Abstract
Many non-human animals are capable of discriminating a group or entity containing more objects from one containing less of the same objects. The capacity for making judgments of numerousness may also allow individuals to discriminate between potential mates. Female meadow voles (Microtus pennsylvanicus) may use judgments of relative numerousness to distinguish between potential suitors by selecting males that signal their interest by depositing more scent marks relative to other males. We used a familiarization-discrimination paradigm in the absence of training to test the hypothesis that female voles will discriminate between the different numerosities of scent marks of two male conspecifics that are similar in features of their phenotype and quality. During the exposure phase, we presented female voles with different ratios of feces scent marks from two males. During the test phase, we presented females with a single, fresh fecal scent mark from each of the two male donors, whose marks they had previously encountered during the exposure phase. In both phases, females spent more time investigating the scent mark(s) of the male that deposited more scent marks than that of the male that deposited fewer scent marks provided the difference in the ratio of scent marks provided by the male donors in the exposure phase was greater than or equal to 2. Our results are consistent with studies on a variety of taxa suggesting that numerosity discriminations are evolutionarily ancient and spontaneously available to non-human animals and humans.
Keywords: Numerosity, scent marks, meadow voles, rodent, discriminations
Introduction
Several studies have shown that a variety of non-human animals in different taxa can discriminate between a group and entity containing more objects from one containing fewer of the same objects (Beran 2001; Boysen 1997; Kilian et al. 2003; Machado and Keen 2002; Pahl et al. 2013; Perdue et al. 2012; Rugani et al. 2011; Uller et al. 2003). This ability to make judgments of relative numerousness may allow animals to assess how much food is available and which patch contains the most food (Barnard et al. 2013; Beran et al. 2008; Hauser et al. 2003; Uller and Lewis 2009), track predators, assist in parenting, prevent brood parasitism, and discriminate between groups of intruders (Benson-Aram et al. 2011; McComb et al. 1994; Pahl et al. 2013; Wilson et al. 2000).
The capacity for making judgments of numerousness may also allow individuals to discriminate between potential mates. Carazo et al. (2009) found that male mealworm beetles (Tenebrio molitor) discriminated between the odors of females if the ratio between the female scent donors exceeded 1:2. By keeping track of the number of females and their odors, males can maximize their mating opportunities. Likewise, mammals may keep track of the odors and scent marks produced by conspecifics to find mates (Brown and Macdonald 1985; Hurst and Beynon 2004; Johnston 1983, 2003). For example, female meadow voles (Microtus pennsylvanicus) may need to keep track of the multiple male suitors who will deposit scent marks in or near their territory (Ferkin 2011; Ferkin et al. 2005). Vaughn and Ferkin (2011) discovered that male meadow voles spent more time investigating the scent mark of a female vole that had more scent marks of male conspecifics adjacent to it relative to the scent mark of a female vole that had fewer scent marks of male conspecifics adjacent to it if the ratio between the two numerosities was greater than 5:1. In another study, male voles were exposed to an area containing the overlapping scent marks of two female conspecifics (over-marks), in which the number of times each female donor provided the top-scent mark versus providing the bottom-scent mark varied (Ferkin et al. 2005). After being exposed to the over-marks, male voles were exposed to single, non-overlapping scent marks of the two scent donors. Male voles could discriminate between moderately large differences in the relative number of over-marks by the two scent donors, distinguishing between ratios in which one donor provided 6 top-scent marks and the other donor provided 1 top-scent mark (Ferkin et al. 2005). Thus, male voles may be more attracted to the female donor that deposited more over-marks. This donor is usually the female that is the current resident in that territory. Male voles may use differences in the amount of scent marks they find in an area to determine which female may be the resident female. Resident female voles have a higher fitness compared to that of female voles that are transients (Wolff 1993). Female meadow voles were able to discriminate between smaller differences in the relative number of over-marks by the two male scent donors, distinguishing between ratios in which one donor provided 4 top-scent marks and the other donor provided 3 top-scent marks (Ferkin et al. 2005). Thus, female voles are better at discriminating between numerosities that differ by smaller amounts compared to male voles. Males that scent mark at higher rates compared to males that mark at lower rates may be of higher quality (Roberts 2007). Accordingly, the ability to discriminate between the number of scent marks that various males deposit in her territory may allow females to select males of higher quality as potential mates (Ferkin 2011). Presumably, males that deposit more scent marks may be more interested in mating with that female or represent higher-quality phenotype (Ferkin 2011; Hurst and Beynon 2004; Roberts 2007).
In the Ferkin et al. (2005) study, we showed that meadow voles were capable of numerosity discrimination. However, in the present study, we quantify a female vole’s numerosity discrimination. Accordingly, we tested two hypotheses. The first hypothesis was that female voles could make numerosity discriminations between scent marks of donors A and B; donors A and B were similar in phenotype and quality to one another. We considered the more investigated mark as being preferred by females (Ferkin and Johnston 1995; Ferkin et al. 2005; Vaughn and Ferkin 2011). During the exposure and test phases we also tested the second hypothesis that the amount of time female voles spent investigating the scent marks of donors A and B followed Weber’s Law as it relates to the just noticeable difference in a stimulus in proportion to the magnitude of the original stimulus (e.g., Beran and Beran 2004; Carazo et al. 2009; Lewis et al. 2005; Rugani et al. 2011). Specifically, as the numerical magnitude increases, a larger difference between the sets is needed to obtain the same level of discrimination.
We also determined if these ratio effects were due to the differences in the proportion of the number of scent marks that they encountered. First, we exposed female meadow voles simultaneously to two sets of scent marks that differed in the number of scent marks within each set, measuring the amount of time they spent investigating each set. We predicted that females would spend more time investigating the larger set of scent marks over the smaller set of scent marks. Next, we presented the females voles with a single scent mark from each of the two male donors. We predicted that female voles would spend more time investigating the scent mark of the male that previously deposited more scent marks during the exposure phase than that of the male that previously deposited fewer scent marks.
We tested these predictions by using a familiarization-discrimination paradigm (Ferkin et al. 2005; Hauser et al. 2003; Vaughn and Ferkin 2011) in the absence of training to determine the capacity of female meadow voles for discriminating between two numerosities of scent marks from two potential mates. Our experimental paradigm consisted of two phases, an exposure phase and a test phase. We chose this format because, during the exposure phase, a female meadow vole may spend more time investigating a set with more scent marks simply because it takes her longer to process the additional information. Including a test phase in which the female encounters only one scent mark from each male allows us to determine if the amount of time she investigates a particular scent mark indicates a preference for that male.
General Methods
Animals
Voles were fourth to sixth generation offspring of field-caught animals captured in Ohio and Kentucky, USA. All voles used in this experiment were weaned at 21 days of age, housed with littermates until 35 days of age, and then housed singly in clear plastic cages (27 x 16.5 x 12.5 cm) thereafter. These cages contained wood chip bedding and cotton nesting material. Voles had continuous access to food (Harlan Teklad Rodent Diet, #8640, Madison, WI, USA) and water. These cages were cleaned once a week and the cotton nesting material was replaced every two weeks.
Female and male meadow voles were used in the following experiments. In each experiment, we used animals born and raised under a long photoperiod (14L: 10D; lights on at 0800 h, CST). This photoperiod simulates a day length typical of the breeding season. Female and male voles born and raised under a long photoperiod reach sexual maturity at 40 and 50 days of age, respectively, and as adults display similar responses to the odors and scent marks of conspecifics as do free-living voles during the spring and summer (Ferkin 2011; Ferkin and Johnston 1995). Female meadow voles do not undergo estrous cycles (Keller 1985) and are induced ovulators (Milligan 1982).
All the voles used in this study were between 65–95 days of age, sexually mature, but sexually inexperienced. Subjects were 132 female voles and scent donors were 64 male voles; subjects and scent donors weighed approximately 48.1 ± 3.9 g. To eliminate litter effects, we used no more than two individuals from the same litter as either subjects or scent donors. During the tests, subjects and scent donors were unfamiliar and unrelated to one another.
Collection of stimulus odors for the exposure and test phases
Fresh fecal scent marks were obtained for each exposure and test phase from each scent donor. The experimenter wore disposable latex gloves to minimize human scent transfer while handling all slides. Feces contain sexually distinct odor cues for meadow voles (Ferkin and Johnston 1995) and are deposited by free-living male voles during their daily travels.
Exposure phase
The exposure phase took place in the female subject’s home cage; each female was exposed to a unique combination of male scent donors. We followed the methods for determining odor preferences that were employed by Vaughn and Ferkin (2011). Briefly, we presented female voles with a plastic slide (2.70 cm x 10.83 cm; w x l) that contained feces scent marks from the following combinations (ratios) of scent marks from two different male conspecifics: 1) 5 scent marks from the first male (donor A) versus (vs.) 4 scent marks from a second male (donor B); 2) 5 vs. 3; 3) 5 vs. 2; 4) 5 vs. 1; 5) 4 vs. 3; 6) 4 vs. 2; 7) 4 vs. 1; 8) 3 vs. 2; 9) 3 vs. 1; 10) 2 vs. 1; and 11) 3 vs. 3.
We divided the plastic slide into three equal sections (each section was 3.61 cm in length); one end section of the slide contained the fresh feces scent marks from donor A and the other end section contained the fresh feces mark(s) from donor B. Except for the control condition (3 vs. 3), donor A always contributed more scent marks than did donor B. The middle section of the slide contained no scent marks. To deposit the scent marks on the slide, we gently rubbed a fecal bolus of one of the male donors against one of the end sections of the slide for 3–5 seconds; we rubbed the feces of the other male donor across the other end section of the same slide and so on until all the scent marks were placed on the slide. One minute separated the deposition of the scent marks on the slide. The scent mark from each male was approximately 0.5 cm x 0.2 cm (l x w) and placed within 5 mm of the center of their respective end section of the slide in a roughly circular pattern; the marks did not touch or overlap. For each trial, we alternated the placement of the male scent donors’ scent marks on the left or right side of the slide.
Five minutes after the last scent marks were placed on the side it was suspended in the home cage of the female subject. A single observer blind to the number of scent marks placed on the slides recorded continuously for 5 minutes the amount of time that female voles investigated (the subject licked the slide or its nose came within 2 cm of the slide) the three sections of the slide. The trial began when the slide was placed into the cage of the subject. Each exposure slide was used once and then cleaned with 70% ethanol and dried prior to use in subsequent trials. In all the exposure trials, females spent little time (1.1 ± 0.2 seconds, mean ± SEM) investigating the clean, middle section of the slide. Thus, we did not include that measure in the data analysis.
Test Phase
One minute after the exposure phase, each female subject underwent a single 5-minute test trial; this test trial duration has been used previously in voles (Ferkin and Johnston 1995). Briefly, females were presented with a clear, glass slide that contained a single, fresh fecal scent mark (1.2 cm x 0.5 cm; l x w) from each of the two male donors whose marks they encountered during the exposure phase. As in the exposure phase, each slide prepared during the test phase was divided into three equal sections. The middle section contained no stimulus odor. One end section contained the scent mark of donor A and the other end section contained the scent mark of donor B; the placement of the scent mark on the right or left side of the slide was alternated for each trial.
The slide was suspended in the female’s cage 5 minutes after the last scent mark was placed on it. During each 5-minute test phase, the observer recorded continuously the amount of time each subject investigated the two scented sections of the slide and the middle section of the slide. Each slide was used in only one trial and discarded. In the test trials, female voles spent little time (0.6 ± 0.3 seconds, mean ± SEM) investigating the clean, middle section of the slide. Thus, we did not include that measure in the data analysis.
Statistics
In both the exposure and test phases, we used separate paired t-tests to determine if females spent different amounts of time investigating the scent marks of donor A and donor B. By doing so, we could test the first hypothesis that female voles make numerosity discriminations between the scent marks of donors A and B. We considered the more investigated mark as being preferred by females (Ferkin and Johnston 1995; Ferkin et al. 2005; Vaughn and Ferkin 2011). We also tested the second hypothesis that the amount of time female voles spent investigating the scent marks of donors A and B followed Weber’s Law during both the exposure and test phases. To do so, we created a continuous variable, the amount of time spent investigating the scent marks from donor A divided by the sum of the amount of time female voles spent investigating the marks of donors A and B. The quotient was arcsine square root transformed. We found that the data were not normally distributed (Shapiro-Wilk test, P > 0.05). Thus, we used a Kruskal-Wallis analysis of variance followed by Student-Newman-Keuls for post-hoc pairwise comparisons. We calculated the correlation between this ratio for both the exposure and test phases and the ratio of scent marks provided by donor A to that of donor B during the exposure phase using Spearman’s rho. Significant differences were accepted at α = 0.05 for all statistical analyses.
Results
Exposure phase
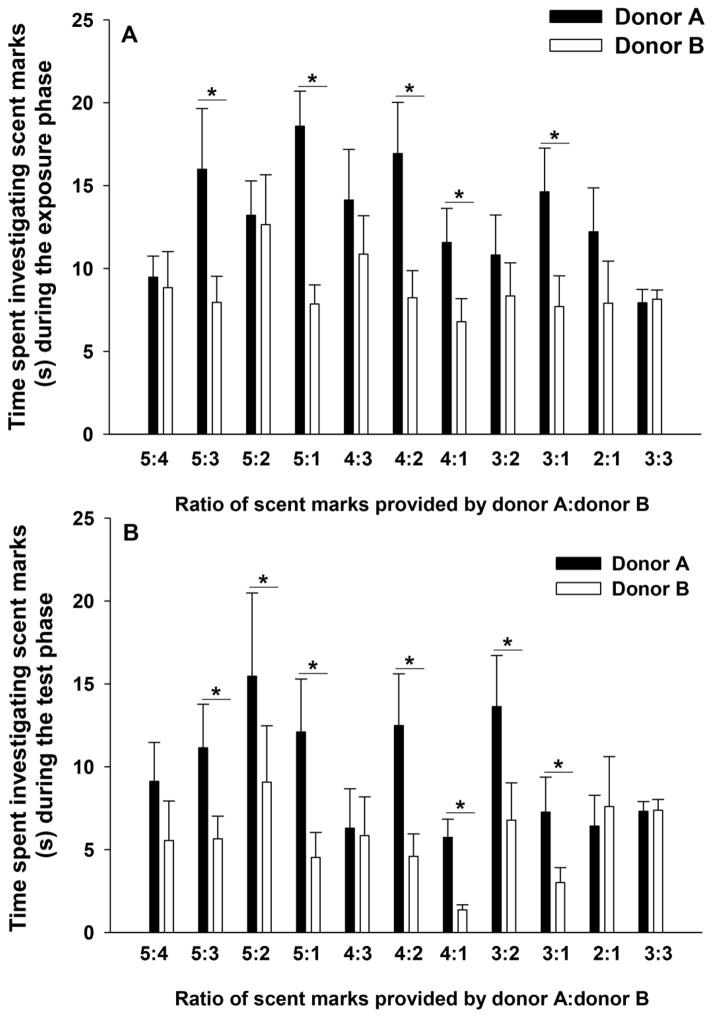
During the exposure phase, female meadow voles spent more time investigating the scent mark(s) of donor A compared to those of donor B when the ratio of scent marks of donor A to donor B was 5:3, 5:1, 4:2, 4:1, or 3:1 (Table 1, Figure 1A). However, females spent similar amounts of time investigating the marks of donor A and donor B when the ratio of their scent marks was 5:4, 5:2, 4:3, 3:2, 3:1, or 3:3 (Table 1, Figure 1A).
Table 1.
Paired t-test values for the exposure and test phases of the 11 ratios tested.
| Ratio | Exposure Phase | Test Phase |
|---|---|---|
| 5:4 | t11 = 0.319, P = 0.755 | t11 = 1.358, P = 0.202 |
| 5:3 | t11 = 2.333, P = 0.040 | t11 = 3.421, P = 0.006 |
| 5:2 | t11 = 0.342, P = 0.739 | t11 = 3.340, P = 0.007 |
| 5:1 | t11 = 5.726, P < 0.001 | t11 = 3.649, P = 0.004 |
| 4:3 | t11 = 1.708, P = 0.116 | t11 = 0.322, P = 0.754 |
| 4:2 | t11 = 3.260, P = 0.008 | t11 = 3.667, P = 0.004 |
| 4:1 | t11 = 2.441, P = 0.033 | t11 = 4.236, P = 0.001 |
| 3:2 | t11 = 1.059, P = 0.312 | t11 = 2.757, P = 0.019 |
| 3:1 | t11 = 5.470, P < 0.001 | t11 = 2.855, P = 0.016 |
| 2:1 | t11 = 1.352, P = 0.204 | t11 = −0.580, P = 0.574 |
| 3:3 | t11 = −0.261, P = 0.799 | t11 = −0.211, P = 0.837 |
Figure 1.
The mean amount of time (+ SEM) in seconds that female meadow voles spent investigating (a) the differing number of scent marks of two male scent donors during the exposure phase and (b) the single scent marks of the same two male donors during the test phase. Asterisk (*) indicate a significant difference (P < 0.05) between the pairs.
During the exposure phase, the ratio of time spent investigating the scent marks of donor A to the total time spent investigating scent marks was significantly correlated with the ratio of the number of scent marks provided by donor A to those provided by donor B (ρ = 0.318, P = 0.0002). As the ratio of the number of scent marks provided by donor A to that of donor B increases, the ratio of time spent investigating donor A’s scent marks to the total time spent investigating scent marks also increases.
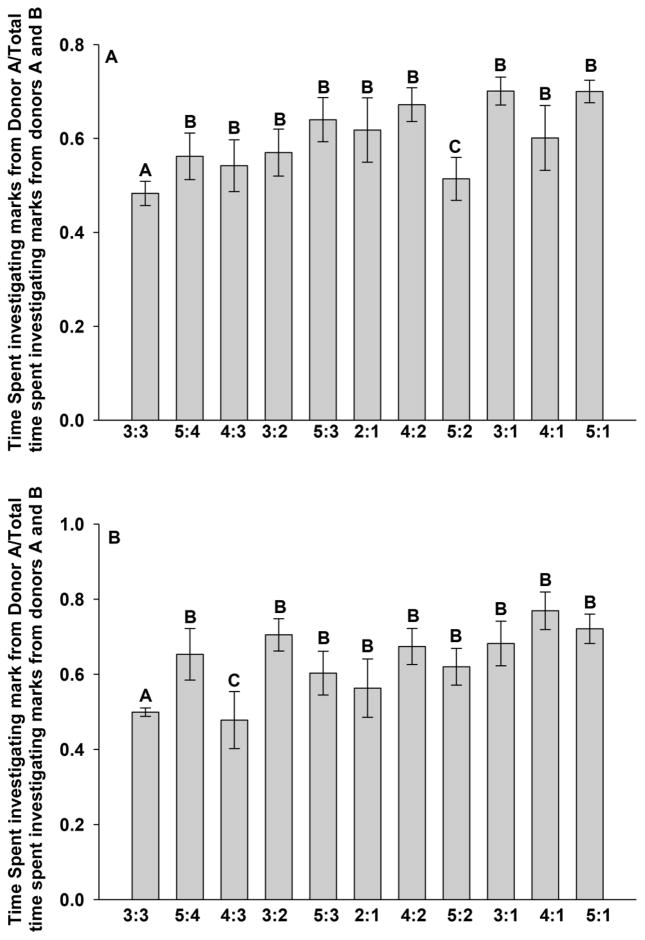
A Kruskal-Wallis Analysis of Variance revealed that group significantly affected the proportion of time spent investigating the marks of donor A to that of time spent investigating the scent marks from donors A and B (H10 = 28.77, P = 0.001). Post-hoc pairwise comparisons using the Student-Newman-Keuls method revealed the following differences: female voles exposed to the ratios of 5:4, 5:3, 5:1, 4:3, 4:2, 4:1, 3:2, 3:1, and 2:1 showed a significantly stronger preference for donor A compared to females exposed to ratios of 3:3 or 5:2 (Figure 2A). Females exposed to a ratio of 5:2 showed a stronger preference for donor A relative to females exposed to the ratio of 3:3 (Figure 2A).
Figure 2.
The mean proportion of time (± SEM) spent investigating either (a) the scent marks of donor A to the total time investigating the scent marks of donors A and B during the exposure phase or (b) the single scent mark of donor A to the total time spent investigating the single scent marks of donors A and B during the test phase. Columns capped by different letters are statistically different from one another (Student-Newman-Keuls method, P < 0.05).
Test phase
During the test phase, females spent more time investigating the single scent mark of donor A compared to donor B when the ratio of scent marks during the exposure phase was 5:3, 5:2, 5:1, 4:2, 4:1, 3:2, and 3:1 (Table 1, Figure 1B). However, female voles spent similar amounts of time investigating the single scent marks of donor A and donor B when the ratio of their scent marks during the exposure phase was 5:4, 4:3, 2:1, or 3:3 (Table 1, Figure 1B).
During the test phase, the ratio of time spent investigating the scent marks of donor A to the total time spent investigating scent marks was significantly correlated with the ratio of the number of scent marks provided by donor A to those provided by donor B (ρ = 0.302, P = 0.0004). As the ratio of the number of scent marks provided by donor A to that of donor B during the exposure phase increases, the ratio of time spent investigating donor A’s scent marks to the total time spent investigating scent marks during the test phase also increases.
Our findings suggest that the amount of time that female voles investigated the scent marks of donors A and B may be following Weber’s Law. That is, as the numerical magnitude increases, a larger difference between the sets is needed to obtain the same level of discrimination. A Kruskal-Wallis Analysis of Variance revealed that group significantly affected the proportion of time spent investigating the mark of donor A to that of time spent investigating the scent marks from donors A and B (H10 = 25.27, P = 0.005). Post-hoc pairwise comparisons using the Student-Newman-Keuls method revealed the following differences: female voles exposed to the ratios of 5:4, 5:2, 5:3, 5:1, 4:2, 4:1, 3:2, 3:1, and 2:1 showed a significantly stronger preference for donor A compared to females exposed to ratios of 3:3 or 4:3 (Figure 2B). Females exposed to a ratio of 3:3 showed a stronger preference for donor A relative to females exposed to the ratio of 4:3 (Figure 2B).
Discussion
The results of our familiarity-discrimination paradigm suggest female voles were able to discriminate between different numerosities of scent marks provided by two male conspecifics that are similar in features of their phenotype and quality. During the exposure phase, female meadow voles were simultaneously exposed to two sets of scent marks that differed in number. We found that female voles spent more time investigating the larger set of scent marks over the smaller set of scent marks in five out of six groups where the difference between numbers in the ratio was greater than or equal to 2 (5:3, 5:1, 4:2, 4:1, and 3:1, with 5:2 being the exception). During the test phase, female voles were exposed simultaneously to a single scent mark from each of the two male donors. We discovered that female voles recalled the donor that provided more scent marks during the exposure phase. During the test phase, females spent more time investigating the single scent mark of the male that previously deposited more scent marks than that of the male that previously deposited fewer scent marks if the difference in the ratio of scent marks provided by the male donors in the exposure phase was greater than or equal to 2 (5:3, 5:2, 5:1, 4:2, 4:1, and 3:1). Interestingly, when exposed to a ratio of 3 scent marks from donor A and two scent marks from donor B (3:2), females spent a similar amount of time investigating the scent marks from both donors. However, during the test phase, females spent more time investigating the mark of donor A than the mark of donor B. Our results provide strong evidence that female meadow voles are capable of numerosity discrimination based on scent marks and that such a system works when an operational ratio of scent marks of male donors is 1.7:1. Overall, these findings support and augment those of Ferkin et al. (2005), who showed previously that voles were capable of discrimination of different numerosities.
Similar ratio effects were reported by Carazo et al. (2009), who found that male beetles discriminated between the odors of females in the ratios 1 vs. 4 and 1 vs. 3 females. However, male beetles could not discriminate between ratios of 1 vs. 2 or 2 vs. 4 females, suggesting that males were capable of chemically discriminating between two odor sources based on the number of females contributing to the odor, if the ratio exceeds 1:2. Vaughn and Ferkin (2011) discovered that male voles spent more time investigating the scent mark of a female vole that had 5 male scent marks adjacent to her scent mark relative to the scent mark of a female vole that had 1 or 0 scent marks adjacent to her scent mark. Male meadow voles also discriminated between the numbers of times an opposite-sex conspecific provided the top-scent mark versus providing the bottom-scent mark if the ratio of providing the top-scent mark to providing the bottom-scent mark was either 7:0 or 6:1 (Ferkin et al. 2005). In contrast, female voles could distinguish between the top- and bottom-scent donors if the ratios were 7:0, 6:1, 5:2, and 4:3, indicating that females could make finer discriminations of relative numerousness compared to male voles (Ferkin et al. 2005). Similarly, cotton-topped tamarins (Saguinus oedipus) presented with auditory stimuli discriminated sequences of 4:8, 4:6, and 8:12 syllables but could not do so if the sequences were 4:5 and 8:10 syllables (Hauser et al. 2003). In these studies, the responses of individuals to the different numerosities were largely due to differences and magnitude changes in the proportion of stimulus intensity and not to differences in the absolute number of stimuli.
It is possible, however, that the numerosity discriminations made by female voles may be due to the total amount of scent marks from each male they encountered, rather than the number of separate scent marks. Quantity estimation can be accomplished using non-numerical continuous cues such as amount estimation, which can co-vary with numerosity (Argillo et al. 2011; Carazo et al. 2012; Shifferman 2011). However, previous work has shown that meadow voles do not select the donor that provided scent marks that covered more surface area than those of another donor. Voles spent similar amounts of time investigating the scent marks and scent marking in response to small and large scent marks of two opposite-sex conspecifics of similar body mass, age, and reproductive condition (Ferkin et al. 1999, 2004a, b; Wolff et al. 2002). Thus, we believe that it is likely that the discrimination by female voles in the present study depended on the difference in the number of scent marks provided by the male scent donors during the exposure phase.
Our results also show that the preference of female meadow voles increases as the ratio between the number of scent marks of the male donors increases and that this numerosity discrimination is independent of absolute size. We suggest that the numerosity discrimination made by female voles in the present study were most likely due to differences and magnitude changes in the proportion of stimulus intensity and not differences in the amount of surface area covered by the scent marks provided by male scent donors. That is, the preferences of female voles for the scent marks of male donors seem to follow Weber’s Law. Thus, the present study using scent marks joins other studies using chemical, visual, and auditory cues (Beran 2001; Hauser et al. 2003; Pahl 2013) that support the view that non-human animals have the capacity for numerical discrimination that is modulated by Weber’s Law (Beran and Beran 2004; Carazo et al. 2009; Lewis et al. 2005; Rugani et al. 2011; Uller and Lewis 2009). Collectively, these studies on a variety of taxa demonstrate that numerosity discriminations are evolutionarily ancient and spontaneously available to non-human animals (Barnhard et al. 2013; Beran 2008; Hauser 2000; Hauser et al. 2003; Pahl et al. 2013; Rugani et al. 2011) and humans (Boysen and Capaldi 1993; Brannon et al. 2006; Lipton and Spelke 2003; Uller et al. 2013).
Also of interest is the extent to which female voles may use judgments of relative numerousness to facilitate decision processing surrounding mate choice. Most terrestrial mammals deposit scent marks along well-traversed paths and on prominent objects in an area, where they can be encountered by conspecifics (Brown and Macdonald 1985; Gosling and Roberts 2001; Thiessen and Rice 1976). Individuals may use the “public information” provided by scent marks to assist them in selecting potential mates (Ferkin 2011; Hurst and Beynon 2004; Johnston 1983, 2003; Roberts 2007). Many terrestrial mammals mate with multiple partners (Birkhead 2000). Thus, more than one male will deposit their scent marks near the scent marks of a sexually receptive female to signal their presence in an area (Brown and Macdonald 1985; Ferkin 2011; Wolff et al. 2002). Female mammals may use judgments of relative numerousness to distinguish between the marks of multiple male scent donors (Ferkin et al. 2005; this study) and identify scent donors, allowing female voles to choose as mates males that are of higher relative quality. Presumably, males of higher quality or phenotype may deposit more scent marks than males of lower quality in the territories of female conspecifics (Hurst and Beynon 2004; Roberts 2007). Females may select the former males as potential mates, which may provide them with indirect fitness benefits such as mating with a vigorous courting male (Kokko et al. 2003).
Acknowledgments
We thank Dr. Chris Vlautin, Lyndsey Pierson, and two anonymous reviewers for providing comments that strengthened the manuscript. This research was supported by NIH grant HD-049525 to MH Ferkin.
Contributor Information
Michael H. Ferkin, Email: mhferkin@memphis.edu, The University of Memphis, Department of Biological Sciences, Ellington Hall, Memphis, TN 38152 USA; –ph# 901-678-3509; fax 901-678-4746
Nicholas J. Hobbs, Email: njhobbs2@gmail.com, Michigan State University, Neuroscience Program, Giltner Hall, East Lansing, MI 48824 USA
References
- Argillo C, Piffer L, Biszarro A. Number versus continuous quantity in numerosity judgments by fish. Cognition. 2011;119:281–287. doi: 10.1016/j.cognition.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Barnard AM, Hughes KD, Gerhardt RR, DiVincenti L, Jr, Bovee JM, Cantlon JF. Inherently analog quantity representations in olive baboons (Papio anubis) Frontiers Psychol. 2013;4:253. doi: 10.3389/fpsyg.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Amram S, Heinen VK, Dryer SL, Holekamp KE. Numerical assessment and individual call discrimination by wild spotted hyaenas, Crocuta crocuta. Anim Behav. 2011;82:743–752. [Google Scholar]
- Beran MJ. Summation and numerousness judgments of sequentially presented sets of items by chimpanzees (Pan troglodytes) J Comp Psych. 2001;115:181–191. doi: 10.1037/0735-7036.115.2.181. [DOI] [PubMed] [Google Scholar]
- Beran MJ. The evolutionary and developmental foundations of mathematics. PLoS Biology. 2008;6:e19. doi: 10.1371/journal.pbio.0060019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Beran MM. Chimpanzees remember the results of one-by-one addition of food items to sets over extended periods of time. Psychol Sci. 2004;15:94–99. doi: 10.1111/j.0963-7214.2004.01502004.x. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Harris EH. Perception of food amounts by chimpanzees based on the number, size, contour length and visibility of items. Anim Behav. 2008;75:1793–1802. doi: 10.1016/j.anbehav.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead TR. Promiscuity: an evolutionary history of sperm competition. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Boysen ST. Representation of quantities by apes. Adv Study Behav. 1997;26:435–462. [Google Scholar]
- Boysen ST, Capaldi EJ. The Development of Numerical Competence: Animal and human models. Erlbaum Publ; Hillsdale, NJ, USA: 1993. [Google Scholar]
- Brannon EM. The representation of numerical magnitude. Curr Opin Neurobiol. 2006;16:222–229. doi: 10.1016/j.conb.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, MacDonald DW. Social odours in mammals. 1 and 2. Clarendon Press; Oxford: 1985. [Google Scholar]
- Carazo P, Fernández-Perea R, Font E. Quantity estimation based on numerical cues in the mealworm beetle (Tenebrio molitor) Frontiers Psychol. 2012;3:502. doi: 10.3389/fpsyg.2012.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo P, Font E, Forteza-Behrendt E, Desfilis E. Quantity discrimination in Tenebrio molitor: evidence of numerosity discrimination in an invertebrate? Anim Cogn. 2009;12:463–470. doi: 10.1007/s10071-008-0207-7. [DOI] [PubMed] [Google Scholar]
- Ferkin MH. Odor-related behavior and cognition in meadow voles, Microtus pennsylvanicus (Arvicolidae, Rodentia) Folia Zool. 2011;60:262–276. [Google Scholar]
- Ferkin MH, Johnston RE. Meadow voles, Microtus pennsylvanicus, use multiple sources of scent for sex recognition. Anim Behav. 1995;49:37–44. [Google Scholar]
- Ferkin MH, Dunsavage J, Johnston RE. What kind of information do meadow voles (Microtus pennsylvanicus) use to distinguish between the top and bottom scent of an over-mark? J Comp Psych. 1999;113:43–51. [Google Scholar]
- Ferkin MH, Lee DN, Leonard ST. The reproductive state of female voles affects their scent marking behavior and the responses of male conspecifics to such marks. Ethology. 2004a;110:257–272. [Google Scholar]
- Ferkin MH, Li HZ, Leonard ST. Meadow voles and prairie voles differ in the percentage of conspecific marks that they over-mark. Acta Ethol. 2004b;7:1–7. [Google Scholar]
- Ferkin MH, Pierce AA, Sealand RO, delBarco-Trillo J. Meadow voles, Microtus pennsylvanicus, can distinguish more over-marks from fewer over-marks. Anim Cogn. 2005;8:182–189. doi: 10.1007/s10071-004-0244-9. [DOI] [PubMed] [Google Scholar]
- Gosling LM, Roberts SC. Scent marking in male mammals: cheat-proof signals to competitors and mates. Adv Study Behav. 2001;30:169–217. [Google Scholar]
- Hauser MD. What do animals think about numbers? Amer Sci. 2000;88:144–151. [Google Scholar]
- Hauser MD, Tsao F, Garcia P, Spelke ES. Evolutionary foundations of number: spontaneous representation of numerical magnitudes by cotton-top tamarins. Proc R Lond B Biol Sci. 2003;270:1441–1446. doi: 10.1098/rspb.2003.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signaling in mice. Bioassays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical signals and reproductive behavior. In: Vandenbergh JG, editor. Pheromones and reproduction in mammals. Academic Press; New York: 1983. pp. 3–37. [Google Scholar]
- Johnston RE. Chemical communication in rodents: from pheromones to individual recognition. J Mamm. 2003;84:1141–1162. [Google Scholar]
- Keller BL. Reproductive patterns. Am Soc Mammal. 1985;8:725–778. [Google Scholar]
- Kilian A, Yana S, von Fersen L, Gunturkun O. A bottlenose dolphin discriminates visual stimuli differing in numerosity. Learn Behav. 2003;31:133–142. doi: 10.3758/bf03195976. [DOI] [PubMed] [Google Scholar]
- Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proc Royal Soc London B, Biol Sciences. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KP, Jaffe S, Brannon EM. Analog number representations in mongoose lemurs (Eulemur mongoz): evidence from a search task. Anim Cogn. 2005;8:247–252. doi: 10.1007/s10071-004-0251-x. [DOI] [PubMed] [Google Scholar]
- Lipton JS, Spelke ES. Origins of number sense. Large-number discrimination in human infants. Psych Sci. 2003;14:396–408. doi: 10.1111/1467-9280.01453. [DOI] [PubMed] [Google Scholar]
- Machado A, Keen R. Relative numerosity discrimination in the pigeon: further tests of the linear-exponential-ratio model. Behav Processes. 2003;57:131–148. doi: 10.1016/s0376-6357(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Madison DM. An integrated view of the social biology of Microtus pennsylvanicus. The Biologist. 1980;62:20–33. [Google Scholar]
- McComb K, Packer C, Pusey A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim Behav. 1994;47:379–387. [Google Scholar]
- Milligan SR. Induced ovulation in mammals. In: Finn CA, editor. Oxford reviews of reproductive biology. Vol. 4. Clarendon Press; London: 1982. pp. 1–46. [Google Scholar]
- Pahl M, Si A, Zhang S. Numerical cognition in bees and other insects. Frontiers Psych. 2013;4:162. doi: 10.3389/fpsyg.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue BM, Talbot CF, Stone A, Beran MJ. Putting the elephant back in the herd: elephant relative quantity judgments match those of other species. Anim Cogn. 2012;15:955–961. doi: 10.1007/s10071-012-0521-y. [DOI] [PubMed] [Google Scholar]
- Roberts SC. Scent marking. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological and evolutionary perspective. The University of Chicago Press; Chicago: 2007. pp. 255–266. [Google Scholar]
- Rugani R, Regolin L, Vallortigara G. Summation of large numerousness by newborn chicks. Frontiers Psychol. 2011;2:179. doi: 10.3389/fpsyg.2011.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifferman EM. It’s all in your head: the role of quantity estimation in sperm competition. Proc R Soc B: Biol Sci. 2011;279:833–840. doi: 10.1098/rspb.2011.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiessen DD, Rice M. Mammalian scent gland marking and social behavior. Psychol Bull. 1976;83:505–539. [PubMed] [Google Scholar]
- Uller C, Lewis J. Horses (Equus caballus) select the greater of two quantities in small numerical contrasts. Anim Cogn. 2009;12:733–738. doi: 10.1007/s10071-009-0225-0. [DOI] [PubMed] [Google Scholar]
- Uller C, Jaegar R, Guidry G, Martin C. Salamanders (Plethodon cinerus) go for more: rudiments of number in an amphibian. Anim Cogn. 2003;6:105–112. doi: 10.1007/s10071-003-0167-x. [DOI] [PubMed] [Google Scholar]
- Uller C, Urquhart C, Lewis J, Berntsen M. Ten-month-old infants’ reaching choices for “more”: the relationship between inter-stimulus distance and number. Frontiers Psych. 2013;4:84. doi: 10.3389/fpsyg.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AA, Ferkin MH. The presence and number of male competitor’s scent marks and female reproductive state affects the response of male meadow voles to female conspecifics’ odours. Behavior. 2011;148:927–943. [Google Scholar]
- Wilson ML, Hauser MD, Wrangham RW. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim Behav. 2001;61:1203–1216. [Google Scholar]
- Wolff JO. Why are female small mammals territorial? Oikos. 1993;68:364–370. [Google Scholar]
- Wolff JO, Mech SG, Thomas SA. Scent marking in female prairie voles: a test of alternative hypotheses. Ethology. 2002;108:483–494. [Google Scholar]