Abstract
Background
Abnormal intrathecal synthesis of IgG, reflected by cerebrospinal fluid (CSF) oligoclonal IgG bands (OBs) and increased IgG index, is much less frequently observed in Japanese multiple sclerosis (MS) cohorts compared with Western cohorts. We aimed to clarify whether genetic and common infectious backgrounds influence CSF IgG abnormality in Japanese MS patients.
Methodology
We analyzed HLA-DRB1 alleles, and IgG antibodies against Chlamydia pneumoniae, Helicobacter pylori, Epstein-Barr virus nuclear antigen (EBNA), and varicella zoster virus (VZV) in 94 patients with MS and 367 unrelated healthy controls (HCs). We defined CSF IgG abnormality as the presence of CSF OBs and/or increased IgG index (>0.658).
Principal Findings
CSF IgG abnormality was found in 59 of 94 (62.8%) MS patients. CSF IgG abnormality-positive patients had a significantly higher frequency of brain MRI lesions meeting the Barkhof criteria compared with abnormality-negative patients. Compared with HCs, CSF IgG abnormality-positive MS patients showed a significantly higher frequency of DRB1*1501, whereas CSF IgG abnormality-negative patients had a significantly higher frequency of DRB1*0405. CSF IgG abnormality-positive MS patients had a significantly higher frequency of anti-C. pneumoniae IgG antibodies compared with CSF IgG abnormality-negative MS patients, although there was no difference in the frequency of anti-C. pneumoniae IgG antibodies between HCs and total MS patients. Compared with HCs, anti-H. pylori IgG antibodies were detected significantly less frequently in the total MS patients, especially in CSF IgG abnormality-negative MS patients. The frequencies of antibodies against EBNA and VZV did not differ significantly among the groups.
Conclusions
CSF IgG abnormality is associated with Western MS-like brain MRI features. DRB1*1501 and C. pneumoniae infection confer CSF IgG abnormality, while DRB1*0405 and H. pylori infection are positively and negatively associated with CSF IgG abnormality-negative MS, respectively, suggesting that genetic and environmental factors differentially contribute to MS susceptibility according to the CSF IgG abnormality status.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) with a supposed autoimmune origin involving T and B cells [1]. Interplay between genetic and environmental factors is assumed to contribute to the pathogenesis of MS [2]. The largest genetic effect on MS susceptibility is conferred by the major histocompatibility complex class II genes. In Caucasians, the HLA-DRB1*1501 allele is most strongly associated with MS, whereas the class I allele HLA-A*0201 allele appears to be a protective allele [3]. In the Japanese population, we and others reported that conventional MS (CMS) is associated with HLA-DRB1*1501, while opticospinal MS (OSMS) is associated with HLA-DPB1*0501 [4], [5], but no associations were found with any HLA class I alleles [6]. Recently, we reported that HLA-DRB1*0405 and HLA-DPB1*0301 are susceptibility alleles, while DRB1*0901 and DPB1*0401 are protective alleles for Japanese MS when neuromyelitis optica (NMO) and NMO spectrum disorder (NMOSD) patients are excluded [7].
Among many potential environmental risk factors, infection is likely to play a significant role in the acquisition of MS susceptibility or resistance. One candidate infectious agent is Epstein-Barr virus (EBV), which is more prevalent in Caucasian MS patients than in healthy controls (HCs), and therefore considered to increase susceptibility to MS [8], [9]. We recently found that the EBV infection rate has increased in a certain subgroup of Japanese MS patients not harboring HLA-DRB1*0405, a genetic risk factor for MS in the Japanese population, compared with HCs [7]. Since the first possible reported association between Chlamydia pneumoniae infection and MS [10], the significance of C. pneumoniae infection in MS has remained a matter of debate. We demonstrated that the anti-C. pneumoniae antibody positivity rate did not differ significantly between MS and HCs in Japanese [7], which is consistent with recent meta-analysis results [11]. We also found that the anti-Helicobacter pylori antibody positivity rate was lower among CMS patients than among HCs and OSMS patients in Japanese [12]. By contrast, the anti-H. pylori and anti-C. pneumoniae antibody positivity rates were increased in Japanese patients with NMO, especially in those with anti-aquaporin 4 (AQP4) antibodies [13], [14].
Abnormal intrathecal synthesis of IgG, reflected by cerebrospinal fluid (CSF) oligoclonal IgG bands (OBs) and increased IgG index, is a significant diagnostic hallmark in MS. More than 90% of MS patients are positive for CSF OBs in Western countries [15], [16]. However, this proportion appears to vary with ethnicity or geographical location, ranging from only 21–56% in Asian countries [17]–[21]. The presence of genetic influences on the OB phenotype is suggested by their associations in several populations. For example, the HLA-DRB1*15 allele is associated with OB-positive MS [19], [22], [23] and the HLA-DRB1*04 allele is associated with OB-negative MS [19], [22]. Although the prognostic significance of OBs is conflicting, the absence of OBs predicted a relatively benign clinical course and lower disease severity in some early studies [24], [25], but not all [18], [26]–[29]. Focusing on MRI findings, some studies have postulated a potentially lower lesion load in OB-negative patients [25], [29].
With this background, we aimed to investigate whether genetic and common infectious profiles influence CSF IgG abnormality in Japanese MS patients. In the present study, we focused on HLA-DRB1 loci that are associated with MS in several populations, including Japanese. Among the infectious factors, we chose C. pneumoniae, H. pylori, EBV, and varicella zoster virus (VZV) infections, which could be potential environmental risk or protective factors for MS.
Methods
Participants
Ninety-four patients examined at the Department of Neurology, Kyushu University Hospital from 2006 to 2010 were enrolled. MS was defined using the 2005 revised McDonald criteria for MS [30]. NMO was defined as cases fulfilling the 2006 revised criteria for NMO [31]. We regarded patients as having an NMOSD when they fulfilled either two absolute criteria plus at least one supportive criterion, or one absolute criterion plus more than one supportive criterion from the 2006 NMO criteria, as previously described [7], [14]. None of the MS patients met the above-mentioned NMO/NMOSD criteria. Patients with primary progressive MS were excluded from the study. Informed consent was obtained from the 94 patients as well as 367 unrelated HCs. Among the MS patients, 84 patients had RRMS and 10 had SPMS. The MS patients were clinically classified into two subtypes, conventional MS (CMS) and OSMS, as described previously [4]. There were 70 patients with CMS and 24 patients with OSMS. We collected demographic data from the patients by retrospective review of their medical records. These data included sex, age at onset, disease duration, Kurtzke's Expanded Disability Status Scale (EDSS) scores [32], annualized relapse rate, Progression Index [33], CSF OBs, IgG index, and brain MRI lesions meeting the Barkhof criteria for MS [34]. CSF IgG was tested in the acute phase in all cases. OBs were determined by isoelectric focusing, as the most sensitive method for OB determination [15], [20]. OBs were considered positive when they were only detected in CSF and comprised at least two bands. The IgG index represents (CSF IgG/serum IgG)/(CSF albumin/serum albumin). The IgG index was considered to be increased if it was >0.658 [4]. Among the 92 MS patients assayed for CSF OBs, 42 were OB-positive and 50 were OB-negative. Among the 90 MS patients whose IgG index was assayed, 42 had elevation and 48 did not. In this study, we defined CSF IgG abnormality as the presence of CSF OBs and/or increased IgG index. This study was approved by the Kyushu University Hospital Ethics Committee. All individuals involved in this study signed a written informed consent.
MRI analysis
All MRI studies were performed using 1.5 T units (Magnetom Vision and Symphony; Siemens Medical Systems, Erlangen, Germany) as previously described [35]. Brain MRI lesions were evaluated according to the Barkhof criteria for MS [34].
HLA-DRB1 genotyping
The genotypes of the HLA-DRB1 alleles from the subjects were determined by hybridization between the products of polymerase chain reaction amplification of the HLA-DRB1 genes and sequence-specific oligonucleotide probes, as described previously [7].
Anti-AQP4 antibody assay
The presence of anti-AQP4 antibodies was assayed as described previously [36], using green fluorescent protein (GFP)-AQP4 (M1 isoform) fusion protein-transfected human embryonic kidney (HEK) cells. Serum samples diluted 1∶4 were assayed for anti-AQP4 antibodies at least twice using identical samples, with the examiners blinded to the origin of the specimens. Samples that gave a positive result twice were deemed positive. When the judgment was equivocal, we measured the anti-AQP4 antibody levels using GFP-AQP4 (M23 isoform)-transfected HEK cells.
Detection of anti-H. pylori, anti-C. pneumoniae, anti-VZV, and anti-EBV nuclear antigen (EBNA) IgG antibodies
Serum anti-C. pneumoniae, anti-H. pylori, anti-EBNA, and anti-VZV IgG antibodies were measured using commercial ELISA kits (Vircell, Granada, Spain) in accordance with the manufacturer's instructions, as described previously [13]. Each antibody index was determined by dividing the optical density (OD) values for the target samples by the OD values for cut-off control samples and then multiplying by ten. As recommended by the manufacturer, an ELISA test index value was considered positive if higher than 11, equivocal if between 9 and 11, and negative if less than 9. According to the manufacturer's instructions, the ELISAs used in the present study have 100% sensitivity and 83% specificity for H. pylori infection and 100% sensitivity and 93% specificity for C. pneumoniae infection. Samples with equivocal results were retested for confirmation, and if the samples were equivocal twice, they were considered negative.
Statistical analyses
The phenotype frequencies of the HLA-DRB1 alleles were compared using the chi-square test, or Fisher's exact probability test when the criteria for the chi-square test were not fulfilled. Uncorrelated p-values (puncorr) were corrected by multiplying them by the number of comparisons, as indicated in the table footnotes (Bonferroni–Dunn's correction), to calculate the corrected p-values (pcorr). Fisher's exact probability test was used to compare sex, brain MRI lesions meeting the Barkhof criteria [34], and frequencies of antibodies against common infectious agents among the groups. Other demographic features were analyzed using the Wilcoxon rank sum test. We analyzed the trends in the proportions of patients among subgroups with advancing year of birth using the Cochran–Armitage trend test. All analyses were performed using JMP 8.0.3 (SAS Institute, Cary, NC). In all assays, values of p<0.05 were considered statistically significant.
Results
Relationships between CSF IgG abnormality status and demographic features in MS
Among the 94 MS patients, 59 (62.8%) were CSF IgG abnormality-positive and 35 were abnormality-negative. CSF IgG abnormality-positive patients had a significantly higher frequency of brain MRI lesions meeting the Barkhof criteria than abnormality-negative patients (46/58, 79.3% versus 16/34, 47.1%, p = 0.0025) (Table 1). The sex distribution, age at onset, disease duration, EDSS score, annualized relapse rate, Progression Index, and frequency of OSMS presentation with short spinal cord lesions (less than three vertebral segments) showed no associations with the presence or absence of CSF IgG abnormality (Table 1). The proportion of patients with CSF IgG abnormality did not change significantly with advancing year of birth (p>0.1) (Figure 1).
Table 1. Comparisons of the demographic features of MS patients according to the CSF IgG abnormality status.
| CSF IgG abnormality (+) (n = 59) | CSF IgG abnormality (−) (n = 35) | P value | |
| Male:female | 18∶41 | 15∶20 | 0.2669 |
| Age at onset (years)a | 30.54±12.12 | 34.06±15.70 | 0.4157 |
| Disease duration (years)a | 10.48±8.24 | 10.29±8.45 | 0.7899 |
| EDSS scorea | 2.74±1.92 | 2.83±1.84 | 0.6420 |
| Annualized relapse ratea | 0.58±0.60 | 0.87±0.90 | 0.0946 |
| Progression indexa | 0.39±0.39 | 0.90±2.00 | 0.4740 |
| Barkhof criteriab | 46/58 (79.3%) | 16/34 (47.1%) | 0.0025 |
| OSMS with short spinal cord lesionsc | 13/59 (22.0%) | 11/35 (31.4%) | 0.3366 |
Values represent the mean ± SD.
Brain MRI lesions meeting the Barkhof criteria [34].
Opticospinal form of MS with short spinal cord lesions extending less than three vertebral segments.
CSF, cerebrospinal fluid; EDSS, Kurtzke's Expanded Disability Status Scale; MS, multiple sclerosis.
Figure 1. Proportions of patients with CSF IgG abnormality by year of birth.
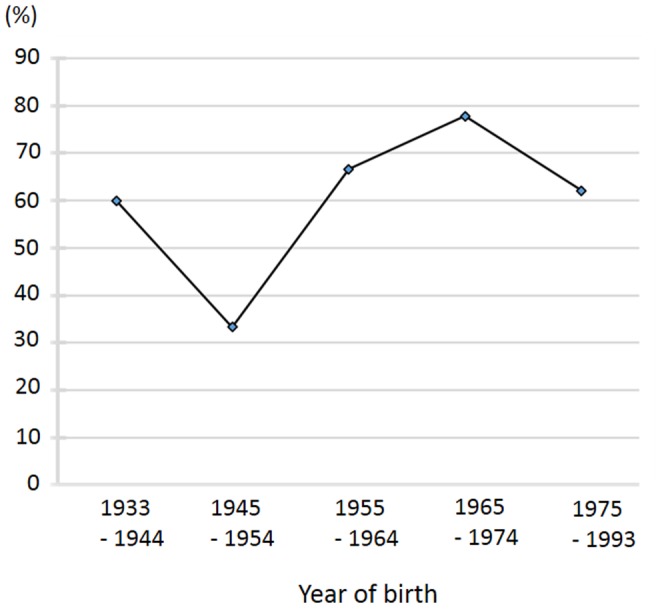
Among the MS patients, the proportion of patients with CSF IgG abnormality did not change significantly with advancing year of birth. CSF, cerebrospinal fluid; MS, multiple sclerosis.
Correlation of CSF IgG abnormality according to clinical subtypes
The frequency of CSF IgG abnormality did not differ significantly between RRMS (52/84, 61.9%) and SPMS (7/10, 70%). Additionally, there was no significant difference in the frequency of CSF IgG abnormality between CMS (46/70, 65.7%) and OSMS (13/24, 54.2%).
HLA-DRB1 alleles in all MS patients
Compared with HCs, MS patients showed a significantly higher frequency of the DRB1*0405 allele (pcorr = 0.0196, OR = 2.217, 95% CI = 1.389–3.539) and a significantly lower frequency of the DRB1*0901 allele (pcorr = 0.0084, OR = 0.279, 95% CI = 0.135–0.575) (Table 2).
Table 2. Comparisons of the phenotype frequencies of the HLA-DRB1 alleles.
| DRB1*X | MS (n = 94) | CSF IgG abnormality (+) (n = 59) | CSF IgG abnormality (−) (n = 35) | HCs (n = 367) |
| 0101 (%) | 13 (13.8) | 7 (11.9) | 6 (17.1) | 51 (13.9) |
| 0301 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) |
| 0401 (%) | 3 (3.2) | 1 (1.7) | 2 (5.7) | 4 (1.1) |
| 0403 (%) | 4 (4.3) | 4 (6.8) | 0 (0.0) | 18 (4.9) |
| 0404 (%) | 2 (2.1) | 2 (3.4) | 0 (0.0) | 0 (0.0) |
| 0405 (%) | 42 (44.7)* a | 22 (37.3) | 20 (57.1)* b | 98 (26.7) |
| 0406 (%) | 13 (13.8) | 8 (13.6) | 5 (14.3) | 23 (6.3) |
| 0407 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) |
| 0410 (%) | 2 (2.1) | 2 (3.4) | 0 (0.0) | 4 (1.1) |
| 0701 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) |
| 0802 (%) | 12 (12.8) | 8 (13.6) | 4 (11.4) | 26 (7.1) |
| 0803 (%) | 11 (11.7) | 9 (15.3) | 2 (5.7) | 58 (15.8) |
| 0901 (%) | 9 (9.5)* c | 6 (10.2) | 3 (8.6) | 101 (27.5) |
| 1001 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.1) |
| 1101 (%) | 4 (4.3) | 2 (3.4) | 2 (5.7) | 16 (4.4) |
| 1106 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| 1201 (%) | 9 (9.6) | 6 (10.2) | 3 (8.6) | 33 (9.0) |
| 1202 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (3.5) |
| 1301 (%) | 1 (1.1) | 1 (1.7) | 0 (0.0) | 1 (0.3) |
| 1302 (%) | 3 (3.2) | 1 (1.7) | 2 (5.7) | 49 (13.4) |
| 1403 (%) | 2 (2.1) | 0 (0.0) | 2 (5.7) | 8 (2.2) |
| 1405 (%) | 3 (3.2) | 2 (3.4) | 1 (2.9) | 14 (3.8) |
| 1406 (%) | 3 (3.2) | 2 (3.4) | 1 (2.9) | 8 (2.2) |
| 1454 (%) | 2 (2.1) | 2 (3.4) | 0 (0.0) | 19 (5.2) |
| 1501 (%) | 24 (25.5) | 20 (33.9)* d | 4 (11.4) | 60 (16.4) |
| 1502 (%) | 14 (14.9) | 8 (13.6) | 6 (17.1) | 80 (21.8) |
| 1601 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| 1602 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.8) |
*aCompared with HCs, pcorr = 0.0196, OR = 2.217, 95% CI = 1.389–3.539.
*bCompared with HCs, pcorr = 0.0056, OR = 3.660, 95% CI = 1.802–7.431.
*cCompared with HCs, pcorr = 0.0084, OR = 0.279, 95% CI = 0.135–0.575.
*dCompared with HCs, pcorr = 0.0392, OR = 2.624, 95% CI 1.432–4.809.
puncorr was corrected by multiplying the value by 28 to calculate pcorr.
CI, confidence interval; CSF, cerebrospinal fluid; HCs, healthy controls; MS, multiple sclerosis; OR, odds ratio; pcorr, corrected p value.
HLA-DRB1 alleles in MS patients according to the presence or absence of CSF IgG abnormality
Compared with HCs, CSF IgG abnormality-positive MS patients showed a significantly higher frequency of the DRB1*1501 allele (pcorr = 0.0392, OR = 2.624, 95% CI = 1.432–4.809), whereas CSF IgG abnormality-negative MS patients showed a significantly higher frequency of the DRB1*0405 allele (pcorr = 0.0056, OR = 3.660, 95% CI = 1.802–7.431) (Table 2). The frequency of CSF IgG abnormality was 83.3% in MS patients with the DRB1*1501 allele compared with 52.4% in MS patients with the DRB1*0405 allele (p = 0.0119).
Relationships between CSF IgG abnormality status and common infectious agents
Anti-C. pneumoniae IgG antibodies were significantly more frequently detected in MS patients with CSF IgG abnormality than in those without CSF IgG abnormality (p = 0.0119) (Table 3), although there was no difference in the frequency of anti-C. pneumoniae IgG antibodies between HCs and total MS patients. Compared with HCs, anti-H. pylori IgG antibodies were detected significantly less frequently in the total MS patients (p = 0.0451) and CSF IgG abnormality-negative MS patients (p = 0.0474) (Table 3). There was no difference in the frequency of anti-H. pylori IgG antibodies between CSF IgG abnormality-positive and abnormality-negative patients. Neither the anti-EBNA nor anti-VZV antibody positivity rates differed significantly among the groups. Among the common infectious agents, only the H. pylori infection rate decreased successively with advancing year of birth (p = 0.0014) (Figure 2).
Table 3. Comparisons of the frequencies of antibodies against common infectious agents.
| MS | CSF IgG abnormality (+) | CSF IgG abnormality (−) | HCs | |
| Chlamydia pneumoniae | 56/90 (62.22%) | 42/58 (72.4%)* | 14/32 (43.8%)* | 92/156 (58.97%) |
| Helicobacter pylori | 26/90 (28.89%)** | 19/58 (32.8%) | 7/32(21.9%)*** | 74/177 (41.81%)** , *** |
| EBV | 86/90 (95.56%) | 56/58 (96.6%) | 30/32 (93.8%) | 143/156 (91.67%) |
| VZV | 89/90 (98.89%) | 57/58 (98.3%) | 32/32 (100%) | 153/156 (98.08%) |
*p = 0.0119, compared with IgG abnormality (−) MS patients.
**p = 0.0451, compared with HCs.
***p = 0.0474, compared with HCs.
*,**,***Significant difference between the linked values (p<0.05).
The age of the patients during examination did not differ significantly among HCs and MS patients, regardless of the presence or absence of IgG abnormality (mean ± SD in years: 37.21±12.54 for MS; 36.19±11.36 for IgG abnormality-positive MS; 39.00±14.39 for IgG abnormality-negative MS; and 38.93±12.11 for HCs).
CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; HCs, healthy controls; MS, multiple sclerosis; pcorr, corrected p value; VZV, varicella zoster virus.
Figure 2. Proportions of patients with Helicobacter pylori infection by year of birth.
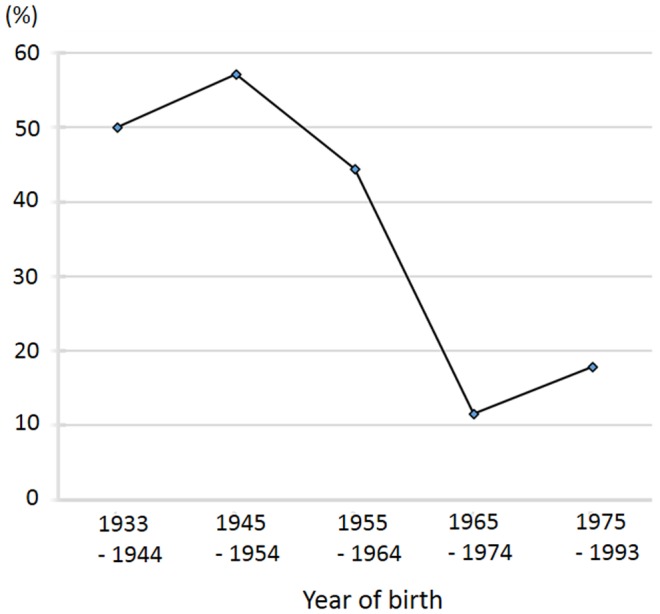
Among the MS patients, the proportion of patients with H. pylori decreased markedly in those born after 1965. MS, multiple sclerosis.
Discussion
The main new findings of the present study are as follows: (1) compared with HCs, CSF IgG abnormality-positive MS patients had a significantly higher frequency of HLA-DRB1*1501, whereas CSF IgG abnormality-negative MS patients had a significantly higher frequency of HLA-DRB1*0405; (2) CSF IgG abnormality-positive MS patients had a significantly higher frequency of anti-C. pneumoniae IgG antibodies compared with abnormality-negative MS patients, although there was no difference in the frequency of anti-C. pneumoniae IgG antibodies between HCs and total MS patients; and (3) compared with HCs, the frequencies of anti-H. pylori IgG antibodies were lower, especially in CSF IgG abnormality-negative MS patients.
The number of enrolled MS patients was not large because of the relative rarity of the disease in the Japanese population, and this could lead to partly inconclusive results. However, this study is the first to investigate the influence of HLA-DRB1 alleles on CSF IgG abnormality in MS patients in Japan when NMO and NMOSD patients were excluded, and is the only study to simultaneously investigate the influence of common infectious agents as environmental risk or protective factors. The ELISAs used in the present study have reasonably high sensitivity and specificity [37], [38], although H. pylori and C. pneumoniae infections should be confirmed by methods other than ELISA in future studies.
In the present study, we demonstrated that the presence or absence of CSF IgG abnormality did not predict the prognosis for the disease course of Japanese MS patients, consistent with relatively larger studies in Western countries and a Japanese study in Hokkaido, the northernmost island of Japan [18], [26]–[28]. Additionally, the frequency of CSF IgG abnormality did not differ according to the clinical subtypes of MS.
In CSF IgG abnormality-positive MS patients, the only significant difference was the more frequent presence of brain MRI lesions meeting the Barkhof criteria compared with CSF IgG abnormality-negative MS patients. Carriage of HLA-DRB1*1501 was associated with an approximately 2.6-fold increased risk for CSF IgG abnormality-positive MS. This is in line with previous findings demonstrating that DRB1*15 is associated with OB-positive MS in Swedish patients [22], Spanish patients [23], and the Japanese population of Hokkaido [19]. Taken together, in this subpopulation, MS was associated with greater brain MRI lesion loads, presence of the HLA-DRB1*1501 allele, and increased humoral immune responses in CSF in Japanese. These features also resemble those of MS in Western people [39], [40]. Therefore, this subgroup of Japanese patients represents a “Western” type of MS in terms of CSF, neuroimaging, and genetic characteristics. In Caucasians, the presence of the DRB1*1501 allele promotes the development of more T2 lesions [41] and intrathecal IgG synthesis [42]. Similar biological mechanisms may occur in Asian patients.
In CSF IgG abnormality-negative MS patients, HLA-DRB1*0405 showed an approximately 3.6-fold increased risk for the condition. In this subgroup, MS was characterized by lower MRI brain lesion loads. This is in line with previous findings that DRB1*04 is associated with OB-negative MS in Swedish patients [22] and the Japanese population of Hokkaido [19]. The low frequency of CSF IgG abnormality is a unique feature in Japanese MS patients, compared with Western MS patients [17], [20]. DRB1*0405 is present in a relatively minor population of Caucasian MS patients [22], while about 60% of MS patients in Northern Europe are positive for HLA-DRB1*15, compared with 30% of HCs [43]. The relatively high frequency of Japanese MS patients carrying the DRB1*0405 allele may be partly responsible for the low prevalence of CSF IgG abnormality in Japanese MS patients. According to the fourth nationwide survey of MS in Japanese people, the most common type of MS had neither Barkhof brain lesions nor longitudinally extensive spinal cord lesions [44]. Hence, CSF IgG abnormality-negative Japanese MS could be a unique subgroup of MS in terms of CSF, neuroimaging, and genetic characteristics. Kuenz et al. [45] showed that CSF B cells were correlated with paraclinical markers such as high numbers of MRI T2 lesions, intrathecal IgG synthesis, and intrathecal production of MMP-9 and B cell chemokine CxCL-13. These findings may suggest that distinct immune mechanisms between IgG abnormality-positive and abnormality-negative patients lead to the differences in their CSF and neuroimaging characteristics.
In the present study, we showed that C. pneumoniae infection was higher in CSF IgG abnormality-positive MS patients than in abnormality-negative MS patients. The mechanism for the abnormal intrathecal IgG synthesis in MS patients infected with C. pneumoniae could be molecular mimicry [46], i.e., cross-reactivity of humoral immune responses against C-pneumoniae antigens and CNS self-antigens. To date, however, OBs have not been found to react highly specifically with any microbial antigens or self-antigens. It is possible that anti-C. pneumoniae IgG antibodies directly confer CSF IgG abnormality in MS patients. In general, however, CSF anti-C. pneumoniae IgG antibodies are only found in a small portion of MS patients, with no differences between MS and controls [47]–[50]. These findings suggest that C. pneumoniae infection may indirectly modify the intrathecal humoral immune functions, leading to CSF IgG abnormality in patients with MS.
It is interesting to note that CSF IgG abnormality-negative MS patients had a lower frequency of H. pylori infection compared with HCs in addition to a lower frequency of C. pneumoniae infection compared with CSF IgG abnormality-positive MS patients. To date, there have been no reports focusing on the association between CSF IgG abnormality and H. pylori infection, and there has been no firm evidence of molecular mimicry between human myelin antigens and H. pylori. Therefore, it is extremely difficult to speculate on the mechanism by which H. pylori infection differentially affects MS subpopulations with and without CSF IgG abnormality. However, these observations extend our previous finding that the rates of H. pylori infection, which occurs in the infantile period and reflects sanitary conditions in younger ages, were lower in Japanese MS patients, compared with HCs [12]. Additionally, the proportion of patients with H. pylori infection decreased successively with advancing year of birth. These findings collectively suggest that CSF IgG abnormality-negative MS patients may have grown up in a relatively clean environment. It is possible that a clean environment at younger ages may confer CSF IgG abnormality-negative MS. We previously reported that HLA-DRB1*0405-positive MS showing a lower frequency of CSF IgG abnormality is increasing in the younger Japanese population [7]. Thus, a modernized clean environment may potentiate susceptibility to this subtype of MS without CSF IgG abnormality in HLA-DRB1*0405 carriers. This possibility should be investigated in future large-scale studies.
In conclusion, DRB1*1501 and C. pneumoniae infection confer CSF IgG abnormality, while DRB1*0405 and H. pylori infection are positively and negatively associated with CSF IgG abnormality-negative MS, respectively, suggesting that genetic and environmental factors differentially contribute to MS susceptibility according to the CSF IgG abnormality status.
Funding Statement
This work was supported in part by a Health and Labour Sciences Research Grant on Intractable Diseases (H20-Nanchi-Ippan-016) from the Ministry of Health, Labour and Welfare, Japan, and a Grant-in-Aid (B; No. 22390178) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Compston A, Coles A (2008) Multiple sclerosis. Lancet 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. Ebers GC (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7: 268–277. [DOI] [PubMed] [Google Scholar]
- 3. Swcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, et al. (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359): 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, et al. (1996) Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol 40: 569–574. [DOI] [PubMed] [Google Scholar]
- 5. Yamasaki K, Horiuchi I, Minohara M, Kawano Y, Ohyagi Y, et al. (1999) HLA-DPB1*0501-associated opticospinal multiple sclerosis: clinical, neuroimaging and immunogenetic studies. Brain 122: 1689–1696. [DOI] [PubMed] [Google Scholar]
- 6. Ono T, Zambenedetti MR, Yamasaki K, Kawano Y, Kamikawaji N, et al. (1998) Molecular analysis of HLA class I (HLA-A and -B) and HLA class II (HLA-DRB1) genes in Japanese patients with multiple sclerosis (Western type and Asian type). Tissue Antigens 52: 539–542. [DOI] [PubMed] [Google Scholar]
- 7. Yoshimura S, Isobe N, Yonekawa T, Matsushita T, Masaki K, et al. (2012) South Japan Multiple Sclerosis Genetics Consortium: genetic and infectious profiles of Japanese multiple sclerosis patients. PLoS ONE 7: e48592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin LI, Munger KL, O'Reilly EJ, Falk KI, Ascherio A (2010) Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 67: 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Handel AE, Giovannoni G, Ebers GC, Ramagopalan SV (2010) Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 6: 156–166. [DOI] [PubMed] [Google Scholar]
- 10. Sriram S, Stratton CW, Yao S, Tharp A, Ding L, et al. (1999) Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol 46: 6–14. [PubMed] [Google Scholar]
- 11. Bagos PG, Nikolopoulos G, Ioannidis A (2006) Chlamydia pneumoniae infection and the risk of multiple sclerosis: a meta-analysis. Mult Scler 12: 397–411. [DOI] [PubMed] [Google Scholar]
- 12. Li W, Minohara M, Su JJ, Matsuoka T, Osoegawa M, et al. (2007) Helicobacter pylori infection is a potential protective factor against conventional multiple sclerosis in the Japanese population. J Neuroimmunol 184: 227–331. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Minohara M, Piao H, Matsushita T, Masaki K, et al. (2009) Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult Scler 15: 1411–1421. [DOI] [PubMed] [Google Scholar]
- 14. Yoshimura S, Isobe N, Matsushita T, Yonekawa T, Masaki K, et al. (2013) Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry 84: 29–34. [DOI] [PubMed] [Google Scholar]
- 15. Link H, Huang YM (2006) Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol 180: 17–28. [DOI] [PubMed] [Google Scholar]
- 16. Lechner-Scott J, Spencer B, de Malmanche T, Attia J, Fitzgerald M, et al. (2012) The frequency of CSF oligoclonal banding in multiple sclerosis increases with latitude. Mult Scler 18: 974–982. [DOI] [PubMed] [Google Scholar]
- 17. Kira J (2003) Multiple sclerosis in the Japanese population. Lancet Neurol 2: 117–127. [DOI] [PubMed] [Google Scholar]
- 18. Fukazawa T, Kikuchi S, Sasaki H, Hamada K, Hamada T, et al. (1998) The significance of oligoclonal bands in multiple sclerosis in Japan: relevance of immunogenetic backgrounds. J Neurol Sci 158: 209–214. [DOI] [PubMed] [Google Scholar]
- 19. Kikuchi S, Fukazawa T, Niino M, Yabe I, Miyagishi R, et al. (2003) HLA-related subpopulations of MS in Japanese with and without oligoclonal IgG bands. Neurology 60: 647–651. [DOI] [PubMed] [Google Scholar]
- 20. Nakashima I, Fujihara K, Sato S, Itoyama Y (2005) Oligoclonal IgG bands in Japanese patients with multiple sclerosis. A comparative study between isoelectric focusing with IgG immunofixation and high-resolution agarose gel electrophoresis. J Neuroimmunol 159: 133–136. [DOI] [PubMed] [Google Scholar]
- 21. Siritho S, Prayoonwiwat N (2007) A retrospective study of multiple sclerosis in Siriraj Hospital, Bankok, Thailand. Can J Neurol Sci 34: 99–104. [DOI] [PubMed] [Google Scholar]
- 22. Imrell K, Landtblom AM, Hillert J, Masterman T (2006) Multiple sclerosis with and without CSF bands: clinically indistinguishable but immunogenetically distinct. Neurology 67: 1062–1064. [DOI] [PubMed] [Google Scholar]
- 23. Romero-Pinel L, Martínez-Yélamos S, Bau L, Matas E, Gubieras L, et al. (2011) Association of HLA-DRB1*15 allele and CSF oligoclonal bands in a Spanish multiple sclerosis cohort. Eur J Neurol 8: 1258–1262. [DOI] [PubMed] [Google Scholar]
- 24. Stendahl-Brodin L, Link H (1980) Relation between benign course of multiple sclerosis and low-grade humoral immune response in cerebrospinal fluid. J Neurol Neurosurg Psychiatry 43: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeman AZ, Kidd D, McLean BN, Kelly MA, Francis DA, et al. (1996) A study of oligoclonal band negative multiple sclerosis. J Neurol Neurosurg Psychiatry 60: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch M, Heersema D, Mostert J, Teelken A, De Keyser J (2007) Cerebrospinal fluid oligoclonal bands and progression of disability in multiple sclerosis. Eur J Neurol 14: 797–800. [DOI] [PubMed] [Google Scholar]
- 27. Siritho S, Freedman MS (2009) The prognostic significance of cerebrospinal fluid in multiple sclerosis. J Neurol Sci 279: 21–25. [DOI] [PubMed] [Google Scholar]
- 28. Lourenco P, Shirani A, Saeedi J, Oger J, Schreiber WE, et al. (2013) Oligoclonal bands and cerebrospinal fluid markers in multiple sclerosis: associations with disease course and progression. Mult Scler 19: 577–584. [DOI] [PubMed] [Google Scholar]
- 29. Nakashima I, Fujihara K, Misu T, Fujimori J, Sato S, et al. (2002) A comparative study of Japanese multiple sclerosis patients with and without oligoclonal IgG bands. Mult Scler 8: 459–462. [DOI] [PubMed] [Google Scholar]
- 30. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 31. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 32. Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 33. Poser S, Ritter G, Bauer HJ, Grosse-Wilde H, Kuwert EK, et al. (1981) HLA-antigens and the prognosis of multiple sclerosis. J Neurol 225: 219–221. [DOI] [PubMed] [Google Scholar]
- 34. Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, et al. (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 35. Matsuoka T, Matsushita T, Osoegawa M, Ochi H, Kawano Y, et al. (2008) Heterogeneity and continuum of multiple sclerosis in Japanese according to magnetic resonance imaging findings. J Neurol Sci 266: 115–125. [DOI] [PubMed] [Google Scholar]
- 36. Isobe N, Yonekawa T, Matsushita T, Kawano Y, Masaki K (2012) Quantitative assays for anti-aquaporin-4 antibody with subclass analysis in neuromyelitis optica. Mult Scler 18: 1541–1551. [DOI] [PubMed] [Google Scholar]
- 37. Laheij RJ, Straatman H, Jansen JB, Verbeek AL (1998) Evaluation of commercially available Helicobacter pylori serology kits: a review. J Clin Microbiol 36: 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hermann C, Graf K, Groh A, Straube E, Hartung T (2002) Comparison of eleven commercial tests for Chlamydia pneumoniaespecific immunoglobulin G in asymptomatic healthy individuals. J Clin Microbiol 40: 1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu JS, Qiu W, Castley A, James I, Joseph J, et al. (2010) Presence of CSF oligoclonal bands (OCB) is associated with the HLA-DRB1 genotype in a West Australian multiple sclerosis cohort. J Neurol Sci 288: 63–67. [DOI] [PubMed] [Google Scholar]
- 40. Romero-Pinel L, Martínez-Yélamos S, Bau L, Matas E, Gubieras L, et al. (2011) Association of HLA-DRB1*15 allele and CSF oligoclonal bands in a Spanish multiple sclerosis cohort. Eur J Neurol 18: 1258–1262. [DOI] [PubMed] [Google Scholar]
- 41. Okuda DT, Srinivasan R, Oksenberg JR, Goodin DS, Baranzini SE, et al. (2009) Genotype-phenotype correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1HMR spectroscopy and MRI measures. Brain 132: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sellebjerg F, Jensen J, Madsen HO, Svejgaard A (2000) HLA DRB1*1501 and intrathecal inflammation in multiple sclerosis. Tissue Antigens 55: 312–318. [DOI] [PubMed] [Google Scholar]
- 43. Masterman T, Ligers A, Olsson T, Andersson M, Olerup O, et al. (2000) HLA-DR15 is associated with lower age at onset in multiple sclerosis. Ann Neurol 48: 211–219. [PubMed] [Google Scholar]
- 44. Ishizu T, Kira J, Osoegawa M, Fukazawa T, Kikuchi S, et al. (2009) Research Committee of Neuroimmunological Diseases: Heterogeneity and continuum of multiple sclerosis phenotypes in Japanese according to the results of the fourth nationwide survey. J Neurol Sci 280: 22–28. [DOI] [PubMed] [Google Scholar]
- 45. Kuenz B, Lutterotti A, Ehling R, Gneiss C, Haemmerle M, et al. (2008) Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS One 3: e2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamradt T, Goggel R, Erb KJ (2005) Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trend Immun 26: 260–267. [DOI] [PubMed] [Google Scholar]
- 47. Layh-Schmitt G, Bendl C, Hildt U, Dong-Si T, Jüttler E, et al. (2000) Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann Neurol 47: 652–655. [PubMed] [Google Scholar]
- 48. Derfuss T, Gürkov R, Then Bergh F, Goebels N, Hartmann M, et al. (2001) Intrathecal antibody production against Chlamydia pneumoniae in multiple sclerosis is part of a polyspecific immune response. Brain 124: 1325–1335. [DOI] [PubMed] [Google Scholar]
- 49. Saiz A, Marcos MA, Graus F, Vidal J, Jimenez de Anta MT (2001) No evidence of CNS infection with Chlamydia pneumoniae in patients with multiple sclerosis. J Neurol 248: 617–618. [DOI] [PubMed] [Google Scholar]
- 50. Sotgiu S, Piana A, Pugliatti M, Sotgiu A, Deiana GA, et al. (2001) Chlamydia pneumoniae in the cerebrospinal fluid of patients with multiple sclerosis and neurological controls. Mult Scler 7: 371–374. [DOI] [PubMed] [Google Scholar]


