Abstract
Mouse models have greatly helped in elucidating the molecular mechanisms involved in hair formation and regeneration. Recent publications have reviewed the genes involved in mouse hair development based on the phenotype of transgenic, knockout and mutant animal models. While much of this information has been instrumental in determining molecular aspects of human hair development and cycling, mice exhibit a specific pattern of hair morphogenesis and hair distribution throughout the body that cannot be directly correlated to human hair. In this mini-review, we discuss specific aspects of human hair follicle development and present an up-to-date summary of human genetic disorders associated with abnormalities in hair follicle morphogenesis, structure or regeneration.
Keywords: Hair follicle, Morphogenesis, Shaft differentiation, Genetic disorders, Alopecia
1. Introduction
1.1. Hair morphogenesis and differentiation
The regulation of hair follicle (HF) development in the embryo and cycling of HF growth during postnatal life are highly conserved processes that involve a similar series of signals between epithelial and mesenchymal cells. These epithelial-mesenchymal interactions are the basis for the formation of the epidermal appendages (hair, nail, and sweat glands). The initial morphological event in HF development is the thickening of the surface ectoderm to form a placode (Fig. 1A). This initial event is in response to a signal from the underlying mesenchyme where critical players involving WNT/βcatenin and epithelial ectodysplasin EDA/EDAR signaling are required for placode formation (see Biggs and Mikkola, in this issue). There is a promotion of the placode stage with a grouping of the dermal cells adjacent to the placode, and this leads to the formation of the hair germ. The hair germ proliferates and invaginates into the dermis to form the hair peg where the condensated mesenchyme becomes the dermal papilla (Fig. 1A).
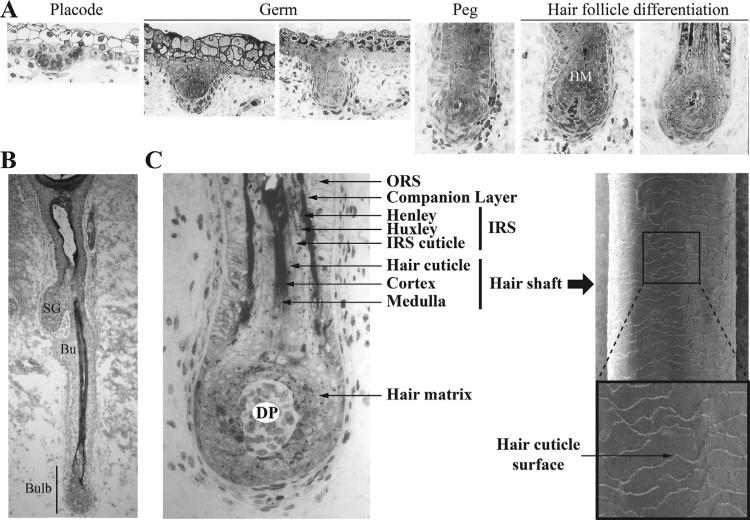
Fig. 1.
Hair morphogenesis, differentiation and structure. (A) Sections of fetal human hair follicles at placode, germ, peg and hair differentiation stages (between 80 and 170 days). (B) Full anagen human hair follicle at 126 day of development. (C) Left panel, Human lower hair follicle and bulb region depicting the different hair compartments. Right panel, Scanning electron microscopy image of terminal human hair shaft. HM, Hair matrix; SG, sebaceous gland; Bu, Bulge; DP, Dermal papilla; ORS, outer root sheath; IRS, inner root sheath. Images of human skin sections were kindly provided by Dr. Karen Holbrook.
A consecutive signal from the dermal papilla to the adjacent epidermal cells induces the differentiation of the inner root sheath (IRS) in which the future hair shaft will develop. When the IRS is formed, the epidermal cells surrounding the dermal papilla, known as the hair matrix cells, start differentiating into distinct lineages to form the different structures of the hair shaft that will grow inside the IRS and eventually protrude through the epidermis. The cellular mechanisms and signaling pathways controlling the process of murine HF development have been recently summarized [1,2].
1.2. Hair structure
The HF, which is usually associated with a sebaceous gland, can be subdivided into four main areas: the infundibulum, spanning from the skin surface to the junction of the sebaceous gland; the isthmus, from the insertion of the arrector pili muscle to the entrance of the sebaceous duct lower portion; the lower follicle, from the bulge to the base of the follicle (hair bulb); and the bulb (Fig. 1B). Within a concavity in the bulb is the dermal papilla; the size and type of hair is related to the volume and shape of the dermal papilla. The bulb also contains the germinative cells of the follicle, the hair matrix cells that give rise to the hair and IRS, and other cells types such as melanocytes.
The bulge is the site of the stem cell population for the follicle (see Rompolas and Greco, in this issue). These bulge stem cells are quiescent and express a unique signature of genes [3]. Results from lineage tracing studies suggest that these bulge stem cells produce all of the cells required for normal hair regeneration but can also repopulate the interfollicular epidermis during wound healing [4]. Additional regions of potential stem cells have been reported [5], with several of these regions being located in the upper isthmus area.
The HF is composed of concentric sheaths each with distinct functions and characteristics. These include supportive sheaths that surround and stabilize the growing hair, and the multilayered hair itself (Fig. 1C) [6]. The outer root sheath (ORS), which is continuous with the basal layer of the epidermis, is the outermost non-keratinizing cell layer of the follicle. Next, from the outside to the inside are the companion layer and the IRS layer. The IRS has several compartments, the most peripheral are the layers of Henle, then the layers of Huxley and finally the IRS cuticle. The IRS ends at the junction between the isthmus and the infundibulum. In the center of the follicle is the hair shaft which consists of the following three layers: the hair cuticle that forms the hair surface; the cortex that forms the bulk of the hair and is composed of keratinized cells which contain pigment from the bulb melanocytes; and the innermost medulla, characterized by the presence of cells that differentiate and are arranged into one or several columns and where follicular keratins are crosslinked with γ-ε(-glutamyl) lysine peptide bonds. Surrounding the HF is the connective tissue sheath which maintains the dermal papilla at the base of the HF.
1.3. Types of hair
While common developmental and structural features are shared in HFs and hair shafts, there are a variety of hair types in a single organism. In the mouse, there are four distinct hair types making up the coat and specialized hairs such as vibrissae and tail hair [7], which play important thermoregulatory and sensory roles. In humans, there are also different types of hair, although by structure and localization, these cannot be directly correlated to any specific hair type in mice. Human hair grows everywhere on the external body except for glabrous skin, which is present on the palms of the hands, soles of the feet and lips. There are specialized types of hair such as the hair that is found on the eyebrows and eyelashes. The density of hair (HFs per area of skin) is determined during development and varies between individuals.
The first hair type is formed in fetal life and is called lanugo hair. This type of hair is characterized as fine, nonmedullated, and lightly pigmented. Vellus hair replaces lanugo hair on a human fetus in late gestation, and is characteristically short, nonmedullated, and not associated to a sebaceous gland. On the scalp, vellus hair is replaced by pigmented terminal hair before or shortly after birth. On the body, vellus hair is present throughout childhood and is replaced by terminal hair in certain areas of the body (arms, legs) during and after puberty. The pituitary gland secretes hormones that trigger the production of androgens in the ovaries and testicles, promoting replacement of vellus hairs with coarse pubic hair in the pubic region and terminal hair in the axillary region. In addition, adult males develop terminal hair on their face and chest.
1.4. Hair follicle regeneration
After the initial follicular morphogenesis, the HF is maintained by cycling through periodic stages which include a growth phase (anagen), a regression phase (catagen), and a quiescent phase (telogen). This cyclic regeneration is a stem-cell dependent process that occurs throughout the life of the individual. In the murine system, the stages have been comprehensively categorized [8]. The molecular signals underlying these processes recapitulate many of the events of follicular morphogenesis, with signals from the dermal papilla proposed to activate a new hair cycle, analogous to placode formation during embryogenesis [2].
During anagen, matrix cells proliferate and differentiate to form the IRS and hair shaft. During catagen the epithelial compartment of the lower follicle, including the matrix cells and the differentiated layers, undergo apoptosis while the dermal papilla remains intact. Consecutively, the follicle enters into the resting telogen phase, after which the dermal papilla cells stimulate stem cell activation and the division of the hair matrix cells for a new cycle of hair growth [1].
In humans, hair also follows a specific growth cycle with three distinct and concurrent phases (anagen, catagen and telogen) that determine the length of the hair. Each phase has several morphologically distinguishable subphases. There is also a shedding exogen phase. The cycle length varies depending on the site on the body. On the scalp, most of the follicles are in the anagen phase which lasts 2–6 years [9], while the catagen and telogen phases last only 2–3 weeks and 3 months, respectively. Hair growth in humans is not synchronized, therefore distinct stages occur simultaneously in different follicles.
2. Genetic basis of human hair disorders
In this section, we summarize the current knowledge related to genetic human hair disorders that have helped to delineate the molecular mechanisms involved in human hair development. Human hair disorders can be pure and non-syndromic or found in combination with other clinical conditions. In particular, hair defects are commonly coincident with defects in other ectodermal appendages (nail, teeth, sweat glands) in a group of rare diseases called ectodermal dysplasias. However, disorders in which hair is the only ectodermal appendage affected, in combination with other unrelated clinical conditions, are also common. Table 1 gives a list of genes that are mutated in human hair disorders causing defects in hair growth and cycling as well as defects in hair structure.
Table 1.
Genes affected in human hair disorders.
| Gene symbol | GeneLoc | Gene description | Disorders | OMIM | Inheritance | Type of hair defects | Refs |
|---|---|---|---|---|---|---|---|
| Signaling and signal transduction | |||||||
| APCDD1 | 18p11.22 | Adenomatosis polyposis coli down-regulated 1 | • Hypotrichosis 1 | 605389 | AD | Alopecia | [37] |
| EDA | Xq13.1 | Ectodysplasin A | • Ectodermal Dysplasia 1, Hypohydrotic, X-linked | 305100 | XL | Alopecia | [29] |
| EDAR | 2q12.3 | Ectodysplasin A receptor | • Ectodermal Dysplasia 10A, Hypohydrotic/Hair/Tooth Type, Autosomal Dominant | 129490 | AD | Alopecia | [30] |
| • Ectodermal Dysplasia 10B, Hypohydrotic/Hair/Tooth Type, Autosomal Recessive | 224900 | AR | Alopecia | [30] | |||
| EDARADD | 1q42-q43 | EDAR-associated death domain | • Ectodermal Dysplasia 11A, Hypohydrotic/Hair/Tooth Type, Autosomal Dominant | 614940 | AD | Alopecia | [32] |
| • Ectodermal Dysplasia 11B, Hypohydrotic/Hair/Tooth Type, Autosomal Recessive | 614941 | AR | Alopecia | [31] | |||
| FGF13 | Xq27.1 | Fibroblast growth factor 13 | • Hypertrichosis, Congenital Generalized | 307150 | XL/PE | Overgrowth | [63] |
| IKBKG | Xq28 | Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma | • Hypohydrotic Ectodermal Dysplasia with immune Deficiency | 300291 | XL | Sparse and thin (variable) | [33] |
| • Incontinentia Pigmenti | 308300 | XL | Alopecia | [34] | |||
| WNT10A | 2q35 | Wingless-type MMTV integration site family, member 10A | • Odontoonychodermal Dysplasia | 257980 | AR | Dry and sparse | [35] |
| • Schopf-Schulz-Passarge Syndrome | 224750 | AR | Sparse | [36] | |||
| Transcription regulation | |||||||
| ALX4 | 11p11.2 | ALX homeobox 4 | • Frontonasal Dysplasia 2 | 613451 | AR | Total alopecia | [16] |
| DLX3 | 17q21.33 | Distal-less homeobox 3 | • Trichodentoosseous Syndrome | 190320 | AD | Kinky | [76] |
| ERCC2 | 19q13.32 | Excision repair cross-complementing rodent repair deficiency, complementation group 2 | • Trichothiodystrophy, Photosensitive | 601675 | AR | Sulfur-deficient, brittle | [82] |
| ERCC3 | 2q14.3 | Excision repair cross-complementing rodent repair deficiency, complementation group 3 | • Trichothiodystrophy, Photosensitive | 601675 | AR | Sulfur-deficient, brittle | [83] |
| FOXC2 | 16q24.1 | Forkhead box C2 | • Lymphedema-distichiasis Syndrome | 153400 | AD | Growth of extra eyelashes | [64] |
| FOXE1 | 9q22.33 | Forkhead box E1 | • Hypothyroidism, Athyroidal, with Spiky Hair and Cleft Palate | 241850 | AR | Spiky hair | [15] |
| FOXN1 | 17q11.2 | Forkhead box N1 | • T-cell Immunodeficiency, Congenital Alopecia, and Nail Dystrophy | 601705 | AR | Total alopecia | [14] |
| GTF2H5 | 6q25.3 | General transcription factor IIH, polypeptide 5 | • Trichothiodystrophy, Complementation Group a | 601675 | AR | Sulfur-deficient, brittle | [84] |
| HOXC13 | 12q13.13 | Homeobox C13 | • Ectodermal Dysplasia 9, Hair/Nail Type | 614931 | AR | Total alopecia | [18] |
| HR | 8p21.3 | Hair growth associated | • Atrichia with Papular Lesions | 209500 | AR | Total alopecia | [11] |
| • Alopecia Universalis | 203655 | AR | Total alopecia | [10] | |||
| • Hypotrichosis 4 | 146550 | AD | Alopecia | [12] | |||
| MBTPS2 | Xp22.12-p22.11 | Membrane-bound transcription factor peptidase, site 2 | • Ichthyosis Follicularis, Atrichia, and Photophobia Syndrome | 308205 | XL | Total alopecia | [20] |
| • Keratosis Follicularis Spinulosa Decalvans | 308800 | XL | Alopecia | [21] | |||
| SOX9 | 17q24 | SRY (sex determining region Y)-box 9 | • Hypertrichosis Terminalis, Generalized, with or without Gingival Hyperplasia | 135400 | AD/PE | Overgrowth | [62] |
| SOX18 | 20q13.33 | SRY (sex determining region Y)-box 18 | • Hypotrichosis-lymphedema-telangiectasia Syndrome | 607823 | AD/AR | Alopecia | [22] |
| TP63 | 3q28 | Tumor protein p63 | • Ankyloblepharon-ectodermal Defects-Cleft Lip/Palate Syndrome | 106260 | AD | Sparse, coarse, wiry | [26] |
| • Ectrodactyly, Ectodermal Dysplasia, and Cleft Lip/Palate Syndrome 3 | 604292 | AD | Sparse, dry | [25] | |||
| • Rapp-Hodgkin Syndrome | 129400 | AD | Dry, coarse, wiry | [27] | |||
| • ADULT Syndrome | 103285 | AD | Sparse, thin | [28] | |||
| TRPS1 | 8q23.3 | Trichorhinophalangeal syndrome I | • Trichorhinophalangeal Syndrome, Type I | 190350 | AD | Sparse hair | [23] |
| • Trichorhinophalangeal Syndrome, Type III | 190351 | AD | Sparse hair | [24] | |||
| • Hypertrichosis Universalis Congenita, Ambras Type | 145701 | AD/PE | Overgrowth | [61] | |||
| VDR | 12q13.11 | Vitamin D (1,25-dihydroxyvitamin D3) receptor | • Vitamin D-Dependent Rickets, Type 2A | 277440 | AR | Alopecia | [19] |
| Cell–cell adhesion and communication | |||||||
| CDH3 | 16q22.1 | Cadherin 3, type 1, P-cadherin (placental) | • Hypotrichosis, Congenital, with Juvenile Macular Dystrophy | 601553 | AR | Short, sparse | [47] |
| • Ectodermal Dysplasia, Ectrodactyly, and Macular Dystrophy | 225280 | AR | Short, sparse | [48] | |||
| CDSN | 6p21.33 | Corneodesmosin | • Hypotrichosis 2 | 146520 | AD | Alopecia | [41] |
| CLDN1 | 3q28 | Claudin 1 | • Ichthyosis, Leukocyte Vacuoles, Alopecia, and Sclerosing Cholangitis | 607626 | AR | Alopecia | [49] |
| DSC3 | 18q12.1 | Desmocollin 3 | • Hypotrichosis and Recurrent Skin Vesicles | 613102 | AR | Sparse | [39] |
| DSG4 | 18q12.1 | Desmoglein 4 | • Hypotrichosis 6 | 607903 | AR | Sparse, fragile | [38] |
| DSP | 6p24.3 | Desmoplakin | • Cardiomyopathy, Dilated, with Woolly hair and Keratoderma | 605676 | AR | Fine and tightly curled | [71] |
| • Skin Fragility-Woolly Hair Syndrome | 607655 | AR | Fine and tightly curled | [72] | |||
| GJB6 | 13q12.11 | Gap junction protein, beta 6, 30 kDa | • Clauston Syndrome | 129500 | AD | Alopecia (variable) | [52] |
| JUP | 17q21.2 | Junction plakoglobin | • Naxos Disease | 601214 | AR | Curly/woolly | [73] |
| PKP1 | 1q32.1 | Plakophilin 1 | • Ectodermal Dysplasia Skin Fragility Syndrome | 604536 | AR | Short, sparse | [40] |
| PVRL1 | 11q23.3 | Poliovirus receptor-related 1 | • Cleft Lip/Palate-Ectodermal Dysplasia Syndrome | 225060 | AR | Sparse, dry | [50] |
| Keratins | |||||||
| KRT71 | 12q13.13 | Keratin 71 | • Woolly Hair, Autosomal Dominant | 194300 | AD | Fine and tightly curled | [74] |
| KRT74 | 12q13.13 | Keratin 74 | • Woolly Hair, Autosomal Dominant | 194300 | AD | Fine and tightly curled | [75] |
| • Hypotrichosis 3 | 613981 | AD | Sparse, fine and tightly curled | [46] | |||
| KRT75 | 12q13.13 | Keratin 75 | • Pseudofolliculitis Barbae | 612318 | AD | Ingrown hairs in shaved areas | [70] |
| KRT81 | 12q13.13 | Keratin 81 | • Monilethrix | 158000 | AD | Constrictions, brittle | [66] |
| KRT83 | 12q13.13 | Keratin 83 | • Monilethrix | 158000 | AD | Constrictions, brittle | [67] |
| KRT85 | 12q13.13 | Keratin 85 | • Ectodermal Dysplasia 4, Hair/Nail Type | 602032 | AR | Total alopecia (extreme brittleness) | [69] |
| KRT86 | 12q13.13 | Keratin 86 | • Monilethrix | 158000 | AD | Constrictions, brittle | [65] |
| Nucleolar proteins and spliceosome | |||||||
| DCAF17 | 2q31.1 | DDB1 and CUL4 associated factor 17 | • Woodhouse-Sakati Syndrome | 241080 | AR | Alopecia | [58] |
| RBM28 | 7q32.1 | RNA binding motif protein 28 | • Alopecia, Neurologic Defects, and Endocrinopathy Syndrome | 612079 | AR | Alopecia | [56] |
| SNRPE | 1q32.1 | Small nuclear ribonucleoprotein polypeptide E | • Hypotrichosis 11 | 615059 | AD | Alopecia | [57] |
| Lipid metabolism and signaling | |||||||
| LIPH | 3q27.2 | Lipase, member H | • Hypotrichosis 7 | 604379 | AR | Sparse, fine and tightly curled | [53] |
| LPAR6 | 13q14.2 | Lysophosphatidic acid receptor 6 | • Hypotrichosis 8 | 278150 | AR | Sparse, fine and tightly curled | [54,55] |
| Transporters | |||||||
| ATP7A | Xq21.1 | ATPase, Cu2+ transporting, alpha polypeptide | • Menkes Disease | 309400 | XL | Kinky hair | [79–81] |
| SLC29A3 | 10q22.1 | Solute carrier family 29, member 3 | • Histiocytosis-Lymphadenopathy Plus Syndrome | 602782 | AR | Hypertrichosis | [60] |
| Transmembrane | |||||||
| ADAM17 | 2p25.1 | ADAM metallopeptidase domain 17 | • Inflammatory Skin and Bowel Disease, Neonatal | 614328 | AR | Short and broken | [42] |
| ANTXR1 | 2p13.3 | Anthrax toxin receptor 1 | • GAPO Syndrome | 230740 | AR | Alopecia | [51] |
| Proteases and inhibitors | |||||||
| SPINK5 | 5q32 | Serine peptidase inhibitor, Kazal type 5 | • Netherton Syndrome | 256500 | AR | “Bamboo hair” | [44] |
| ST14 | 11q24.3 | Suppression of tumorigenicity 14 | • Ichthyosis with hypotrichosis, Autosomal Recessive | 610765 | AR | Sparse, curly, brittle | [43] |
| Cell cycle | |||||||
| MPLKIP | 7p14.1 | M-phase specific PLK1 interacting protein | • Trichothiodystrophy, Nonphotosensitive 1 | 234050 | AR | Sulfur-deficient, brittle | [85] |
| Membrane trafficking | |||||||
| RIN2 | 20p11.23 | Ras and Rab interactor 2 | • Macrocephaly, Alopecia, Cutis Laxa, and Scoliosis | 613075 | AR | Alopecia | [59] |
Note: AD, autosomal dominant; AR, autosomal recessive; PE, position effect; XL, X-linked.
2.1. Atrichia: transcription factors as master regulators of human hair development
The most severe form of alopecia is known as atrichia or total alopecia which is characterized by the complete absence of all types of hair. This type of alopecia is caused by early defects in hair morphogenesis. So far, cases of total alopecia have been associated with mutations in genes involved in transcriptional regulation. In these cases, the regulation of a complete developmental/differentiation program is affected. Atrichia is the main feature of Alopecia Universalis that was associated with mutations in HR, the human homolog of the murine gene, hairless [10]. HR encodes a zinc finger transcription co-repressor. Mutations in HR were also associated with Atrichia with Papular Lesions [11], and was more recently found in patients with Hypotrichosis 4 commonly known as Marie Unna Hereditary Hypotrichosis [12]. While Alopecia Universalis and Atrichia with popular lesions are autosomal recessive disorders, Hypotrichosis 4 has an autosomal dominant mode of inheritance and is characterized by the development of sparse, coarse and wiry hair during childhood and progressive hair loss at puberty. Interestingly, mutations in HR lead to clinically distinct forms of hair disorders, suggesting that the domains mutated in each disorder alter distinct functions of the protein. Moreover, mutations in Hypotrichosis 4 result in an increased translation of the main HR open reading frame (ORF), suggesting that strict regulation of HR protein levels is essential for normal hair development. Atrichia is also found in patients with T-cell Immunodeficiency, Congenital Alopecia, and Nail Dystrophy, caused by autosomal recessive mutations in FOXN1, the human homolog of the gene mutated in nude mice [13]. FOXN1 encodes a forkhead transcription factor and is expressed in the precortex and the innermost cells of the outer root sheath [14]. In contrast, mutations in the FOXE1 gene, another member of the forkhead transcription factor family, leads to Hypothyroidism, Athyroidal, with Spiky Hair and Cleft Palate, featuring structural hair defects but not alopecia [15]. Mutations in the ALX4 gene, coding for a homeodomain transcription factor, were found in patients with Frontonasal Dysplasia, another disorder characterized by total alopecia [16]. More recently, an ectodermal dysplasia with congenital atrichia and severe nail dystrophy was associated with mutations in the HOXC13 gene, a homeobox gene long known for its essential role in hair development in mice [17,18]. Mutations in the Vitamin D Receptor, which works as a nuclear receptor, were the first to be associated with atrichia in patients suffering from Vitamin D-Dependant Rickets, Type 2A [19]. However, some patients with this syndrome show partial to no alopecia.
Atrichia can be linked to defects in the regulation of the release of membrane-bound transcription factors. Indeed, deficiency in a membrane bound transcription factor peptidase encoded by the MBTPS2 gene causes Ichthyosis Follicularis Atrichia Photophobia [20] and Keratosis Follicularis Spinulosa Decalvans [21], the latter exhibiting a milder form of alopecia. MBTPS2 cleaves transcription factors that are attached to the nuclear envelope or endoplasmic reticulum membranes and allows them to translocate to the nucleus and activate their downstream targets. Sterol Regulatory Element-Binding Proteins (SREBPs) controlling the expression of sterol synthesizing enzymes, and the ATF6 transcription factor involved in the unfolded protein response (ER stress), are know substrates of MBTPS2. These proteins are therefore potentially involved in hair development.
2.2. Hypotrichosis: mutations leading to sparse hair in humans
The type of hair loss commonly called hypotrichosis is characterized by normal to sparse hair at birth, gradual hair loss during childhood, and sparse hair in adulthood. In many cases of hypotrichosis, hair morphogenesis is not affected (normal hair density at birth). However, in hypotrichosis, hair regeneration is impaired as a result of defects in various mechanisms including hair cycling and anchoring of the hair shaft in the skin. Mutations in different type of genes have been associated with a non-syndromic form of alopecia known as Hereditary Hypotrichosis Simplex. However, hypotrichosis can also be a feature of numerous syndromes.
2.2.1. Hypotrichosis related to defects in transcription regulation and signaling pathways
Similarly to the cases of atrichia described above, several cases of hypotrichosis result from the dysregulation of a developmental/differentiation program. However, in addition to transcriptional regulators, components of signaling pathways are also mutated in cases of Hypotrichosis.
Autosomal recessive and dominant mutations in SOX18 are found in patients with Hypotrichosis-Lymphedema-Telangiectasia Syndrome [22]. Hypotrichosis is a feature of Trichorhinophalangeal Syndrome, Type I and Type III, both caused by mutations in the transcription factor TRPS1 [23,24]. Sparse hair is a feature of several ectodermal dysplasias caused by mutations in the transcription factor TP63: Ectrodactyly, Ectodermal Dysplasia, and Cleft Lip/Palate Syndrome [25], Ankyloblepharon-Ectodermal Defects-Cleft Lip/PalateSyndrome 3 [26], Rapp-Hodgkin Syndrome [27] and ADULT Syndrome [28]. In these syndromes, the structure of the hair is also affected (see below).
Hypotrichosis is also found in association with mutations in components of important signaling pathways. Prominently affected in human hair disorders is the EDA/EDAR pathway. Hypohidrotic Ectodermal Dysplasia (HED) was the first ectodermal dysplasia to be genetically characterized. Mutations in the EDA gene, located on the X chromosome and encoding the signaling molecule ectodysplasin-A, were first identified in an X-linked form of HED [29]. Subsequently, autosomal dominant and recessive forms of HED were mapped to the EDAR locus, encoding the receptor of EDA [30]. Mutations in the EDARADD locus, which encodes a death domain adaptor of EDAR, were also found in autosomal recessive [31] and autosomal dominant [32] forms of HED. Activation of NF-κB is an essential component of the downstream effects of EDA/EDAR signaling. The IKBKG gene, encoding IKKγ (also known as NEMO), a subunit of the IKK complex (inhibitor of NF-κB), is mutated in a form of HED associated with immune deficiency and variable degrees of alopecia [33]. Mutations in the same gene were linked to Incontinentia Pigmenti, also featuring alopecia [34]. The EDA/EDAR pathway is a great example of a pathway in which most components are mutated in syndromes with strongly related phenotypes.
The WNT signaling pathway is also affected in cases of hypotrichosis. Mutations in the WNT10A locus were found in Odontoonychodermal Dysplasia [35] and Schopf-Schulz-Passarge Syndrome [36], both resulting in sparse hair. The APCDD1 gene, encoding a Wnt inhibitor, is mutated in Hypotrichosis 1 (hereditary hypotrichosis simplex), a pure hair disorder characterized by reduced hair density and length [37].
2.2.2. Hypotrichosis related to defects with anchoring the hair follicle in the skin
Hypotrichosis can be triggered by defects in specific structural components of the HF that are essential for anchoring the hair. In this regard, cell–cell adhesion molecules are commonly involved. In particular, mutations related to hair disorders have been found in all components of the desmosomes (Fig. 2A). Desmosomes are junctional complexes establishing strong connections between epithelial cells. The two adhesion molecules involved in the formation of desmosomes are specialized cadherins called desmoglein and desmocollin. The extracellular domain of desmogleins and desmocollins are involved in a calcium-dependent interaction, while their cytoplasmic domains interact with desmoplakin via plakoglobin and plakophilin. Desmoplakin establishes the connection with intermediate filament proteins such as keratins. Mutations in the DSG4 (desmoglein 4) and the DSC3 (desmocollin 3) genes are associated with Hypotrichosis 6 [38] and Hypotrichosis with Recurrent Skin Vesicles [39], respectively. Mutations in the PKP1 locus, coding for plakophilin, also result in sparse hair in Ectodermal Skin Fragility Syndrome [40]. Interestingly, mutations in the JUP (plakoglobin) and the DSP (desmoplakin) loci lead to defects in hair shaft structure with no sign of hypotrichosis. During epithelial cornification and in the IRS of the HF, desmosomes evolve into corneodesmosomes. This process involves the accumulation of corneodesmosin, a glycoprotein encoded by CDSN, into the extracellular core of desmosomes (Fig. 2B). Autosomal dominant mutations in CDSN are responsible for Hypotrichosis 2 [41]. Desmosome attachment can be affected by metallopro-teases cleaving components of the adhesion complex (Fig. 2C). In this regard, mutations in the ADAM17 gene, encoding a desintegrase/metalloprotease that has the potential of cleaving desmogleins, lead to structural hair defects in patients with Inflammatory Skin and Bowel Disease [42]. Similarly, Matriptase, a type II transmembrane serine protease encoded by the ST14 gene, has been suggested to be involved in corneodesmosome degradation. Mutations in ST14 lead to Ichthyosis with Hypotrichosis [43]. Mutations in the SPINK5 locus, encoding the serine protease inhibitor LEKTI, lead to Netherton Syndrome featuring a characteristic hair shaft defect called chorrexis invaginata or “bamboo hair” [44]. LEKTI may affect the activity of Matriptase and be also involved in the regulation of desmosome degradation.
Fig. 2.
Desmosomes and human genetic hair disorders. Schematic representation of desmosome (A) and corneodesmosome (B) indicating components mutated in different human hair disorders. Panel C indicates mutations in factors (proteases and inhibitors) involved in desmosome degradation and associated hair disorders. mb, plasma membranes of cell 1 and cell 2.
Desmosomes are closely connected with cytoskeletal components that are potential players in anchoring the hair shaft. Keratins are the major cytoskeletal components of epithelial cells and have been related to numerous skin blistering disorders [45]. Mutations in the KRT74 gene, encoding a keratin expressed in the IRS of the HF, cause Hypotrichosis 3 [46]. Other mutations in keratins have been associated with hair disorders mostly featuring defects in hair shaft structure (see section below; Fig. 3).
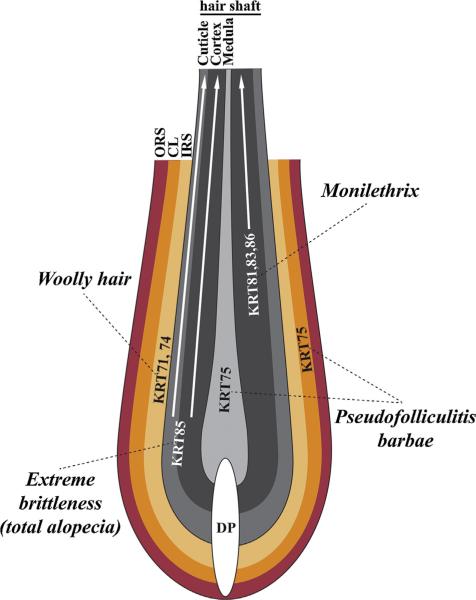
Fig. 3.
Keratins and human genetic hair disorders. Schematic representation of hair follicle indicating the compartment-specific keratin expression and mutations associated with different human hair disorders. DP, dermal papilla; ORS, outer root sheath; CL, companion layer; IRS, inner root sheath.
Other types of adhesion molecules have been associated with hypotrichosis. Mutations in CDH3, coding for P-cadherin, lead to Hypotrichosis, Congenital, with Juvenile Macular Dystrophy [47], as well as Ectodermal Dysplasia, Ectrodactyly, Macular Dystrophy [48]. Both syndromes feature short and sparse hair. The CLDN1 locus, encoding the tight junction protein claudin, is mutated in Ichthyosis, Leukocyte Vacuoles, Alopecia, and Sclerosing Cholangitis [49]. Hypotrichosis is also a feature of Cleft Lip/Palate-Ectodermal Dysplasia Syndrome due to mutations in the PVRL1 gene encoding a Ca2+-independent cell–cell adhesion molecule [50]. Transmembrane proteins involved with the interaction and remodeling of the extracellular matrix could play a role in anchoring the HF in the skin. This may be the case for the trans-membrane protein encoded by the ANTXR1 gene that is mutated in GAPO Syndrome [51].
2.2.3. Additional mechanisms involved in hypotrichosis
In the previous section, we summarized the importance of cell–cell adhesion factors in hair development. Cell–cell communication through gap junctions, which allows the passive transfer of metabolites and ions between neighboring cells, also plays an important role in hair development. Mutations in GJB6, which encodes a gap junction subunit known as connexin 30, are responsible for Clauston Syndrome [52]. This autosomal dominant disorder features variable degrees of alopecia.
Lipids are essential components of hair shaft protection (cuticle surface) and are also active players in hair shaft differentiation. So far, two related genes involved in lipid synthesis and signaling have been associated with hypotrichosis. Hypotrichosis 7 and 8 are caused by mutations in LIPH [53] and LPAR6 [54,55], respectively. Both genes are expressed in the IRS of the HF, where LIPH encodes lipase H that catalyzes the synthesis of lysophosphatidic acid. The latter subsequently binds to P2RY5, a G-protein coupled receptor encoded by the LPAR6 gene. The downstream effects of this signaling have not been completely elucidated. Lipid synthesis in the HF may be controlled by SREBPs that, as mentioned earlier, control the expression of sterol synthesizing enzymes and can be activated by the membrane-bound transcription factor peptidase MBTPS2 [20,21].
It is interesting that hair defects, either isolated or in combination with defects in a specific subset of other tissues, can be caused by mutations in ubiquitously expressed genes. Two nucleolar components of the spliceosomal small nuclear ribonucleoprotein (snRNPs) have been associated with cases of hypotrichosis. The proteins endoded by the RBM28 and the SNRPE genes are mutated in Alopecia, Neurologic Defects, and Endocrinopathy Syndrome [56], and in Hypotrichosis 11 [57], respectively. Both syndromes display similar hair defects but mutations in RBM28 are autosomal recessive while mutations in SNRPE are autosomal dominant. Auto-somal recessive mutations in the DCAF17 gene encoding a different nucleolar protein lead to Woodhouse-Sakati Syndrome which also features alopecia [58]. The function of DCAF17 remains unknown. The RIN2 gene encodes a ubiquitously expressed protein involved in endocytic trafficking. Mutations in RIN2 lead to Macrocephaly, Alopecia, Cutis Laxa, and Scoliosis, an autosomal recessive disorder featuring sparse hair [59].
2.3. Hypertrichosis and ectopic hair follicle formation
Hypertrichosis is a condition characterized by excessive hair growth. Different degrees of hypertrichosis can be found in combination with a large variety of other clinical conditions, but few syndromes featuring hair overgrowth have been genetically characterized. Among them, Histiocystosis-Lymphadenopathy Plus Syndrome is caused by mutations in the SLC29A3 gene encoding a nucleoside transporter [60]. In this condition, localized hair over-growth is observed. In a small family of rare disorders known as Congenital Generalized Hypertrichoses, the primary clinical feature is hypertrichosis. Interestingly, no specific mutations have been related to these conditions so far. The genetic defects that have been associated with these severe forms of hypertrichosis are chromo-some rearrangements and copy number variations, some of which were shown to lead to position effects. Hypertrichosis Universalis Congenita, Ambras Type is due to a rearrangement of chromosome 8 that leads to a position effect on the TRPS1 gene, resulting in reduced expression of the transcription factor [61]. A copy number variation upstream of the SOX9 gene that encodes another transcription factor known for its role in hair development significantly decreases the expression of the gene in the HF of patients with Hypertrichosis Terminalis, Generalized, with Gingival Hyper-plasia [62]. Moreover, SOX9 was found to be a target of TRPS1. More recently, an interchromosomal insertion causing a position effect on FGF13 and reduced expression of the growth factor, was associated to an X-linked form of Congenital Generalized Hypertrichosis [63].
In some cases, HFs can form in areas not expected to develop follicles. This type of ectopic hair formation is found in patients with Lymphedema-Distichiasis Syndrome who exhibit a double row of eyelashes. This syndrome is caused by mutations in FOXC2, encoding a forkhead box transcription factor [64]. In this case, the extra eyelashes grow from the Meibomian glands, suggesting that the mutation leads to a defect in the specification of this ectodermal appendage.
2.4. Alteration of hair structure
In most cases of hypotrichosis, the hair is not only sparse but the structure of the hair shaft is often altered (thin, brittle, coarse), suggesting that the mechanisms involved in the morphogenesis and cycling of the hair are closely related to the mechanisms controlling the proper assembly of the hair shaft. However, several hair disorders affect the structure of the hair shaft without significantly reducing HF density.
2.4.1. Defects in hair thickness and integrity
Monilethrix is a hair disorder characterized by a beaded necklace-like structure of the hair shaft due to intermittent constrictions separating elliptical nodes of normal thickness. Even though the brittleness of the hair leads to variable degrees of dystrophic alopecia, hair density is not directly affected in Monilethrix. The mutations leading to this condition were located in three loci in an epithelial keratin cluster on chromosome 12q13.13: the KRT86 [65], KRT81 [66] and KRT83 [67] loci. All three keratins are expressed in the cortex of the HF and incorporated into the hair shaft (Fig. 3). The hair shaft structure alterations caused by these mutations suggest that these keratins play essential roles in building a proper shaft and they follow a cyclic pattern of incorporation. Moreover, all mutations are autosomal dominant, meaning one copy of the mutated keratin is enough to impair hair structure. Monilethrix-like hair is also found in combination with alopecia in Hypotrichosis 6, which is caused by autosomal recessive mutations in DSG4 [68]. Interestingly, autosomal recessive mutations in the KRT85 gene, located in the same keratin cluster and encoding another keratin expressed in the hair cortex and cuticle (Fig. 3), lead to Ectodermal Dysplasia 4, Hair/Nail Type featuring extreme brittleness of the hair leading to total hair loss [69]. In this case, contrary to mutations leading to Monilethrix, one copy of mutated KRT85 is not enough to impair hair shaft formation, but homozygosity of the mutation leads to complete impairment of hair shaft formation.
A polymorphism in the KRT75 gene was shown to be associated with a common hair disorder called Pseudofolliculitis Barbae which primarily affects the African Americans [70]. The presence of this mutation, in combination with a curly hair type, significantly increases the risk for ingrown hair leading to inflamed pustular formations in shaved areas (Fig. 3).
2.4.2. Defects in the curvature of the hair
Hair disorders featuring abnormal curvature of the hair shaft have been related to different types of genes. One condition commonly found is the woolly hair phenotype characterized by the formation of fine and tightly curled hair. Mutations in several components of desmosomal junctions have been associated to a woolly hair phenotype (Fig. 2A). Mutations in the DSP locus, encoding desmoplakin, are responsible for two syndromes with woolly hair: Cardiomyopathy, Dilated, with Woolly Hair and Keratoderma [71]; and Skin Fragility-Woolly Hair Syndrome [72]. Woolly hair is also a feature of Naxos Disease caused by mutations in the JUP gene that encodes plakoglobin [73]. Both syndromes are autosomal recessive. Desmoplakin and plakoglobin are involved in the connection between the adhesion plaque and keratin filaments. Interestingly, mutations in KRT71 and KRT74, encoding two keratins expressed in the IRS of the HF (Fig. 3), cause Autosomal Dominant Woolly Hair [74,75]. Patients with Hypotrichosis 3 also have mutations in KRT74 and exhibit woolly hair in combination with alopecia [46]. As mentioned earlier, mutations in LIPH and LPAR6 lead to hypotrichosis 6 and 7, respectively, also featuring a woolly hair phenotype [53–55]. This suggests that the production of lysophosphatidic acid in the IRS and its signaling effects through the G-protein coupled receptor P2RY5 play a role in controlling the curvature of the hair shaft.
Patients with Trichodentoosseous syndrome, an ectodermal dysplasia caused by autosomal dominant mutations in the DLX3 gene, exhibit a kinky hair phenotype [76]. DLX3 encodes a home-odomain transcription factor expressed in the hair matrix and most layers of the HF except for the outer root sheath [77]. Some of the mutations in the transcription factor TP63 (shown to be an upstream regulator of DLX3) result in a wiry hair phenotype with a large range of defects in the structure of the hair shaft [26,28,78]. Kinky hair is also a feature of Menkes Disease, caused by mutations in the ATP7A gene encoding an ATP-dependent copper transporter [79–81].
2.4.3. Hair dystrophy: defects in hair chemical composition leading to brittleness
Brittleness of the hair can be due to the structure of the shaft as in Monilethrix, but can also be related to the chemical composition of the shaft. The hair shaft is composed primarily of keratins and associated proteins that are highly crosslinked through the formation of disulfide bonds, and having sufficient sulfur is essential to this process. Trichothiodystrophy is a hair condition characterized by a sulfur deficiency leading to brittleness. Forms of Photosensitive Trichothiodystrophy are associated with autosomal recessive mutations in three genes coding for different subunits of the basal transcription factor 2 (TFIIH): ERCC2 [82], ERCC3 [83] and GTF2H5 [84]. TFIIH has a central role in the transcription of RNA polymerase I and II, nucleotide excision repair and transcription-coupled repair. ERCC2 and ERCC3 encode two TFIIH helicase subunits commonly known as XPD and XPB (in relation to their role in Xeroderma Pigmentosum), respectively. GTF2H5 is a subunit of TFIIH involved in nucleotide excision repair. The connection between TFIIH and the levels of sulfur in the hair shaft remains unknown. A non-photosensitive form of Trichothiodystrophy has been related to mutations in the MPLKIP gene encoding M-phase specific PLK1 interacting protein involved in the control of cell cycle integrity [85].
3. Spatial-temporal specificity of human hair disorders
As mentioned earlier, humans develop different types of hair (scalp hair, eyebrows, eyelashes, facial hair, axillary hair, pubic hair) in different parts of their body and at different times of their life. Hair disorders can affect all hair types or a specific subset of hairs. In all cases of total alopecia, all hair types are affected, suggesting that the genes associated with these syndromes (FOXN1, ALX4, HOXC13, MBTPS2, VDR, HR, ANTXR1) play a universal role in hair development, independent of their location on the body and time of induction.
Hypotrichosis shows a large panel of variations with regards to the hair types affected. In Hypotrichosis 2 and 3, hair loss is restricted to the scalp, implying that the mutations in CDSN and KRT74 in these syndromes have no direct effect or are compensated by other factors in other hair types on the body. Mutations in CDH3 in Hypotrichosis, Congenital, with Juvenile Macular Dystrophy also lead to scalp-specific alopecia. However, mutations in the same gene in Ectodermal Dysplasia, Ectrodactyly, and Macular Dystrophy affect not only the scalp but also eyelashes and eyebrows, demonstrating that the range of hair types affected by mutations in P-cadherin is dependent on the domain of the protein affected by the mutation. Patients with Hypotrichosis 1 (APCDD1 mutations) develop sparse hair on the scalp, body, axilla and pubis, but grow normal eyebrows, eyelashes and beard. Hypotrichosis 6 (DSG4 mutations) features sparse hair on the scalp, chest, arms and legs, mildly affected eyebrows and beard, but normal eyelashes, axillary and pubic hair. In Hypotrichosis 7, all hair types are affected except for the beard that develops normally in males, which suggests that mutations in LIPH have no effect on facial hair growth. Interestingly, this is not found in Hypotrichosis 8, despite the functional link between LIPH and LPAR6. Similarly, mutations in SNRPE in Hypotrichosis 11 affect all hair types, except for pubic hair which grows normally. In Hypotrichosis 4, all hair types are affected, confirming the universal role of HR in hair development.
4. Ectodermal appendage defects and clinical conditions associated with hair disorders
Hair defects can be found in human disorders that are exclusively characterized by hair anomalies, but in most cases, hair defects are found in combination with other symptoms. Other ectodermal appendages such as nails, teeth and sweat glands share common developmental processes with HFs. Therefore, it is common to find human disorders in which two or more of these structures are affected, as is the case in a large family of rare diseases called Ectodermal Dysplasias. Table 2 presents a classification of the genes mutated in human hair disorders, showing if the hair defects are found in combination with anomalies in other ectodermal appendages. Also, defects in other organs are often found in combination with hair disorders. Table 3 presents a classification of some clinical conditions that are found in combination with two or more human hair disorders. Given that the mechanisms involved in epidermal differentiation and HF development are closely related, it is not surprising to find genes that are mutated in disorders affecting both hair and skin even though a large proportion of hair disorders are not accompanied by epidermal anomalies. However, it is interesting that many genes mutated in hair disorders are also associated with skeletal defects or neurological defects.
Table 2.
Ectodermal appendages affected in human hair disorders.
| Appendages affected | Mutated genes |
|---|---|
| Hair only | ALX4, APCDD1, ATP7A, CDSN, DCAF17, DSC3, DSG4, DSP, FOXC2, FOXE1, HR, JUP, KRT71, KRT74, KRT75, LIPH, LPAR6, SLC29A3, SNRPE, SOX18, SPINK5, ST14, TRPS1 |
| Hair and nail | ADAM17, ERCC2, ERCC3, FOXN1, GJB6, GTF2H5, HOXC13, KRT81, KRT83, KRT85, KRT86, MBTPS2, MPLKIP |
| Hair and teeth | ANTXR1, CDH3, CLDN1, PVRL1, RBM28, RIN2, VDR |
| Hair, nail and teeth | DLX3, TP63, WNT10A |
| Hair, teeth and sweat gland | EDA, EDAR, EDARADD, IKBKG |
| Hair, nail and sweat gland | PKP1 |
Note: Bolded genes are associated exclusively with hair defects (no other clinical condition).
Table 3.
Common clinical conditions associated with hair disorders.
| Clinical condition | Mutated genes |
|---|---|
| Skin defects | ADAM17, ALX4, DSC3, DSP, ERCC2, ERCC3, GJB6, GTF2H5, IKBKG, JUP, MBTPS2, PKP1, SLC29A3, SPINK5, ST14,TP63 |
| Skeletal defects | ALX4, ANTXR1, DLX3, FOXE1, MBTPS2, PVRL1, RBM28, RIN2, SLC29A3, TRPS1, TP63, VDR |
| Neurologic defects | ATP7A, DCAF17, ERCC2, ERCC3, GTF2H5, IKBKG, MBTPS2, MPLKIP, PVRL1, RBM28 |
| Heart defects | ANTXR1, DSP, JUP, SLC29A3 |
| Immune defects | FOXN1, IKBKG |
| Liver defects | CLDN1, SLC29A3 |
| Hearing defects | DCAF17, GJB6, MBTPS2, SLC29A3 |
| Gonad defects | DCAF17, RBM28, SLC29A3 |
| Vascular defects | FOXC2, SOX18 |
| Vision defects | ANTXR1, CDH3, MBTPS2 |
5. Summary and future perspectives
In this review we have summarized the present understanding of causative genes associated to human hair genetic disorders. Great advances in this regard have been achieved by correlations of phenotypes and genetics. New methods in molecular biology (genome sequencing, RNASeq) should facilitate future identification of causative genes for a wide variety of hereditary hair disorders that are still undetermined. Many questions remain to be explored: What is the effect of modifier genes that influence the phenotypic outcome of mutations on a specific gene (i.e. CDH3 mutations can lead to EEM or HJMD)? Why is there spatial specificity in the hair diseases (i.e. DSG4 mutations associate with HF defects in the scalp, chest, arms, legs and eyebrows, but no effect on axillary, pubic hair or eyelashes)?
Thorough knowledge of the molecular and cellular mechanisms regulating HF formation and regeneration will be of upmost importance in the quest to elucidate targets that can be used in translational therapeutics of hair loss.
Acknowledgments
The authors thank Ms. Julie Erthal and Ms. Meghan Kellett for comments on the manuscript. We also thank Dr. Karen Holbrook for providing human hair follicle images. This work is supported by funding by the Intramural Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health.
References
- 1.Lee J, Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol. 2012;23:906–16. doi: 10.1016/j.semcdb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23:917–27. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohyama M, Vogel JC, Amagai M. Gene ontology analysis of human hair follicle bulge molecular signature. J Dermatol Sci. 2007;45:147–50. doi: 10.1016/j.jdermsci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 5.Jaks V, Kasper M, Toftgard R. The hair follicle – a stem cell zoo. Exp Cell Res. 2010;316:1422–8. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol. 2005;243:1–78. doi: 10.1016/S0074-7696(05)43001-6. [DOI] [PubMed] [Google Scholar]
- 7.Duverger O, Morasso MI. Epidermal patterning and induction of different hair types during mouse embryonic development. Birth Defects Res C Embryo Today. 2009;87:263–72. doi: 10.1002/bdrc.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg. 2006;25:2–10. doi: 10.1016/j.sder.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279:720–4. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- 11.Sprecher E, Lestringant GG, Szargel R, Bergman R, Labay V, Frossard PM, et al. Atrichia with papular lesions resulting from a nonsense mutation within the human hairless gene. J Invest Dermatol. 1999;113:687–90. doi: 10.1046/j.1523-1747.1999.00723.x. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Liu Y, Xu Y, Zhao Y, Hua R, Wang K, et al. Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat Genet. 2009;41:228–33. doi: 10.1038/ng.276. [DOI] [PubMed] [Google Scholar]
- 13.Mecklenburg L, Nakamura M, Sundberg JP, Paus R. The nude mouse skin pheno-type: the role of Foxn1 in hair follicle development and cycling. Exp Mol Pathol. 2001;71:171–8. doi: 10.1006/exmp.2001.2386. [DOI] [PubMed] [Google Scholar]
- 14.Frank J, Pignata C, Panteleyev AA, Prowse DM, Baden H, Weiner L, et al. Exposing the human nude phenotype. Nature. 1999;398:473–4. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- 15.Clifton-Bligh RJ, Wentworth JM, Heinz P, Crisp MS, John R, Lazarus JH, et al. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet. 1998;19:399–401. doi: 10.1038/1294. [DOI] [PubMed] [Google Scholar]
- 16.Kayserili H, Uz E, Niessen C, Vargel I, Alanay Y, Tuncbilek G, et al. ALX4 dys-function disrupts craniofacial and epidermal development. Hum Mol Genet. 2009;18:4357–66. doi: 10.1093/hmg/ddp391. [DOI] [PubMed] [Google Scholar]
- 17.Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Chen Q, Shi L, Lee M, Giehl KA, Tang Z, et al. Loss-of-function mutations in HOXC13 cause pure hair and nail ectodermal dysplasia. Am J Hum Genet. 2012;91:906–11. doi: 10.1016/j.ajhg.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, et al. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988;242:1702–5. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- 20.Oeffner F, Fischer G, Happle R, Konig A, Betz RC, Bornholdt D, et al. IFAP syndrome is caused by deficiency in MBTPS2, an intramembrane zinc metalloprotease essential for cholesterol homeostasis and ER stress response. Am J Hum Genet. 2009;84:459–67. doi: 10.1016/j.ajhg.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aten E, Brasz LC, Bornholdt D, Hooijkaas IB, Porteous ME, Sybert VP, et al. Keratosis Follicularis Spinulosa Decalvans is caused by mutations in MBTPS2. Hum Mutat. 2010;31:1125–33. doi: 10.1002/humu.21335. [DOI] [PubMed] [Google Scholar]
- 22.Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–8. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momeni P, Glockner G, Schmidt O, von Holtum D, Albrecht B, Gillessen-Kaesbach G, et al. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet. 2000;24:71–4. doi: 10.1038/71717. [DOI] [PubMed] [Google Scholar]
- 24.Ludecke HJ, Schaper J, Meinecke P, Momeni P, Gross S, von Holtum D, et al. Genotypic and phenotypic spectrum in tricho-rhino-phalangeal syndrome types I and III. Am J Hum Genet. 2001;68:81–91. doi: 10.1086/316926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–53. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- 26.McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR, et al. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet. 2001;10:221–9. doi: 10.1093/hmg/10.3.221. [DOI] [PubMed] [Google Scholar]
- 27.Kantaputra PN, Hamada T, Kumchai T, McGrath JA. Heterozygous mutation in the SAM domain of p63 underlies Rapp-Hodgkin ectodermal dysplasia. J Dent Res. 2003;82:433–7. doi: 10.1177/154405910308200606. [DOI] [PubMed] [Google Scholar]
- 28.Amiel J, Bougeard G, Francannet C, Raclin V, Munnich A, Lyonnet S, et al. TP63 gene mutation in ADULT syndrome. Eur J Hum Genet. 2001;9:642–5. doi: 10.1038/sj.ejhg.5200676. [DOI] [PubMed] [Google Scholar]
- 29.Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–16. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- 30.Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–9. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- 31.Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–6. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 32.Bal E, Baala L, Cluzeau C, El Kerch F, Ouldim K, Hadj-Rabia S, et al. Autosomal dominant anhidrotic ectodermal dysplasias at the EDARADD locus. Hum Mutat. 2007;28:703–9. doi: 10.1002/humu.20500. [DOI] [PubMed] [Google Scholar]
- 33.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am J Hum Genet. 2000;67:1555–62. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405:466–72. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 35.Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81:821–8. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohring A, Stamm T, Spaich C, Haase C, Spree K, Hehr U, et al. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet. 2009;85:97–105. doi: 10.1016/j.ajhg.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimomura Y, Agalliu D, Vonica A, Luria V, Wajid M, Baumer A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2010;464:1043–7. doi: 10.1038/nature08875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O'Shaughnessy R, Mahoney MG, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–60. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 39.Ayub M, Basit S, Jelani M, Ur Rehman F, Iqbal M, Yasinzai M, et al. A homozygous nonsense mutation in the human desmocollin-3 (DSC3) gene underlies hereditary hypotrichosis and recurrent skin vesicles. Am J Hum Genet. 2009;85:515–20. doi: 10.1016/j.ajhg.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, et al. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet. 1997;17:240–4. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- 41.Levy-Nissenbaum E, Betz RC, Frydman M, Simon M, Lahat H, Bakhan T, et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151–3. doi: 10.1038/ng1163. [DOI] [PubMed] [Google Scholar]
- 42.Blaydon DC, Biancheri P, Di WL, Plagnol V, Cabral RM, Brooke MA, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365:1502–8. doi: 10.1056/NEJMoa1100721. [DOI] [PubMed] [Google Scholar]
- 43.Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, Lurie R, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–77. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 45.Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Wasif N, Naqvi SK, Basit S, Ali N, Ansar M, Ahmad W. Novel mutations in the keratin-74 (KRT74) gene underlie autosomal dominant woolly hair/hypotrichosis in Pakistani families. Hum Genet. 2011;129:419–24. doi: 10.1007/s00439-010-0938-9. [DOI] [PubMed] [Google Scholar]
- 47.Sprecher E, Bergman R, Richard G, Lurie R, Shalev S, Petronius D, et al. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet. 2001;29:134–6. doi: 10.1038/ng716. [DOI] [PubMed] [Google Scholar]
- 48.Kjaer KW, Hansen L, Schwabe GC, Marques-de-Faria AP, Eiberg H, Mundlos S, et al. Distinct CDH3 mutations cause ectodermal dysplasia, ectrodactyly, macular dystrophy (EEM syndrome). J Med Genet. 2005;42:292–8. doi: 10.1136/jmg.2004.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–90. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, et al. Mutations of PVRL1, encoding a cell–cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–30. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 51.Stranecky V, Hoischen A, Hartmannova H, Zaki MS, Chaudhary A, Zudaire E, et al. Mutations in ANTXR1 cause GAPO syndrome. Am J Hum Genet. 2013;92:792–9. doi: 10.1016/j.ajhg.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, et al. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet. 2000;26:142–4. doi: 10.1038/79851. [DOI] [PubMed] [Google Scholar]
- 53.Kazantseva A, Goltsov A, Zinchenko R, Grigorenko AP, Abrukova AV, Moliaka YK, et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science. 2006;314:982–5. doi: 10.1126/science.1133276. [DOI] [PubMed] [Google Scholar]
- 54.Pasternack SM, von Kugelgen I, Al Aboud K, Lee YA, Ruschendorf F, Voss K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–34. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 55.Shimomura Y, Wajid M, Ishii Y, Shapiro L, Petukhova L, Gordon D, et al. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat Genet. 2008;40:335–9. doi: 10.1038/ng.100. [DOI] [PubMed] [Google Scholar]
- 56.Nousbeck J, Spiegel R, Ishida-Yamamoto A, Indelman M, Shani-Adir A, Adir N, et al. Alopecia, neurological defects, and endocrinopathy syndrome caused by decreased expression of RBM28, a nucleolar protein associated with ribosome biogenesis. Am J Hum Genet. 2008;82:1114–21. doi: 10.1016/j.ajhg.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasternack SM, Refke M, Paknia E, Hennies HC, Franz T, Schafer N, et al. Mutations in SNRPE, which encodes a core protein of the spliceosome, cause autosomal-dominant hypotrichosis simplex. Am J Hum Genet. 2013;92:81–7. doi: 10.1016/j.ajhg.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alazami AM, Al-Saif A, Al-Semari A, Bohlega S, Zlitni S, Alzahrani F, et al. Mutations in C2orf37, encoding a nucleolar protein, cause hypogonadism, alopecia, diabetes mellitus, mental retardation, and extrapyramidal syndrome. Am J Hum Genet. 2008;83:684–91. doi: 10.1016/j.ajhg.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basel-Vanagaite L, Sarig O, Hershkovitz D, Fuchs-Telem D, Rapaport D, Gat A, et al. RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am J Hum Genet. 2009;85:254–63. doi: 10.1016/j.ajhg.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molho-Pessach V, Lerer I, Abeliovich D, Agha Z, Abu Libdeh A, Broshtilova V, et al. The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am J Hum Genet. 2008;83:529–34. doi: 10.1016/j.ajhg.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fantauzzo KA, Tadin-Strapps M, You Y, Mentzer SE, Baumeister FA, Cianfarani S, et al. A position effect on TRPS1 is associated with Ambras syndrome in humans and the Koala phenotype in mice. Hum Mol Genet. 2008;17:3539–51. doi: 10.1093/hmg/ddn247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fantauzzo KA, Kurban M, Levy B, Christiano AM. Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet. 2012;8:e1003002. doi: 10.1371/journal.pgen.1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeStefano GM, Fantauzzo KA, Petukhova L, Kurban M, Tadin-Strapps M, Levy B, et al. Position effect on FGF13 associated with X-linked congenital generalized hypertrichosis. Proc Natl Acad Sci USA. 2013;110:7790–5. doi: 10.1073/pnas.1216412110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–8. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winter H, Rogers MA, Langbein L, Stevens HP, Leigh IM, Labreze C, et al. Mutations in the hair cortex keratin hHb6 cause the inherited hair disease monilethrix. Nat Genet. 1997;16:372–4. doi: 10.1038/ng0897-372. [DOI] [PubMed] [Google Scholar]
- 66.Winter H, Labreze C, Chapalain V, Surleve-Bazeille JE, Mercier M, Rogers MA, et al. A variable monilethrix phenotype associated with a novel mutation, Glu402Lys, in the helix termination motif of the type II hair keratin hHb1. J Invest Dermatol. 1998;111:169–72. doi: 10.1046/j.1523-1747.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- 67.van Steensel MA, Steijlen PM, Bladergroen RS, Vermeer M, van Geel M. A mis-sense mutation in the type II hair keratin hHb3 is associated with monilethrix. J Med Genet. 2005;42:e19. doi: 10.1136/jmg.2004.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zlotogorski A, Marek D, Horev L, Abu A, Ben-Amitai D, Gerad L, et al. An auto-somal recessive form of monilethrix is caused by mutations in DSG4: clinical overlap with localized autosomal recessive hypotrichosis. J Invest Dermatol. 2006;126:1292–6. doi: 10.1038/sj.jid.5700251. [DOI] [PubMed] [Google Scholar]
- 69.Naeem M, Wajid M, Lee K, Leal SM, Ahmad W. A mutation in the hair matrix and cuticle keratin KRTHB5 gene causes ectodermal dysplasia of hair and nail type. J Med Genet. 2006;43:274–9. doi: 10.1136/jmg.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter H, Schissel D, Parry DA, Smith TA, Liovic M, Birgitte Lane E, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652–7. doi: 10.1111/j.0022-202X.2004.22309.x. [DOI] [PubMed] [Google Scholar]
- 71.Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–6. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 72.Whittock NV, Wan H, Morley SM, Garzon MC, Kristal L, Hyde P, et al. Compound heterozygosity for non-sense and mis-sense mutations in desmoplakin underlies skin fragility/woolly hair syndrome. J Invest Dermatol. 2002;118:232–8. doi: 10.1046/j.0022-202x.2001.01664.x. [DOI] [PubMed] [Google Scholar]
- 73.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet. 2000;355:2119–24. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 74.Fujimoto A, Farooq M, Fujikawa H, Inoue A, Ohyama M, Ehama R, et al. A missense mutation within the helix initiation motif of the keratin K71 gene underlies autosomal dominant woolly hair/hypotrichosis. J Invest Dermatol. 2012;132:2342–9. doi: 10.1038/jid.2012.154. [DOI] [PubMed] [Google Scholar]
- 75.Shimomura Y, Wajid M, Petukhova L, Kurban M, Christiano AM. Autosomal-dominant woolly hair resulting from disruption of keratin 74 (KRT74), a potential determinant of human hair texture. Am J Hum Genet. 2010;86:632–8. doi: 10.1016/j.ajhg.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price JA, Bowden DW, Wright JT, Pettenati MJ, Hart TC. Identification of a mutation in DLX3 associated with tricho-dento-osseous (TDO) syndrome. Hum Mol Genet. 1998;7:563–9. doi: 10.1093/hmg/7.3.563. [DOI] [PubMed] [Google Scholar]
- 77.Hwang J, Mehrani T, Millar SE, Morasso MI. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135:3149–59. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radoja N, Guerrini L, Lo Iacono N, Merlo GR, Costanzo A, Weinberg WC, et al. Homeobox gene Dlx3 is regulated by p63 during ectoderm development: relevance in the pathogenesis of ectodermal dysplasias. Development. 2007;134:13–8. doi: 10.1242/dev.02703. [DOI] [PubMed] [Google Scholar]
- 79.Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, et al. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993;3:14–9. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- 80.Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993;3:20–5. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 81.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 82.Takayama K, Salazar EP, Broughton BC, Lehmann AR, Sarasin A, Thompson LH, et al. Defects in the DNA repair and transcription gene ERCC2(XPD) in trichothiodystrophy. Am J Hum Genet. 1996;58:263–70. [PMC free article] [PubMed] [Google Scholar]
- 83.Weeda G, Eveno E, Donker I, Vermeulen W, Chevallier-Lagente O, Taieb A, et al. A mutation in the XPB/ERCC3 DNA repair transcription gene, associated with trichothiodystrophy. Am J Hum Genet. 1997;60:320–9. [PMC free article] [PubMed] [Google Scholar]
- 84.Giglia-Mari G, Coin F, Ranish JA, Hoogstraten D, Theil A, Wijgers N, et al. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat Genet. 2004;36:714–9. doi: 10.1038/ng1387. [DOI] [PubMed] [Google Scholar]
- 85.Nakabayashi K, Amann D, Ren Y, Saarialho-Kere U, Avidan N, Gentles S, et al. Identification of C7orf11 (TTDN1) gene mutations and genetic heterogeneity in nonphotosensitive trichothiodystrophy. Am J Hum Genet. 2005;76:510–6. doi: 10.1086/428141. [DOI] [PMC free article] [PubMed] [Google Scholar]