Summary
Vemurafenib and dabrafenib block MEK-ERK1/2 signaling and cause tumor regression in the majority of advanced-stage BRAFV600E melanoma patients; however, acquired resistance and paradoxical signaling have driven efforts for more potent and selective RAF inhibitors. Next generation RAF inhibitors, such as PLX7904 (PB04), effectively inhibit RAF signaling in BRAFV600E melanoma cells without paradoxical effects in wild-type cells. Furthermore, PLX7904 blocks the growth of vemurafenib-resistant BRAFV600E cells that express mutant NRAS. Acquired resistance to vemurafenib and dabrafenib is also frequently driven by expression of mutation BRAF splice variants; thus, we tested the effects of PLX7904 and its clinical analog, PLX8394 (PB03), in BRAFV600E splice variant-mediated vemurafenib-resistant cells. We show that paradox breaker RAF inhibitors potently block MEK-ERK1/2 signaling, G1/S cell cycle events, survival and growth of vemurafenib/PLX4720-resistant cells harboring distinct BRAFV600E splice variants. These data support the further investigation of paradox-breaker RAF inhibitors as a second-line treatment option for patients failing on vemurafenib or dabrafenib.
Keywords: BRAF, paradox breaker RAF inhibitor, splice variant, vemurafenib resistance
Introduction
The RAF inhibitors, vemurafenib and dabrafenib, are FDA-approved for the treatment of BRAFV600E-harboring melanoma but most patients who respond will ultimately develop progressive disease (Chapman et al., 2011; Flaherty et al., 2010; Hauschild et al., 2012; Sosman et al., 2012). This acquired/secondary resistance is frequently driven by ERK1/2 pathway re-activation and is mediated by expression of alternative splice forms of BRAFV600E, secondary mutations in forms of RAS, or amplification of BRAFV600E (Nazarian et al., 2010; Poulikakos et al., 2011; Shi et al., 2012). In contrast to effects in BRAFV600E melanoma cells, vemurafenib and other RAF inhibitors hyperactivate the ERK1/2 pathway in wild-type BRAF cells that exhibit high RAS activity (Halaban et al., 2010; Heidorn et al., 2010; Kaplan et al., 2011; Poulikakos et al., 2010). This paradoxical ERK1/2 hyperactivation promotes the proliferation of keratinocytes that harbor initiating mutations and is associated with the development of cutaneous squamous cell carcinomas/keratoacanthomas (Oberholzer et al., 2012; Su et al., 2012), adenomas (Chapman et al., 2012), and leukemia (Callahan et al., 2012) in RAF inhibitor-treated patients. The paradoxical action of RAF inhibitors is linked to altered heterodimerization of RAF proteins and is directing the development of next generation RAF inhibitors.
Paradox breaker compounds from Plexxikon Inc. are new selective RAF inhibitors that do not hyperactivate MEK-ERK1/2. PLX7904 (PB04) potently blocks MEK-ERK1/2 signaling in mutant BRAF melanoma cells but does not elicit paradox signaling in cells expressing mutant forms of RAS (Le et al., 2013). PLX7904 also blocks ERK1/2 signaling in BRAFV600E-expressing melanoma cells that have acquired resistance to PLX4720 (the tool compound for vemurafenib) via co-expression of mutant NRAS. In this form of resistance, ERK1/2 re-activation is dependent on BRAF and CRAF, likely through their hetero-dimerization (Kaplan et al., 2012). BRAFV600E alternative splice variants are also frequently associated with acquired resistance to RAF inhibitor (31% of resistant patients in one study) and mediate their actions through enhanced homo-dimerization (Poulikakos et al., 2011). Thus, we sought to determine the effect of PLX7904 and its clinical grade analog, PLX8394 (PB03), on resistance to PLX4720 mediated by BRAFV600E splice variants.
Results and Discussion
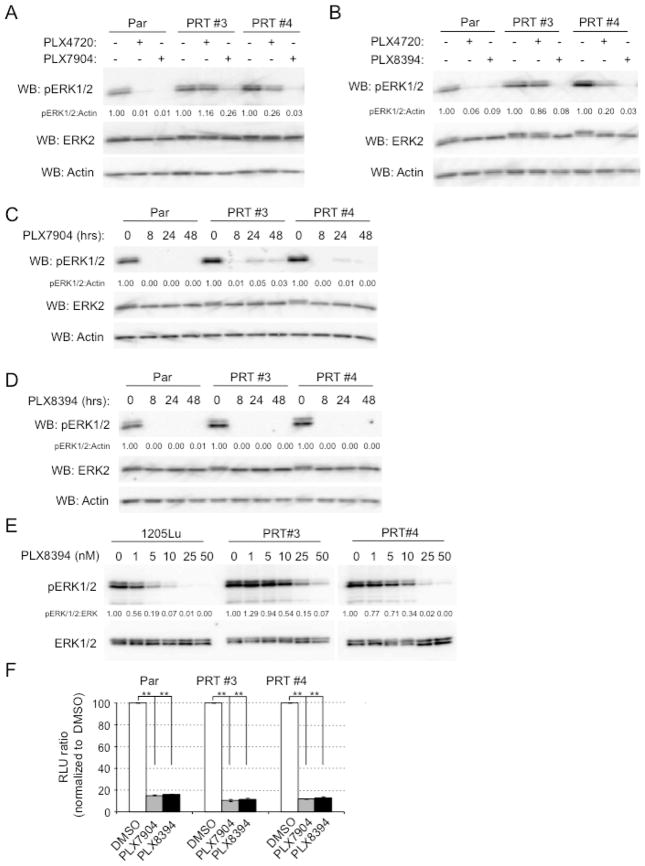
We utilized two RAF-inhibitor resistant cell lines that were generated in vivo through long-term (6 week) treatment of 1205Lu xenografts with PLX470 (Basile et al., 2013). PRT #3 cells express the human splice variant BRAFV600E ΔEx 3–10, whereas PRT #4 cells express BRAFV600E ΔEx 2–8 ((Basile et al., 2013) and Supplemental Fig. 1A). PRT #3 and PRT #4 exhibited IC50s of approximately 5 μM for PLX4720 (tool compound for vemurafenib) in growth assays compared to an IC50 of 150 nM in parental cells (Supplemental Fig. 1B). PLX7904 and PLX8394 have similar properties and IC50s against BRAFV600E of ~5 nM and do not elicit paradoxical ERK1/2 activation in mutant RAS expressing cells ((Le et al., 2013) and Supplemental Fig. 2). As expected, PLX4720 blocked ERK1/2 phosphorylation in parental 1205Lu cells but not in PRT #3 and PRT #4 cells (Fig. 1A). By contrast, PLX7904 and PLX8394 potently inhibited phosphorylation of ERK1/2 in PRT #3 and PRT #4 cells in addition to parental cells (Fig. 1A and 1B). Effects of PLX7904 and PLX8394 were persistent in that they were observed through 48 hours of treatment (Fig. 1C–D). A degree of differential sensitivity was observed between parental and PRT cells with phosphorylation of ERK1/2 effectively inhibited (>80%) in parental cells at 10 nM PLX8394 but PRT #3 and PRT #4 required drug concentrations of >25 nM (Fig. 1E). Highlighting effects on ERK1/2 activity, PLX7904 and PLX8394 potently inhibited ERK1/2-driven GAL4-Elk1 reporter activity in PRT cells as well as parental cells (Fig. 1F).
Figure 1. Paradox breaker RAF inhibitors inhibit phosphorylation of ERK1/2 in mutant BRAF splice variant-expressing cells.
(A) Parental 1205LuTR reporter cells (Par) or PRTs #3 and #4 cells were treated with DMSO (−), PLX4720 (1 μM) or PLX7904 (1 μM) for 24 h. Cells were then lysed and analyzed by Western blotting for phospho-ERK1/2, total ERK1/2 and actin. (B) Similar to A, except that cells were treated with PLX8394. (C) Parental 1205LuTR reporter cells (Par) or PRTs #3 and #4 cells were treated with PLX7904 (1 μM) for 0, 8, 24 and 48 h. Cell lysates were analyzed by Western blotting as indicated. (D) Similar to C, except that cells were treated with PLX8394. (E) Cells were treated for 1 hr with the indicated dose of PLX8394. Cell lysates were analyzed by Western blotting, as indicated. (F) Parental 1205LuTR reporter cells or PRTs #3 and #4 cells were treated with DMSO, PLX7904 (1 μM) or PLX8394 (1 μM) for 24 h. Dual-luciferase assays were performed. Columns represent mean. n=3. Error bars, s.e.m. **p-value<0.01 by unpaired, student t-test.
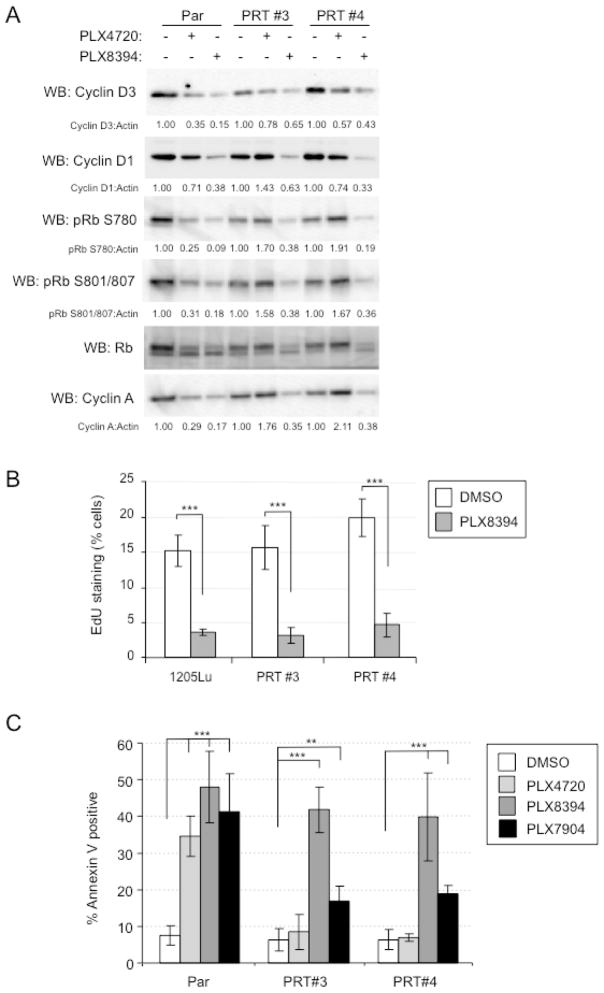
We next determined whether paradox breaker inhibitors altered cell cycle progression and apoptosis in RAF inhibitor-resistant cells. Both PLX4720 and PLX8394 effectively reduced cyclin D3 and cyclin D1 (an early G1 markers), phosphorylation of retinoblastoma protein (a restriction point marker in G1), and expression of cyclin A2 (a marker of G1 to S transition) in parental cells. However, PLX8394, but not PLX4720, reduced levels of these cell cycle markers in PRT #3 and PRT #4 cells (Fig. 2A). Consistent with these effects, PLX8394 inhibited EdU incorporation into PRT #3 and PRT #4 cells in S phase entry assays (Fig. 2B). By analyzing annexin V staining in cells cultured in 3D collagen, we observed that PLX8394 and to a lesser extent PLX7904 induced apoptosis in PRT #3 and PRT #4 cells, as well as parental cells (Fig. 2C). By contrast, PRT #3 and #4 cells were resistant to PLX4720.
Figure 2. Inhibition of G1/S cell cycle events in mutant BRAF splice variant-expressing cells treated with PB inhibitors.
(A) Parental 1205LuTR reporter cells and PRTs #3 and #4 were treated with DMSO (−), PLX4720 (1 μM) or PLX8394 (1 μM) for 24 h. Cells were then lysed and analyzed by Western blotting for the cell cycle proteins indicated. (B) 1205Lu, PRT #3, and PRT #4 cells were treated with DMSO (vehicle) or 1 μM PLX8394 for 48 h with EdU added for the last 16 h. EdU incorporation was analyzed by flow cytometry. (C) Parental reporter, PRT #3, and PRT #4 cells were plated in 3D collagen gels and treated with DMSO (control), 1 μM PLX4720, 1 μM PLX8394 or 1 μM PLX7904 for 48 hours. Cells were collected and analyzed by annexin V staining. Shown is the mean and SD from three independent experiments. * p<0.05, ** p<0.01, *** p<0.001, based on two-tail student T-test.
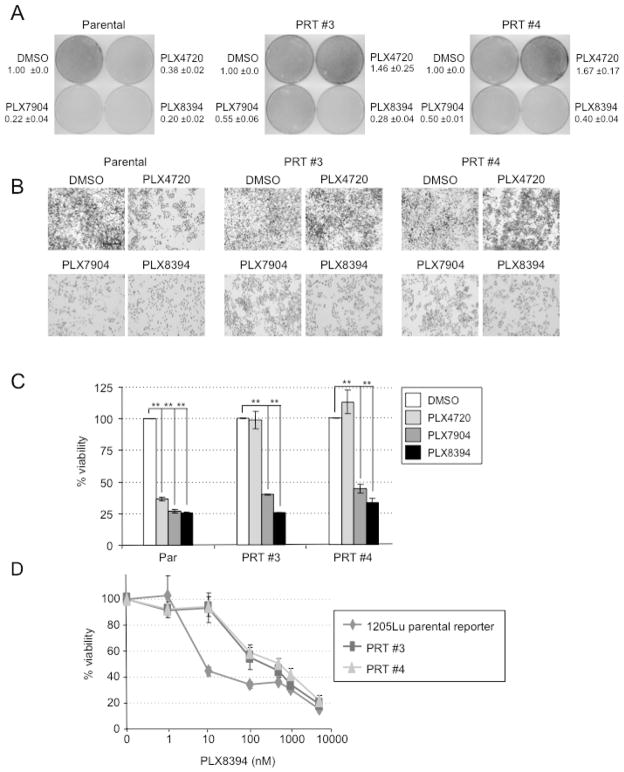
To assess effects on cell growth, we performed colony formation and viability assays. PLX7904 and PLX8394 treatment at 1 μM concentration reduced colony formation and viability in parental cells to a similar level as PLX4720 (Fig. 3A–C). Both paradox breaker inhibitors also reduced colony formation and viability in parental cells to a similar level as PLX4720. Dose concentration experiments showed that PRT #3 and PRT #4 cells were more resistant than parental cells at low (<500 nM) concentrations of PLX8394 but showed comparable drug sensitivity at concentrations >500 nM (Fig. 3D).
Figure 3. Paradox breaker RAF inhibitors block growth of mutant BRAF splice variant-expressing cells.
(A) Parental 1205LuTR reporter cells or PRTs #3 and #4 cells were plated at clonal density and treated with DMSO, PLX4720, PLX7904 or PLX8394, as indicated. Cells were processed for crystal violet staining. Mean and standard deviation are shown, n=2. (B) High magnification images from the experiment in A. (C) Same as (A), except cells were processed for AlamarBlue® staining. Columns represent mean, n=3. Error bars, s.e.m. **p-value<0.01 by unpaired, student t-test. (D) Parental, PRT #3 and PRT #4 cells were treated with increasing doses response of PLX8394 and a MTT viability assay was performed. Each point represents the mean of 3 technical replicates. Error bars, s.e.m.
These data show that paradox breaker inhibitors block BRAFV600E splice variant signaling and, thus, may offer therapeutic benefit to vemurafenib-resistant disease mediated by these alternatively spliced forms of mutant BRAF. These findings add to our previous data showing that PLX7904 inhibits phosphorylation of ERK1/2 and growth of mutant BRAF WM793 cells that have acquired resistance to PLX4720 through secondary mutation in NRAS (Le et al., 2013). Through our in vivo generation of PLX4720-resistant 1205Lu cells, we also obtained one PRT line in which a mutant form of HRAS was required for resistance. Parallel experiments showed that PLX7904 and PLX8394 were effective at inhibiting the phosphorylation of ERK1/2 and growth in this mutant BRAF/HRAS cell line (PRT #6), although higher inhibitor concentrations were required for effective growth suppression compared to PRT #3 and #4 (Supplementary Figure 3). Furthermore, our findings are consistent with those from another group, which showed that PLX8394 inhibited ERK1/2 phosphorylation and the growth of vemurafenib-resistant cells harboring either a BRAF V600K/L505H double mutation or an transposon-induced, N-terminal truncated form of BRAF (Choi et al., 2013).
Notably, the susceptibility of resistant cells to PLX7904 and PLX8394 was demonstrated in the absence of PLX4720. This offers hope in a situation that MEK inhibitors have shown minimal clinical activity. When given as a sequential treatment, trametinib/GSK1120212 monotherapy achieved median progression free survival of 1.8 months in patients previously treated with RAF inhibitor (Kim et al., 2013). Since PLX8394 is progressing towards the clinic and >10 μM mean plasma level is achieved using in vivo efficacy models (Gideon Bollag and Chao Zhang, Plexxikon Inc., personal communication), it will be interesting to test the extent to which PLX8394 monotherapy is able to effectively inhibit ERK1/2 signaling in patient-derived xenografts from individuals progressing on vemurafenib and dabrafenib. In summary, we show that new selective RAF inhibitors that do not elicit paradoxical actions inhibit the signaling and growth of BRAFV600E splice variant expressing, vemurafenib-resistant melanoma cells.
Materials and Methods
Inhibitors
PLX7904 (PB04), PLX8394 (PB03) and PLX4720 were provided by Dr. Gideon Bollag (Plexxikon Inc., Berkeley, CA). AZD6244 was purchased from Selleck Chemicals LLC (Houston, TX).
Cell lines
1205Lu and PRT cells were cultured in MCDB 153 containing 20% Leibovitz L-15 medium, 2% FBS, 5 μg/ml insulin and penicillin/streptomycin (Hu and Aplin, 2010).
In vivo generation of PLX4720-resistant cell lines
The generation of cell lines is described elsewhere (Basile et al., 2013). Briefly, 1205LuTR reporter cells expressing a GAL4-ELK1 driven firefly luciferase reporter were injected into athymic, nude mice and allowed to form tumors. After tumors reached a size of ~100 mm3, mice were fed chow containing 417 ppm PLX4720. Tumors acquired resistance to PLX4720 were harvested and cell lines were established in the presence of 1 μM PLX4720.
Western blotting
Primary antibodies utilized were: phospho-ERK1/2 (Thr202/Tyr204, #4377), Rb (#9309), phospho-Rb (Ser780, #9307) and phospho-Rb (Ser807/811, #9308) from Cell Signaling Technology, Beverley, MA; cyclin D3 (DCS-22) from Lab Vision/NeoMarkers, Fremont, CA; ERK2 (sc-154), ERK1/2 (sc-094), cyclin D1 (sc-718) and cyclin A (sc-751) from Santa Cruz Biotechnology, Inc., Santa Cruz, CA; and β-actin (A5316) from Sigma-Aldrich, Inc., St Louis MO. Blots were quantitated using a Versadoc Imaging system (BioRad, Hercules, CA).
Dual luciferase assay
Firefly and renilla luciferase activities measured in cell lysates using the Dual-Luciferase® Assay System kit (Promega). Luciferase readings were measured on a Glomax luminometer (Promega).
EdU (5-ethynyl-2′-deoxyuridine) incorporation assays
Parental 1205Lu cells and PRT #3 and PRT #4 cells were treated with DMSO or PLX8394 for 32 hours before the addition of 10 μM EdU for another 16 h. Cells were then processed using the Click-iT™ EdU Alexa Fluor 647 Flow Cytometry Assay kit (Invitrogen, Carlsbad, CA) for flow cytometry analysis.
Annexin V assays
Cells were seeded in 3D gels of bovine type 1 collagen (Advanced BioMatrix Inc., San Diego, CA), as previously described (Le et al., 2013). Gels were incubated with inhibitors at 37°C for 48 hours, after which cells were released with 1 mg/ml collagenase type 1A (Sigma-Aldrich, Inc.) and stained for annexin V (Le et al., 2013).
Growth assays
For MTT assays, 2 × 103 cells were seeded in triplicate in 96 wells in their regular culture medium (containing PLX4720 for PRT lines). Next day, cells were washed twice with PBS and then the medium was replenished containing the indicated RAF inhibitor. Medium was changed 48 hours later and after a further 48 hours, 10 μL of 5 mg/mL MTT reagent (Sigma-Aldrich, Inc) was added to wells, and incubated for three hours. Formazan crystals were then solubilized overnight with a 1:10 dilution of 0.1 M glycine (pH 10.5) in DMSO. Wells were then analyzed at 450 nM in a Multiskan® Spectrum spectrophotometer (Thermo Scientific). Results depicted are normalized to DMSO conditions and are a composite of three independent experiments. Error bars shown are representative of the standard error of mean (SEM).
In AlamarBlue® viability assays, cells were plated at a confluency of 4 × 104 cells per well and treated as indicated. Medium and drugs were replenished once. After 5 days, 1x AlamarBlue® (Invitrogen) was added to each well and allowed to reduce for approximately 30 minutes. Medium was collected in triplicate from each condition and the absorbance of oxidized and reduced AlamarBlue® were measured at wavelengths 600 nM and 570 nM respectively in a spectrophotometer. The change in viability was calculated from the resulting absorbance using the manufacturer’s guidelines. All conditions were normalized to DMSO control.
For colony growth assays, cells (1.5 × 105 per 10 cm dish) were treated with the RAF inhibitors indicated. After 7 days of culture, including two medium/drug replenishments, cells were stained with crystal violet in formalin. Colonies were imaged on a Nikon™ Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) with NIS-Elements AR 3.00 software (Nikon). The percent plate coverage is indicated as determined from 5 independent areas per plate using ImageJ software.
Statistical analysis
Statistical analysis of the data was performed using an unpaired student t-test assuming unequal variance.
Supplementary Material
Significance.
The treatment options for mutant BRAF melanoma patients failing on vemurafenib and dabrafenib are ineffective. Here, we show that next generation, selective RAF inhibitors effectively block signaling and growth in a subset of resistant melanomas mediated by BRAFV600E splice variants.
Acknowledgments
We thank Dr. Gideon Bollag and Plexxikon Inc. (Berkeley, CA) for providing PLX7904 (PB04), PLX8394 (PB03) and PLX4720. 1205Lu cells were obtained directly from Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). This work was supported by National Institutes of Health (CA160495) and by a grant from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Kevin Basile and Dr. Edward Hartsough were supported in part by the Joanna M. Nicolay Melanoma Foundation and National Cancer Center, respectively. Core facilities in the Kimmel Cancer Center are funded by National Cancer Institute Support Grant P30CA56036.
Abbreviations
- ERK
extracellular signal-regulated kinase
- MEK1/2
MAPK/ERK kinases 1 and 2
References
- Basile KJ, Abel EV, Dadpey N, Hartsough EJ, Fortina P, Aplin AE. In vivo MAPK reporting reveals the heterogeneity in tumoral selection of resistance to RAF inhibitors. Cancer Res. 2013;73:7101–10. doi: 10.1158/0008-5472.CAN-13-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Rampal R, Harding JJ, Klimek VM, Chung YR, Merghoub T, Wolchok JD, Solit DB, Rosen N, Abdel-Wahab O, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med. 2012;367:2316–21. doi: 10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P, Metz D, Sepulveda A, Uehara T, Rustgi A, Nathanson KL, Kim K, Puzanov I, Flaherty K, Sosman JA, et al. Development of colonic adenomas and gastric polyps in BRAF mutant melanoma patients treated with vemurafenib. Pigment Cell Melanoma Res. 2012;25:847. [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Eng J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Landrette S, Wang T, Evans P, Bacchiocchi A, Bjornson R, Cheng E, Stiegler AL, Gathiaka S, Acevedo O, et al. Identification of PLX4032-resistance mechanisms and implications for novel RAF inhibitors. Pigment Cell Melanoma Res. 2013 doi: 10.1111/pcmr.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, Mcarthur GA, Sosman JA, O’dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Eng J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, Mccusker JP, Kluger Y, Sznol M. PLX4032, a selective BRAF V600E kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF WT melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Aplin AE. alphaB-crystallin is mutant B-RAF regulated and contributes to cyclin D1 turnover in melanocytic cells. Pigment Cell Melanoma Res. 2010;23:201–9. doi: 10.1111/j.1755-148X.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FM, Kugel CH, Dadpey N, Shao Y, Abel EV, Aplin AE. SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J Biol Chem. 2012;287:41797–807. doi: 10.1074/jbc.M112.390906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2011;30:366–71. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, Fecher LA, Millward M, Mcarthur GA, Hwu P, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–9. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K, Blomain E, Rodeck U, Aplin AE. Selective RAF inhibitor impairs ERK1/2 phosphorylation and growth in mutant NRAS, vemurafenib-resistant melanoma cells. Pigment Cell Melanoma Res. 2013;26:2155–68. doi: 10.1111/pcmr.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, Roden C, Chalk CJ, Ardlie K, Palescandolo E, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated With RAF inhibitors. J Clin Oncol. 2012;30:316–21. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, Mcarthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Eng J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Eng J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.