Patients with SCC or adenocarcinoma had similar outcome and lived longer than patients with other histologies (P<0.001). No survival difference was observed according to gender, smoking history or pathological grade.
Table 1. Patient characteristics.
| Characteristics | n | %a |
| Total | 447 | 100 |
| Sex | ||
| Male | 370 | 82.8 |
| Female | 77 | 17.2 |
| Smoking history | ||
| Never | 40 | 8.9 |
| Former | 238 | 53.2 |
| Current | 161 | 36.0 |
| Unknown | 8 | 1.8 |
| Histology | ||
| Adenocarcinoma | 244 | 54.6 |
| SCC | 138 | 30.9 |
| Other | 65 | 14.5 |
| Stage | ||
| I | 166 | 37.1 |
| II | 99 | 22.1 |
| III | 146 | 32.7 |
| IV | 36 | 8.1 |
| Grade | ||
| I | 32 | 7.2 |
| II | 255 | 57.0 |
| III | 158 | 35.3 |
| Not reported | 2 | 0.4 |
percentages have been rounded and may not total 100%.
Abbreviation: SCC = squamous cell carcinoma.
SOX2 and PIK3CA Copy Number Gain
SOX2 and PIK3CA gene status was successfully determined in 445 (99.6%) and 435 (97.3%) patients, respectively. Overall, SOX2 increased gene copy number was detected in 105 cases (23.6%), with true gene amplification observed in 19 patients (4.3%). PIK3CA gene gain was found in 29.2% of patients, including 21 cases (4.8%) with true gene amplification. As expected given their physical proximity, a statistically significant association was observed for copy number gain between SOX2 and PIK3CA (Pearson correlation coefficient: 0.78, P<0.001), with discordant FISH results between the two genes in only 9.9% of cases. This association was maintained across all histological subtypes (data not shown). As summarized in table 2, both SOX2+ and PIK3CA+ status significantly associated with male gender, former/current smoking history, and SCC histology. Additionally, PIK3CA gene gain was more commonly observed in grade III tumors (P = 0.040). All cases with SOX2 true gene amplification were SCC, for an overall prevalence of 13.8% SOX2-amplified tumors in patients with SCC histology. Similarly, PIK3CA true gene amplification was found in 12.8% of cases with SCC, while only 4 patients with non-squamous tumors were PIK3CA-amplified, including 3 adenocarcinomas and one pleomorphic carcinoma.
Table 2. Associations between FISH status and patient characteristics.
| SOX2 | PIK3CA | FGFR1 | BRF2 | |||||||||||||||||
| (N = 445) | (N = 435) | (N = 445) | (N = 435) | |||||||||||||||||
| − | + | − | + | − | + | − | + | |||||||||||||
| n | % | n | % | P value | n | % | n | % | P value | n | % | n | % | P value | n | % | n | % | P value | |
| Total | 340 | 76.4 | 105 | 23.6 | 308 | 70.8 | 127 | 29.2 | 371 | 83.4 | 74 | 16.6 | 370 | 85.1 | 65 | 14.9 | ||||
| Sex | ||||||||||||||||||||
| Male | 273 | 74.0 | 96 | 26.0 | 0.012 | 243 | 67.7 | 116 | 32.3 | 0.003 | 305 | 82.7 | 64 | 17.3 | 0.469 | 300 | 83.6 | 59 | 16.4 | 0.085 |
| Female | 67 | 88.2 | 9 | 11.8 | 65 | 85.5 | 11 | 14.5 | 66 | 86.8 | 10 | 13.2 | 70 | 92.1 | 6 | 7.9 | ||||
| Smoking History | ||||||||||||||||||||
| Never | 37 | 92.5 | 3 | 7.5 | 0.010 | 35 | 89.7 | 4 | 10.3 | 0.005 | 37 | 92.5 | 3 | 7.5 | 0.121 | 38 | 97.4 | 1 | 2.6 | 0.018 |
| Former + Current | 296 | 74.4 | 102 | 25.6 | 265 | 68.3 | 123 | 31.7 | 327 | 82.2 | 71 | 17.8 | 324 | 83.5 | 64 | 16.5 | ||||
| Histology | ||||||||||||||||||||
| SCC | 69 | 50.0 | 69 | 50.0 | <0.001a | 55 | 41.3 | 78 | 58.7 | <0.001a | 99 | 71.7 | 39 | 28.3 | <0.001a | 95 | 71.4 | 38 | 28.6 | <0.001a |
| Adenocarcinoma | 217 | 89.3 | 26 | 10.7 | 199 | 84.0 | 38 | 16.0 | 215 | 88.5 | 28 | 11.5 | 215 | 90.3 | 23 | 9.7 | ||||
| Other | 54 | 84.4 | 10 | 15.6 | 54 | 83.1 | 11 | 16.9 | 57 | 89.1 | 7 | 10.9 | 60 | 93.8 | 4 | 6.2 | ||||
| Stage | ||||||||||||||||||||
| I–II | 200 | 75.5 | 65 | 24.5 | 0.654 | 179 | 69.4 | 79 | 30.6 | 0.495 | 222 | 83.8 | 43 | 16.2 | 0.883 | 221 | 85.7 | 37 | 14.3 | 0.773 |
| III–IV | 140 | 77.8 | 40 | 22.2 | 129 | 72.9 | 48 | 27.1 | 149 | 82.8 | 31 | 17.2 | 149 | 84.2 | 28 | 15.8 | ||||
| Grade | ||||||||||||||||||||
| I–II | 226 | 79.0 | 60 | 21.0 | 0.089 | 207 | 74.2 | 72 | 25.8 | 0.040 | 241 | 84.3 | 45 | 15.7 | 0.545 | 239 | 85.4 | 41 | 14.6 | 0.881 |
| III | 112 | 71.3 | 45 | 28.7 | 99 | 64.3 | 55 | 35.7 | 128 | 81.5 | 29 | 18.5 | 129 | 84.3 | 24 | 15.7 | ||||
SCC vs non-squamous histology.
Abbreviation: SCC = squamous cell carcinoma.
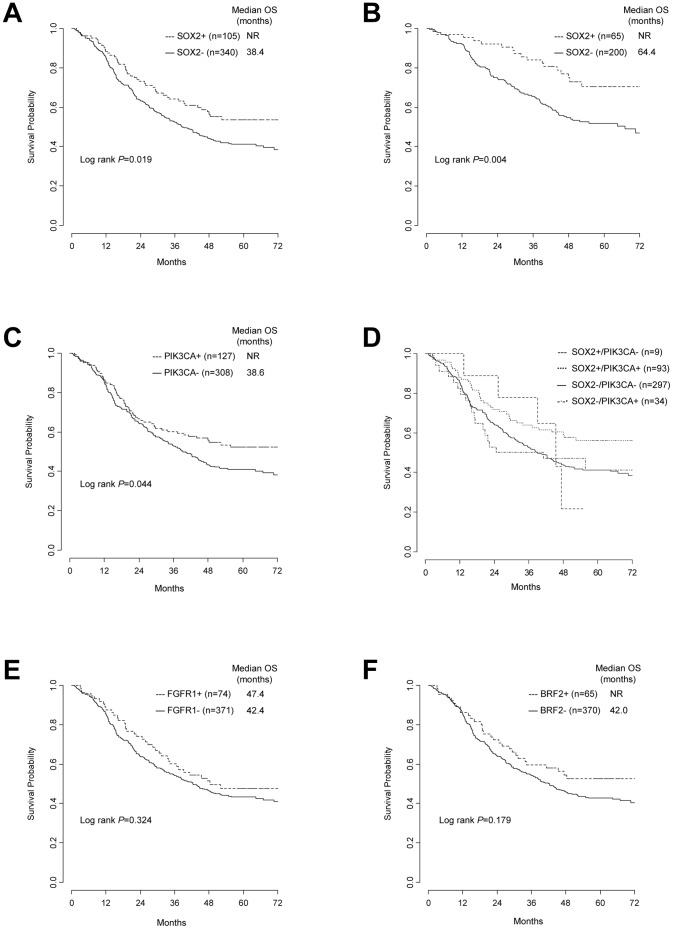
SOX2+ patients had a significantly longer survival than SOX2- subjects (53.7% vs 41.1% 5-year OS, P = 0.019) (figure 2A), with no difference in outcome between cases with ≥4 mean gene copies/cell and those with true gene amplification (P = 0.937). Subgroup analyses according to single clinico-pathological characteristics, including stage, histology, sex, smoking status and grade, detected a significant survival advantage for SOX2+ patients in the subset of early stage (70.4% vs 51.8% 5-year OS, P = 0.004, figure 2B) and grade 3 tumors (52.3% vs 37.4% 5-year OS, P = 0.037). In patients with stage III–IV disease, no survival difference was detected between SOX2+ and SOX2- cases (P = 0.993), while a trend for a better outcome in favor of SOX2+ subjects was observed in the SCC subgroup (P = 0.053).
Figure 2. Kaplan-Meier overall survival estimates according to gene copy number.
(A) SOX2, whole cohort. (B) SOX2, stage I-II. (C) PIK3CA, whole cohort. (D) SOX2/PIK3CA, whole cohort. (E) FGFR1, whole cohort. (F) BRF2, whole cohort. Abbreviations: OS = overall survival; NR = not reached.
Survival data according to PIK3CA gene status were similar to SOX2, likely as a result of the substantial overlap between the FISH status of these two genes. In fact, a statistically significant survival advantage was observed for PIK3CA+ patients when compared with PIK3CA- patients in the whole population (52.2% vs. 40.9% 5-year OS, P = 0.044) (figure 2C). The survival advantage for PIK3CA+ patients remained statistically significant only in the subset of early stage tumors (69.5% vs 51.5% 5-year OS, P = 0.005), with a trend for improved survival in patients with SCC (P = 0.064).
We further aimed to explore the survival patterns of patients with discordant SOX2/PIK3CA gene status (n = 43). As shown in figure 2D, there was similar outcome between SOX2-/PIK3CA+ (n = 34) and double negative (n = 297) patients, while survival of SOX2+/PIK3CA- individuals (n = 9) appeared to be similar to that of the double positive group (n = 93).Those findings suggested that PIK3CA gene gain was not per se prognostically relevant.
FGFR1 and BRF2 Copy Number Gain
A total of 445 (99.6%) patients were assessable for FGFR1 gene copy number, with 16.6% FISH+ patients, including 37 cases (8.3%) with true gene amplification. FGFR1 gene gain was significantly more common in patients with SCC than in those with adenocarcinoma or other histologies (SCC vs non-SCC: 28.3% vs 11.4%, P<0.001,) while no association with gender, smoking status, stage or grade was found (table 2). FGFR1 true gene amplification occurred in 17.4% of SCC but was also observed in 3/26 (11.5%) tumors with neuroendocrine differentiation and 2/28 (7.1%) never-smokers with lung adenocarcinoma, including a female with a concurrent EGFR L858R mutation.
BRF2 gene status was evaluated by FISH in 435 (97.3%) patients and increased gene copy number was observed in 14.9% of cases, including 27 patients (6.2%) with gene amplification. As shown in table 2, former/current smoking history and SCC histology were significantly associated with BRF2+ status. Likely in consequence of their close physical location on chromosome 8p11-12, a significant association was observed between FGFR1 and BRF2 gene copy number (Pearson correlation coefficient: 0.78, P<0.001), with only 5.3% of cases with discordant FISH results. The association between the two genes was independent of histology (data not shown). Furthermore, significant associations were observed between copy number gains of genes located on different chromosomes (FGFR1 or BRF2 with SOX2 or PIK3CA, P<0.001 for each association), regardless of histology. Particularly, SOX2 and PIK3CA gene gains were observed in 55.4% and 75.7%, respectively, of FGFR1+ tumors.
The FGFR1+ or BRF2+ patients survived longer than those with no or low copy number gain, although this difference did not reach statistical significance (FGFR1, P = 0.324; BRF2, P = 0.179) (figures 2E and 2F). FGFR1 gene copy number failed to show a significant prognostic impact when analysing survival according to clinico-pathological characteristics, including sex, smoking status, stage, histology and grade (data not shown). Conversely, a significant survival advantage was observed for BRF2+ patients with early stage disease when compared with the BRF2- group (74.6% vs 54.0% 5-year OS, P = 0.040), while no prognostic relevance was observed for BRF2 gene copy number in other patient subsets (data not shown). Due to the substantial overlap between FGFR1 and BRF2 FISH status, no survival differences were found in the whole population when grouping patients according to both genes (data not shown). Similarly, no survival impact was observed when combining FGFR1 or BRF2 with SOX2 or PIK3CA FISH results in the whole population or in clinically-defined subgroups (data not shown).
Multivariate Analysis
As previously mentioned, factors associated with survival were stage, histology, SOX2 and PIK3CA gene copy number. Considering the strong association between SOX2 and PIK3CA gene status, the two genes were assessed in two separate models. In the SOX2 model, SOX2 gene gain, early stage and adenocarcinoma/SCC histology emerged as independent prognostic factors for improved survival (HRSOX2+ vs SOX2- 0.48, 95% CI: 0.28–0.83, P = 0.008; HRstage I-II vs III-IV 0.49, 95% CI: 0.37–0.65; P<0.001; HR adenocarcinoma/SCC vs other 0.67, 95% CI: 0.46-0.93; P = 0.016). Importantly, a statistically significant interaction was observed between stage and SOX2 gene status (P = 0.021), indicating that the prognostic impact of increased SOX2 gene copy number differed according to tumor stage and was limited to patients with early stage disease (HR 0.44, 95% CI: 0.25–0.77; P = 0.004, adjusted for histology). In contrast, in the multivariate model considering PIK3CA gene copy number, stage and histology, PIK3CA gene gain failed to predict a significant longer survival (P = 0.135).
The survival impact observed for BRF2 gene gain in patients with early stage disease was not confirmed in the multivariate model after correction for histology (P = 0.135), although the small number of BRF2+ cases precluded robust conclusions.
Discussion
This study evaluated the prognostic relevance of copy number alterations of four oncogenes previously associated with lung SCC - SOX2, PIK3CA, FGFR1 and BRF2 - in a large cohort of surgically resected NSCLC patients. For the first time, we report increased SOX2 gene copy number assessed by FISH as an independent favorable prognostic factor in patients with stage I and II NSCLC, regardless of histology.
SOX2, a transcription factor that plays a key role in embryonic development, has recently emerged as an attractive therapeutic target in lung cancer. In fact, amplification and/or overexpression of SOX2 have been reported in lung SCC, lung adenocarcinoma and, more recently, small-cell lung cancer [5], [20]–[21]. Preclinical reports have been consistent in showing that SOX2 silencing leads to significant impairment of cell growth in lung cancer models, supporting SOX2 inhibition as a promising anticancer strategy in lung malignancies [5], [22].
Defining FISH+ status as mean ≥4 gene copies/cell or presence gene clusters (true gene amplification), SOX2 gene gain was observed in about one fourth of NSCLCs and was significantly associated with male gender, exposure to tobacco smoke and SCC histology, as previously reported by others [5]–[7]. Particularly, half of lung SCC categorized as FISH+, with true gene amplification occurring in 13.8% of cases, while lung adenocarcinomas presented increased SOX2 gene copy number in 10.7% of samples with no cases harbouring true gene amplification. These findings are roughly in line with other FISH studies in NSCLC, in which different scoring criteria were used [7]–[8].
The prognostic role of SOX2 has been investigated in retrospective NSCLC series. Despite data from a recent meta-analysis suggesting a favorable prognostic impact for SOX2 expression in NSCLC regardless of histology,[23] most studies showed that SOX2 protein overexpression predicted prolonged survival in surgically resected lung SCC [7]–[8], while its survival impact in lung adenocarcinoma remains controversial [21], [24]. Two studies have shown a significant association between SOX2 protein expression evaluated by immunohistochemistry and SOX2 gene gain assessed by FISH, and suggested a favorable prognostic role for increased SOX2 gene copy number in lung SCC, although statistical significance was not reached [7]–[8]. Particularly, Wilbertz et al. detected a trend for improved survival in lung SCC patients with ≥10 SOX2 copies/cell compared with tumors with no or low level amplification, but this effect was lost in the multivariate model [7].
For the first time, we reported a statistically significantly improved overall survival for SOX2 FISH+ patients with stage I and II NSCLC. Importantly, the favorable prognostic effect of SOX2 gene gain observed in our study, where non-SCC patients accounted for about 38% of SOX2+ cases, was independent of histology and PIK3CA gene gain. Our findings contrast with those of another study where a negative survival impact for SOX2 low level amplification, compared to lack of amplification, was observed in lung adenocarcinoma [7]. Additionally, our data indicate that the survival benefit observed in unselected early stage NSCLC was not limited to patients with true gene amplification, but was also extended to those with mean ≥4 SOX2copies/cell and no gene clusters. The discrepancies between our study and that of Wilbertz et al. might be explained by differences in FISH scoring criteria and patient clinical characteristics. The reason for the lack of a survival impact for SOX2 gene gain in patients with stage III and IV disease in our cohort is unclear and should be further investigated. We speculate that SOX2 gene gain is an early event in lung tumorigenesis and that tumor progression leads to additional molecular abnormalities that affect patient outcome.
FGFR1 has recently emerged as a promising target in NSCLC after the gene was reported as amplified in about 20% of lung SCC [13]–[14], [25], leading to early phase clinical trials of anti-FGFR1 agents in FGFR1-amplified NSCLC. Our data confirmed the previously reported association between increased FGFR1 gene copy number and SCC histology. Importantly, FGFR1 gene gain was also found in 11.4% of non-squamous tumors, including true gene amplification in about 7% of adenocarcinoma patients with never smoking history - the same population with an increased likelihood of harboring EGFR mutations or ALK rearrangements - and 11% among neuroendocrine tumors. Although the small numbers of these subgroups preclude any firm conclusion, our data suggest that FGFR1 gene copy number assessment should be pursued in selected patients with non-squamous tumors to identify candidates for anti-FGFR1 agents.
In the present study, we observed that concurrent copy number gains of SOX2, PIK3CA, FGFR1 or BRF2 were common. While the strong correlation between SOX2 and PIK3CA and between FGFR1 and BRF2 gene status was anticipated because of their proximity on chromosomes 3q26 and 8p11-12, respectively, our study is the first to report statistically significant associations between copy number gains in both genomic regions, regardless of histology. In contrast, Weiss et al. described that FGFR1 and SOX2 amplifications were mutually exclusive in lung SCC, although in that study the genomic gain was investigated by single-nucleotide polymorphism arrays and different thresholds for copy number changes were used [13]. Our findings should raise caution in the development of PI3K or FGFR1 inhibitors as single agents in NSCLCs with PIK3CA or FGFR1 gene gain, respectively. In fact, in presence of concurrent genomic gain of multiple oncogenes concomitant inhibition of more than one target could be required to effectively impair tumor growth. This concept is supported by the observation that PI3K inactivation with RNA interference technology, in lung SCC cell lines with 3q26 amplification, produced only limited effects on cell proliferation as opposed to SOX2 knockdown [5]. Moreover, an intriguing observation was the coexistence of FGFR1 true gene amplification and EGFR L858R mutation in a female, never smoker patient with lung adenocarcinoma, suggesting that some NSCLCs could be co-dependent on FGFR1 and EGFR for survival. Overall, these data suggest that in some tumors the assessment of a single drug target may not be sufficient to predict drug sensitivity, addressing the need for a deeper understanding of NSCLC biology.
Our study showed no statistically significant prognostic role for FGFR1 or BRF2 gene copy number in surgically resected NSCLC patients when using our predefined FISH scoring criteria, although a trend for improved survival for FGFR1+ or BRF2+ patients could be observed in the whole population. Other investigators have recently explored whether FGFR1 gene gain affects survival in NSCLC with inconclusive results [13], [26]–[29]. In fact, while some authors reported a survival advantage for NSCLCs with increased FGFR1 gene copy number [28], a recent Korean study showed that FGFR1 high level amplification negatively affects survival in surgically resected lung SCC [27]. Different techniques, scoring methods, and criteria for patient selection (i.e. histology, ethnicity, stage, perioperative treatments) might account for the differences observed across studies, encouraging the assessment of the prognostic role of FGFR1 gene copy number in uniformly selected cohorts.
In conclusion, we showed for the first time that increased SOX2 gene copy number is significantly associated with improved survival in surgically resected stage I and II NSCLC patients, regardless of histology. We also found that SOX2 gene gain is associated with copy number gains of other actionable oncogenes, including FGFR1 and PIK3CA. Our findings confirm the complexity of NSCLC biology and encourage the exploration of novel therapeutic combinations.
Acknowledgments
We thank the Molecular Pathology/Cytogenetics Shared Resource of the University of Colorado for technical assistance.
Funding Statement
This work was supported by private donation from Mr. Gerry Scotti, Colorado Lung Cancer SPORE NCI P50CA058187, NCI CCSG P30CA046934 and NIH/NCATS Colorado CTSI KL2TR000156 grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- 2. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 3. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, et al. (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamachi Y, Uchikawa M, Kondoh H (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet 16: 182–187. [DOI] [PubMed] [Google Scholar]
- 5. Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, et al. (2009) SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 41: 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussenet T, Dali S, Exinger J, Monga B, Jost B, et al. (2010) SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 5: e8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, et al. (2011) SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol 24: 944–953. [DOI] [PubMed] [Google Scholar]
- 8. Brcic L, Sherer CK, Shuai Y, Hornick JL, Chirieac LR, et al. (2012) Morphologic and clinicopathologic features of lung squamous cell carcinomas expressing Sox2. American journal of clinical pathology 138: 712–718. [DOI] [PubMed] [Google Scholar]
- 9. Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto H, Shigematsu H, Nomura M, Lockwood WW, Sato M, et al. (2008) PIK3CA mutations and copy number gains in human lung cancers. Cancer Res 68: 6913–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spoerke JM, O'Brien C, Huw L, Koeppen H, Fridlyand J, et al. (2012) Phosphoinositide 3-Kinase (PI3K) Pathway Alterations Are Associated with Histologic Subtypes and Are Predictive of Sensitivity to PI3K Inhibitors in Lung Cancer Preclinical Models. Clin Cancer Res 18: 6771–6783. [DOI] [PubMed] [Google Scholar]
- 12. Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149. [DOI] [PubMed] [Google Scholar]
- 13. Weiss J, Sos ML, Seidel D, Peifer M, Zander T, et al. (2010) Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2: 62ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, et al. (2011) Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One 6: e20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Zhang L, Su X, Li M, Xie L, et al. (2012) Translating the Therapeutic Potential of AZD4547 in FGFR1-Amplified Non-Small Cell Lung Cancer through the Use of Patient-Derived Tumor Xenograft Models. Clin Cancer Res 18: 6658–6667. [DOI] [PubMed] [Google Scholar]
- 16. Cabarcas S, Schramm L (2011) RNA polymerase III transcription in cancer: the BRF2 connection. Mol Cancer 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lockwood WW, Chari R, Coe BP, Thu KL, Garnis C, et al. (2010) Integrative genomic analyses identify BRF2 as a novel lineage-specific oncogene in lung squamous cell carcinoma. PLoS Med 7: e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, et al. (2009) Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 27: 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arcaroli JJ, Quackenbush KS, Powell RW, Pitts TM, Spreafico A, et al. (2012) Common PIK3CA mutants and a novel 3' UTR mutation are associated with increased sensitivity to saracatinib. Clin Cancer Res 18: 2704–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, et al. (2012) Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL (2010) Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol 34: 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiang R, Liao D, Cheng T, Zhou H, Shi Q, et al. (2011) Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer 104: 1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Huang Y, Chen J, Wang S, Zhou J (2013) The Prognostic Value of SOX2 Expression in Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS One 8: e71140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, et al. (2013) High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One 8: e61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schildhaus HU, Heukamp LC, Merkelbach-Bruse S, Riesner K, Schmitz K, et al. (2012) Definition of a fluorescence in-situ hybridization score identifies high- and low-level FGFR1 amplification types in squamous cell lung cancer. Mod Pathol 25: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heist RS, Mino-Kenudson M, Sequist LV, Tammireddy S, Morrissey L, et al. (2012) FGFR1 Amplification in Squamous Cell Carcinoma of The Lung. J Thorac Oncol 7: 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim HR, Kim DJ, Kang DR, Lee JG, Lim SM, et al. (2012) Fibroblast Growth Factor Receptor 1 Gene Amplification Is Associated With Poor Survival and Cigarette Smoking Dosage in Patients With Resected Squamous Cell Lung Cancer. J Clin Oncol 31: 731–737. [DOI] [PubMed] [Google Scholar]
- 28. Tran TN, Selinger CI, Kohonen-Corish MR, McCaughan BC, Kennedy CW, et al. (2013) Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer 81: 462–467. [DOI] [PubMed] [Google Scholar]
- 29. Craddock KJ, Ludkovski O, Sykes J, Shepherd FA, Tsao MS (2013) Prognostic value of fibroblast growth factor receptor 1 gene locus amplification in resected lung squamous cell carcinoma. J Thorac Oncol 8: 1371–1377. [DOI] [PubMed] [Google Scholar]