Abstract
Purpose
To assess whether an increase in a subvolume of intrahepatic tumor with elevated arterial perfusion during radiation therapy (RT) predicts tumor progression post RT.
Methods and Materials
Twenty patients with unresectable intrahepatic cancers undergoing RT were enrolled in a prospective IRB-approved study. Dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) were performed prior to RT (pre-RT), after delivering ~60% of the planned dose (mid-RT) and one month after completion of RT to quantify hepatic arterial perfusion. The arterial perfusions of the tumors at pre-RT were clustered into low-normal and elevated perfusion by a fuzzy clustering-based method, and the tumor subvolumes with elevated arterial perfusion were extracted from the hepatic arterial perfusion images. The percentage changes in the tumor subvolumes and means of arterial perfusion over the tumors from pre-RT to mid-RT were evaluated for predicting tumor progression post-RT.
Results
Of the 24 tumors, 6 tumors in 5 patients progressed 5–21 months after RT completion. Neither tumor volumes nor means of tumor arterial perfusion at pre-RT were predictive of treatment outcome. The mean arterial perfusion over the tumors increased significantly at mid-RT in progressive tumors comparing to the responsive ones (p=0.006). From pre-RT to mid-RT, the responsive tumors had a decrease in the tumor subvolumes with elevated arterial perfusion (median: −14%, range: −75% – 65%), while the progressing tumors had an increase of the subvolumes (median: 57%, range: −7% – 165%) (p=0.003). Receiver operating characteristic (ROC) analysis of the percentage change in the subvolume for predicting tumor progression post-RT had an area under the curve (AUC) of 0.90.
Conclusion
The increase in the subvolume of the intrahepatic tumor with elevated arterial perfusion during RT has the potential to be a predictor for tumor progression post-RT. The tumor subvolume could be a radiation boost candidate for response-driven adaptive RT.
Keywords: Intrahepatic cancer, Tumor subvolume, Dose boosting, Hepatic Arterial perfusion
Introduction
High-dose, conformal radiation therapy (RT) can control intrahepatic cancers (1). Conventional RT delivers uniformly-distributed dose to a target volume. Considering the heterogeneity of tumors, specific subvolumes in the tumors may be more active or aggressive; thus, targeting these subvolumes with higher dose of RT could improve the rates of local tumor control (2). Technological advancements in RT allow delivery of a high-precision, non-uniform dose painting in the target volume. To take advantage of this, one would need to detect and visualize the active or aggressive target for dose intensification.
The concept of biological target volumes determined by physiological, metabolic and molecular imaging has been proposed by Ling et al (2). In the last several years, physiological and metabolic imaging have shown the potential for assessment and prediction of tumor response and/or outcome to RT in the tumors of various types (3, 4). However, the heterogeneous distributions of physiological and biological parameters within the tumors were largely ignored in most of these studies. Recently, a globally-initiated fuzzy clustering technique has been proposed to extract tumor subvolumes from perfusion images in head and neck cancers (HNC) and brain metastases (5, 6). Both studies found that the early change in the subvolumes extracted from blood-volume images by the techniques and assessed during RT, predicted post-RT response, better than using the change in the mean blood volume over the whole tumor (5, 6). Tumor subvolumes that correlated with local failure could represent the active or aggressive subvolume of the tumor, and could potentially be targeted by high-precision and high-dose RT to improve treatment outcome.
Arterial-vascularization is a common characteristic of the intrahepatic cancers, which represents as an abnormal increase in arterial perfusion and a decrease in portal venous perfusion (7–9). An effective therapy would lead to disruption of tumor vasculature resulting in early reduction of blood perfusion (10, 11). Quantitative hepatic perfusion derived from dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) has been evaluated for assessment of tumor response to anti-angiogenic therapy and other treatment regimens for intrahepative cancers (12–14). However, these studies only assessed changes in the means of arterial perfusion over the tumors, and neglected regional tumor perfusion heterogeneity. Given the heterogeneous tumor biology, response of the aggressive tumor component to therapy could be a better indicator of the whole tumor response to treatment. Thus, an intensified treatment of the subvolume of the heterogeneous intrahepatic cancer, which is at high-risk for progression, could yield a better tumor control.
In this study of RT, we hypothesized that an early increase in the subvolume of the intrahepatic cancer with elevated arterial perfusion during RT could predict tumor progression post-RT. We extracted tumor subvolumes with elevated arterial perfusion from quantitative hepatic arterial perfusion images in the patients who had unresectable intrahepatic cancers and were treated with RT, and then evaluated a change in the tumor subvolume from pre-RT to mid-RT for predicting post-RT tumor progression.
Methods and Materials
Patients and Treatment
Twenty patients (5 women and 15 men, 43–80 years of age) with intrahepatic cancers were enrolled in an institutional review board (IRB)-approved liver DCE MRI study (Table 1). Nine patients had hepatocellular carcinoma (HCC), four had cholangiocarcinoma, and seven had metastasis to the livers. Patients were treated using 3-dimensional (3D) conformal RT (n=14) or stereotactic body RT (SBRT) (n=6) as clinically indicated. Three patients had more than one tumor treated, resulting in a total of 24 tumors considered in this study. The GTV was defined on contrast-enhanced CT for metastatic patients with well-demarcated tumors, and on the gadolinium-enhanced arterial and/or portal venous phase of the diagnostic MRI for all other patients. Then the diagnostic MRI was registered to the planning CT. The treatment was planned on a CT obtained at end-exhalation using active breathing control as previously described (15). The dose was prescribed to have a 10% or less risk of radiation-induced liver disease according to the normal tissue complication probability (NTCP) model (16). The median prescribed dose was 59 Gy in 1.8–3.0 Gy per fraction for 3D conformal RT. For SBRT, four patients received 50 Gy in 5 fractions, one received 30 Gy in 3 fractions, and the other one received 60 Gy in 3 fractions as clinically indicated. After completion of RT, patients were typically followed every three months by history and physical exam, laboratories, and imaging, as per routine clinical practice. RECIST criteria were used to determine progression. Lack of progression was considered response.
Table 1.
Patient Information
| Patient No. | Gender/Age | Cancer | Number of Treated Tumors | Mid-RT accumulated dose/Total accumulated dose/Fraction dose (Gy) | Treatment outcome |
|---|---|---|---|---|---|
| 1 | M/55 | HCC | 1 | 28/62/2 | Progressive |
| 2 | M/55 | cholangiocarcinoma | 1 | 36/62/2 | Responsive |
| 3 | M/65 | HCC | 1 | 30/50/10 | Responsive |
| 4 | M/79 | colon metastases | 1 | 25/50/2.5 | Responsive |
| 5 | M/77 | cholangiocarcinoma | 1 | 24/48/2 | Responsive |
| 6 | M/54 | HCC | 1 | 30/50/10 | Progressive |
| 7 | F/45 | colorectal metastases | 3 | 39/63/3 | Responsive |
| 8 | M/62 | anal metastases | 1 | 32.5/52.5/2.5 | Responsive |
| 9 | M/43 | HCC | 1 | 30/64/2 | Responsive |
| 10 | M/58 | colorectal metastases | 1 | 42/82/2 | Responsive |
| 11 | M/72 | colorectal metastases | 1 | 45/75/3 | Responsive |
| 12 | M/65 | HCC | 1 | 30/30/10 | Progressive |
| 13 | M/80 | neuroendocrine metastases | 1 | 45/55/2.5 | Responsive |
| 14 | F/76 | cholangiocarcinoma | 1 | 48/70/2 | Progressive |
| 15 | M/56 | gastric metastases | 1 | 20/60/20 | Responsive |
| 16 | M/72 | HCC | 1 | 30/50/2.5 | Responsive |
| 17 | F/44 | HCC | 2 | 21.6/43.2/1.8 | Responsive |
| 18 | F/74 | cholangiocarcinoma | 1 | 35.2/48.4/2.2 | Responsive |
| 19 | F/79 | HCC | 1 | 30/50/10 | Responsive |
| 20 | M/77 | HCC | 2 | 10/50/10 | Progressive |
HCC: hepatocellular carcinoma
MR Imaging and Processing
The patients had liver DCE MRI scans within 2 weeks prior to RT (pre-RT), after delivery of ~60% of the planned dose (mid-RT), and 1 month after completion of RT (post-RT). The volumetric DCE MRI was acquired on a 3T scanner (Philips Achieva 3.0T; Philips Healthcare, The Netherlands) using a 3D gradient echo pulse sequence (TR/TE/FA: 4.48ms/2.15ms/20º; Matrix: 320×320×66; FOV: 33×33×19.8 cm; SENSE factor: 2 in 2 different directions) in a coronal or oblique-coronal orientation. The dynamic image volumes were acquired approximately 2.4 secs per volume for a total of 2 mins, during which a breathing control paradigm was used (15). After DCE MRI, a high-resolution T1-weighted MR image volume (Matrix: 320×320×104; Resolution: 1×1×2 mm) was obtained using a gradient echo pulse sequence (TR/TE: 4.1 ms/2.1 ms) during a single breath holding.
Hepatic arterial perfusion and portal venous perfusion in the liver were quantified voxel-by-voxel by fitting the DCE data to a dual-input single compartment model (17), which was implemented on a graphic process unit (GPU) to achieve fast computation (18). The high-resolution T1-weighted MR images at each scanning session were rigidly registered to the treatment planning CT using the functional image analysis tool (FIAT). The hepatic arterial perfusion images were then co-registered to the planning CT by applying the same transformation. The GTV was transferred onto the co-registered T1-weighted MR images pre-RT, and then edited by a radiation oncologist to compensate for any mis-registration between the image datasets. Finally, the edited GTV was applied to both the co-registered hepatic arterial perfusion images pre-RT and mid-RT. We inspected the alignment between mid-RT and pre-RT images, and evaluated the pre-RT GTV for coverage of the tumor on the mid-RT scan. For two patients (#12 and #19), region-limited image registration that focused on the tumor area was performed between the anatomical MR images pre-RT and mid-RT to ensure the pre-RT GTV reliably covered the tumor at the mid-RT scan.
Tumor Subvolume Defined by Elevated Arterial Perfusion
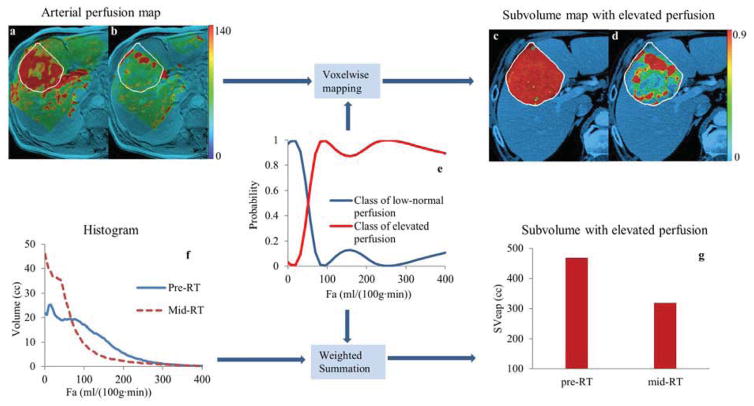
We aimed to extract the tumor subvolumes with elevated arterial perfusion (i.e., higher than the arterial perfusion in normal liver tissue) and relate their early changes during RT to treatment outcome post-RT. The subvolumes of the tumors were extracted from the hepatic arterial perfusion images by using the fuzzy clustering-based method that was recently published (6). In brief, first, we generated a histogram of hepatic arterial perfusion of a tumor, which consisted of 100 evenly spaced points over 0–600 ml/(100g· min) that covered the range of arterial perfusion of all the tumors in this study. Then, the pre-RT histograms of all the tumors, after normalization, were pooled together as representative samples of hepatic atrial perfusion of the intrahepatic cancers. A fuzzy c-means clustering analysis was used to classify the pooled histograms into three classes corresponding to low-normal, normal-high, and high arterial perfusion. Through this analysis, a probabilistic membership (P(Fa, classj) of an arterial perfusion value Fa belonging to a perfusion class j (low-normal, normal-high, or high perfusion) was established, which allowed us to classify any voxel in a tumor at any time point according to its arterial perfusion value (Figure 1). Finally, a subvolume of a tumor with elevated arterial perfusion (SVeap), including normal-high and high arterial perfusion, was calculated by a summation of the probabilities of a voxel belonging to the normal-high and high perfusion classes over all the voxels in the tumor (Figure 1f).
Figure 1.
Generation of tumor subvolume with elevated arterial perfusion. Color-coded hepatic arterial perfusion images (unit: ml/(100g·min)) of a patient at pre-RT (a) and mid-RT (b) were transformed to probability maps of the subvolumes with elevated arterial perfusion (c and d), respectively, by applying the probabilistic membership function (e). The white curves contoured the gross tumor volumes. The volume of the tumor subvolume with elevated arterial perfusion was sum of the arterial perfusion histogram (f) weighted by the probability function (e). Note that there was 32% reduction in the tumor subvolume from pre-RT to mid-RT (g), suggesting early tumor response.
Spatial Overlap of the Tumor Subvolumes
We examined the spatial overlap of the derived tumor subvolumes between pre-RT and mid-RT. The physical subvolumes were obtained from the voxels belonging to the class of elevated arterial perfusion with the probability greater than 0.3, 0.4, 0.5, 0.6 or 0.7. Then, the overlap fraction, the ratio of the overlap volume between the pre-RT and mid-RT subvolumes to the smallest subvolume of the two, was calculated (19). In this calculation, only contiguous voxels in the thresholded subvolumes were considered.
Prediction of Treatment Outcome
To evaluate the predictive value of SVeap for treatment outcome, a percentage change in SVeap from pre-RT to mid-RT (ΔSVeap) was calculated. We tested whether there was a significant difference of the changes in SVeap between responsive and progressive tumor groups using the Mann-Whitney U test. We further tested the SVeap change from pre-RT to mid-RT for predicting tumor progression post RT by receiver operating characteristic (ROC) analysis using the ROCKIT software package, in which the area under the ROC curve (AUC) and the standard error (SE) of the AUC were calculated. Similar analyses were applied to a percentage change in the means of the arterial perfusions over the whole tumors from pre-RT to mid-RT for prediction of post-RT tumor progression. Also, clinical parameters, such as age, tumor type (HCC vs other non-HCC tumor) and pre-RT tumor volume were tested for prediction of progression using Mann-Whitey U test for continuous variables and Fisher’s Exact test for dichotomous variables. All statistical computations were done by statistical analysis package R.
Results
Patient Outcome
Twenty-four intrahepatic cancers from the 20 patients were followed up after the completion of RT by routine clinical MRI. Six tumors (5 HCC and 1 Cholangiocarcinoma) from 5 patients progressed 5–21 months after the completion of RT with a median of 15 months followup (Table 1). Prior to RT, the median gross tumor volume was 140 cc (3–1644 cc) for responsive tumors, and 37 cc (4–1324 cc) for progressive ones (p=0.31). There were no significant differences in patient age (p=0.44) and tumor type (p=0.06) between responsive and progressive groups.
Hepatic Arterial Perfusion Prior to RT
Prior to RT, the average arterial perfusion in the gross tumor volume ranged from 15 to 207 ml/(100g·min), with a median of 55 ml/(100g·min) for the responsive tumors, and from 26 to 93 ml/(100g·min)) with a median of 30 ml/(100g min) for the progressive ones, with no significant difference (p=0.37) but great inter-subject heterogeneity in both groups. A median volume of 28 cc for responsive tumors and 6 cc for progressive ones had elevated arterial perfusion before RT (p=0.2) (Table 2). These subvolumes accounted for a median of 38% (3% – 90%) and 16% (15% – 46%) of the entire gross tumor volumes for responsive and progressive groups (p=0.31), respectively.
Table 2.
Gross tumor volumes (GTVs) and subvolumes with elevated arterial perfusion
| Patient No. | Tumor No. | GTV (cc) | Subvolume with elevated arterial perfusion
|
||
|---|---|---|---|---|---|
| Pre-RT (cc) | Mid-RT (cc) | % Change | |||
| 1 | 1* | 1324 | 197.9 | 328.0 | 66 |
| 2 | 1 | 580 | 413.8 | 328.5 | −21 |
| 3 | 1 | 33 | 11.1 | 16.5 | 49 |
| 4 | 1 | 1118 | 515.4 | 189.4 | −63 |
| 5 | 1 | 729 | 467.9 | 318.3 | −32 |
| 6 | 1* | 21 | 3.4 | 8.9 | 165 |
| 7 | 1 | 14 | 1.4 | 1.5 | 10 |
| 2 | 73 | 2.1 | 2.7 | 28 | |
| 3 | 18 | 1.1 | 1.1 | −8 | |
| 8 | 1 | 348 | 92.8 | 83.7 | −10 |
| 9 | 1 | 151 | 10.4 | 15.0 | 44 |
| 10 | 1 | 496 | 123.9 | 60.5 | −51 |
| 11 | 1 | 119 | 16.4 | 11.3 | −31 |
| 12 | 1* | 53 | 8.8 | 12.9 | 48 |
| 13 | 1 | 133 | 101.9 | 83.7 | −18 |
| 14 | 1* | 141 | 64.6 | 60.0 | −7 |
| 15 | 1 | 3 | 1.6 | 1.0 | −35 |
| 16 | 1 | 246 | 134.5 | 104.7 | −22 |
| 17 | 1 | 1644 | 710.2 | 723.3 | 2 |
| 2 | 27 | 24.1 | 6.0 | −75 | |
| 18 | 1 | 147 | 32.8 | 44.2 | 35 |
| 19 | 1 | 16 | 8.1 | 13.4 | 65 |
| 20 | 1* | 4 | 1.2 | 1.8 | 42 |
| 2* | 5 | 0.7 | 1.8 | 140 | |
tumor progressed post-RT
Change in the Subvolume of the Tumor with Elevated Arterial Perfusion
After receiving approximately 60% of planned doses, the tumor subvolumes with elevated arterial perfusion increased for progressive tumors, with a median of 57% (−7% – 165%) but decreased for responsive tumors with a median of −14% (−75% – 65%) (Table 2 and Figure 2. Left), and the difference between the two groups was significant (p=0.003). Figure 3 shows examples of tumor subvolumes with elevated arterial perfusion for two patients prior to and during treatment. From pre-RT to mid-RT, the volumetric averaged hepatic arterial perfusion values in the tumor increased significantly in the progressive group comparing to the responsive group (66% vs −9%, p=0.006).
Figure 2.
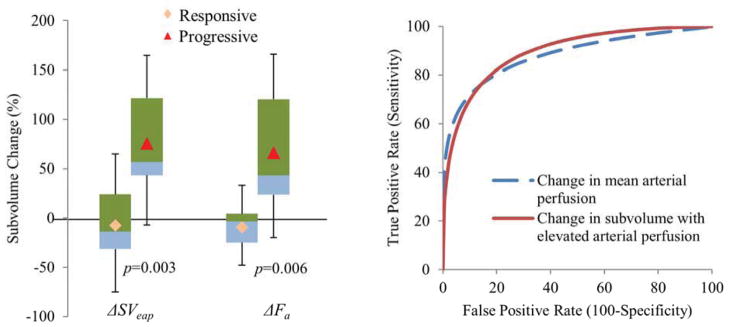
Left: Percentage changes in tumor subvolumes with elevated arterial perfusion ( SVeap) and means of arterial perfusion over the tumors ( Fa) from pre-RT to mid-RT. Right: ROC curves of predicting tumor progression post-RT using the percentage changes in the tumor subvolumes and means of arterial perfusion over the tumors.
Figure 3.
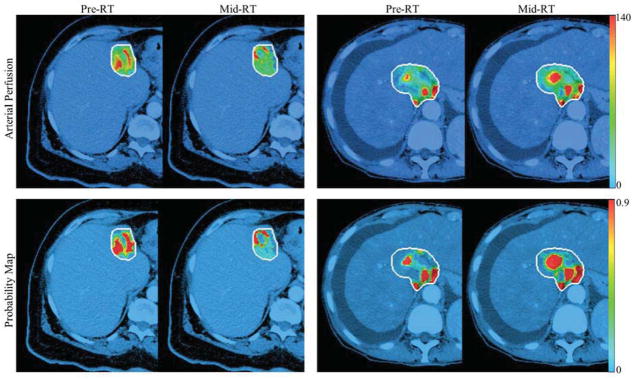
Examples of hepatic arterial perfusion maps (first row) and probability maps (second row) of a responsive tumor (first two columns) and a progressive tumor (last two columns) at pre-RT and mid-RT. The maps were overlapped on treatment planning CT using the colorbars of perfusion (first row) and probability (second row). The white curves contoured the gross tumor volumes.
Spatial Overlap of the Tumor Subvolumes
Using the probability threshold values of 0.3, 0.4, 0.5, 0.6, and 0.7, we obtained respective overlap fractions of the physical subvolumes between pre-RT to mid-RT of 70±24%, 68±23%, 65±24%, 63±24%, 60±24%, indicating the relative stability of the subvolume. With the threshold from 0.3 to 0.7, the mean ratios of the thresholded subvolumes to their GTVs were 49%, 46%, 43%, 41%, and 38%, respectively. Note that varying the threshold values from 0.3 to 0.7 only changes the subvolume by 11% (see Figure 4). A probability of 0.5 that represents perfusion of 52 ml/(100g·min) could be chosen as a threshold value to define the physical subvolume.
Figure 4.
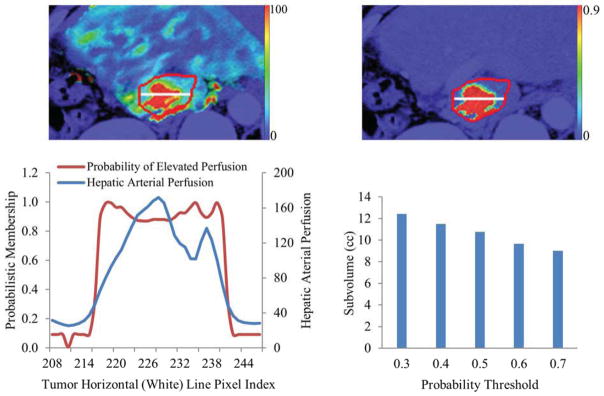
An example of a color-coded arterial perfusion map (left top), corresponding probability map of the red curve-contoured tumor (top right), a plot of the probabilities and perfusion values along a horizontal (white) line placed in the tumor (left bottom), and a plot of the tumor subvolumes at different probability thresholds (right bottom). Note that the physical subvolume is insensitive to the probability threshold values between 0.3 and 0.7.
Predictive Value of the Perfusion Imaging-Defined Subvolume
The ROC analysis showed that the percentage change in the probabilistic subvolumes of the elevated-perfusion tumor from pre-RT to mid-RT predicted post-RT tumor progression with an area under the curve (AUC) of 0.90 ± 0.08 (±SE). The ROC curve showed that choosing 85% of sensitivity resulted in 75% of specificity (Figure 2. Right). Using a probability threshold of 0.5, the change in the physically-defined subvolume at mid-RT predicted post-RT progression with an AUC of 0.90 ± 0.07. The percentage change in the mean arterial perfusion of the tumors from pre-RT to mid-RT resulted in the AUC of 0.88 ± 0.09.
Discussion
This study evaluated the change in the arterial perfusion of tumors from prior to RT to midway through a course of intrahepatic cancer treatment for prediction of ultimate tumor progression using DCE MRI. We found that an increase in the subvolumes of tumors exhibiting elevated arterial perfusion predicted progression. Unlike the analysis of a mean arterial perfusion change in the tumor for treatment response, the defined subvolume of the tumor reveals the spatial distribution of the active and potentially progressive portion, which would drive ultimate outcome. Personalized treatment based on patient-specific genetic, biological and physiological information prior to treatment has been extensively studied in clinical research. This study suggests that physiological imaging-defined tumor subvolumes measured during treatment provide information on tumor response, and furthermore, that aggressive subvolumes (those with a significant increase in perfusion during treatment) could be targeted with higher radiation doses to achieve better local control.
Perfusion in a solid tumor is characterized by heterogeneous distribution that might lead to heterogeneous treatment response. Using DCE-MRI, the highly arterial-vascularized area of the intrahepatic cancer, which is commonly considered as an indicator of active tumor (7), was extracted as tumor subvolume with elevated arterial perfusion. Meanwhile, the tortuous, chaotic tumor vasculature might have poor function to carry normal level of oxygen (20–22). Therefore, the subvolume of the intrahepatic cancer with arterial vascularization could be aggressive and/or resistant to radiotherapy (23). Our study generates the hypothesis that persistence or an increase in the subvolume of the intrahepatic tumor with elevated arterial perfusion after receiving a significant fraction of the treatment has the potential to early predict tumor progression.
It is a great challenge to measure the heterogeneous changes in tumors to identify a sensitive metric for response assessment. Assessing a voxel-level change in a physiological, metabolic or molecular imaging parameter depends upon high accuracy of voxel-to-voxel registration of a pair of images acquired at different times, which is a very complicated task when a tumor grows or shrinks and normal liver may shrink or hypertrophy. In this study, we extracted the feature subvolumes of the tumor from a heterogeneously distributed physiological imaging parameter using a fuzzy clustering-based method, and tested the feature subvolume defined metrics for response assessment (6). This approach does not require voxel-level accuracy of image registration of physiological images acquired over time, yet it still preserves the spatial location of the feature information. The representation of the continuously distributed probability function within the subvolume of tumor, instead of sporadic hot and cold areas, might be useful for dose re-distribution using adaptive re-planning for local boosting.
Consistent and accurate contouring of intrahepatic tumors on MR images was complicated by previous liver-directed therapies (e.g., transcatheter arterial chemoembolization [TACE], radiofrequency ablation [RFA], and RT) in some patients. In this study, the physician manually contoured a tumor on the co-registered pre-RT MR images by referring to the tumor volume that was determined previously for treatment planning based on diagnostic images. The pre-RT tumor contours were transferred onto the co-registered mid-RT images to define the tumor subvolumes with elevated arterial perfusion. If there was tumor shrinkage during RT, although the mid-RT tumor contours could include some areas with normal tissue or subclinical diseases, the inclusion of non-gross tumor tissue with low arterial perfusion into the tumor contours has little effect on our subvolume analysis. Future investigation of automatic tumor segmentation on multiple-parameter MR images may allow independent determination of tumor volumes at each imaging session without the need of image registration.
This study is limited by the small number of patients and the heterogeneity of their tumors in pathologic diagnosis, previous treatments and received radiotherapies. It is well known that RT controls metastases well but it is not the case for HCC and cholangiocarcinoma. We also observed the change in the tumor subvolume with elevated arterial perfusion at mid-RT was significantly different between HCC and non-HCC cases (p=0.005). It is necessary to evaluate the findings in this study in a cohort of patients with a same tumor type in the future. Furthermore, DCE-MRI has several limitations, including lack of standardization of imaging acquisition and post-processing, B1 field inhomogeneity at a 3T machine, and quantification of contrast concentration. Nevertheless, our findings show the potential for physiological adaptation of RT based upon early individual tumor response by assessing the change in the tumor subvolume with elevated arterial perfusion. Further validation of the present study in a large population may enable design of clinical trials to adaptively boost the “aggressive” subvolumes of intrahepatic tumors for better tumor control rates.
Conclusions
Our study provides preliminary evidence that the subvolume of a tumor with persistently elevated arterial perfusion during RT may predict local control failure after RT for unresectable intrahepatic cancer. The physiological imaging-defined subvolume could be a candidate target for focal dose boosting and allow therapy adaption based on tumor response.
SUMMARY.
This study extracted subvolumes of tumors from hepatic arterial images obtained before and during radiation therapy for intrahepatic tumors. The change in the tumor subvolumes with elevated arterial perfusion during treatment was tested for predicting tumor progression after therapy. The arterial perfusion imaging-defined subvolumes of tumors could provide candidates for dose boosting in irradiation for intrahepatic cancer.
Acknowledgments
The study was supported by RO1CA132834.
Footnotes
This work was presented at the 55th Annual Meeting of AAPM, August 2013.
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 2.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–560. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 3.Dirix P, Vandecaveye V, De Keyzer F, et al. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50:1020–1027. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 4.Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Popovtzer A, Eisbruch A, et al. An approach to identify, from DCE MRI, significant subvolumes of tumors related to outcomes in advanced head-and-neck cancer. Med Phys. 2012;39:5277–5285. doi: 10.1118/1.4737022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farjam R, Tsien CI, Feng FY, et al. Physiological Imaging-Defined, Response-Driven Subvolumes of a Tumor. Int J Radiat Oncol Biol Phys. 2013;85:1383–1390. doi: 10.1016/j.ijrobp.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande PV, Krinsky GA, Rusinek H, et al. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661–673. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 8.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 9.Hecht EM, Holland AE, Israel GM, et al. Hepatocellular carcinoma in the cirrhotic liver: gadolinium-enhanced 3D T1-weighted MR imaging as a stand-alone sequence for diagnosis. Radiology. 2006;239:438–447. doi: 10.1148/radiol.2392050551. [DOI] [PubMed] [Google Scholar]
- 10.Ceelen W, Smeets P, Backes W, et al. Noninvasive monitoring of radiotherapy-induced microvascular changes using dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) in a colorectal tumor model. Int J Radiat Oncol Biol Phys. 2006;64:1188–1196. doi: 10.1016/j.ijrobp.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Chen FH, Chiang CS, Wang CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. 2009;15:1721–1729. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 15.XXX
- 16.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 17.Materne R, Van Beers BE, Smith AM, et al. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 2000;99:517–525. [PubMed] [Google Scholar]
- 18.XXX
- 19.Aerts HJ, Bosmans G, van Baardwijk AA, et al. Stability of 18F-deoxyglucose uptake locations within tumor during radiotherapy for NSCLC: a prospective study. Int J Radiat Oncol Biol Phys. 2008;71:1402–1407. doi: 10.1016/j.ijrobp.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 21.Gillies RJ, Schornack PA, Secomb TW, et al. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1:197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 23.Chapman JD, Engelhardt EL, Stobbe CC, et al. Measuring hypoxia and predicting tumor radioresistance with nuclear medicine assays. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1998;46:229–237. doi: 10.1016/s0167-8140(97)00186-2. [DOI] [PubMed] [Google Scholar]