SUMMARY
Faithful genome transmission during cell division requires precise, coordinated action of DNA metabolic enzymes, including proteins responsible for DNA damage detection and repair. Dynamic phosphorylation plays an important role in controlling repair enzymes during the DNA damage response (DDR). Cdc14 phosphatases oppose cyclin-dependent kinase (Cdk) phosphorylation and have been implicated in the DDR in several model systems. Using new insight into Cdc14 specificity we identified the budding yeast Holliday junction resolvase Yen1 as the first DNA repair target of Cdc14. Cdc14 activation at anaphase triggers nuclear accumulation and enzymatic activation of Yen1, likely to resolve persistent recombinational repair intermediates. Consistent with this, expression of a phosphomimetic Yen1 mutant increased sister chromatid non-disjunction. In contrast, lack of Cdk phosphorylation resulted in constitutive activity and elevated crossover-associated repair. The precise timing of Yen1 activation, governed by core cell cycle regulators, helps coordinate DNA repair with chromosome segregation and safeguards against genome destabilization.
INTRODUCTION
Robust regulatory systems have evolved to maximize faithful transmission of genetic information from one cell generation to the next. Genome instability arising from a variety of sources contributes to human disease. In many cases, such as the repair of DNA damage, genome stability requires rapid and reversible cellular responses to environmental challenges, and therefore cells often make use of dynamic regulatory protein modifications, such as phosphorylation. Reversible phosphorylation by opposing kinase and phosphatase activities not only controls the activation of DNA repair pathways, but also plays a critical role in coordinating repair with cell division. Cyclin-dependent kinases (Cdks) directly regulate most cell cycle events and also are required for an effective response to various types of DNA damage and maintenance of genome stability (Enserink et al., 2009; Trovesi et al., 2013). Reversal of Cdk phosphorylation events is essential for completion of mitosis and initiation of cytokinesis and can be achieved by several different phosphatases, including the Cdc14 family (Queralt and Uhlmann, 2008).
Cdc14 phosphatases contribute to genome stability in a variety of ways through the dephosphorylation of key Cdk substrates. Work in yeasts has implicated Cdc14 in promoting proper chromosome segregation, mitotic spindle function, DNA replication, and cytokinesis (Mocciaro and Schiebel, 2010). Cdc14 has been implicated in a variety of cell division functions in metazoans as well, based primarily on knockdown and/or overexpression studies. These include centriole duplication, centrosome separation, cytokinesis, spindle assembly, and mitotic exit (Mocciaro and Schiebel, 2010). However, the specific biological functions of metazoan Cdc14 proteins remain unclear and few direct substrates have been identified outside of yeast model systems. The lack of knowledge of direct targets hampers our mechanistic understanding of Cdc14 contributions to genome stability.
In addition to the biological functions described above, several recent studies have provided evidence linking Cdc14 to the DNA damage response (DDR) and DNA repair. Human Cdc14B is activated by nucleolar release in response to genotoxic stress to promote the G2 DNA damage checkpoint (Bassermann et al., 2008). In fission yeast, release of Clp1 from the nucleolus in response to replication stress contributes to full activation of DNA damage checkpoint signaling (Broadus and Gould, 2012; Diaz-Cuervo and Bueno, 2008). Interestingly, deletion of the CDC14A or CDC14B genes from chicken or human cultured cells was recently reported to cause defective repair of DNA damage without compromising checkpoint activation (Mocciaro et al., 2010). Likewise, Cdc14B-deficient mice exhibit premature aging, and embryo fibroblasts from these animals have defective DNA damage repair but an apparently normal checkpoint response (Wei et al., 2011). Collectively, these studies clearly indicate that Cdc14 proteins in multiple organisms are activated by DNA damage and contribute in some way to the cellular DDR. Specific targets of Cdc14 involved in DNA repair or DNA damage checkpoints have not been identified.
Cdc14 enzymes historically have been thought to possess general specificity for proline-directed Ser/Thr phosphorylation sites (Gray et al., 2003; Kaiser et al., 2002; Visintin et al., 1998), matching the minimal consensus of Cdks and other proline-directed kinases. We recently found that Cdc14 enzymes from diverse eukaryotes are actually highly specific for a subset of Cdk-type phosphorylation sites containing phosphoserine (pSer) and basic amino acids immediately downstream from pSer-Pro (Bremmer et al., 2012). The unexpected, strict enzymatic specificity of Cdc14 raises the possibility that novel substrates can be predicted bioinformatically.
In this study, we further refined the features of optimal Cdc14 substrates, and using this information identified the Holliday junction (HJ) resolvase Yen1 as a novel early anaphase Cdc14 substrate. Yen1 was previously identified as a preferential S phase cyclin-Cdk target in vitro (Loog and Morgan, 2005) and its enzymatic activity was shown to be inhibited by phosphorylation until anaphase in vivo (Matos et al., 2011). Here, we demonstrate that Cdk and Cdc14 are responsible for controlling the enzymatic activation and nucleocytoplasmic localization of Yen1, effectively restricting its DNA repair function during cell division to anaphase. We provide evidence that this regulation contributes to genome stability during the mitotic cell cycle, as proposed previously (Matos et al., 2011). This work provides the first link between Cdc14 and DNA repair in budding yeast, identifies the first DNA damage repair substrate of Cdc14 in any organism, and reveals a mechanism by which DNA repair events are coordinated with the cell division machinery to ensure faithful segregation of chromosomes.
RESULTS
Refinement of Cdc14 specificity and prediction of Yen1 as a substrate
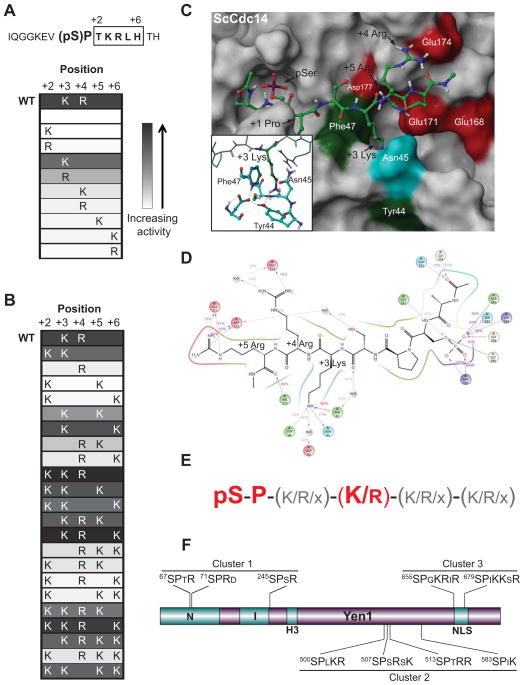
Cdc14 enzymes display a strong preference for basic amino acids downstream from pSer-Pro (Bremmer et al., 2012). Phosphopeptides lacking basic residues in the +2 to +6 region relative to pSer-Pro are poor substrates. However, we did not previously explore the preference for Lys and Arg in enough detail to define which specific positions are critical for Cdc14 activity. We reasoned that identification of additional strong consensus site features might allow us to discover novel Cdc14 substrates using motif prediction algorithms. To define the basic amino acid requirement in more detail we generated a small phosphopeptide library based on the previously defined Cdc14 substrate site at residue 71 in the securin ortholog Pds1 (Holt et al., 2008). We first tested the contribution of an individual Lys or Arg at positions +2 through +6 to Cdc14 catalytic efficiency. The results are displayed as a heat map in Figure 1A (see Table S1 for rates). Consistent with our previous results, Cdc14 was unreactive towards the phosphopeptide lacking basic amino acids in this region. Only a basic amino acid at +3 provided activity close to the wild-type substrate. Moreover, Lys provided approximately 4-fold higher activity than Arg.
Figure 1. Refinement of Cdc14 enzymatic specificity.
A–B) The peptide sequence from Pds1 was synthesized with a central phosphoserine (pS), and variants contained different combinations of basic amino acids in the +2 to +6 region (boxed). WT, wild-type. Heat map cells show positions and identity of basic residues, Lys (K) or Arg (R), in each peptide. Shading indicates relative activity (white = low, black = high) in Cdc14 assays conducted under steady-state conditions that approximate kcat/Km. C) Representative frame from MD simulation of the ScCdc14 homology model (surface representation) with bound optimal peptide substrate Ac-A(pSer)PSKRR-NHMe (ball and stick). Residues that contact the substrate KRR are colored (red, acidic; green, hydrophobic; cyan, polar neutral) and labeled in white. Key residues of the substrate are labeled in black. Inset highlights the local arrangement of +3 Lys engaged in cation-pi contacts with Tyr44 and Phe47, stabilized by hydrogen bonding (dashed lines). See also Fig. S1. D) 2-dimensional representation of stable (≥ 40% of simulation time) contacts (direct or water-bridged) between peptide (black) and ScCdc14 residues obtained from an MD experiment. Percentages represent the fraction of time that the contact was maintained. Purple arrows are hydrogen bonds, red lines are cation-pi interactions, residue colors are as in panel C with basic residues in blue. E) Summary of Cdc14 substrate features used for substrate prediction. Essential requirements are in large red font. See additional information in Tables S1-S3. F) Diagram of relevant Yen1 protein features. All nine Ser-Pro Cdk site positions with downstream basic residues are shown and divided into the 3 clusters used for the purpose of mutagenesis throughout the paper. N, I, and H3 (helix-hairpin-helix) are conserved domains of the XPG nuclease family.
We also tested combinations of basic amino acids to determine which patterns provided the best catalytic efficiency. Of the multi-basic peptides, only those with Lys at +3 produced significant activity (Fig. 1B and Table S1). For several of these, activity was substantially higher than with a single Lys at +3. Enhanced activity in the multi-basic peptides correlated best with a basic residue at +4, although this effect was not universal. We conclude that a +3 Lys (Arg to a lesser extent) represents an additional critical feature of Cdc14 substrates, and that optimal activity is achieved with additional basic residues in the immediate vicinity of +3.
A stretch of conserved acidic residues lines the peptide binding groove in the human Cdc14B (hCdc14B) crystal structure and was proposed to contribute to recognition of phosphosubstrates containing downstream basic sequences (Gray et al., 2003). This extended acidic patch is insufficient though to explain the strong preference for Lys/Arg specifically at +3. To understand the strong +3 effect we performed docking and molecular dynamics (MD) simulations with the optimal substrate sequence A(pSer)PSKRR and either the hCdc14B structure or a homology model of the budding yeast Cdc14 (ScCdc14) catalytic domain. The ScCdc14 model revealed extensive amino acid conservation for the substrate binding channel (Fig. S1C) and resulted in similar positions and contacts for the -1 Ala, pSer and +1 Pro compared to the empirical structure. Interestingly, the MD simulations with both ScCdc14 (Fig. 1C) and hCdc14B (Fig. S1B) suggested that the +3 Lys does not contact the acidic groove. Instead, the Lys side chain sits in a conserved pocket adjacent to the +1 Pro cage where it engages in cation-pi contacts, stabilized by a hydrogen bond network (Fig. 1C and 1D). In ScCdc14 the invariant Phe47 and Tyr44 both contribute strongly to this interaction. Cation-pi interactions at solvent-exposed protein surfaces generally contribute much greater binding energy than ionic contacts (Gallivan and Dougherty, 2000), providing rationale for the +3 requirement. Although both Lys and Arg engage in cation-pi interactions, only the longer aliphatic side chain of Lys allowed for a favorable orientation with Tyr44/Phe47. The shorter Arg side chain prevented the preferred guanidinium-pi stacking arrangement (Nandi et al., 1993), explaining the Lys preference. The +4 and +5 Arg both interact with acidic residues in the binding groove through most of the MD simulation. The models are consistent with our biochemical data defining the critical contribution of a +3 basic amino acid and the auxiliary contributions of surrounding basic residues to substrate selectivity.
Using pSer, +1 Pro, and +3 Lys or Lys/Arg as fixed consensus features, and additional Lys/Arg residues as variable consensus features (Fig. 1E), we scanned the yeast proteome for proteins enriched in candidate Cdc14 target sites (Tables S2 and S3). These searches produced just over 60 hits, of which 13 were previously reported Cdc14 substrates. To identify novel substrates, we first focused on proteins with clusters of optimal Cdc14 sites since regulatory Cdk phosphorylation commonly occurs in clusters on disordered loops (Holt et al., 2009; Moses et al., 2007). Yen1 caught our eye because it contains more optimal Cdc14 sites than any other yeast protein (Table S3). The optimal consensus sites are clustered within a 180 amino acid stretch near the C-terminus (Fig. 1F), and are well conserved across the order Saccharomycetales. Yen1 is likely a direct Cdk substrate (Loog and Morgan, 2005) and contains a predicted Cdk-regulated NLS (Kosugi et al., 2009). We therefore proceeded to test if it is a regulatory target of the opposing Cdk and Cdc14 activities.
Yen1 is phosphorylated in vivo by Cdk
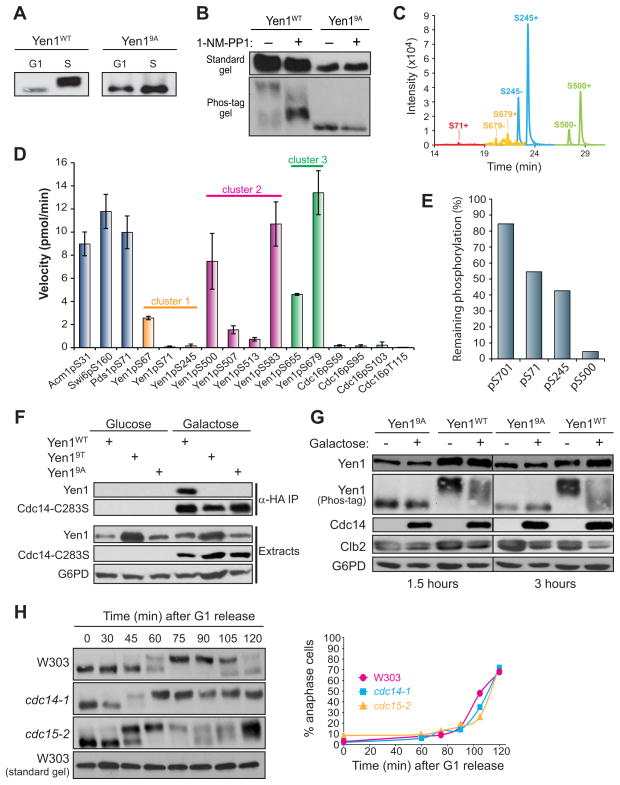
We first tested if Yen1 is an in vivo substrate of the cell cycle Cdk, Cdc28. Phosphorylated Yen1 species can be effectively separated from unphosphorylated Yen1 using Phos-tag™ SDS-PAGE and immunoblotting (Matos et al., 2011). We compared the migration of Yen1-TAP extracted from cells arrested in G1 and S phase. Mobility was strongly retarded when cells were arrested in S phase, a time when Cdc28 activity is high, compared to G1, when Cdc28 is inactive (Fig. 2A). Mutation of the 9 Ser-Pro sequences in Yen1 to Ala-Pro (Yen19A) completely abolished the slow mobility species in S phase cells, demonstrating that Yen1 phosphorylation is dependent on Cdk consensus sites. To test if phosphorylation is dependent on Cdc28 activity, we used a cdc28-as1 strain in which a Cdc28 active site mutation renders the kinase uniquely sensitive to inhibition by the ATP analog 1-NM-PP1 (Bishop et al., 2000). Cdc28 inhibition resulted in rapid loss of slow mobility phosphorylated Yen1-TAP but did not affect migration of Yen19A-TAP (Fig. 2B). To directly detect phosphorylation we purified Yen1-3FLAG from cdc14-1 cultures arrested in late mitosis with high Cdk activity and analyzed it by mass spectrometry (MS). Four peptides containing Ser-Pro sequences, representing each cluster depicted in Figure 1F, were convincingly identified. All were detected predominantly in phosphorylated form (Fig. 2C and S2). We conclude that Yen1 is heavily phosphorylated in vivo by Cdc28 at multiple Ser-Pro sites throughout the primary sequence.
Figure 2. Yen1 is an in vivo substrate of Cdk and Cdc14.
A) Anti-protein A immunoblots from Phos-tag SDS-PAGE. Extracts from cells arrested in G1 or S and expressing integrated YEN1-TAP or yen19A-TAP from PYEN1 were compared. B) Same as A except asynchronous cdc28-as1 cells were treated with 20 μM 1-NM-PP1 or an equal volume of DMSO for 30 min. Immunoblots using standard and Phos-tag gels are shown to emphasize the phosphorylation-dependence of the mobility shift. C) Ion chromatograms for Yen1 tryptic peptides containing the indicated Cdk phosphorylation sites in both unmodified (“−“) and phosphorylated (“+”) forms detected by LC-MS. Identity of the peak in each case was verified by product ion spectra (Fig. S2). Unmodified S71 peptide was undetectable D) Activity of Cdc14 towards the indicated phosphopeptide substrates was measured under identical steady state conditions. Data are the average of three trials and error bars are standard deviations. Peptide sequences shown in Table S4. E) Yen1-3FLAG purified from yeast was treated with 250 nM recombinant Cdc14 for 10 min and analyzed by LC-MS. Ion signals for peptides phosphorylated at the indicated residues were integrated and percent phosphorylation remaining relative to untreated Yen1-3FLAG was calculated using unmodified peptides for normalization. F) Co-IP of the substrate trap 3HA-Cdc14-C283S expressed from PGAL1 and Yen1-TAP, Yen19A-TAP, or Yen19T-TAP expressed from PYEN1 in S phase cells. 3HA-Cdc14-C283S was isolated on anti-HA affinity resin and co-purification of Yen1 variants monitored by immunoblotting. G6PD is a loading control. G) 3HA-Cdc14 was ectopically overexpressed from PGAL1 for either 1.5 or 3 hours in S phase cells expressing Yen1-TAP or Yen19A-TAP from PYEN1. H) W303, cdc14-1, and cdc15-2 strains, each expressing Yen1-TAP from PYEN1 were arrested at 23 °C in G1, then released into fresh medium at 37 °C. Cells were harvested at the indicated times for analysis by Phos-tag immunoblotting and nuclear staining. The graph shows similar cell cycle kinetics based on percentage of anaphase cells (large-budded with two DNA masses).
Yen1 is a novel early anaphase Cdc14 substrate
To determine if Yen1 is a Cdc14 substrate we first synthesized phosphopeptides containing each minimal Cdk consensus site and surrounding sequence and measured the rate of Cdc14-catalyzed dephosphorylation under steady-state conditions. kcat/Km for pSer-Pro sites can vary by as much as 3 orders of magnitude (Bremmer et al., 2012). For comparison, we analyzed phosphopeptides representing known in vivo Cdc14 substrate sites that exhibit high activity and phosphopeptides from a Cdk site cluster on Cdc16 that are poor substrates. The Yen1 phosphopeptides exhibited a range of activities reflecting our current understanding of Cdc14 specificity (Fig. 2D). The four best peptides displayed dephosphorylation rates comparable to our “gold standards”, and all contain the optimal SPxK sequence. Three of these also have at least one additional basic amino acid at +4. The remaining peptides contained either a +3 Arg or no basic amino acid at +3 and two were very weak substrates. We also evaluated the ability of Cdc14 to dephosphorylate intact affinity-purified Yen1 at several phosphorylation sites that gave high quality MS signals (Fig. 2E). Consistent with our peptide work, dephosphorylation of the cluster 1 S71 and S245 sites was only slightly better than a non-Cdk site. In contrast, activity towards the optimal sequence at S500 was high.
To determine if Cdc14 can recognize Yen1 as a substrate in vivo we performed co-immunoprecipitation (co-IP) experiments with a Cdc14 substrate trap variant, Cdc14-C283S, which is catalytically inactive but retains high affinity substrate binding (Hall et al., 2008). Wild-type Yen1 co-purified with 3HA-Cdc14-C283S (Fig. 2F). In contrast, Yen19A and another mutant, Yen19T, containing Thr substitutions at all nine Ser-Pro sites were not detected in 3HA-Cdc14-C283S immunoprecipitates despite being expressed at similar levels. Thus, Cdc14 physically associates with Yen1 in vivo, dependent on Yen1 Cdk phosphorylation sites.
We next ectopically overexpressed Cdc14 in S phase cells and monitored the phosphorylation status of Yen1-TAP by immunoblotting of whole cell extracts. After Cdc14 expression, Yen1-TAP mobility increased substantially, demonstrating that Cdc14 promotes the dephosphorylation of Yen1 (Fig. 2G). We monitored the stability of the mitotic cyclin Clb2 to ensure that under these experimental conditions the overexpressed Cdc14 had not induced mitotic exit conditions that terminate Cdk activity via cyclin proteolysis.
To test if Yen1 dephosphorylation in mitosis is dependent on Cdc14 function we synchronized W303, cdc14-1, or cdc15-2 strains expressing YEN1-TAP in G1 with α-factor at 23 °C and released them from arrest at 37 °C. The phosphorylation status of Yen1-TAP was monitored by immunoblotting (Fig. 2H). In W303, Yen1-TAP became phosphorylated roughly 45 minutes after release and was dephosphorylated as cells entered anaphase and segregated their chromosomes, consistent with previous results (Matos et al., 2011). cdc14-1 and cdc15-2 strains both arrest in late anaphase at 37°C with segregated DNA, the only difference being that cdc15-2 cells transiently release active Cdc14 from nucleolar sequestration in early anaphase via the FEAR network (Stegmeier et al., 2002) whereas cdc14-1 cells completely lack Cdc14 function. Yen1-TAP was phosphorylated with similar kinetics in both. In contrast, dephosphorylation was absent in cdc14-1 but occurred similar to W303 in cdc15-2. Thus, Cdc14 function is essential for Yen1 dephosphorylation during mitosis. The lack of dependence on Cdc15 argues strongly that Yen1 is a target of FEAR-activated Cdc14 at anaphase onset.
Cdk sites regulate Yen1 biological function
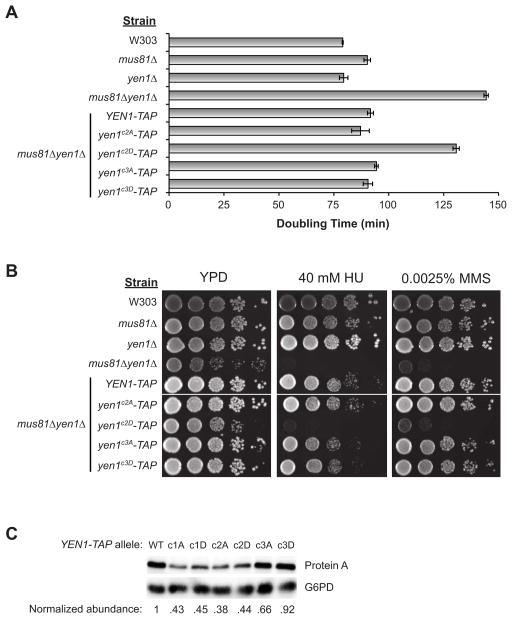
To determine if regulation of Yen1 at Cdk phosphorylation sites is functionally important we performed complementation experiments with Yen1 phosphosite mutants. We split the Yen1 Cdk sites into 3 clusters as depicted in Figures 1F and 2D. For example, Yen1c2A refers to mutation of the four Ser-Pro sites in cluster 2 to Ala-Pro to prevent Cdk phosphorylation, whereas Yen1c2D contains Asp-Pro substitutions at the same sites to mimic constitutive Cdk phosphorylation. Clusters c2 and c3 were of the most interest because they contained all of the optimal Cdc14 substrate sites and contained the sites that exhibited highest activity in our peptide assays. While we also evaluated c1 mutations, no strong effects on Yen1 regulation were observed and therefore we focus on the results from c2 and c3. All YEN1 variants were integrated as TAP fusions into mus81Δ yen1Δ strains under control of the YEN1 promoter. The Mus81-Mms4 endonuclease performs an overlapping function with Yen1 in HR-based DNA repair and strong phenotypes of yen1Δ require a mus81Δ background.
mus81Δ yen1Δ strains were reported to exhibit a significant slow growth phenotype (Agmon et al., 2011; Blanco et al., 2010; Ho et al., 2010). Consistent with this, yen1Δ growth was indistinguishable from the parental W303 strain, whereas mus81Δ yen1Δ took nearly twice as long to double (Fig. 3A). Wild-type Yen1, Yen1c2A, Yen1c3A, and Yen1c3D all complemented the yen1Δ growth defect in the mus81Δ background. In contrast, Yen1c2D almost completely failed to complement.
Figure 3. Phosphomimetic Cdk site mutants block Yen1 function.
A) OD600 of each strain was measured during log phase growth to calculate doubling times. B) Serial dilutions of each strain were spotted and grown on YPD agar containing the indicated chemicals. Similar results were obtained with 25 mM HU and 0.005 % MMS (not shown). Results for c1 alleles were removed (white line) and are shown in Fig. S3. C) Anti-protein A immunoblot analysis of extracts from mus81Δ yen1Δ strains expressing the Yen1-TAP variants used in A and B. G6PD-corrected abundance values relative to wild-type (WT) Yen1-TAP are shown.
Yen1 functions in the repair of double-strand DNA breaks (DSBs) by homologous recombination (HR), and strong sensitivity to chemicals that promote DSB formation is observed in mus81Δ yen1Δ strains (Agmon et al., 2011; Blanco et al., 2010; Ho et al., 2010). We therefore examined the ability of the YEN1 alleles to complement the acute sensitivity of mus81Δ yen1Δ to methyl methanesulfonate (MMS) and hydroxyurea (HU). Similar to the growth experiments, Yen1, Yen1c2A, Yen1c3A, and Yen1c3D all provided full Yen1 function and eliminated the chemical sensitivity (Fig. 3B & 3C). Yen1c2D failed to complement, demonstrating it is non-functional. In both assays, Yen1c1D failed to complement and Yen1c1A only partially complemented (Fig. S3). Together, the growth and DNA damage sensitivity results are consistent with Cdk phosphorylation within cluster 2 inhibiting the function of Yen1 in repair of DNA damage and Cdc14-mediated dephosphorylation of these clusters being required to activate Yen1 function. Cluster 1 sites also affect Yen1 function. However, the dependence on phosphorylation status is unclear since Yen1c1A was only partly functional.
Cdc14 is essential for Yen1 nuclear accumulation
The apparently normal function of Yen1c3D was somewhat surprising given the prior evidence that Cdk phosphorylation of S679 prevented Yen1 nuclear localization (Kosugi et al., 2009). To directly test if Cdc14 is required for nuclear accumulation of Yen1, we examined cdc14-1 cells expressing a Yen1-GFP fusion from the constitutive GPD promoter by fluorescence microscopy (we could not detect Yen1-GFP expressed from its natural promoter). As expected, Yen1-GFP was evenly distributed throughout the cytoplasm of pre-mitotic cells in an asynchronous culture grown at permissive temperature, but exhibited strong nuclear signal in large-budded late mitotic cells that had segregated their DNA (Fig. 4A). In some cases, we observed Yen1-GFP accumulation in the nucleus of cells that appeared to be undergoing anaphase and had not fully separated their two chromosome sets (Fig. 4B). At 37 °C, cdc14-1 cells arrest in late mitosis with segregated DNA, however Yen1-GFP failed to accumulate in the nucleus under these conditions (Fig. 4A). The requirement for Cdc14 function to accumulate Yen1 in the nucleus was completely bypassed by expressing Yen1c3A-GFP. Neither Yen1c1A (Fig. S4A) nor Yen1c2A (Fig. 4C) bypassed this Cdc14 requirement, and cell cycle-dependent nuclear localization was not altered by the c1D or c2D mutations (Fig. S4B). Thus, the cluster 3 Cdk sites near the putative NLS uniquely control the nucleocytoplasmic distribution of Yen1. Oddly, Yen1c3D-GFP behaved similar to Yen1c3A-GFP (Fig. S4B). Instead of being constitutively cytoplasmic, as would be expected if the Ser to Asp mutations mimicked the effect of phosphorylation, Yen1c3D-GFP signal was detected in the nucleus at all cell cycle stages. The Asp mutations clearly do not effectively mimic the inhibitory effect of Cdk phosphorylation on nuclear localization, likely explaining why Yen1c3D appeared fully functional in the complementation assays. Nonetheless, our results clearly show that Cdc14-mediated dephosphorylation of one or both cluster 3 Cdk sites is essential for nuclear accumulation of Yen1 at anaphase onset. The West lab found that mutation of S679 to Ala alone causes less nuclear accumulation than mutation of all Cdk consensus sequences (Stephen West, personal communication), consistent with S655 influencing localization as well. Both S655 and S679 lie immediately upstream of consensus classical NLS sequences (KRIR and KKSR, respectively), which could conceivably function as a bipartite NLS.
Figure 4. Cdc14 is required for nuclear accumulation of Yen1 in anaphase.
A) cdc14-1 containing a CEN plasmid expressing Yen1-GFP or Yen1c3A-GFP from PGPD was grown in selective medium at 23 °C. Where indicated, growth temperature was shifted to 37 °C for 4 hours. Cells were briefly fixed and stained with DAPI prior to microscopic imaging. At 37 °C less than 5% of cells expressing Yen1-GFP exhibited detectable nuclear fluorescence above the cytoplasmic signal. Greater than 90% of cells expressing Yen1c3A-GFP exhibited strong accumulation of nuclear fluorescence (n>100). B) Select images from cultures used in A showing cells in early anaphase with nuclear accumulation of Yen1-GFP signal. C) Similar to A, cdc14-1 cells expressing Yen1c2A-GFP were arrested at 37 °C and imaged. Less than 10% of cells exhibited detectable nuclear signal (N>65). See also Fig. S4.
Cdc14 and Cdk1 directly regulate Yen1 enzymatic activity
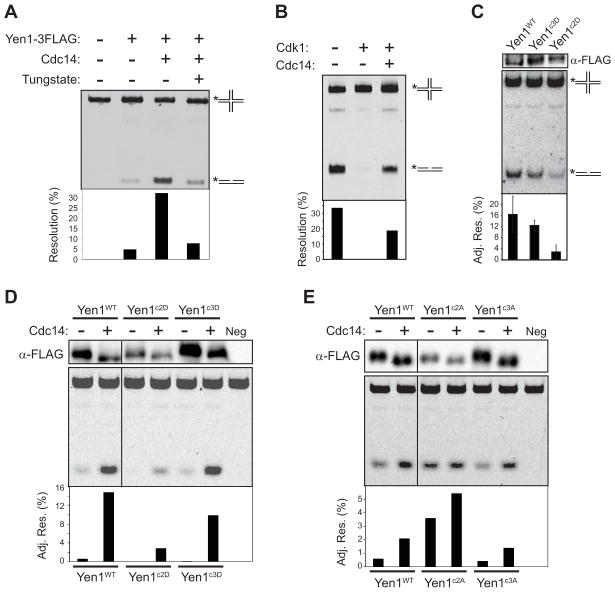
Since Yen1 resolvase activity is influenced by phosphorylation (Matos et al., 2011), we used an in vitro HJ resolution assay to explore the effects of purified Cdc14 and Cdk1. We isolated Yen1-3FLAG from cdc14-1 cells arrested at 37 °C to obtain phosphorylated, inactive protein. We first confirmed that treatment of isolated Yen1 with recombinant Cdc14 caused dephosphorylation and that subsequent treatment with purified Cdk1 (Clb2-Cdc28) restored phosphorylation, based on gel mobility (Fig. S5C). Pre-treatment of Yen1-3FLAG with Cdc14 strongly enhanced cleavage of the fluorescent HJ substrate into nicked duplex products (Fig. 5A). This effect was suppressed by sodium tungstate, a potent inhibitor of Cdc14. We next isolated Yen1-3FLAG from cycling cells without using phosphatase inhibitors and found that it was highly active (Fig. 5B). Pre-treatment with Cdk1 completely inhibited HJ resolution and additional treatment of the Cdk-inhibited Yen1 with Cdc14 restored activity. These results demonstrate that Cdc14 and Cdk directly activate and inhibit the Yen1 resolvase activity, respectively.
Figure 5. Cdc14 and Cdk directly control Yen1 endonuclease activity.
In all panels, Yen1-3FLAG resin was added to reactions containing the fluorescent synthetic HJ substrate (see Fig. S5). Yield differences in independent Yen1 preparations likely account for apparent fluctuations in activity between panels, however all activities of Yen1 variants within panels are appropriately normalized to recovered protein levels. Products were resolved by native PAGE and quantified by fluorescence imaging. A) Yen1-3FLAG was isolated from cdc14-1 cells arrested at 37 °C. Equal fractions of Yen1-bound resin were either untreated or treated with recombinant Cdc14 (+ or - sodium tungstate) prior to HJ resolution assays. B) Yen1-3FLAG isolated under permissive conditions without phosphatase inhibitors was treated with purified Cdk1. Half of the Cdk1-treated resin was washed and additionally treated with recombinant Cdc14. C) The indicated proteins were prepared as in B. anti-FLAG immunoblots of reaction mixtures were used to normalize product levels and account for differences in protein recovery (shown as adjusted resolution % in bottom graph). The graph shows averages of 3 trials with standard deviations. D) Yen1-3FLAG and Asp variants were isolated as in A. Prior to reactions, Yen1-bound resins were divided, and one half treated with recombinant Cdc14. Signals normalized as in C. Results for Yen1c1D were cropped (black line) E) Same as D, comparing Yen1-3FLAG and Ala variants.
We next tested if the cluster 2 or 3 mutations affected Yen1 enzymatic activity and response to Cdc14. First, we compared Yen1, Yen1c2D, and Yen1c3D purified from asynchronous cultures without phosphatase inhibitors (Fig. 5C). Yen1 and Yen1c3D displayed statistically indistinguishable activity, but Yen1c2D activity was significantly lower, suggesting that phosphorylation of the cluster 2 Cdk sites might inhibit Yen1. Consistent with this, Yen1c2D was much less responsive to Cdc14-induced activation compared to Yen1 and Yen1c3D when isolated from the inactivating conditions of cdc14-1 cells (Fig. 5D). Finally, we examined the Ala mutants isolated from cdc14-1 cells at restrictive temperature. Whereas Yen1c3A behaved similar to wild-type Yen1, exhibiting low basal activity and strong stimulation upon Cdc14 treatment, Yen1c2A was highly active in the absence of Cdc14 treatment and only slightly more active after Cdc14 treatment (Fig. 5E). These results suggest that Cdk and Cdc14 primarily control Yen1 enzymatic activity by regulating the phosphorylation status of one or more sites within cluster 2, and that the cluster 3 sites that regulate nuclear localization do not influence activity. Cluster 1 mutations had a very minor effect on activity compared to cluster 2 (data not shown).
Activation of Yen1 by Cdc14 influences chromosome segregation during mitosis
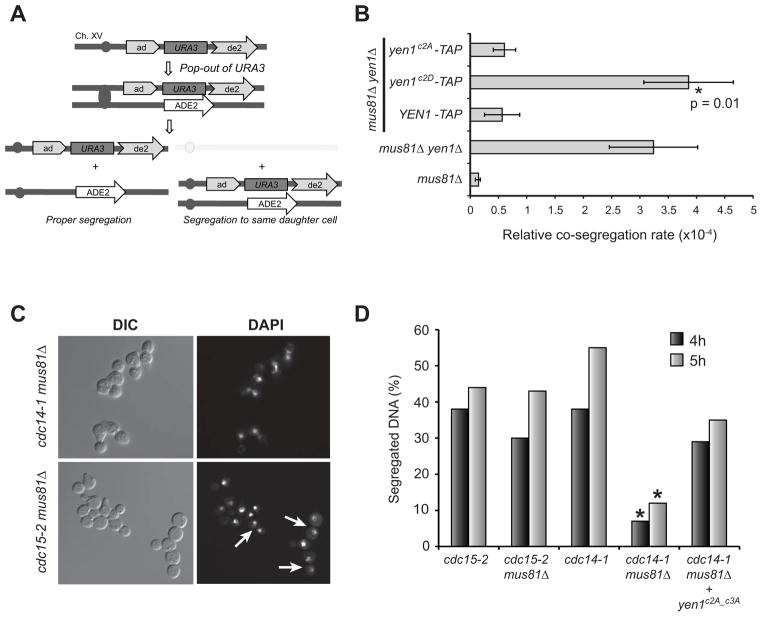
Matos et al. proposed that activation of Yen1 during mitosis might be important to resolve persistent recombination intermediates that had escaped non-crossover (NCO) HR repair pathways and thereby ensure all sister chromatids can be segregated during anaphase (Matos et al., 2011). In support of this idea, mus81Δ yen1Δ cells exhibit a significantly elevated rate of sister chromatid non-disjunction that is largely dependent on HR (Ho et al., 2010). We tested if expression of the non-functional Yen1c2D mutant that mimics constitutive cluster 2 Cdk phosphorylation would also cause elevated sister chromatid non-disjunction using this same assay (Fig. 6A). The rate of sister chromatid non-disjunction in yen1c2D-TAP cells was indistinguishable from yen1Δ and significantly higher than in YEN1-TAP and yen1c2A-TAP cells (Fig. 6B). This result reveals that activation of Yen1 by Cdc14 contributes to genome stability by ensuring complete segregation of sister chromatids during mitosis.
Figure 6. Activation of Yen1 by Cdc14 helps prevent chromosome segregation defects.
A) Schematic of sister chromatid co-segregation assay (Ho et al., 2010). Spontaneous recombination between the direct repeats of ade2 alleles in the ade2–5′::URA3::ade2–3′ construct on one sister generates a chromatid pair with URA3 and ADE2 markers that are monitored for co-segregration on medium lacking uracil and adenine. B) Co-segregation of sister chromatids in strain LSY2307-2C harboring integrated YEN1-TAP, yen1c2A-TAP, and yen1c2D driven by PYEN1 was monitored using the assay from A. The method of the median was used to calculate co-segregation rates and data are the average of 3 independent experiments. Error bars are standard deviations. Asterisk and p value indicate a statistically significant difference compared to the YEN1-TAP complementing strain determined using Student’s t-test. C) Representative fields of view of the indicated strains collected by either DIC or fluorescence microscopy 4 hours after release from G1 arrest at 37 °C in the presence of 0.001% MMS. White arrows show examples of fully segregated DNA. D) Quantification of images from experiments in C at 4 and 5 hours after release. A minimum of 120 cells were counted for each strain at each time point. Asterisks indicate a significant difference compared to both cdc14-1 and cdc14-1 mus81Δ + yen1c2A_c3A in a chi-square test (p < 0.0001).
mus81Δ yen1Δ cells exhibit a defect in bulk DNA segregation and an associated G2/M cell cycle delay when grown in low concentrations of MMS (Blanco et al., 2010). If Cdc14 is critical for Yen1 activation, then a similar effect should be observed in mus81Δ cdc14-1 cells at the restrictive temperature. To test this, we released G1-arrested cells at 37 °C in the presence of 0.001% MMS and monitored DNA segregation cytologically by DAPI staining (Fig. 6C & 6D). We used the cdc15-2 background for comparison due to its similar late mitotic arrest at 37 °C. All strains released and reached G2/M as large-budded cells with similar kinetics, although slower than at lower temperatures (data not shown). At 4 and 5 hours post-release, the cdc14-1, cdc15-2, and cdc15-2 mus81Δ strains exhibited statistically indistinguishable fractions of cells with clearly segregated DNA masses. In contrast, a much smaller fraction of cdc14-1 mus81Δ cells had fully segregated DNA masses (Fig. 6D). This difference was highly significant, and was eliminated by expression of a Yen1c2A_c3A mutant containing Ala substitutions at all 6 C-terminal Ser-Pro sequences that should be constitutively active and nuclear based on the experiments described above. We interpret this phenomenon as failure to properly resolve MMS-induced damage repair intermediates leading to a persistent DNA damage checkpoint, a physical barrier to segregation, or a combination of both. This provides additional evidence that Cdc14 function is required for Yen1 activation and proper chromosome segregation.
Constitutively active Yen1 increases the crossover (CO) frequency during mitotic DSB repair
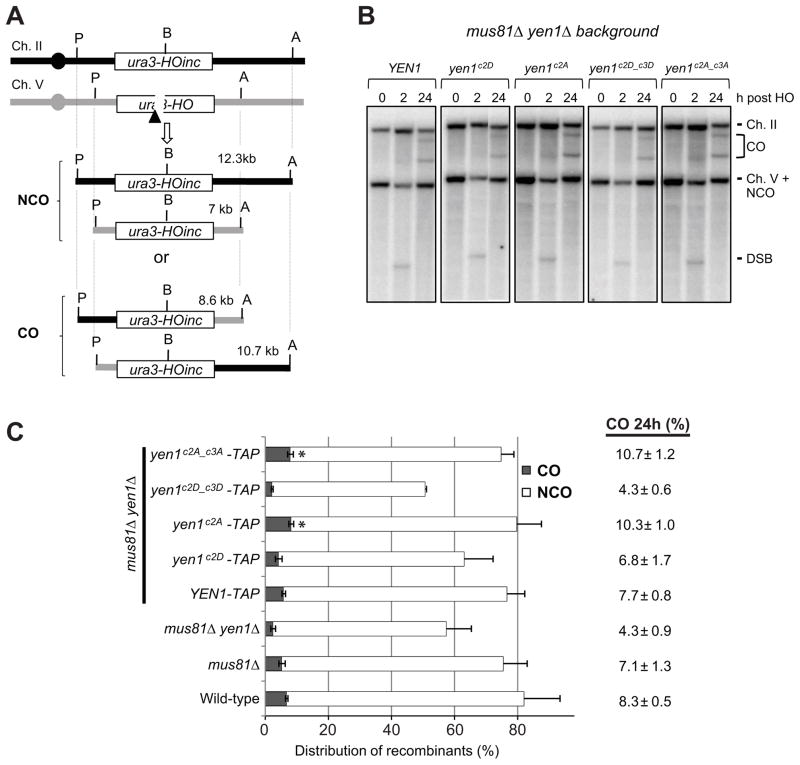
We predicted that the combined Yen1c2A_c3A mutant might result in constitutive Yen1 function that could influence the CO outcome of DSB repair by HR. To address the roles of the Yen1 Cdk site mutants in DSB-induced recombination we used a well-characterized system to monitor CO formation between dispersed chromosomal repeats (Agmon et al., 2011; Mazon et al., 2012). A haploid strain expressing a galactose-inducible HO endonuclease was used to monitor repair by recombination between a DSB generated at the HO endonuclease cut site (HOcs) inserted within the URA3 locus on chromosome (Ch) V and a 5.6kb URA3 donor sequence inserted at the LYS2 locus on Ch II (Fig. 7A). After galactose induction, a DSB is continuously generated at the Ch V locus until repair by gene conversion transfers the HOcs-inc sequence from the donor to the recipient locus. CO products can be monitored and quantified physically by using asymmetrically located restriction sites flanking both the URA3 regions on Ch V and Ch II.
Figure 7. Mis-regulation of Cdk sites on Yen1 influences CO formation.
A) Schematic of the ectopic recombination assay used to monitor CO formation between ura3 repeats (Mazon et al., 2012). P and A mark the PvuII and ApaLI restriction sites used to monitor CO formation by Southern blot and B indicates the HO cut site. B) CO products are detected as ApaLI (A)/PvuII (P) restriction fragments of 10.7 and 8.6 kb in Southern blot analysis of DNA extracted from cells after HO-endonuclease induction. The chromosome V band represents entirely NCO products by 24 h. Only the larger of the two DSB fragments is present under the gel conditions used. C) Quantitation of CO and NCO products from gels in B normalized to the plating efficiency of the indicated strains. Values for the raw estimate of % COs at 24 h are shown and are averages of at least 3 independent trials. Error bars and values are standard deviations. Asterisks indicate p-values < 0.05 from a Students t-test comparing CO to that from the YEN1-TAP strain.
As described previously, the Yen1 effect on CO formation can only be seen in a mus81Δ background (Mazon et al., 2012). We therefore evaluated CO levels and plating efficiency (competence to repair the DSB) in mus81Δ yen1Δ complemented by YEN1-TAP or mutant derivatives (Fig. 7B & 7C). Expression of yen1c2D only partially complemented the decreased CO level while yen1c2D_c3D completely failed to complement the mus81Δ yen1Δ CO defect (6.8% and 4.3% CO, respectively). These results are mostly consistent with the lack of function of the Asp mutants in the DNA damage sensitivity assays. In contrast, expression of both yen1c2A and yen1c2A_c3A not only fully complemented the CO defect but resulted in a modest, but statistically significant, elevation in CO levels compared to the strain complemented with YEN1 (10.3 and 10.7% CO, respectively, compared to 7.7% CO; p=0.044 and p=0.028, respectively).
DISCUSSION
A novel function for Cdc14 in DNA repair
A daunting challenge in the functional characterization of kinases and phosphatases is the identification of bona fide physiological substrates. For Ser/Thr phosphatases, this problem is exacerbated by the structural complexity of phosphatase families and lack of strong active site specificity. Cdc14 enzymes, members of the protein tyrosine phosphatase family despite their pSer specificity, do not share this problem. Cdc14 phosphatases function independent of accessory or regulatory subunits. Importantly, as shown here and previously (Bremmer et al., 2012; Gray et al., 2003), the Cdc14 active site region imposes strict substrate selection requirements on the enzyme. This includes Ser as the phosphoamino acid, a +1 Pro, and a +3 Lys (or to a lesser extent Arg). Absence of any one of these features renders a substrate nearly unreactive in vitro using phosphopeptides. We used this strict enzymatic specificity to predict optimal Cdc14 substrates in silico and identified a novel role for Cdc14 in activating the DNA repair enzyme Yen1 that contributes to genome stability. Such an approach may be a useful companion tool to substrate trap methodologies to facilitate direct substrate identification, or even as a stand-alone approach to identification of novel substrates in metazoan systems where very little is known about Cdc14 functions.
Cdc14 has been implicated in both DNA damage checkpoint signaling and repair in several model systems (Bassermann et al., 2008; Diaz-Cuervo and Bueno, 2008; Mocciaro et al., 2010; Wei et al., 2011). Consistent with this, several Cdc14 orthologs are activated by nucleolar release upon genotoxic stress (Bassermann et al., 2008; Broadus and Gould, 2012; Diaz-Cuervo and Bueno, 2008; Mocciaro et al., 2010). However, little evidence exists directly linking Cdc14 to specific DNA repair functions. Aside from a report that Cdc14 activates the anaphase-promoting complex to facilitate DNA damage checkpoint arrest in human cells (Bassermann et al., 2008), the direct targets of Cdc14 in the DDR are not known. To our knowledge, Yen1 is the first reported DNA repair substrate of Cdc14 and the first connection between Cdc14 function and DNA repair in budding yeast. The evidence for Cdc14 involvement in the repair of DNA damage in budding yeast, fission yeast, and metazoans suggests that it may be a conserved function for this phosphatase family.
Cdc14 enzymatic specificity and the DDR
Cdk phosphorylation is required for an effective DDR but also must be suppressed to prevent cell cycle progression until damage is repaired (Trovesi et al., 2013). While Cdk is a positive regulator of CO pathways of HR in budding yeast (Trovesi et al., 2011), our work suggests it suppresses the CO-promoting activity of Yen1. How the pro- and anti-HR effects of Cdk (and likely other kinases) are coordinated to yield an appropriate DDR is unclear. Phosphatase specificity may be one contributing factor. The strict specificity of Cdc14 for a distinct class of Cdk substrates could act to selectively reverse phosphorylation on optimal substrates at times when overall Cdk activity is high. This would be an effective strategy to selectively activate repair proteins that are otherwise kept dormant in the absence of DNA damage. It also may underlie Cdc14 substrate selection in late mitosis as cells execute the ordered dephosphorylation of Cdk substrates to coordinate chromosome segregation, mitotic exit, and cytokinesis (Bouchoux and Uhlmann, 2011).
Yen1 is a new regulatory target of the FEAR network
Yen1 appears to be a target of the transient nuclear pool of Cdc14 activated by the FEAR network in anaphase, as it was independent of the other major Cdc14 activation pathway, the mitotic exit network (Fig. 2H). The timing of Yen1 activation is consistent with it being a FEAR target because FEAR signaling originates at the metaphase-anaphase transition when separase is activated via proteolysis of its inhibitor, securin, to promote nucleolar release of Cdc14 (Azzam et al., 2004; Stegmeier et al., 2002; Sullivan and Uhlmann, 2003). FEAR-released Cdc14 selectively dephosphorylates a handful of Cdk substrates to ensure proper spindle function and chromosome segregation during anaphase (Stegmeier and Amon, 2004). Since cells expressing the Yen1c2D phosphomimetic mutant exhibited a sister chromatid segregation defect similar to yen1Δ strains (Fig. 6B), dephosphorylation of Yen1 by Cdc14 represents another mechanism by which the FEAR pathway promotes faithful chromosome segregation and safeguards genome integrity.
Strict regulation of Yen1 function contributes to genome stability
Yen1 was identified as a HJ resolvase several years ago, along with its human ortholog GEN1, based on detection of its enzymatic activity (Ip et al., 2008). Since then, several studies have shown that the HJ cleavage activity of Yen1 functions in HR-based repair of DSBs during mitosis (Agmon et al., 2011; Blanco et al., 2010; Ho et al., 2010; Tay and Wu, 2010). Cells lacking YEN1 seem to be mostly proficient for DNA repair, and strong phenotypes are typically only observed in combination with deletion of other structure-selective endonucleases. However, yen1Δ cells do exhibit a modest defect in the repair of replication-derived DSBs by sister chromatid recombination (Munoz-Galvan et al., 2012). Collectively, these studies suggest that Yen1 function in resolving HR intermediates overlaps heavily with other structure-selective nucleases and implies a highly robust network of repair pathways that ensure genome integrity during cell division.
Yen1 was proposed to serve as a last line of defense against sister chromatid non-disjunction caused by unresolved recombination intermediates that arise during the repair of DSBs (Matos et al., 2011). The phospho-inhibition of Yen1 from S phase through early mitosis would encourage non-CO pathways of DSB repair, which are preferred during mitotic divisions to limit loss of heterozygosity. In support of the model of Matos et al. our results show that Cdc14-mediated dephosphorylation of Yen1 promotes faithful chromosome segregation, likely by removing residual branched structures that would otherwise prevent sister chromatid disjunction. Furthermore, lack of Cdk phosphorylation on Yen1 results in constitutive activity and elevates the fraction of HR repair events resulting in CO formation (Fig. 7), potentially leading to an increase in loss of heterozygosity. This phenotype is modest, (although statistically significant), likely because mechanisms exist to limit COs associated with homology-directed repair of DSBs during mitotic cell divisions.
Multiple mechanisms of Yen1 inhibition by Cdk
The opposing activities of Cdk and Cdc14 enforce specific activation of Yen1 at anaphase by two independent mechanisms. Cdk phosphorylation of the cluster 2 Cdk sites directly inhibits Yen1 enzymatic activity, whereas phosphorylation of the cluster 3 sites near the C-terminal NLS restricts Yen1 to the cytoplasm. FEAR network-released Cdc14 dephosphorylates Yen1 to allow its accumulation in the nucleus in a fully active state. The existence of two independent regulatory mechanisms may be necessary to completely silence Yen1 function until anaphase onset. Phosphorylated Yen1 may retain minimal catalytic activity or interfere with other repair factors or pathways if not sequestered in the cytoplasm. On the other hand, Yen1 may actively shuttle between the cytoplasm and nucleus with phosphorylation status shifting the equilibrium between export and import. The small amount of Yen1 present at any given time in the nucleus prior to anaphase could potentially function in repair without inhibition of its enzymatic activity. Our observation that Yen1c2A leads to increased COs (Fig. 7), despite a normal localization pattern, is consistent with this idea. The mechanism by which the cluster 2 Cdk sites contribute to Yen1 inhibition remains an open question. These sites are distant from the catalytic domain in the primary sequence, but may interact with it in the folded, native protein. The West Lab found evidence that phosphorylation at Yen1 Cdk sites inhibits binding to its DNA substrates (Stephen West, personal communication) and the region around cluster 2 may be important for recognition of HJs and other DNA structures that Yen1 acts on. Another possibility is that cluster 2 sites regulate interaction with another protein that influences Yen1 activity. Additional experiments will be required to define the detailed molecular mechanism of Yen1 inhibition by Cdk.
EXPERIMENTAL PROCEDURES
Reagents
Yeast strains are listed in Table S5 and plasmids in Table S6. Phosphopeptides were synthesized by New England Peptide. Phos-tag™ reagent was from Wako Pure Chemical Industries.
Enzyme and interaction assays
Phosphatase assays were conducted as described (Bremmer et al., 2012). The synthetic X0 Holliday junction (Fig. S5) and resolution assay conditions were described previously (Ip et al., 2008). Co-IP experiments were performed as described (Bremmer et al., 2012).
Fluorescence microscopy
Fluorescence and DIC images were acquired using a 100x oil objective on an Olympus BX51 microscope equipped with a Hamamatsu Orca-R2 digital camera.
Functional assays
Cell doubling times were calculated as described (Bernstein et al., 2009). DNA damage sensitivity was measured by spotting serial culture dilutions on YPD plates with 40 mM HU or 0.0025% MMS and incubating at 30° for 48 or 72 hours. Rates of sister chromatid co-segregation were measured as described (Ho et al., 2010). Recombination assays were performed as described (Mazon et al., 2012).
Molecular modeling
Homology modeling, docking, and molecular dynamics simulations were conducted using Prime 3.3 and Glide 6.0 (Schrödinger, LLC), and Desmond Molecular Dynamics System 3.5 (D. E. Shaw Research).
Detailed methods are provided in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Cdc14 specificity can be used to identify novel Cdc14 substrates.
The Holliday junction resolvase Yen1 is a novel early anaphase Cdc14 substrate.
Cdk phosphorylation restrains Yen1 activity to limit mitotic crossovers.
Cdc14 triggers Yen1 activation to ensure proper chromosome segregation.
Acknowledgments
We acknowledge support from the Purdue Center for Cancer Research small grants and shared resource funding programs, and the NIH (R01 GM041784 and R01 GM094386 to L.S.S.). C.L.E. was supported by fellowships from Purdue Research Foundation and Purdue Graduate School. We thank Harry Charbonneau for helpful comments on the project and manuscript, Scott Briggs for plate reader access, and Laurie Parker and Frank Ankudey for help with peptide synthesis. We thank Hana Hall, Wai Kit Ma, Chris Petell, Ryan Chaparian, and Nikki Lautensack for technical help.
References
- Agmon N, Yovel M, Harari Y, Liefshitz B, Kupiec M. The role of Holliday junction resolvases in the repair of spontaneous and induced DNA damage. Nucleic Acids Res. 2011;39:7009–7019. doi: 10.1093/nar/gkr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam R, Chen SL, Shou W, Mah AS, Alexandru G, Nasmyth K, Annan RS, Carr SA, Deshaies RJ. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305:516–519. doi: 10.1126/science.1099402. [DOI] [PubMed] [Google Scholar]
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KA, Shor E, Sunjevaric I, Fumasoni M, Burgess RC, Foiani M, Branzei D, Rothstein R. Sgs1 function in the repair of DNA replication intermediates is separable from its role in homologous recombinational repair. EMBO J. 2009;28:915–925. doi: 10.1038/emboj.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Blanco MG, Matos J, Rass U, Ip SC, West SC. Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae. DNA Repair (Amst) 2010;9:394–402. doi: 10.1016/j.dnarep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Bouchoux C, Uhlmann F. A Quantitative Model for Ordered Cdk Substrate Dephosphorylation during Mitotic Exit. Cell. 2011;147:803–814. doi: 10.1016/j.cell.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Bremmer SC, Hall H, Martinez JS, Eissler CL, Hinrichsen TH, Rossie S, Parker LL, Hall MC, Charbonneau H. Cdc14 Phosphatases Preferentially Dephosphorylate a Subset of Cyclin-dependent kinase (Cdk) Sites Containing Phosphoserine. J Biol Chem. 2012;287:1662–1669. doi: 10.1074/jbc.M111.281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus MR, Gould KL. Multiple protein kinases influence the redistribution of fission yeast Clp1/Cdc14 phosphatase upon genotoxic stress. Mol Biol Cell. 2012;23:4118–4128. doi: 10.1091/mbc.E12-06-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cuervo H, Bueno A. Cds1 controls the release of Cdc14-like phosphatase Flp1 from the nucleolus to drive full activation of the checkpoint response to replication stress in fission yeast. Mol Biol Cell. 2008;19:2488–2499. doi: 10.1091/mbc.E07-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Hombauer H, Huang ME, Kolodner RD. Cdc28/Cdk1 positively and negatively affects genome stability in S. cerevisiae. J Cell Biol. 2009;185:423–437. doi: 10.1083/jcb.200811083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Dougherty DA. A Computational Study of Cation–π Interactions vs Salt Bridges in Aqueous Media: Implications for Protein Engineering. Journal of the American Chemical Society. 2000;122:870–874. [Google Scholar]
- Gray CH, Good VM, Tonks NK, Barford D. The structure of the cell cycle protein Cdc14 reveals a proline-directed protein phosphatase. EMBO J. 2003;22:3524–3535. doi: 10.1093/emboj/cdg348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MC, Jeong DE, Henderson JT, Choi E, Bremmer SC, Iliuk AB, Charbonneau H. Cdc28 and Cdc14 Control Stability of the Anaphase-promoting Complex Inhibitor Acm1. J Biol Chem. 2008;283:10396–10407. doi: 10.1074/jbc.M710011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CK, Mazon G, Lam AF, Symington LS. Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Mol Cell. 2010;40:988–1000. doi: 10.1016/j.molcel.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, Krutchinsky AN, Morgan DO. Positive feedback sharpens the anaphase switch. Nature. 2008;454:353–357. doi: 10.1038/nature07050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, West SC. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–361. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- Kaiser BK, Zimmerman ZA, Charbonneau H, Jackson PK. Disruption of Centrosome Structure, Chromosome Segregation, and Cytokinesis by Misexpression of Human Cdc14A Phosphatase. Mol Biol Cell. 2002;13:2289–2300. doi: 10.1091/mbc.01-11-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci U S A. 2009;106:10171–10176. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- Matos J, Blanco MG, Maslen S, Skehel JM, West SC. Regulatory control of the resolution of DNA recombination intermediates during meiosis and mitosis. Cell. 2011;147:158–172. doi: 10.1016/j.cell.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon G, Lam AF, Ho CK, Kupiec M, Symington LS. The Rad1-Rad10 nuclease promotes chromosome translocations between dispersed repeats. Nat Struct Mol Biol. 2012;19:964–971. doi: 10.1038/nsmb.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocciaro A, Berdougo E, Zeng K, Black E, Vagnarelli P, Earnshaw W, Gillespie D, Jallepalli P, Schiebel E. Vertebrate cells genetically deficient for Cdc14A or Cdc14B retain DNA damage checkpoint proficiency but are impaired in DNA repair. J Cell Biol. 2010;189:631–639. doi: 10.1083/jcb.200910057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocciaro A, Schiebel E. Cdc14: a highly conserved family of phosphatases with non-conserved functions? J Cell Sci. 2010;123:2867–2876. doi: 10.1242/jcs.074815. [DOI] [PubMed] [Google Scholar]
- Moses AM, Heriche JK, Durbin R. Clustering of phosphorylation site recognition motifs can be exploited to predict the targets of cyclin-dependent kinase. Genome Biol. 2007;8:R23. doi: 10.1186/gb-2007-8-2-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Galvan S, Tous C, Blanco MG, Schwartz EK, Ehmsen KT, West SC, Heyer WD, Aguilera A. Distinct roles of Mus81, Yen1, Slx1-Slx4, and Rad1 nucleases in the repair of replication-born double-strand breaks by sister chromatid exchange. Mol Cell Biol. 2012;32:1592–1603. doi: 10.1128/MCB.00111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi CL, Singh J, Thornton JM. Atomic environments of arginine side chains in proteins. Protein Engineering. 1993;6:247–259. doi: 10.1093/protein/6.3.247. [DOI] [PubMed] [Google Scholar]
- Queralt E, Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr Opin Cell Biol. 2008;20:661–668. doi: 10.1016/j.ceb.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Amon A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay YD, Wu L. Overlapping roles for Yen1 and Mus81 in cellular Holliday junction processing. J Biol Chem. 2010;285:11427–11432. doi: 10.1074/jbc.M110.108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovesi C, Falcettoni M, Lucchini G, Clerici M, Longhese MP. Distinct Cdk1 requirements during single-strand annealing, noncrossover, and crossover recombination. PLoS Genet. 2011;7:e1002263. doi: 10.1371/journal.pgen.1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovesi C, Manfrini N, Falcettoni M, Longhese MP. Regulation of the DNA Damage Response by Cyclin-Dependent Kinases. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- Wei Z, Peddibhotla S, Lin H, Fang X, Li M, Rosen J, Zhang P. Early-onset aging and defective DNA damage response in cdc14b-deficient mice. Mol Cell Biol. 2011;31:1470–1477. doi: 10.1128/MCB.01330-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.