Abstract
Purpose
High meat consumption, especially red and processed meat consumption is associated with an increased risk of several cancers, however, evidence for oral cavity and oropharynx cancer is limited. Thus, we performed this meta-analysis to determine the association between intakes of total meat, processed meat, red meat, and white meat, and the risk of oral cavity and oropharynx cancer.
Methods
Electronic search of Pubmed, Embase, and Cochrane Library Central database was conducted to select relevant studies. Fixed-effect and random-effect models were used to estimate summary relative risks (RR) and the corresponding 95% confidence intervals (CIs). Potential sources of heterogeneity were detected by meta-regression. Subgroup analyses and sensitivity analysis were also performed.
Results
12 case–control studies and one cohort study were included in the analyses, including 501,730 subjects and 4,104 oral cavity and oropharynx cancer cases. Pooled results indicated that high consumption of total meat, red meat, and white meat were not significantly associated with increased risk of oral cavity and oropharynx cancer (RR = 1.14, 95% CI[0.78–1.68]; RR = 1.05, 95% CI[0.66, 1.66] and RR = 0.81, 95% CI[0.54, 1.22], respectively), while the high consumption of processed meat was significantly associated with a 91% increased risk of oral cavity and oropharynx cancer (RR = 1.91, 95% CI [1.19–3.06]). Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results.
Conclusions
The present meta-analysis suggested that high consumption of processed meat was significantly associated with an increased risk of oral cavity and oropharynx cancer, while there was no significantly association between total meat, red meat or white meat and the risk of oral cavity and oropharynx cancer. More prospective cohort studies are warranted to confirm these associations.
Introduction
Oral cavity and oropharynx cancer are the tenth most common cancer and seventh most common cause of cancer-related mortality worldwide [1], [2]. The primary risk factors for oral cavity and oropharynx cancer have been well documented, including betel-quid chewing, tobacco smoking, and alcohol consumption [2]. However, the roles of many putative risk factors in etiology of oral cavity and oropharynx cancer remain unclear. Numerous studies have shown that diet may also be of etiologic importance. As we know, meat plays an important part in a healthy, balanced diet, and high meat consumption, especially red and processed meat consumption has been found to be associated with an increased risk of several malignancies, such as colorectal cancer [3], esophageal cancer [4], lung cancer [5], bladder cancer [6], and renal cancer [7]. The association of meat consumption as a potential risk for oral cavity and oropharynx cancer has been studied in several observational studies, however, these studies yielded different or even controversial results. For example, Lissowska J et al found that consumption of total meat, processed meat were inversely associated with the risk of oral cavity and oropharynx cancer [8], however, other investigators found that meat consumption was significantly associated with increased risk of oral cavity and oropharynx cancer [9], [10], [11]. Therefore, to better characterize the association between meat consumption and the risk of oral cavity and oropharynx cancer, we conducted a comprehensive meta-analysis of the current observational studies.
Methods
Literature Search
The present meta-analysis was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines(PRISMA) [12], and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [13]. A literature search was carried out using Pubmed, Embase, and Cochrane Library Central database between January 1966 and May 2013. There was no restriction of origin and language. Search terms included: “meat” or “lamb” or “beef” or “pork” or “bacon” or “poultry” or “chicken” and ‘‘cancer(s)’’ or ‘‘neoplasm(s)’’ or ‘‘malignancy(ies)’’ and “oral” or “mouth” or “pharynx” or “pharyngeal” or “oropharyngeal”. The reference lists of each study included in this meta-analysis and previous reviews were manually examined to identify additional relevant studies.
Study selection
Two reviewers independently selected eligible studies. Disagreement between the two reviewers was settled by discussing with the third reviewer. Studies were selected if they met our criteria (i) had a case-control or cohort design; (ii) evaluated the association between meat (total meat, red meat, processed meat, or white meat) consumption and the risk of oral cavity and oropharynx cancer, and (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimates with its 95% confidence interval (CI).When there were multiple publications from the same population, only data from the most recent report was included in the meta-analysis and the others were excluded. Studies reporting different measures of RR like risk ratio, rate ratio, hazard ratio, and odds ratio were included in the meta-analysis. In practice, these measures of effect yield a similar estimate of RR, since the absolute risk of oral cavity and oropharynx cancer is low.
Data extraction and methodological quality assessment
The following data was collected by two reviewers independently using a purpose-designed form: name of the first author, publishing time, study region, study design, study period, number of cancer cases and subjects, dietary assessment method, the exposure of meat intake, quantity of intake, the study-specific adjusted ORs, RRs, or HRs with their 95% CIs for the highest category of meat consumption versus the lowest, confounding factors for matching or adjustments.
We used Newcastle-Ottawa scale to assess the methodologic quality of cohort and case-control studies. The Newcastle-Ottawa Scale contains eight items that are categorized three categories: selection (four items, one star each), comparability (one item, up to two stars), and exposure/outcome (three items, one star each). A ‘‘star’’ presents a ‘‘high-quality’’ choice of individual study. The full score was 9 stars, and the high-quality study was defined as a study with≥6 awarded stars.
Data synthesis and analysis
Heterogeneity was assessed using the Cochran Q and I2 statistics. For the Q statistic, a P value<0.10 was considered statistically significant for heterogeneity; for the I2 statistic, heterogeneity was interpreted as absent (I2: 0%–25%), low (I2: 25.1%–50%), moderate (I2: 50.1%–75%), or high (I2: 75.1%–100%) [14]. The overall analysis including all eligible studies was performed first, and subgroup analyses were performed according to (i) Study location(South America, North America, Europe, and Asia), and (ii)number of confounding factors (n≥7, n≤6), adjustment for alcohol intake (yes, no), adjustment for BMI (yes, no), adjustment for education(yes, no), adjustment for fruit and/or vegetable intake(yes, no), to examine the impact of these factors on the associations. When substantial heterogeneity was detected, the summary estimate based on the random-effect model (DerSimonian –Laird method) [15] was reported, which assumed that the studies included in the meta-analysis had varying effect sizes. Otherwise, the summary estimate based on the fixed-effect model (the inverse variance method) [16] was reported, which assumed that the studies included in the meta-analysis had the same effect size. To test the robustness of the associations and characterize possible sources of statistical heterogeneity, sensitivity analysis was carried out by excluding studies one-by-one and analyzing the homogeneity and effect size for all of the rest studies. To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed [17]. An univariate model was established, and then variables with P values ≥0.1 were entered into a multivariable model. Publication bias was assessed using Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test [18], [19]. All analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
Results
Literature search and study characteristics
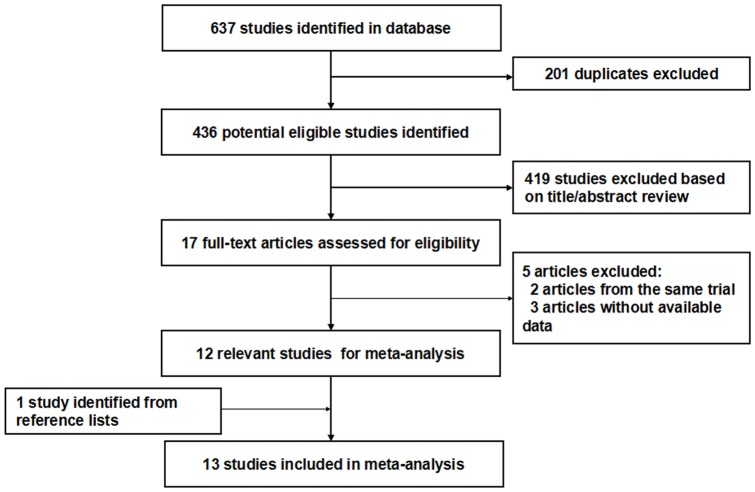
The detailed steps of our literature search are shown in Figure 1. The search strategy generated 637 citations. On the basis of the titles and abstracts, we identified 17 relevant articles. After further evaluation, three studies were excluded for lack of available data, and two studies were excluded because they were from the same population. One study was identified from the reference lists. At last, a total of 13 eligible studies published between 1992 and 2012 were identified, including 12 case–control studies [8], [9], [10], [11], [20], [21], [22], [23], [24], [25], [26], [27] and one cohort study [28](Baseline data and other details of included studies are shown in Table 1). A total of 501,730 subjects, including 4,104 oral cavity and oropharynx cancer cases were involved. Of the 13 included studies, six studies were conducted in Europe [8], [21], [22], [23], [24], [25], three studies in Asia [11], [26], [27], three studies in South America [9], [10], [20], and the remaining one study in North America [28]. Among the 12 case-control studies, only one study was population based [27], and the others were hospital based. Most studies used food frequency questionnaires(FFQ) for the assessment of meat consumption. All studies adjusted for smoking, and most studies adjusted for some potential confounders, including age, sex, and alcohol consumption. The NOS scores for the included studies ranged from 4 to 8; nine studies were deemed to be of a high quality (≥6) (shown in Table 1).
Figure 1. Flow diagram of screened, excluded, and analysed publications.
Table 1. Characteristics of observational studies of the relation between meat intakes and risk of oral cavity and oropharynx cancer included in the meta-analysis.
| Author | Publication year | Country | Study design | Study period | Dietary assessments | Cases/Subjects | Type of meat | Units and comparison groups | Confounders for adjustment | NOS score |
| De Stefani E | 2012 | Uruguay | hospital-based case–control study | 1996–2004 | FFQ 64 items | 56/940 | processed meat | g/day 11.5–28.2 vs ≤11.4 ≥28.3 vs ≤11.4 | age, residence, BMI, smoking status, alcohol drinking, mate consumption, total energy, total vegetables and fruits, total white meat, and red meat intakes. | 7 |
| Daniel CR | 2011 | USA | cohort study | 1995–1996 | FFQ 124 items | 1,305/492,186 | Poultry | Q5 vs Q1 | age, sex, education, marital status, family history of cancer, race, BMI, smoking status, frequency of vigorous physical activity, menopausal hormone therapy in women, intake of alcohol, fruit, vegetables, fish, red meat, and total energy | 8 |
| Sapkota A | 2008 | central and eastern Europe | hospital-based case–control study | 1999–2003 | FFQ 23 items | 378/1,606 | Poultry, red meats | Tertile 3 vs Tertile 1 | age, country, gender, tobacco pack-years, education, BMI, frequency of alcohol consumption, tertiles of total vegetable consumption, and tertiles of total fruit consumption | 7 |
| Levi F | 2004 | Switzerland | hospital-based case–control study | 1992–2002 | FFQ 79 items | 316/1,587 | Processed meat | Frequency/week 0.8–1.5 vs <0.8 1.6–3.2 vs <0.8 >3.2 vs <0.8 | age, sex, education, tobacco smoking, alcohol drinking, total energy intake, fruit and vegetable intake,BMI, and physical activity . | 7 |
| Toporcov TN | 2004 | Brazil | hospital-based case–control study | 2003 | FFQ 41 items | 70/140 | pepperoni,bacon, red meat | high vs low | sex, age, smoking status, frequency for the use of dental prosthesis | 6 |
| Lissowska J | 2003 | Poland | hospital-based case–control study | 1997–2000 | FFQ 25 items | 122/246 | Total meat, processed meat | Tertile 3 vs Tertile 1 | gender, age, residence, drinking and smoking habits | 6 |
| Rajkumar T | 2003 | India | hospital-based case–control study | 1996–1999 | FFQ 21 items | 591/1,773 | Total meat, processed meat | Servings/week 1-2 vs <1 >2 vs <1 | sex, age, centre, education, chewing, smoking and drinking habits | 6 |
| Sánchez MJ | 2003 | Spain | hospital-based case–control study | 1996–1999 | FFQ 25 items | 375/750 | Total meat, processed meat | Servings/week 2-5 vs <1 6 vs <1 | gender, age, centre, years of schooling, smoking and drinking habits | 6 |
| Escribano Uzcudun A | 2002 | Spain | hospital-based case–control study | 1990–1995 | interview-administered questionnaire | 232/464 | Total meat | high vs low | tobacco smoking, and alcohol consumption | 5 |
| Petridou E | 2002 | Greece | hospital-based case–control study | N/A | FFQ 110 items | 106/212 | Meats and meat products | high vs low | energy intake, tobacco smoking, and alcohol consumption | 5 |
| Garrote LF | 2001 | Cuba | hospital-based case–control study | 1996–1999 | dietary questionnaire | 200/400 | Total meat, processed meat | Servings/week 3-5 vs <3 >6 vs <3 | gender, age, area of residence, education, smoking and drinking habits | 6 |
| Zheng T | 1993 | China | hospital-based case–control study | 1989 | FFQ 63 items | 404/808 | Total meat | Servings/month 1-2 vs <1 >3 vs <1 | tobacco smoking, alcohol drinking, inadequate dentition, years of education, Quetelet Index, sex and age | 4 |
| Zheng W | 1992 | China | population-based case-control study | 1988–1990 | FFQ 42 items | 204/618 | Salted meat | daily/weekly vs seldom | smoking and education. | 5 |
BMI = body mass index; FFQ = Food Frequency Questionnaire.
Total meat intake and the risk of oral cavity and oropharynx cancer
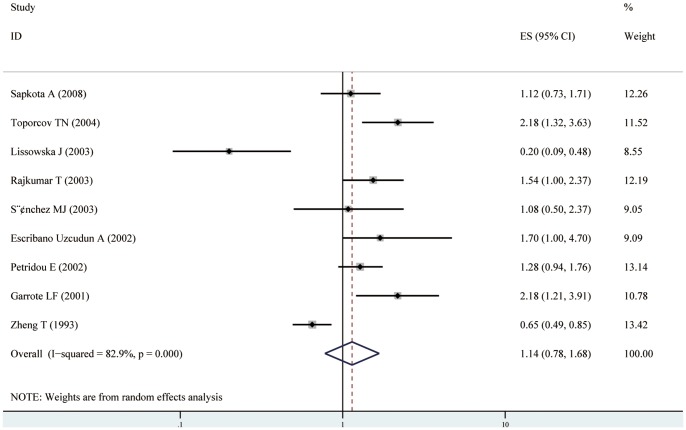
Nine case–control studies of total meat distinction were included in the meta-analysis [8], [9], [10], [11], [21], [23], [24], [25], [26]. We found that the high consumption of total meat was not significantly associated with the risk of oral cavity and oropharynx cancer (RR = 1.14, 95% CI[0.78–1.68]) (shown in Table 2, Figure 2). Statistically significant heterogeneity was detected (I2 = 82.9%, Q = 46.87, P<0.001). There was no indication of a publication bias, either from Egger ’s test (P = 0.780) or from Begg ’ s test (P = 0.835 )(shown in Figure 4 A). In subgroup analyses, when stratified the various studies by study location, no significant association was noted among studies conducted in Europe (RR = 0.93, 95%CI [0.55, 1.59]), and Asia (RR = 0.98, 95%CI [0.42, 2.29]), however, high consumption of total meat was significantly associated with a 118% increased risk of oral cavity and oropharynx cancer in South America (RR = 2.18, 95%CI [1.49, 3.20]). When we examined whether the associations were affected by adjustment for alcohol, BMI, education, and fruit and/or vegetable intake, the associations were not affected by these factors(shown in Table 2). Further, it was observed that studies with higher control for potential confounders (n≥7) as well as studies with lower control (n≤6) presented no significant association (RR = 1.02, 95% CI[0.59, 1.74] and RR = 1.21, 95% CI[0.70, 2.09], respectively). Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results. Meta-regression analysis was performed to investigate the possible sources of between-study heterogeneity. Geographic area, sex, age, study quality, publication year, control for confounding factors(alcohol, BMI, education, and fruit and/or vegetable intake) which may be potential sources of heterogeneity, were tested by a meta-regression method. We found that study quality( P = 0.029) had statistical significance in a multivariate model.
Table 2. Summary relative risks of the association between meat consumption and risk of oral cavity and oropharynx cancer.
| No. of studies | RR (95% CI) | P value for heterogeneity | I2 value (%) | |
| Overall studies | ||||
| Total meat | 9 | 1.14 (0.78–1.68) | <0.001 | 82.90 |
| Processed meat | 9 | 1.91 (1.19–3.06) | <0.001 | 85.90 |
| Red meat | 3 | 1.05 (0.66–1.66) | 0.12 | 49.40 |
| White meat | 3 | 0.81(0.54–1.22) | 0.09 | 59.40 |
| Subgroup analyses for total meat | ||||
| Continent | ||||
| Europe | 5 | 0.93 (0.55–1.59) | <0.001 | 77.80 |
| South America | 2 | 2.18 (1.49–3.20) | 0.99 | 0.00 |
| Asia | 2 | 0.98 (0.42–2.29) | <0.001 | 90.90 |
| Adjusted for confounders | ||||
| n≥7 confounders | 3 | 1.02 (0.59–1.74) | <0.001 | 83.90 |
| n≤6 confounders | 6 | 1.21 (0.70–2.09) | <0.001 | 81.30 |
| Major confounders adjusted | ||||
| BMI | ||||
| yes | 1 | 1.12(0.73–1.71) | / | / |
| no | 8 | 1.14(0.73–1.78) | <0.001 | 85.10 |
| Alcohol | ||||
| yes | 7 | 1.15(0.77–1.73) | <0.001 | 74.80 |
| no | 2 | 1.17(0.36–3.84) | <0.001 | 94.10 |
| Education | ||||
| yes | 5 | 1.18(0.75–1.88) | <0.001 | 80.50 |
| no | 4 | 1.04(0.48–2.28) | <0.001 | 87.30 |
| Fruit and/or vegetable intake | ||||
| yes | 1 | 1.12(0.73–1.71) | / | / |
| no | 8 | 1.14(0.73–1.78) | <0.001 | 85.10 |
| Subgroup analyses for processed meat | ||||
| Continent | ||||
| Europe | 3 | 1.64(0.59–4.60) | <0.001 | 91.00 |
| South America | 3 | 1.93(1.25–3.00) | 0.15 | 47.90 |
| Asia | 3 | 2.09(0.70–6.29) | <0.001 | 91.30 |
| Adjusted for confounders | ||||
| n≥7 confounders | 5 | 1.94(0.97–3.88) | <0.001 | 89.10 |
| n≤6 confounders | 4 | 1.86(0.92–3.74) | <0.001 | 82.60 |
| Major confounders adjusted | ||||
| BMI | ||||
| yes | 3 | 2.00(0.94–4.24) | <0.001 | 83.50 |
| no | 6 | 1.86(0.96–3.23) | <0.001 | 88.70 |
| Alcohol | ||||
| yes | 7 | 1.71(0.97–3.00) | <0.001 | 88.50 |
| no | 2 | 2.91(1.81–4.67) | 0.86 | 0.00 |
| Education | ||||
| yes | 7 | 1.88(1.02–3.46) | <0.001 | 88.60 |
| no | 2 | 1.98(0.95–4.13) | 0.06 | 72.70 |
| Fruit and/or vegetable intake | ||||
| yes | 3 | 2.00(0.94–4.24) | <0.001 | 83.50 |
| no | 6 | 1.86(0.96–3.23) | <0.001 | 88.70 |
BMI = body mass index; CI = confidence interval; RR = relative risk.
Figure 2. Forest plot: estimates (95% CIs) of total meat consumption and risk of oral cavity and oropharynx cancer.
Squares indicated study-specific risk estimates (size of square reflects the study-statistical weight, i.e. inverse of variance); horizontal lines indicate 95% confidence intervals; diamond indicates summary relative risk estimate with its corresponding 95% confidence interval.
Figure 4. Begger's funnel plot of publication.
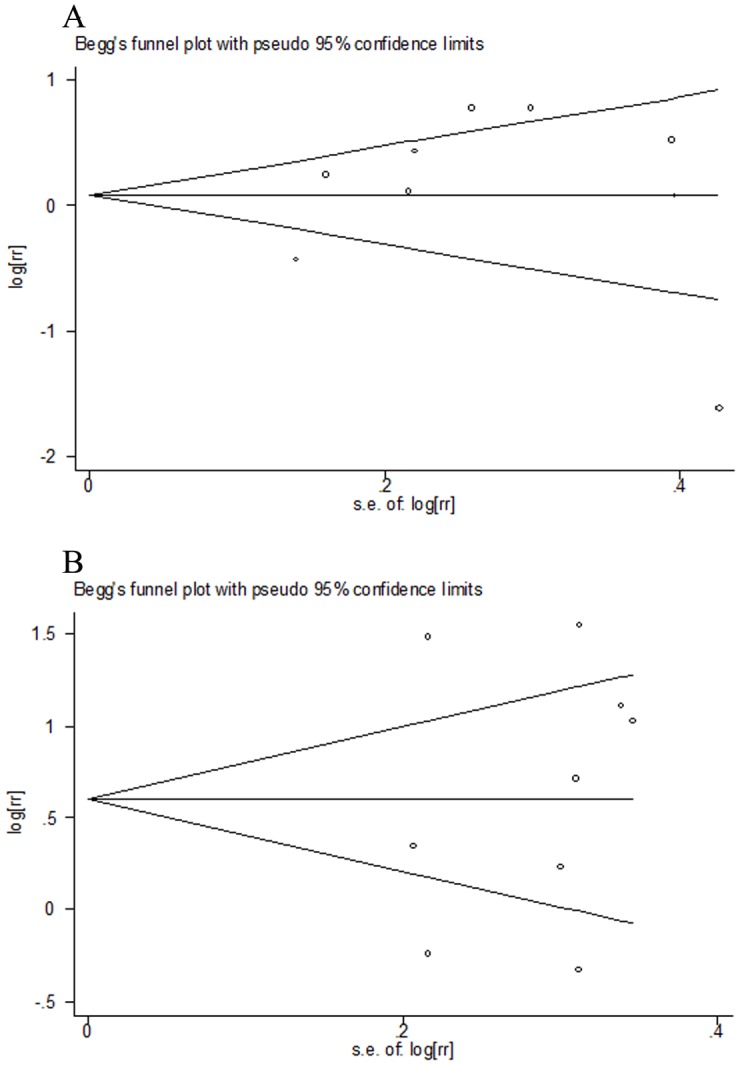
A: Funnel plot for studies investigating total meat consumption and risk of oral cavity and oropharynx cancer; B: Funnel plot for studies investigating processed meat consumption and risk of oral cavity and oropharynx cancer.
Processed meat intake and risk of oral cavity and oropharynx cancer
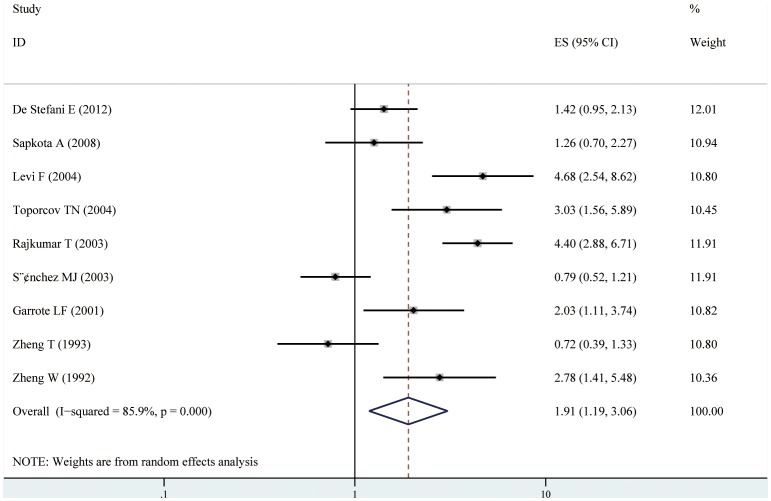
Nine case–control studies of processed meat distinction were included in the meta-analysis [9], [10], [11], [20], [22], [24], [25], [26], [27]. We found that the high consumption of processed meat was significantly associated with a 91% increased risk of oral cavity and oropharynx cancer (RR = 1.91, 95% CI [1.19–3.06]) (shown in Table 2, Figure 3). Statistically significant heterogeneity was detected (I2 = 85.9%, Q = 46.87, P<0.001). There was no indication of a publication bias, either from Egger ’s test ( P = 0.999) or from Begg ’ s test ( P = 0.297) (shown in Figure 4 B). In subgroup analyses, when stratified the various studies by study location, high consumption of total meat was significantly associated with a 93% increased risk of oral cavity and oropharynx cancer in South America (RR = 1.93, 95%CI [1.25, 3.00]), however, no significant association was noted among studies conducted in Europe (RR = 1.64, 95%CI [0.59, 4.60]), and Asia (RR = 2.09, 95%CI [0.70, 6.29]). When we examined if thorough adjustment of potential confounders could affect the combined RR, it was observed that studies with higher control for potential confounders (n≥7) as well as studies with lower control (n≤6) presented no significant association (RR = 1.94, 95% CI[0.97, 3.88] and RR = 1.86, 95% CI[0.92, 3.74], respectively). However, we found that the associations were significantly affected by adjustment for alcohol consumption and education (shown in Table 2). Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results. Meta-regression analysis was performed to investigate the possible sources of between-study heterogeneity. Geographic area, sex, age, study quality, publication year, control for confounding factors(alcohol, BMI, education, and fruit and/or vegetable intake) which may be potential sources of heterogeneity, were tested by a meta-regression method. However, meta-regression revealed that none of the above factors was responsible for the between-study heterogeneity.
Figure 3. Forest plot: estimates (95% CIs) of processed meat consumption and risk of oral cavity and oropharynx cancer.
Squares indicated study-specific risk estimates (size of square reflects the study-statistical weight, i.e. inverse of variance); horizontal lines indicate 95% confidence intervals; diamond indicates summary relative risk estimate with its corresponding 95% confidence interval.
Red, white meat intake and the risk of oral cavity and oropharynx cancer
Three case–control studies of red meat distinction were included in the meta-analysis [9], [25], [26]. And two case–control studies [25], [26] and one cohort study [28] of white meat distinction were included in the meta-analysis. It was founded that neither red meat nor white meat was associated with an increased risk of oral cavity and oropharynx cancer(RR = 1.05, 95% CI[0.66, 1.66] and RR = 0.81, 95% CI[0.54, 1.22], respectively).
Discussion
To our knowledge, it was the first meta-analysis evaluating the association between meat consumption and oral cavity and oropharynx cancer risk. Twelve case-control studies and one cohort study were included in the present analysis, involving 501,730 participants and 4,104 oral cavity and oropharynx cancer cases. In the present study, we found that the total meat consumption was not significantly associated with the risk of oral cavity and oropharynx cancer. When we investigated different types of meat and the risk of oral cavity and oropharynx cancer, we found that processed meat consumption was significantly associated with an increased risk of oral cavity and oropharynx cancer, however, no statistically significant association was observed between red meat or white meat intake and the risk of oral cavity and oropharynx cancer.
Processed meat (meat preserved by smoking, curing, salting, or by addition of chemical preservatives) has been found to be associated with several tumors, such as colorectal adenomas [3], esophageal cancer [4], bladder cancer [6], and renal cancer [7]. The finding of our meta-analysis was in line with these meta-analyses. Processed meat has been hypothesized to play a important role in carcinogenesis, owing to their high levels of saturated fat and heme iron content, and potent mutagens produced during high temperature cooking and meat processing or preservation, including N –nitroso compounds (NOCs) [29], polycyclic aromatic hydrocarbons(PAHs) [30], [31], and heterocyclic amines (HCAs) [32], [33]. NOCs intake may contribute to carcinogenesis at a variety of anatomic sites in animals [34]. PAHs and HCAs, which can form DNA adducts and induce genetic alterations characteristic of tumors, have been shown to be carcinogens in animal studies [35]. During subgroup analyses, after being stratified by study location, it demonstrated that processed meat consumption had a significant association with increased risk of oral cavity and oropharynx cancer in south Americans, however, it could not be validated in Asians or Europeans. So, the strength of the association varied greatly across ethnic groups. The possible reasons were considerable differences in genetic background, life styles and environmental factors. In addition, we should notice that there were only two included studies investigating meat intake and the risk of oral cavity and oropharynx cancer among Asians, and limited number of patients may limit us to detect stable effects in this population. Additional studies are warranted to further validate ethnic difference in the effect of meat intake on oral cavity and oropharynx cancer risk, especially in Asians.
Red meat is a source of heme iron, which is more bioavailable than non-heme iron. Heme iron is contributed to carcinogenesis in rodents by generating free radicals and inducing oxidative stress [36]. Heme iron has also been shown to induce endogenous formation of NOCs [37]. Previous meta-analyses have shown that red meat intake was associated with an increased risk of colorectal adenomas [3], esophageal cancer [4], lung cancer [5], bladder cancer [6], and renal cancer [7]. However, the result of the present meta-analysis showed that red meat consumption was not significantly associated with an increased risk of oral cavity and oropharynx cancer. We should notice that there were only three studies investigating the relationship between red meat intake and oral cavity and oropharynx cancer risk, which limited us to get a narrow confidence interval and detect stable effects. So, more studies focusing on red meat and oral cavity and oropharynx cancer risk are needed in the future.
The strength of the present meta-analysis lies in a large sample size (501,730 subjects and 4,104 oral cavity and oropharynx cancer cases) and no significant evidence of publication bias. All studies adjusted for smoking, and most studies adjusted for some potential confounders, including age, sex, and alcohol consumption. Furthermore, our findings were stable and robust in sensitivity analyses. However, several limitations to this meta-analysis should be noted. Firstly, as a meta-analysis of observational data, the possibility of recall and selection biases can’t be ruled out. Compared with case-control studies, cohort studies are less susceptible to bias due to their nature. However, the present meta-analysis included only one cohort study, so more prospective cohort studies are needed to confirm the association in the future. Secondly, we did not search for unpublished studies, so only published studies were included in our meta-analysis. Therefore, publication bias may have occurred although no publication bias was indicated from both visualization of the funnel plot and Egger’s test. Thirdly, none of the included studies adjusted for betel-quid chewing, which is one of the primary risk factors for oral cavity and oropharynx cancer. Lastly, due to different methods used to report meat intake among studies, we failed to carry out a dose-response analysis between meat intake and the risk of oral cavity and oropharynx cancer.
In conclusion, the present meta-analysis suggested that a high intake of processed meat was significantly associated with an increased risk of oral cavity and oropharynx cancer, while there was no significantly association between total meat, red meat or white meat and the risk of oral cavity and oropharynx cancer. More prospective cohort studies are warranted to confirm these associations.
Supporting Information
(DOC)
Funding Statement
The present work was supported by Shandong Provincial Natural Science Foundation of China (No. ZR2010HL045, ZR2010HL057), Jining city science and technology development plan program of jining science and technology bureau (No. 2012jnfs02; 2012jnfs11; 2012jnfs13), Youth Fund of Jining Medical University (No. JYQ2011KZ005), and a Project of Shandong Province Higher Educational Science and Technology Program (No. J12LE10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Mehanna H, Paleri V, West CM, Nutting C (2011) Head and neck cancer-part 1: epidemiology, presentation, and preservation. Clin Otolaryngol 36: 65–68. [DOI] [PubMed] [Google Scholar]
- 3. Xu X, Yu E, Gao X, Song N, Liu L, et al. (2013) Red and processed meat intake and risk of colorectal adenomas: a meta-analysis of observational studies. Int J Cancer 132: 437–448. [DOI] [PubMed] [Google Scholar]
- 4. Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F (2013) Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev 71: 257–267. [DOI] [PubMed] [Google Scholar]
- 5. Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, et al. (2012) Meat consumption and risk of lung cancer: evidence from observational studies. Ann Oncol 23: 3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang C, Jiang H (2012) Meat intake and risk of bladder cancer: a meta-analysis. Med Oncol 29: 848–855. [DOI] [PubMed] [Google Scholar]
- 7. Faramawi MF, Johnson E, Fry MW, Sall M, Zhou Y (2007) Consumption of different types of meat and the risk of renal cancer: meta-analysis of case-control studies. Cancer Causes Control 18: 125–133. [DOI] [PubMed] [Google Scholar]
- 8. Lissowska J, Pilarska A, Pilarski P, Samolczyk-Wanyura D, Piekarczyk J, et al. (2003) Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev 12: 25–33. [DOI] [PubMed] [Google Scholar]
- 9. Toporcov TN, Antunes JL, Tavares MR (2004) Fat food habitual intake and risk of oral cancer. Oral Oncol 40: 925–931. [DOI] [PubMed] [Google Scholar]
- 10. Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, et al. (2001) Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer 85: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajkumar T, Sridhar H, Balaram P, Vaccarella S, Gajalakshmi V, et al. (2003) Oral cancer in Southern India: the influence of body size, diet, infections and sexual practices. Eur J Cancer Prev 12: 135–143. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Woolf B (1955) On estimating the relation between blood group and disease. Ann Hum Genet 19: 251–253. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG (2004) Controlling the risk of spurious findings from meta-regression. Stat Med 23: 1663–1682. [DOI] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Stefani E, Boffetta P, Ronco AL, Deneo-Pellegrini H, Correa P, et al. (2012) Processed meat consumption and risk of cancer: a multisite case-control study in Uruguay. Br J Cancer 107: 1584–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Escribano Uzcudun A, Rabanal Retolaza I, Garcia Grande A, Miralles Olivar L, Garcia Garcia A, et al. (2002) Pharyngeal cancer prevention: evidence from a case—control study involving 232 consecutive patients. J Laryngol Otol 116: 523–531. [DOI] [PubMed] [Google Scholar]
- 22. Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C (2004) Processed meat and the risk of selected digestive tract and laryngeal neoplasms in Switzerland. Ann Oncol 15: 346–349. [DOI] [PubMed] [Google Scholar]
- 23. Petridou E, Zavras AI, Lefatzis D, Dessypris N, Laskaris G, et al. (2002) The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer 94: 2981–2988. [DOI] [PubMed] [Google Scholar]
- 24. Sanchez MJ, Martinez C, Nieto A, Castellsague X, Quintana MJ, et al. (2003) Oral and oropharyngeal cancer in Spain: influence of dietary patterns. Eur J Cancer Prev 12: 49–56. [DOI] [PubMed] [Google Scholar]
- 25. Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, et al. (2008) Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control 19: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 26. Zheng T, Boyle P, Willett WC, Hu H, Dan J, et al. (1993) A case-control study of oral cancer in Beijing, People's Republic of China. Associations with nutrient intakes, foods and food groups. Eur J Cancer B Oral Oncol 29B: 45–55. [DOI] [PubMed] [Google Scholar]
- 27. Zheng W, Blot WJ, Shu XO, Diamond EL, Gao YT, et al. (1992) Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol Biomarkers Prev 1: 441–448. [PubMed] [Google Scholar]
- 28. Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, et al. (2011) Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila) 4: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haorah J, Zhou L, Wang X, Xu G, Mirvish SS (2001) Determination of total N-nitroso compounds and their precursors in frankfurters, fresh meat, dried salted fish, sauces, tobacco, and tobacco smoke particulates. J Agric Food Chem 49: 6068–6078. [DOI] [PubMed] [Google Scholar]
- 30. Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res 443: 139–147. [DOI] [PubMed] [Google Scholar]
- 31. Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N (2001) Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 39: 423–436. [DOI] [PubMed] [Google Scholar]
- 32. Puangsombat K, Gadgil P, Houser TA, Hunt MC, Smith JS (2011) Heterocyclic amine content in commercial ready to eat meat products. Meat Sci 88: 227–233. [DOI] [PubMed] [Google Scholar]
- 33. Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, et al. (1998) Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol 36: 289–297. [DOI] [PubMed] [Google Scholar]
- 34. Mirvish SS (1995) Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93: 17–48. [DOI] [PubMed] [Google Scholar]
- 35. Ohgaki H, Kusama K, Matsukura N, Morino K, Hasegawa H, et al. (1984) Carcinogenicity in mice of a mutagenic compound, 2-amino-3-methylimidazo[4,5-f]quinoline, from broiled sardine, cooked beef and beef extract. Carcinogenesis 5: 921–924. [DOI] [PubMed] [Google Scholar]
- 36. Huang X (2003) Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res 533: 153–171. [DOI] [PubMed] [Google Scholar]
- 37. Cross AJ, Sinha R (2004) Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen 44: 44–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)