Abstract
Apolipoprotein E (APOE) ε4 allele is the most important genetic risk factor for Alzheimer’s disease (AD) and it is thought to do so by modulating levels of the its product, apolipoprotein E (Apo-E), and regulating amyloid-β (Aβ) clearance. However, information on clinical and biomarker correlates of Apo-E proteins is scarce. We examined the relationship of cerebrospinal fluid (CSF) and plasma Apo-E protein levels, and APOE genotype to cognition and AD biomarker changes in 311 AD Neuroimaging Initiative (ADNI) subjects with CSF Apo-E measurements and 565 subjects with plasma Apo-E measurements. At baseline, higher CSF Apo-E levels were associated with higher total and phosphorylated CSF tau levels. CSF Apo-E levels were associated with longitudinal cognitive decline, MCI conversion to dementia, and grey matter atrophy rate in total tau/Aβ1–42 ratio and APOE genotype adjusted analyses. In analyses stratified by APOE genotype, our results were only significant in the group without the ε4 allele. Baseline CSF Apo-E levels did not predict longitudinal CSF Aβ or tau changes. Plasma Apo-E levels show a mild correlation with CSF Apo-E levels, but were not associated with longitudinal cognitive and MRI changes. Based on our analyses, we speculate that increased CSF Apo-E2 or -E3 levels might represent a protective response to injury in AD and may have neuroprotective effects by decreasing neuronal damage independent of tau and amyloid deposition in addition to its effects on amyloid clearance.
Keywords: cerebrospinal fluid, plasma, dementia, beta amyloid, tau, MRI, dementia, neurodegeneration, Alzheimer’s Disease, APOE
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia. Most AD cases are late onset AD (LOAD), and the strongest genetic risk factor for LOAD is the apolipoprotein E (APOE, referring to the gene) ε4 allele which also is associated with an earlier AD onset [15], and higher brain Aβ plaque burden [38], although there are conflicting results regarding the association with disease progression in symptomatic subjects and Aβ biomarker changes [18,53,57]. The APOE gene product is the apolipoprotein E (Apo-E) protein which is expressed by three different APOE alleles (i,e, APOE ε2, ε3, and ε4). The presence of the APOE ε4 allele has also been reported to be associated with lower plasma Apo-E protein levels as well as with a distinct cognitive profile, and brain atrophy pattern compared to subjects with the ε2, and ε3 [29,47,62]. In the periphery, Apo-E is mainly, but not exclusively, synthesized in the liver, and by macrophages, whereas in the central nervous system (CNS) astrocytes are the main source of Apo-E protein synthesis, and release under normal conditions [4,36].
The different Apo-E isoforms have been associated with different rates of brain Aβ clearance [7], and there are several mechanisms that have been proposed to explain the association between APOE, and AD [34]. A previous biomarker study from our group using a multi-analyte panel to interrogate plasma from three different cohorts of cognitively normal (CN), mild cognitive impairment (MCI), and AD subjects found that lower plasma Apo-E protein levels were associated with a diagnosis of MCI, and AD in analyses that were not adjusted for APOE genotype [24]. Studies of another separate cohort found an association of lower plasma Apo-E with clinical diagnosis of MCI, and AD [13], but not with amyloid positron emission tomography (PET) imaging positivity [6]. A small cross-sectional study including Lewy body disease subjects has described increased cerebrospinal (CSF) Apo-E levels associated with APOE ε4 presence and association between higher CSF Apo-E levels and worse cognitive and neuroimaging measures [56]. Finally, one further study described the association between CSF Apo-E levels and CSF Aβ 1–42, clinical diagnosis and its genetic associations [11]. However, no studies have described longitudinal neuropsychological or structural imaging associations with CSF levels of Apo-E protein in AD, MCI and CN subjects. In our study we wanted to test if plasma CSF Apo-E protein levels were associated with clinical and longitudinal biomarker and cognitive changes and evaluate if the associations of Apo-E protein levels went beyond the ones expected based on the APOE genotype.
Subjects and Methods
Subjects
Data used in the current study were downloaded on November 7th, 2013 from the AD Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). The ADNI has been extensively described elsewhere [59]; see supplementary material (SM). 311 subjects had CSF Apo-E levels measured at baseline, although one subject had no clinical information and was therefore excluded from the study (Table 1). 565 subjects had plasma Apo-E measurements at baseline (Supplementary table 2). We reviewed the medications of all subjects and grouped the cholesterol drugs into statins, fibrates, resins, niacin and ezetimibe to test if these drugs were associated with Apoe-E levels (Supplementary table 3).
Table 1.
Characteristics of the ADNI subjects studied here.
| CN (n=92) |
MCI (n=149) |
AD (n=69) |
p-value | |
|---|---|---|---|---|
| Age at baseline (years)* |
75.7 (5.4) | 74.8 (7.2) | 75.0 (7.7) | 0.61 |
| Gender (% male) | 50% | 64.5% | 57.0% | 0.0070 |
| Education (years)* | 15.6 (2.9) | 16.0 (2.9) | 15.1 (2.9) | 0.14 |
|
APOE ε4 presence (%) |
22 (23.9%) | 80 (53.7%) | 49 (71.0%) | <0.0001 |
| ADAS* | 9.4(4.2) | 19.2 (6.1) | 29.1 (8.3) | <0.0001 |
| APOE CSF levels (µg/ml)† |
6.9 (6.1–8.5) | 7.2 (5.4–8.5) | 6.1 (4.8–8.0) | 0.065 |
| Aβ1–42 (pg/ml)† | 220.0 (161.0–252.5) | 144.0 (126.0–185.5) | 134.0 (122.0–162.0) | <0.0001 |
| T-tau (pg/ml)† | 61.0 (48.0–84.5) | 87.0 (65.0–121.5) | 116.0 (81.0–157.8) | <0.0001 |
| P-tau181 (pg/ml)† | 20.0 (16.5–29.5) | 35.5 (23.0–46.3) | 36.0 (29.0–49.0) | <0.0001 |
Mean (standard deviation);
Median (1st quartile-3rd quartile).
Sample collection and biomarker measurements
CSF samples were obtained in the morning after an overnight fast [43](see ADNI procedures manual (http://www.adni-info.org/ and SM). All but 3 subjects had CSF Aβ 1–42 and tau baseline measurements. In addition, 127 subjects had longitudinal CSF Aβ 1–42 and tau measurements on a yearly basis for a total of 589 measurements. Longitudinal CSF data has been analyzed and described previously in more detail [53].
Aβ 1–42, t-tau, and p-tau181 were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNO-BIA AlzBio3; Ghent, Belgium; for research use–only reagents) immunoassay kit–based reagents. Capture monoclonal antibodies used were 4D7A3 for Aβ 1–42, AT 120 for total tau (t-tau), and AT270 for phosphorylated tau (p-tau181). The analyte-specific detector antibodies were HT7, for tau, and 3D6, for the N-terminus of Aβ [44] (see Supplementary methods). Hemoglobin was measured in CSF samples as an indication of blood contamination [23,50,30] using a human hemoglobin ELISA quantitation kit from Bethyl Lab Inc (Montgomery, TX, USA).
571 plasma samples from 571 individual subjects and 327 CSF samples from 311 subjects, including 16 replicates, of never before thawed aliquots were interrogated by Rules Based Medicine (RBM, Austin, TX) using the multiplex Human DiscoveryMAP™ panel on a Luminex 100 platform (see papers on methods, and procedures available in http://www.adni-info.org/). Apo-E was measured as a part of a multiplexed panel using a flow-based laser apparatus to detect polysterene beads loaded with different ratios of two spectrally distinct fluorochromes. The beads are coated with antibodies and serve as a solid phase coating matrix to detect the analytes of interest, in this case CSF Apo-E. The beads are read one at a time as they pass through a flow cell on the Luminex laser instrument using a dual laser system. Two sets of quality control (QC) measures are available. The first one was obtained by spiking human plasma with cell culture extracts expressing the human analytes, which were run in duplicate. Based on this analysis, the least detectable dose for Apo-E was 0.00128 µg/ml. Coefficients of variation (CV) were calculated by running three standards in duplicate for five runs. The three standards had a mean concentration of 7.26 µg/ml, 13.6 and 35.3 with a CV of 9.4%, 7.97% and 8.48%, respectively (average CV=8.6%). For the second QC measure, two different CSF aliquots were sent to Myriad RBM for 16 cases, blinded to the Myriad RBM analytical staff. All of these analytes were successfully measured; the mean percent difference was 18.2 and neither the Bland-Altman intercept (−0.11, p=0.93) nor slope (0.20, p=0.24) were significantly different from 0.
Structural MRI acquisition and processing
Subjects with a 1.5 T MRI, and a sagittal volumetric 3D MPRAGE with variable resolution around the target of 1.2 mm isotropically were included in the analysis. The scans have gone through certain correction methods such as gradwarp, B1 calibration, N3 correction, and (in-house) skull-stripping. See (www.loni.ucla.edu/ADNI), and for detail.[27] Processed cortical grey matter (GM) volumes from that were processed using free-surfer software package version 4.4 longitudinal image processing framework (http://surfer.nmr.mgh.harvard.edu/)(“ucsffsl” file) were used [39,40]. Only CN and MCI subjects were included in the MRI analysis. MRI scans were performed at baseline, 6 months, one year and then on a yearly basis for the CN subjects. The MCI subjects had an additional MRI scan at 18 months. Only MRIs which passed the quality control for all the areas were included in the analysis.
Statistical Analysis
For analyses included in the descriptive tables, ANOVAs, Kruskall-Wallis test, and Chi-square tests were applied. For further analyses power transformations were applied if necessary and biomarker levels were standardized (mean=0, standard deviation=1) to be able to compare effect sizes (regression coefficient (β) and hazard ratio (HR)). Tukey honestly significance difference was applied for post-hoc comparison of significant ANOVA analyses. Associations between Apo-E levels, and other variables were tested in covariate-adjusted multivariable linear regression models (note that β refers to the transformed values). Gender and APOE genotype differed between the clinically defined groups and age has been shown to be associated with changes in biomarkers [2]. Therefore these variables were included as covariates. We tested the conditions necessary to apply the regression model (normal distribution of the residuals and absence of multicollinearity (variance inflation factor<5)), which were fulfilled by all the models A Cox model was used to study the conversion of MCI to AD for Apo-E levels. The CSF Apo-E mismatch (Apo-E-Mis) was calculated as the residuals from the regression model that included age, gender, APOE ε4 presence and t-tau as predictors. We analyzed longitudinally different quantitative outcome measures (MRI volumetric measures and neuropsychological measures) using mixed-effects models to assess the association with CSF Apo-E levels. All the AD subjects were excluded from longitudinal analyses due to short follow-up. For the ADAS-Cog analysis, age, gender, education, APOE ε4 allele presence, CSF t-tau/Aβ1–42 ratio, and clinical diagnosis at baseline were included in the model as fixed effects. Three random effects were included: an intercept, follow-up time measured in years and the squared time. An interaction between time, and clinical diagnosis, time and CSF t-tau/Aβ1–42 ratio, and time and Apo-E levels was also included. A significant value for any of these interactions would indicate that the longitudinal change differs between groups, i.e. an interaction between clinical diagnosis and time would indicate that MCI subjects show a higher increase of ADAS-Cog score than CN subjects during follow-up. A similar model was applied for the association with volumetric MRI changes that also included intracranial volume (ICV) as a covariate. Statistical tests were two-sided and significance was set at the p<0.05. Benjamini-Hochberg correction was applied for the MRI comparisons and Bonferroni correction when multiple comparisons were performed (except the descriptive values presented in table 1). Only corrected p-values are presented. Analyses were performed using R v. 3.0.1.
Results
Association of CSF Apo-E protein levels with AD CSF biomarkers and clinical variables
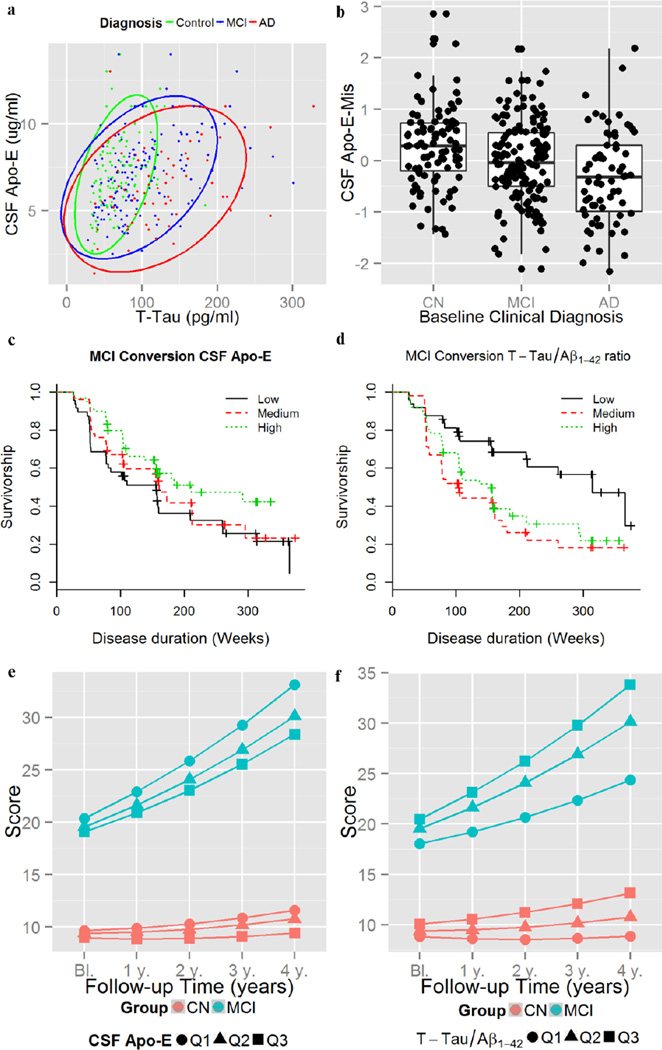
We tested the association of CSF Apo-E protein levels with APOE ε4 presence, age, gender, clinical diagnosis, and CSF hemoglobin (to evaluate the effect of blood contamination), Aβ 1–42, t-tau and p-tau181 levels in independent univariate models and p-values were adjusted for multiple comparisons. Female compared to male gender (β=−0.34, t308=−2.94, p=0.025) and APOE ε4 presence (β=−0.35, t308=3.13, p=0.014) were associated with decreased CSF Apo-E levels, whereas increasing age (β=0.022, t308=2.72, p=0.048) was associated with higher CSF Apo-E levels. Hemoglobin showed no association with CSF Apo-E levels (β=−0.001, t308=−0.47, p=0.64). Higher CSF Apo-E levels were associated with higher t-tau (β=0.33, t302=6.09, p<0.0001) (Figure 1a) and p-tau181 (β=0.20, t305=3.51, p=0.0037), but not with Aβ 1–42 (β=0.13, t305=2.33, p=0.14). Whereas there were no significant CSF Apo-E differences between the clinical groups in the univariate analysis (Table 1), MCI (β=−0.40, t297=−3.38, p=0.0008) and AD (β=−0.74, t297=−5.0, p<0.0001) patients showed decreased CSF Apo-E levels (Figure 1a) in the analysis adjusted for t-tau levels, age, gender, clinical diagnosis and APOE ε4. This would indicate that Apo-E increases with higher t-tau levels but that MCI and AD subjects show for similar levels of t-tau lower levels of CSF Apo-E. For visual display we calculated the Apo-E-Mis that showed differences between clinical groups in the ANOVA analysis (F301=9.8, p=0.0001) (Figure 1b). The post-hoc comparison found that MCI (β=−0.29, p=0.021) and AD (β=−0.58, p<0.0001) subjects had lower CSF Apo-E-Mis than the CN subjects and that AD subjects (β=−0.29, p=0.048) had also lower CSF Apo-E-Mis than MCI subjects.
Figure 1. Biomarker and clinical associations of CSF Apo-E levels.
Association of CSF t-tau with CSF Apo-E levels (a). CSF Apo-E-Mis values in the different clinical groups (b). Conversion from MCI to dementia for CSF Apo-E (c) and CSF CSF t-tau/Aβ1–42 ratio (d) tertiles. ADAS-Cog changes based on CSF Apo-E (e) and CSF CSF t-tau/Aβ1–42 ratio (f) tertiles.
To test the association with longitudinal CSF changes we studied CSF Aβ 1–42, t-tau and p-tau181 changes in a subset of 127 subjects who had a total of 429 CSF measurements. The model that studied longitudinal CSF changes was adjusted for gender, clinical diagnosis age, and APOE ε4 presence. We found no association between baseline CSF Apo-E levels and longitudinal changes in Aβ 1–42 (t336=−1 .62, p=0.11), t-tau (t335=−0.86, p=0.39) or p-tau181 (t339=0.68, p=0.49).
We tested if any of the cholesterol lowering drugs was associated with CSF Apo-E values, but none of the drugs affected CSF Apo-E levels (Supplementary table 3).
Association of CSF Apo-E protein levels and APOE genotype with diagnosis and cognitive decline
The following analyses had clinical diagnosis, neuropsychological score or MRI GM volumes as dependent variables (outcome) and included age, gender, education, APOE ε4 presence, t-tau and CSF Apo-E protein levels as independent/predictor variables. Clinical diagnosis was included as a predictor in the mixed-effects model that studied ADAS-Cog changes and MRI atrophy, but not the Cox hazard model that studied MCI conversion to AD.
149 MCI subjects had CSF Apo-E level measurements with a median follow-up of 158.8 weeks (1st quartile: 105.6 weeks; 3rd quartile: 312.8 weeks) and we found that 88 converted to dementia. Lower CSF Apo-E and higher CSF t-tau/Aβ1–42 ratio, were associated with MCI conversion to dementia in the fully adjusted models (Table 2)(Figure 1c). For comparison we present the association with the CSF t-tau/Aβ 1−42 ratio including CSF Apo-E as a covariate (Figure 1d). The proportional hazard model that included CSF Apo-E levels showed a better predictive value that the one that excluded CSF Apo-E levels (χ2=6.85, p=0.0089, 1 d.f.). After the exclusion of CSF Apo-E from the model APOE ε4 presence remained not associated with MCI progression (data not shown) and there was a decrease of the CSF t-tau/Aβ1–42 ratio HR (HR=1.33, z=2.23, p=0.026).
Table 2.
Association of APOE ε4 status and CSF Apo-E levels with clinical outcomes. All models were adjusted for age, gender, education and clinical diagnosis at baseline (if two or more diagnostic groups were included). Additional covariates included in the model are detailed in the table.
| Outcome | Statistical Model | APOE ε4 status | CSF Apo-E | T-tau/Aβ1–42 |
|---|---|---|---|---|
| MCI to AD conversion |
Cox proportional hazards model |
HR=1.01 z=0.05 p=0.96 |
HR=0.70 z=−2.6 p=0.0086 |
HR =1.65 z=3.2 p=0.0012 |
| ADAS-Cog longitudinal changes |
Mixed effects models |
β=0.017 (0.032) t1333=0.53 p=0.60 |
β=−0.050 (0.015) t1333=−3.26 p=0.0011 |
β=0.10 (0.018) t1333=5.80 p<0.0001 |
β: standardized coefficient (standard error); HR: Hazard ratio.
For the longitudinal ADAS-Cog analysis 91 CN subjects with 626 visits and 144 MCI subjects with 949 visits were included. Low CSF Apo-E levels and high CSF t-tau/Aβ1–42 ratio, but not APOE ε4 presence, were associated with a greater cognitive decline as measured by ADAS-Cog scale (Table 2, Figures 1e–f). When CSF Apo-E levels were excluded from the model, APOE ε4 presence remained not significantly associated with ADAS-Cog changes (data not shown).
Figure 2. Proposed models for CSF Apo-E effects in AD.
CSF Apo-E as a neuroprotective mechanism that fails in advanced disease stages (a) and CSF Apo-E levels as a neuroprotective mechanism that modulates disease progression (b).
Association with structural MRI
Finally, we tested the association of CSF Apo-E levels with longitudinal brain atrophy in an analysis that included age, gender, clinical diagnosis, APOE ε4 presence, CSF t-tau/Aβ 1–42 ratio and intracranial volume as covariates. Low baseline CSF Apo-E levels and high CSF t-tau/Aβ1–42 ratio were associated with faster rate of atrophy in several cortical areas (Table 3, Supplementary Figure 1). When we excluded CSF Apo-E levels from the model, APOE ε4 presence remained not significantly associated with MRI longitudinal changes (data not shown).
Table 3.
Association of CSF Apo-E levels and t-tau/Aβ1–42 ratio with longitudinal MRI volume changes in a model adjusted for gender, APOE ε4presence, clinical diagnosis and intracranial volume.
| Areas | CSF Apo-E | T-tau/Aβ1–42 | ||||||
|---|---|---|---|---|---|---|---|---|
| β | S.E. | t-value | p-value | β | S.E. | t-value | p-value | |
| Fusiform | 39.06 | 11.90 | 3.28 | 0.011 | −74.64 | 12.87 | −5.80 | <0.0001 |
| Inferior Parietal | 51.59 | 15.80 | 3.26 | 0.011 | −58.18 | 16.99 | −3.43 | 0.0014 |
| Inferior Temporal | 43.48 | 14.09 | 3.09 | 0.014 | −69.17 | 15.28 | −4.53 | <0.0001 |
| Superior Frontal | 70.40 | 23.51 | 2.99 | 0.014 | −62.78 | 25.34 | −2.48 | 0.0191 |
| Precuneus | 31.72 | 11.08 | 2.86 | 0.017 | −36.10 | 11.95 | −3.02 | 0.0047 |
| Middle Frontal | 66.07 | 23.93 | 2.76 | 0.019 | −55.81 | 25.76 | −2.17 | 0.0358 |
| Entorhinal | 8.09 | 3.15 | 2.57 | 0.027 | −18.87 | 3.38 | −5.59 | <0.0001 |
| Inferior Frontal | 28.62 | 11.19 | 2.56 | 0.027 | −14.85 | 11.99 | −1.24 | 0.2161 |
| Lateral Orbitofrontal | 19.65 | 8.24 | 2.39 | 0.038 | −21.10 | 8.88 | −2.38 | 0.0220 |
| Middle Temporal | 34.43 | 14.77 | 2.33 | 0.040 | −79.70 | 16.06 | −4.96 | <0.0001 |
| Superior Parietal | 30.53 | 14.55 | 2.10 | 0.066 | −41.40 | 15.61 | −2.65 | 0.0125 |
| Medial Orbitofrontal | 10.30 | 5.02 | 2.05 | 0.067 | −23.18 | 5.38 | −4.31 | <0.0001 |
| Superior Temporal | 21.19 | 11.67 | 1.82 | 0.107 | −54.94 | 12.58 | −4.37 | <0.0001 |
| Anterior Cingulate | 7.39 | 4.27 | 1.73 | 0.120 | −11.03 | 4.57 | −2.41 | 0.0213 |
| Lingual | 9.06 | 5.82 | 1.56 | 0.159 | −17.60 | 6.22 | −2.83 | 0.0079 |
| Posterior Cingulate | 5.54 | 3.99 | 1.39 | 0.206 | −13.21 | 4.29 | −3.08 | 0.0042 |
| Parahippocampal | 3.74 | 2.83 | 1.32 | 0.219 | −15.61 | 3.03 | −5.15 | <0.0001 |
| Cuneus | 3.07 | 2.97 | 1.03 | 0.335 | −5.52 | 3.17 | −1.74 | 0.0906 |
| Transverse Temporal | 1.22 | 1.52 | 0.80 | 0.446 | −2.06 | 1.62 | −1.27 | 0.2133 |
| Temporal Pole | 1.33 | 2.18 | 0.61 | 0.544 | −9.48 | 2.32 | −4.09 | 0.0001 |
β: standardized coefficient; S.E.: Standard error.
P-values are adjusted for multiple comparisons.
Stratification of CSF Apo-E analyses based on APOE ε4 presence
We stratified our analysis based on the presence or absence of one or more APOE ε4 alleles (Supplementary tables 4 – 6). In these analyses, results remained significant only in the subgroup of subjects with no APOE ε4 alleles, although the coefficients of the variables with significant associations changed overall less than 12% in the subgroup with APOE ε4 alleles. In these analyses, CSF the t-tau/Aβ 1–42 ratio was not associated with conversion from MCI to AD and similarly associations with longitudinal MRI changes were decreased.
Lack of association of plasma Apo-E levels with clinical and neuroimaging outcomes
Plasma and CSF Apo-E levels showed a mild correlation (r=0.23, p=0.004, Supplementary Figure 2) in an analysis that included 202 subjects who had the plasma and CSF samples drawn the same day. There was no association between plasma Apo-E levels and AD CSF biomarkers (Aβ 1–42: t341=−0.75, p=0.45; t-tau: t336=1.35, p=0.18; p-tau181: t342=1.14, p=0.26). Statin treatment was associated with decreased plasma Apo-E levels (Supplementary table 3). Plasma Apo-E levels were associated with CSF Aβ 1–42 and p-tau181 levels only if APOE genotype was not included as a covariate (data not shown). The only clinical association of plasma Apo-E levels was with baseline clinical diagnosis; plasma Apo-E levels showed a stronger association with MCI diagnosis (β=−0.57, t343=−4.03. p=0.0001) than with AD diagnosis (β=−0.41, t343=−2.48. p=0.014). There was no association with progression from MCI to AD (HR=0.92, z=−0.68, p=0.50, n=192), changes in ADAS-Cog (β=−0.018, S.E.=0.016, t1301=−1.31, p=0.26, n=248, visits=1556) or longitudinal MRI atrophy (t≤1.62, p≥0.998, n=209, 1676 MRI scans). Results did not change when statin treatment was included as a covariate (data not shown).
Discussion
In our study, we showed the association of CSF Apo-E levels with CSF AD biomarkers, MCI conversion, structural MRI changes and longitudinal cognitive decline in models that were adjusted for baseline clinical diagnosis, age, gender, presence of APOE ε4, and the tau/Aβ 1–42 ratio. Our results suggest that increased CSF Apo-E levels could represent a protective response to neuronal injury and that decreased CSF Apo-E levels are associated with a worse longitudinal outcome. Whereas the presence of APOE ε4 has been shown to be associated with the risk of AD and Aβ amyloid deposition decades ago [15,38], reported data on plasma and CSF Apo-E levels are scarce and mainly reported as compared group-based differences [13,24,47,33,19,11,56], despite the fact that the initial report on measures of CSF Apo-E levels was published over 30 years ago [42].
Interestingly, CSF Apo-E levels were positively correlated with CSF t-tau levels independent of APOE ε4 presence, indicating that Apo-E synthesis might be also increased in response to injury (which is thought to cause the t-tau increase in CSF). Increased CSF Apo-E would facilitate neuronal repair as a response to neuron damage [3,1,63]. As seen in Figure 1b although the correlation between CSF t-tau and CSF Apo-E levels was present across the three clinical groups, CSF Apo-E levels were significantly decreased in MCI and AD subjects. Plausibly, this could indicate that as AD progresses, there is an insufficient response to neuronal injury and that there is an impaired repair response. Accordingly, those subjects with lower CSF Apo-E levels progressed faster. Alternatively, truncated CSF Apo-E4 fragments (A272–299) haven been associated with tau phosphorylation and therefore increased CSF Apo-E4, which is more susceptible to truncation that the Apo-E3 isoform, could contribute to increased CSF p-tau levels [26]. Finally, it also can be expected that neuronal loss might be responsible for the decreased CSF Apo-E levels as atrophy might be associated with a lower demand for CSF Apo-E levels. In addition, since neurons produce Apo-E in response to neuronal insults, even in situations where there is increased astrocytosis and gliosis, neuronal loss might be associated with a reduced production of Apo-E. We tested baseline association of CSF Apo-E and cortical GM atrophy, but the association was not significant (β=0.091, t=1.65, p=0.10). Therefore, it is not clear that baseline atrophy or neuronal loss can explain the association with longitudinal changes.
In our analysis, lower CSF Apo-E levels were associated with increased conversion of MCI subjects to dementia, longitudinal cognitive decline in CN and MCI subjects and longitudinal GM atrophy in all clinical groups. In addition, models that included CSF Apo-E protein levels showed a better prediction of the outcome variable than those that did not include CSF Apo-E, indicating that CSF Apo-E is an independent predictor of cognitive decline and that the clinical and MRI changes associated with CSF Apo-E levels go beyond the effect of APOE ε4 presence. A previously published small cross-sectional study included patients with Lewy body disease and the authors analyzed clinical and biomarker correlations of CSF Apo-E levels [56]. In this study the authors described a positive correlation between CSF Apo-E levels and CSF t-tau and PiB PET binding and an inverse correlation with cognitive scores. That study reported increased CSF Apo-E levels in APOE ε4 carriers. On the other hand, our study and a previous study with CSF Apo-E measurements in over 700 subjects [11] showed decreased levels CSF Apo-E levels in APOE ε4 carriers. One important difference, besides the lack of inclusion of AD subjects and the younger age of the subjects, is that the study by Vijayaraghavan et al only included 6 subjects (mainly with APOE ε4) with Pittsburgh compound B (PiB) PET who had standardized uptake value ratios above 1.5 (compatible with amyloid deposition) and that analyses were not adjusted for covariates [56].
It is unclear why increased CSF levels might have the associations we report here and appear to protect against cognitive decline, AD and the conversion from MCI to AD. The association with the clinical and biomarker outcomes in models adjusted for CSF t-tau/Aβ1–42 ratio may imply that mechanisms beyond Aβ amyloid plaques and tau neurofibrillary tangles might be linked to the cognitive changes associated with CSF Apo-E levels or that Apo-E is involved in injury repair mechanisms that are independent of amyloid clearance [25]. In line with this hypothesis, neither plasma nor CSF Apo-E baseline visit levels predicted longitudinal changes in CSF Aβ 1–42, t-tau or p-tau181 levels. Nevertheless, larger longitudinal early stage samples are needed to confirm these results. Apo-E is known to act as a scaffold for the formation of high density lipoprotein-like particles that are thought to bind to soluble Aβ and promote the proteolytic degradation of soluble Aβ [28,14]. Although the capacity of Apo-E to promote Aβ degradation depends not only on the levels of Apo-E but also on the Apo-E isoform and the lipidation status of these proteins, we did not measure specific Apo-E2, Apo-E3 and Apo-E4 isoforms nor we do know the lipidation state of the Apo-E isoforms we measured here as our assay measures total Apo-E. In addition, several Aβ independent protective mechanisms have been suggested for CSF Apo-E [25]. Based on our results an increase in CSF Apo-E levels could be a response to initial neuronal injury which decreases when patients show cognitive changes and do not respond to the potential protective effects of Apo-E and stabilize or even decrease during disease progression (Figures 1a and 2a). Alternatively, other factors may regulate CSF Apo-E levels and differences in CSF Apo-E levels (not related to disease stage) may modify the cognitive decline. Therefore, high CSF Apo-E levels would delay the onset of cognitive decline (right-shifted dark green curve)(Figure 2b).These are preliminary hypotheses and further studies with longitudinal biomarker data are needed to develop the model. In line with this modifier effect, we found that the inclusion of CSF Apo-E levels in our model was associated with a stronger association of the CSF t-tau/Aβ 1–42 ratio and a better fit of the model.
Therapies such as bexarotene that increase Apo-E levels using liver X receptor (LXR) agonists have been reported to enhance behavioral test performance in AD mouse models engineered to overexpress Aβ and form Aβ plaques [10,14,17,54] in addition to reducing the number of plaques after acute but not chronic treatment in one study [10]. However, the reduction of plaques has not been replicated in other studies [17,31,37,54,55,48], although one study showed some trends [14]. Of these studies, only five tested behavior in mice; two found an improvement [54,17,14], one had conflicting results [48] whereas the last one did not find an improvement [31]. All studies showed target engagement with an increase of ABCA 1 and/or Apo-E [55,14,17,37,31,54,48]. Differences, between studies have been attributed to the formulation of bexarotene [32] and gender differences between groups [55,31]. CSF Apo-E levels before treatment together with CSF Apo-E levels after treatments could be used to monitor baseline Apo-E levels, target engagement and correlations between the extent of target engagement and observed response in studies involving LXR.
In the different models APOE ε4 presence was not associated with longitudinal clinical outcomes or GM atrophy. This observation would indicate that either APOE ε4 presence is not associated with the rate of decline. There are consistent results of APOE ε4 presence being associated with an earlier age of onset of AD [8], however the association with cognitive decline is inconsistent across different studies [64,9,61,5]. Recently, a study that included neuropathological data regarding Aβ and tau deposition showed that the effect of APOE genotype on cognition is mediated by Aβ and tau deposits and that adjusting for these variables leads to a lack of association between APOE genotype and cognitive decline [65]. This last study is in line with our results; when the CSF t-tau/Aβ1–42 ratio was included there was no association between cognitive decline and APOE genotype, but after excluding the CSF t-tau/Aβ 1–42 ratio was excluded APOE genotype was associated with cognitive decline (data not shown).
We found a weak correlation between the levels of Apo-E in each of these compartments when we analyzed subjects from whom both CSF and plasma samples were obtained on the same day as previously reported [11]. This is in agreement with animal studies that found that the blood brain barrier was not permeable to peripherally labeled Apo-E [35]. The association between lower plasma and CSF Apo-E levels in subjects with APOE ε4 presence has been previously reported in the ADNI cohort [29,47] and other studies [20,21], although other studies did not find this association or found higher CSF Apo-E levels in the presence of an APOE ε4 [58,12]. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study described a mild correlation with amyloid deposition measured by PiB PET, although this associated decreased when the analysis was adjusted for APOE genotype [21]. Similarly, Cruchaga et al reported that the significant decrease of plasma Apo-E values observed in AD subjects disappeared once APOE genotype was included in the analysis [11]. The association of lower CSF Apo-E levels in APOE ε4 carriers might be related to a faster degradation Apo-E4 isoforms which has been observed in animal and cell models [41] and also a faster metabolism in the periphery [20]. Alternatively, changes in plasma Apo-E may early events in AD onset pathologically and reach a plateau before the onset of cognitive symptoms and this timing difference could explain the lack of association with cognitive changes, however the lack or largely decreased association with AD when the analysis is adjusted for APOE genotype makes this hypothesis less probable. The more complex origin and metabolism of plasma biomarkers and the presence of the blood brain barrier may account for a weak association of plasma biomarkers, like plasma Aβ, with cognitive decline [52,16,51].
In our study we were not able to measure the different Apo-E isoforms and the limited age-span of the subjects and lack of longitudinal CSF Apo-E measurements prevents us from predicting how CSF Apo-E levels changes during disease history. Since Apo-E4 shows different lipophilicity than Apo-E3 and therefore future studies should consider if the difference in lipophilicity may alter the measurements based on the diluents used in the assay since it has been reported that Apo-E3 forms dimers and heterodimers whereas Apo-E4 does not [60]. Hence the presence of these dimers might also affect the masurements of different Apo-E isoforms. When we stratified our results based on the presence APOE genotype results, they were only significant in subjects without APOE ε4 alleles. There are at least three potential explanations to account for this: (1) Our assay might not be adequately measuring Apo-E levels in the APOE ε4 group, (2) Apo-E levels might not be protective in APOE ε4 subjects (or only the Apo-E3 isoform might be protective) or (3) the stratification reduced the sample size in each of the APOE groups with the APOE ε4 group having a smaller sample size for the cognitive and MRI analysis. In line with the third hypothesis, some of the t-tau/Aβ1–42 associations were not significant or their association decreased in the APOE ε4 group. Therefore, further independent validation of the longitudinal results in an independent cohort including a different assay is needed. Whereas the mean value of the highest standard (35.5 µg/ml) was higher than the values in the CSF, it was below the median Apo-E plasma value of each of the three clinical groups. Therefore, our plasma results should be considered cautiously.
Finally cholesterol levels are modified by statin treatment and there are conflicting results regarding the protective effect of statin treatment [45,46] thereby prompting suggestions that statins are neuroprotective [22]. Based on our analyses, statins (and other cholesterol lowering drugs) affected plasma but not CSF Apo-E levels independently of the lipophilicity of these drugs so CSF Apo-E levels might not be affected by usual statin doses. Statins may exert their protective effects by reducing abnormal vascular changes and cerebrovascular pathology that are commonly found in AD and other neurodegenerative diseases, and which lower the dementia threshold [49].
In summary, we found that CSF Apo-E protein levels are decreased in association with neuronal damage, as measured by t-tau, in cognitively impaired subjects compared to CN subjects and that baseline CSF Apo-E levels are associated with cognitive decline and brain atrophy which is independent of CSF t-tau/Aβ 1–42 ratio. These results provide clinical evidence that CSF Apo-E levels might represent a response to neuronal insults with beneficial effects and would prompts us to infer that a therapy targeted to increase Apo-E3 levels in the brain as reflected by CSF measures of Apo-E might improve cognitive performance and GM MRI volumetric measures in MCI patients or even in later stages of AD. Finally, it is possible that inclusion of repeated CSF Apo-E measures in clinical trials could be used to measures target engagement for drugs targeting Apo-E and as a covariate in the analysis of the clinical outcomes.
Supplementary Material
Acknowledgements
Data collection and sharing for this project was funded by (ADNI (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514. JQT is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology.
Footnotes
Conflicts of interest
Dr. Arnold reports grants from NIH, the American Health Assistance Foundation and the Marian S Ware Alzheimer’s Programseveral pharmaceutical companies , other from Universities, pharmaceutical companies and advisory/speaking honoraria from Universities, pharmaceutical companies and law firms. Dr. Trojanowski reports grants from NIH, Bristol Myer Squib, Astra Zeneca and several non-profits, outside the submitted work; In addition, Dr. Trojanowski has a patent Modified avidin-biotin technique, Method of stabilizing microtubules to treat Alzheimer's disease, Method of detecting abnormally phosphorylated tau, Method of screening for Alzheimer's disease or disease associated with the accumulation of paired helical filaments, Compositions and methods for producing and using homogeneous neuronal cell transplants, Rat comprising straight filaments in its brain, Compositions and methods for producing and using homogeneous neuronal cell transplants to treat neurodegenerative disorders and brain and spinal cord injuries, Diagnostic methods for Alzheimer's disease by detection of multiple MRNAs, Methods and compositions for determining lipid peroxidation levels in oxidant stress syndromes and diseases, Compositions and methods for producing and using homogenous neuronal cell transplants, Method of identifying, diagnosing and treating alpha-synuclein positive neurodegenerative disorders, Mutation-specific functional impairments in distinct tau isoforms of hereditary frontotemporal dementia and parkinsonism linked to chromosome-17: genotype predicts phenotype, Microtubule stabilizing therapies for neurodegenerative disorders, and Treatment of Alzheimer's and related diseases with an antibody pending, and a patent Amyloid plaque aggregation inhibitors and diagnostic imaging agents sold to Avid to Eli Lily.
Dr. Weiner reports stock/stock options from Elan, Synarc, travel expenses from Novartis, Tohoku University, Fundacio Ace, Travel eDreams, MCI Group, NSAS, Danone Trading, ANT Congress, NeuroVigil, CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer’s Disease, Pfizer, AD PD meeting, Paul Sabatier University, board membership for Lilly, Araclon, Institut Catala de Neurociencies Aplicades, Gulf War Veterans Illnesses Advisory Committee, VACO, Biogen Idec, Pfizer, consultancy from AstraZeneca, Araclon, Medivation/Pfizer, Ipsen, TauRx Therapeutics, Bayer Healthcare, Biogen Idec, ExonHit Therapeutics, Servier, Synarc, Pfizer, Janssen, honoraria from NeuroVigil, Insitut Catala de Neurociencies Aplicades, PMDA/Japanese Ministry of Health, Labour, and Welfare, Tohoku University, commercial research support from Merck, Avid; government research support, DOD, VA, outside the submitted work. Other authors report no conflicts of interest.
References
- 1.Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34(4):875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- 2.Baird GS, Nelson SK, Keeney TR, Stewart A, Williams S, Kraemer S, Peskind ER, Montine TJ. Age- dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am J Pathol. 2012;180(2):446–456. doi: 10.1016/j.ajpath.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999;6(6):508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- 4.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63(5):816–821. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 6.Burnham SC, Faux NG, Wilson W, Laws SM, Ames D, Bedo J, Bush AI, Doecke JD, Ellis KA, Head R, Jones G, Kiiveri H, Martins RN, Rembach A, Rowe CC, Salvado O, Macaulay SL, Masters CL, Villemagne VL. A blood-based predictor for neocortical Abeta burden in Alzheimer's disease: results from the AIBL study. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 7.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89) doi: 10.1126/scitranslmed.3002156. 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M, Blacker D, Stern Y. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Pt 2):1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM, Morris JC, Goate AM. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21(20):4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darreh-Shori T, Modiri N, Blennow K, Baza S, Kamil C, Ahmed H, Andreasen N, Nordberg A. The apolipoprotein E epsilon4 allele plays pathological roles in AD through high protein expression and interaction with butyrylcholinesterase. Neurobiol Aging. 2011;32(7):1236–1248. doi: 10.1016/j.neurobiolaging.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Bush AI, Brown B, De Ruyck K, Ellis KA, Fowler C, Gupta VB, Head R, Macaulay SL, Pertile K, Rowe CC, Rembach A, Rodrigues M, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Ames D, Masters CL, Martins RN. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69(10):1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donkin JJ, Stukas S, Hirsch-Reinshagen V, Namjoshi D, Wilkinson A, May S, Chan J, Fan J, Collins J, Wellington CL. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285(44):34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 16.Figurski MJ, Waligorska T, Toledo J, Vanderstichele H, Korecka M, Lee VM, Trojanowski JQ, Shaw LM. Improved protocol for measurement of plasma beta-amyloid in longitudinal evaluation of Alzheimer's Disease Neuroimaging Initiative study patients. Alzheimers Dement. 2012;8(4):250–260. doi: 10.1016/j.jalz.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340(6135):924–c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster JK, Albrecht MA, Savage G, Lautenschlager NT, Ellis KA, Maruff P, Szoeke C, Taddei K, Martins R, Masters CL, Ames D. Lack of reliable evidence for a distinctive {varepsilon}4-related cognitive phenotype that is independent from clinical diagnostic status: findings from the Australian Imaging, Biomarkers and Lifestyle Study. Brain. 2013;136(Pt 7):2201–2216. doi: 10.1093/brain/awt127. [DOI] [PubMed] [Google Scholar]
- 19.Fukuyama R, Mizuno T, Mori S, Yanagisawa K, Nakajima K, Fushiki S. Age-dependent decline in the apolipoprotein E level in cerebrospinal fluid from control subjects and its increase in cerebrospinal fluid from patients with Alzheimer's disease. Eur Neurol. 2000;43(3):161–169. doi: 10.1159/000008157. [DOI] [PubMed] [Google Scholar]
- 20.Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78(3):815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta VB, Laws SM, Villemagne VL, Ames D, Bush AI, Ellis KA, Lui JK, Masters C, Rowe CC, Szoeke C, Taddei K, Martins RN. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76(12):1091–1098. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 22.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80(1):13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 23.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133(Pt 3):713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, Grossman M, Xiong C, Craig-Schapiro R, Clark CM, Pickering E, Kuhn M, Chen Y, Van Deerlin VM, McCluskey L, Elman L, Karlawish J, Chen-Plotkin A, Hurtig HI, Siderowf A, Swenson F, Lee VM, Morris JC, Trojanowski JQ, Soares H. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79(9):897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y. Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer's disease. Trends Mol Med. 2010;16(6):287–294. doi: 10.1016/j.molmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A. 2001;98(15):8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J LW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Swaminathan S, Inlow M, Risacher SL, Nho K, Shen L, Foroud TM, Petersen RC, Aisen PS, Soares H, Toledo JB, Shaw LM, Trojanowski JQ, Weiner MW, McDonald BC, Farlow MR, Ghetti B, Saykin AJ. Influence of genetic variation on plasma protein levels in older adults using a multi-analyte panel. PLoS One. 2013;8(7):e70269. doi: 10.1371/journal.pone.0070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korff A, Liu C, Ginghina C, Shi M, Zhang J. α-Synuclein in cerebrospinal fluid of Alzheimer's disease and mild cognitive impairment. J. 2013 doi: 10.3233/JAD-130458. Alzheimers Dis (epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaClair KD, Manaye KF, Lee DL, Allard JS, Savonenko AV, Troncoso JC, Wong PC. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol Neurodegener. 2013;8:18. doi: 10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landreth GE, Cramer PE, Lakner MM, Cirrito JR, Wesson DW, Brunden KR, Wilson DA. Response to comments on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340(6135):924–g. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehtimaki T, Pirttila T, Mehta PD, Wisniewski HM, Frey H, Nikkari T. Apolipoprotein E (apoE) polymorphism and its influence on ApoE concentrations in the cerebrospinal fluid in Finnish patients with Alzheimer's disease. Hum Genet. 1995;95(1):39–42. doi: 10.1007/BF00225071. [DOI] [PubMed] [Google Scholar]
- 34.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Kuhel DG, Shen L, Hui DY, Woods SC. Apolipoprotein E does not cross the blood- cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R903–R908. doi: 10.1152/ajpregu.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11(2):97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 37.Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340(6135):924–d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- 38.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28(45):11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, Siemers E, Potter W, Lee VM, Trojanowski JQ. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121(5):597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011;68(10):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68(11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM, Ferm M, Dean RA, Simon AJ, Swenson F, Siuciak JA, Kaplow J, Thambisetty M, Zagouras P, Koroshetz WJ, Wan HI, Trojanowski JQ, Shaw LM. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69(10):1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D'Hooge R, De Strooper B. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340(6135):924–e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- 49.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136(Pt 9):2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer's disease. Acta Neuropathol. 2013;126(5):683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements - a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther. 2013;5(2):8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW, Jack CR, Jr, Jagust W, Decarli C, Toga AW, Toledo E, Xie SX, Lee VM, Trojanowski JQ, Shaw LM. Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122(4):401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013;126(5):659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanmierlo T, Rutten K, Dederen J, Bloks VW, van Vark-van der Zee LC, Kuipers F, Kiliaan A, Blokland A, Sijbrands EJ, Steinbusch H, Prickaerts J, Lutjohann D, Mulder M. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol Aging. 2011;32(7):1262–1272. doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340(6135):924–f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- 56.Vijayaraghavan S, Maetzler W, Reimold M, Lithner CU, Liepelt-Scarfone I, Berg D, Darreh-Shori T. High apolipoprotein E in cerebrospinal fluid of patients with Lewy body disorders is associated with dementia. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 58.Wahrle SE, Shah AR, Fagan AM, Smemo S, Kauwe JS, Grupe A, Hinrichs A, Mayo K, Jiang H, Thal LJ, Goate AM, Holtzman DM. Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol Neurodegener. 2007;2:7. doi: 10.1186/1750-1326-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisgraber KH, Shinto LH. Identification of the disulfide-linked homodimer of apolipoprotein E3 in plasma. Impact on receptor binding activity. J Biol Chem. 1991;266(18):12029–12034. [PubMed] [Google Scholar]
- 61.Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of apolipoprotein E epsilon4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry. 2002;72(6):794–797. doi: 10.1136/jnnp.72.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolk DA, Dickerson BC. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107(22):10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26(19):4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54(9):1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 65.Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA. Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.