Abstract
The growing prevalence of nanotechnology in the fields of biology, medicine and the pharmaceutical industry is confounded by the relatively small amount of data on the impact of these materials on the immune system. In addition to concerns surrounding the potential toxicity of nanoparticle (NP)-based delivery systems, there is also a demand for a better understanding of the mechanisms governing interactions of NPs with the immune system. Nanoparticles can be tailored to suppress, enhance, or subvert recognition by the immune system. This “targeted immunomodulation” can be achieved by delivery of unmodified particles, or by modifying particles to deliver drugs, proteins/peptides or genes to a specific site. In order to elicit the desired, beneficial immune response, considerations should be made at every step of the design process: the NP platform itself, ligands and other modifiers, the delivery route, and the immune cells that will encounter the conjugated NPs can all impact host immune responses.
INTRODUCTION
Nanomaterials were reportedly first alluded to as “molecular machines” in 1959 by Nobel Prize winner Richard Feynman. Fifteen years later, Norio Taniguchi coined the term “nanotechnology”. By 1996, the first conference in nanobiology took place, and the use of nanoparticles for the advancement of biomedicine was on the forefront of scientific research. 1 Today, the applications for nanotechnology in biomedicine continue to broaden in scope, encompassing drug delivery, diagnostic imaging, vaccine development and tissue regeneration. The majority of research concerning the use of nanoparticles as vehicles for antigen delivery focuses on encapsulated antigens. Here, we discuss the considerations, advantages and disadvantages of using various types of nanoparticle platforms for antigenic display on the particle surface, and the types of immune responses those various formulations can provoke.
FACTORS AFFECTING NANOPARTICLE RECOGNITION AND UPTAKE BY APCs
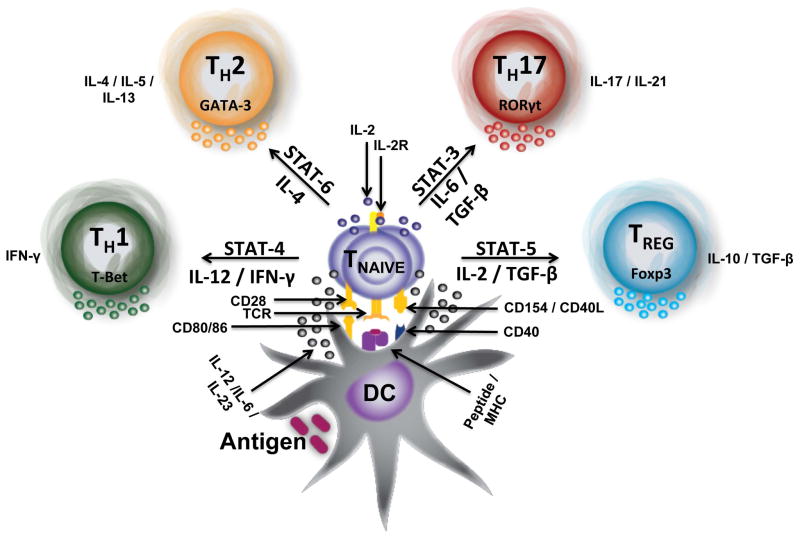
The induction of T cell-mediated immunity requires the presentation of antigen to T cells by mature and activated dendritic cells (DCs) in vivo.2,3 The ability of DCs to promote T cell activation and differentiation is dependent upon multiple factors, including 1) antigen-capture, 2) DC maturation, and 3) the efficient processing and presentation of antigen to T cells. Under steady-state conditions, tissue resident DCs are quiescent, constantly surveying the environment for antigens to acquire and present to T cells. Under these conditions, the functional outcome of DCs presenting self-antigens to T cells is the maintenance of peripheral tolerance through anergy or deletion.4 However, the acquisition of foreign antigen by DCs in the presence of inflammatory signals elicited by cytokines such as TNF-α and IL-1β5, the ligation of TLRs and other pattern-recognition receptors,6 or tissue injury and cell necrosis7 are critical steps in the activation and functional maturation of dendritic cells. These signals induce phenotypic and morphological changes in dendritic cells and their precursors that facilitate their migration to the lymph nodes via the CCR7-CCL19/21 axis5 where they are able to initiate contact with a dense number of T cells,8 allowing for the priming of an effective T cell response. Ultimately CD4+ and CD8+ T cell activation requires multiple signals supplied by activated DCs (Figure 1) – 1) display of processed antigenic peptides in the context of major histocompatibility complex class I and class II molecules which are recognized by the T cell antigen receptor (TCR); 2) delivery of APC-derived costimulatory signals (e.g., CD80 or CD86) which interact with receptors (e.g., CD28) on naïve T cells; and 3) delivery of cytokines (e.g. IL-12, IL-4, IL-1/IL-6/IL-21, and TGF-β) which instruct the differentiation of naïve T cells to become TH1, TH2, TH17 or iTreg effector cells, respectively.9 Since viruses and tumors have developed mechanisms that evade recognition by the innate immune system, and therefore escape elimination by the adaptive immune system, nanoparticle vaccine strategies that effectively target and activate dendritic cell pathways are an area of intense interest. As such, multiple parameters of nanoparticle fabrication have been manipulated to enhance targeting of the vaccine-bearing nanoparticles towards DCs. Indeed, the chemical composition of nanoparticles can inform such inherent characteristics as size, solubility, and charge, which in turn can dictate their applicability as biocompatible agents, as can the route by which they are administered.
FIGURE 1.
Dendritic cells initiate T cell immunity in the lymph nodes. A mature DC encounters antigen in the lymph nodes where it processes and cross-presents the antigen in the context of peptide/MHC molecules to T cells. A T cell bearing a receptor (TCR) of cognate specificity to the presented peptide/MHC is induced by TCR stimulation (signal 1) to express CD40L, leading to activation of the DC through CD40 engagement. DC activation results in increased costimulation to the T cell through the B7/C28 pathway (signal 2). TCR stimulation in the presence of CD28 signaling results in T cell activation, IL-2 dependent proliferation, and differentiation into an effector T cell. The cytokine milieu directs T cell differentiation along one of the helper T cell (TH) lineages.
Route
Classically, antigen delivered subcutaneously leads to the induction of immunity while antigen delivered intravenously leads to the induction of immunological tolerance. Strategies designed to elicit immunity with nanoparticle delivery platforms have targeted similar routes of immunization with suboptimal effect: nanoparticles administered subcutaneously or intradermally may be taken up by tissue resident APCs or their precursors including monocytes, macrophages, and DCs; due to the low frequency of DCs in peripheral tissues, antigens delivered subcutaneously via nanoparticle platforms may not be the most efficient method for priming optimal T cell responses. Indeed, observations from a study utilizing 0.5–1.0 μm latex or polystyrene particles delivered intracutaneously suggest that inflammatory blood-derived monocytes that were recruited to the site of particle administration are the dominant phagocytic population present in the skin during inflammation.10 Interestingly, the particle-bearing monocytes were demonstrated to migrate to the draining lymph nodes where they differentiated into CD11clowMHC class IIhigh DCs that were capable of priming allogeneic T cell responses to a similar capacity as the lymph node resident population of DCs. Nonetheless, the monocyte derived DCs comprised only 2.0% of the DC population, and only 0.04% of the total cell population in the draining lymph node. Compared to the number of LN resident CD11chigh DCs, this monocyte-derived DC population represents an insignificant number of cells. Due to this observation, many strategies are being developed that target NP vaccines directly to the LN where they can interact with LN resident DCs.
Nevertheless, the skin, lungs, gut and LN are common tissue targets for the delivery of immunomodulatory agents, due to the presence of large numbers of immune cells and because the magnitude and duration of the immune response can differ depending on which tissue was targeted11,12. In addition to the use of targeting molecules on the surface of particles, the route of administration can help direct materials to the desired tissue compartments. The mucosal immune system is highly compartmentalized, and while immunization by parenteral routes does not induce mucosal antibodies, delivery by the mucosal route can induce both mucosal and systemic antibodies.13 For example, pluronic-stabilized polypropylene sulfide (PPS) nanoparticles conjugated to ovalbumin induced cytotoxic T cell responses in the lung as well as remotely in the spleen following intranasal and pulmonary administration, indicating the induction of both systemic and mucosal immune responses.14,15 Co-conjugation of ovalbumin and flagellin, a TLR5 ligand, enhanced humoral and cytotoxic T cell responses in mucosal airways, as well as remotely in the vaginal and rectal mucosal compartments when administered intranasally.15 In contrast, following intradermal injection, these same particles were shown to preferentially traffic to the lymph node, and although both the cellular and humoral arms of the immune system were activated, these responses were not noted in mucosal compartments.16 Lastly, pulmonary immunization with PPS nanoparticles expressing surface-linked tuberculosis antigen was reported to induce systemic TH17 responses while intradermal administration of the same PPS nanoparticle conjugates did not induce any detectable TH17 response, suggesting that the route can also dictate the type of T cell mediated immune response that is formed.17
Size and Shape
Nanoparticles can also be preferentially targeted to lymph node resident DCs simply by modifying the size of the nanoparticles. Smaller nanoparticles (20–40nm in size) are able to cross tissue barriers and traffic directly to the lymph node via interstitial flow, whereas larger nanoparticles (>100nm) require uptake by APCs to access the lymph node.18 For instance, an investigation by Makino et al. confirmed that larger polystyrene particles 1.0 μm in diameter were preferentially phagocytosed by alveolar macrophages whereas smaller particles (0.2 μm)were not phagocytosed.19 Uptake by macrophages in this study was also dependent on additional surface properties including charge and “softness” of the particle, emphasizing that no single property determines particle fate, but rather that nanoparticle size, shape, composition, and other physical and chemical properties together will define the biological effect of nanoparticles. Fifis et al. compared particle size within both a viral (<0.5 μm) and a bacterial (>0.5 μm) range, and examined antigen uptake efficiency and in vivo localization of antigen-decorated carboxylated polystyrene particles in the lymph node (LN) after injection. They showed that lymph node-resident DCs most efficiently took up particles in the viral range, between 20 and 100 nm in diameter (optimally 40 nm).20 Importantly, this “nanovaccine” elicited both antibody and CD8 T cell immune responses comparable to the adjuvants alum and monophosphoryl lipid A, and cleared established tumor masses in mice within 2 weeks after a single injection. A different study using 25nm PPS nanoparticles observed that up to 50% of the lymph node resident DCs were positive for the nanoparticles 24 hours after intradermal administration. In contrast, less than 10% of the lymph node DCs were positive for the co-injected, 100 nm sized PPS nanoparticles. Furthermore, the 25 nm PPS nanoparticles were capable of promoting the expansion of CFSE-labeled OT-II CD4+ T cells conjugated to the model antigen ovalbumin, which subsequently led to induction of both cytotoxic and humoral immune responses.16
Interestingly, a study by Champion et al. reported that while size of the nanoparticle determined the ability of the APC to complete phagocytosis, it was the shape of the biomaterial that mediated the initiation of phagocytosis. Alveolar macrophages initiated phagocytosis of polystyrene particles of varying sizes and shapes, however shape was the sole determinant of whether the particle was successfully ingested.21 A separate report also showed that ligand-coated gold NPs of similar size, charge, and hydrophobicity could either penetrate the plasma membrane without disruption or be trapped by endosomes depending on their homogenous versus random surface structure, respectively. Indeed, these NPs were designed as chemical isomers on a nanoscale, differing only in the molecular arrangement of surface chemical groups. Together, these studies indicate that shape and surface structure play a role in determining the uptake and subcellular fate of nanoparticles.22
Charge
Manipulation of nanoparticle surface properties can also modulate how the conjugated particles react with the immune system. Surfactants such as polyethylene glycol (PEG), phenylethylmalonamide (PEMA) and polyvinyl alcohol (PVA) can alter the NP’s charge and solubility, thereby modifying overall biocompatibility. Attaching hydrophilic polymers (PEG, for example) to the NP surface greatly increases its solubility and can protect attached proteins from enzymatic degradation, thereby allowing enhanced delivery of the drug or protein of interest to the target population.23 “Pegylated” NPs can also be used as platforms for lipophilic molecules, wherein insoluble molecules can be attached or adsorbed to the hydrated NPs.24
Surfactants are also commonly used to alter the charge of the NP. Charge can influence a nanoparticle’s ability to navigate through biological barriers, including the stringent blood brain barrier (BBB).25,26 Lockman et al. showed that in nanoparticles comprised of emulsifying wax and Brij 78 (a common nonionic surfactant): 1) the NP surface could be correspondingly changed by using neutral, anionic, or cationic surfactants, and 2) while BBB integrity was not affected by neutral or low concentrations of anionic NPs, an immediate toxic effect and disruption of the BBB was caused by cationic NPs or high concentrations of anionic NPs. Further investigation of NP uptake in the brain demonstrated that thiamine-coated nanoparticles facilitated binding and/or association with BBB thiamine transporters over uncoupled particles, introducing a viable mechanism for nanoparticle ligand-mediated drug delivery to the brain.26
Upon intravenous injection, charged particles can either prevent or enhance the adsorption of serum proteins and opsonins. Coating nanoparticles with hydrophilic polymers has been shown to prolong their half-life by decreasing opsonization that results in rapid nanoparticle clearance by macrophages.27–29 For example, an enhanced negative charge on PVA-coated PLG NPs affected recognition by APCs.29,30 In contrast, dendrimer particles with positively charged surface amine groups were significantly more cytotoxic than their anionic counterparts.31 Moreover, the charge of the nanoparticle can have a direct effect on antigen processing once captured by the intended APC population. Acid-degradable cationic OVA-encapsulating NPs composed of acrylamide and the amine monomer AETMAC were shown to increase the delivery of antigens into the MHC class I cross-presentation pathway of bone marrow-derived DCs at lower concentrations when compared to acid-degradable nanoparticles bearing a neutral charge,32 and hepatitis C virus DNA adsorbed to the surface of cationic PLG nanoparticles was demonstrated to induce both cytotoxic T cell responses and seroconversion in treated animals.33 The potent ability of cationic nanoparticles to deliver antigen to the MHC class I loading pathway was suggested to be the result of endosome disruption by the phagocytosed NP, and leakage of the antigen into the cytosol where it could then interact with the cross-presentation machinery.34 Surface charge has also been implicated in NP distribution between the vascular and extravascular compartments of tumors.35 Finally, increased charge may also influence the size and structure of conjugated NPs, resulting in NP-antigen-NP conjugates and potential agglomeration.36
One method that utilizes the surface charge of a platform to enhance vaccine delivery to DCs is the conjugation of DC-specific receptor antibodies to the surface of the nanoparticle. Use of a chemical cross-linker such as 1-(3-dimethylamiopropyl)-3-ethylcarbodiimide hydrochloride (ECDI) can be used to covalently link the antibody of interest to the free amines or carboxyl groups present on the surface of the nanoparticle. The conjugation of anti-DEC205 and anti-CD11c antibodies to PLG nanoparticles has been demonstrated to enhance nanoparticle uptake by DC and macrophage-mediated phagocytosis by as much as 50% in vitro and in vivo.37 Similar effects on particle uptake have also been reported in a model using an ICAM-1 specific peptide conjugated to PLG nanoparticles in a HUVEC culture system.38 However, such methods have been reported to have a non-stimulatory effect on DCs, even when injected into the footpad of mice,37 and may therefore require additional factors to promote DC maturation and activation where protective immunity is the desired outcome. Thus, nanoparticle platforms for vaccine delivery may require the incorporation of danger signals into their design for the optimal induction cell mediated immunity.
Protein Corona
The exposure of nanoparticles to biological fluids such as blood, serum, and interstitial fluid leads to the formation of the protein corona, a layer of serum proteins and other biological factors that adsorb to the surface of the nanoparticle. Formation of the protein corona has become a topic of significant interest to investigators, as the corona is known to affect nanoparticle distribution, recognition, and uptake in biological environments.39 The type of proteins that constitute the protein corona is largely dependent on the same factors that determine nanoparticle uptake by the immune system, such as charge, shape, structure, and size. One study observed that while the protein composition of the corona surrounding 100 nm and 50 nm neutrally-charged polystyrene particles shared 80% homology, protein homology was at 50% when the corona of negatively-charged 100 nm and 50 nm polystyrene particles where compared. Furthermore, the same study reported that 50 nm polystyrene particles of positive, neutral, and negative charge shared only 40% protein homology in their coronas, suggesting an important role for both charge and size in determining the composition of the protein corona.40 Previous models of the protein corona suggested that the corona was composed of 2 layers: 1) the ‘hard’ corona which contains up to 100 proteins that become irreversibly adsorbed to the nanoparticle as a function of time, and 2) the ‘soft’ corona that is hypothesized to be in a state of dynamic exchange with free proteins in the serum as a result of binding kinetics. However, a recent study by Stauber and colleagues suggest that this model may require revision. The authors observed that the protein corona surrounding silica and polystyrene NPs was rapidly formed within less that 1.0 minute, was composed over 300 proteins, and that the composition of the protein corona changed qualitatively little as a function of time. Rather, the amount of a given protein adsorbed to the nanoparticle fluctuated between the early and late corona, and that significant changes to the corona were most likely to occur within 2 minutes of its formation. Thus, the stable composition of the corona, and its rapid development call into question the existence of a dynamic ‘soft corona’.41 Moreover, the authors reported that the proteins making up the corona had a net negative charge irrespective of the surface charge of the nanoparticle, suggesting that the corona may form independently of the surface properties of the nanoparticles, or that no particular property of the NP could dictate formation of the corona. Nonetheless, the authors observed that formation of the corona could affectively mask the identity of the nanoparticle, and this is in agreement with other publications. The transferrin receptor is overexpressed by tumor cells due to their excessive metabolic requirements, and can be used to target drug delivery to the tumor on the basis of transferrin expression.42 Salvati et al. found that when transferrin was conjugated to silica nanoparticles in an attempt to mediate specific uptake by transferrin receptor expressing A549 lung cell line, the level of uptake was not diminished even when transferrin receptor expression by the A549 tumor cell line had been silenced. Upon further investigation, the authors observed that following exposure to biological media containing serum, the ability to target uptake of the particles through the transferrin receptor was lost due to masking of the conjugated transferrin molecules by the protein corona.43 Thus, the protein corona may limit the ability of the nanoparticle to target specific populations, thereby allowing accumulation in non-desired cell types or tissues. Lastly, the protein corona may differentially affect uptake by the immune system. Using bovine serum albumin (BSA) as a model serum protein to examine the effects of the protein corona, a study by Caruso and colleagues reported that the adsorption of BSA onto nanoparticles composed of poly(methacrylic acid) (PMA) inhibited uptake in a monocytic cell line, but promoted uptake in a macrophage cell line through the scavenger receptor-A mediated phagocytosis.44 To delay the uptake of opsonized nanoparticles by macrophages, Rodriguez et al. conjugated human CD47 (hCD47) to the polystyrene nanoparticle. CD47 is a glycoprotein found on all mammalian cells that signals through the SIRPα receptor on macrophages to identify the cell as ‘self’, thereby preventing phagocytic clearance.45,46 Conjugation of hCD47 or a peptide derivative of hCD47 to the nanoparticle significantly delayed clearance and enhanced circulation when administered to humanized mice, and treatment of the mice with an anti-CD47 antibody reversed this effect to restore clearance of the nanoparticle.27 This strategy may offer an attractive approach to minimize the clearance of nanoparticles used in diagnostic imaging or other drug delivery applications, and it will be interesting to see how this strategy may be incorporated into nanovaccines for immunotherapy. Thus while few studies have examined the effect of protein adsorption on nanoparticle delivery in vivo, it is becoming increasingly apparent that the protein corona is a factor that must be taken into consideration by the investigator when designing a nanovaccine to optimize recognition and uptake of the nanoparticle by the appropriate cell type.
NANOPARTICLE ADJUVANTS AND DC MATURATION
A common goal in the design of vaccine strategies is how to elicit a long-lasting immune response that sufficiently induces the development of effector and memory T cells. The generation of an effector T cell response requires the presentation of cognate peptide/MHC molecules to the T cell by a mature DC bearing T cell costimulatory receptor ligands, such as CD80 (B7-1) and CD86 (B7-2) (Figure 1). DC maturation occurs upon activation, and this can be induced by a number of external and/or internal danger signals such as the presence of TLR agonists, inflammatory cytokines and T cell-derived costimulation.47 Although some NP platforms may inherently posses some adjuvant-like properties, many strategies have attempted to incorporate some form of DC activation/maturation signal to enhance the immunogenicity of the NP vaccine. In one study, the incorporation of Poly I:C (a TLR3 ligand) and resiquimod into OVA-PLG NPs significantly enhanced the activation, proliferation, and effector function of OVA-specific CTLs when the nanoparticles were targeted to DCs via the DEC205 receptor in vivo.48 The CD40/CD154 costimulatory pathway is a potent activator of innate immune function and has been shown to be critical for the induction of adaptive immune responses in the absence of TLR signals. CD40 is promiscuously expressed throughout the hematopoietic compartment, while its ligand CD154 is primarily expressed by CD4+ T cells following TCR stimulation. Ligation of the CD40 receptor by CD154 or an agonist mAb has been shown to exert potent phenotypic changes in DCs, leading to the enhanced expression of CD80 and CD86, the upregulation of MHC molecules, and production of the pro-inflammatory cytokine, IL-12.49 Although the administration of an agonist anti-CD40 mAb can lead to severe side effects due to the promiscuous expression of CD40, one study reported that adsorption of an agonist anti-CD40 mAb to a nanoparticle composed of poly(γ-glutamic acid) minimized the systemic effects of anti-CD40 and synergized with the NP to enhance the stimulatory effect of anti-CD40 treatment.50 Another study that used porous silicon nanoparticles expressing avidin, found that the potency of a biotinylated agonistic anti-CD40 mAb (FGK) was increased over 30-fold when conjugated to the nanoparticles. This platform led to the enhanced delivery of the nanoparticles to DCs, and significantly promoted the activation and proliferation of B cells when compared to the effect of soluble FGK treatment.51 Alternatively, the local release of DC maturation signals, such as GM-CSF, can also be used to enhance the adjuvanticity of nanovaccines. Chou, et al. reported that the incorporation of GM-CSF and hepatitis B virus surface antigen into a copolymer hydrogel led to the recruitment and activation of DCs that were able to stimulate potent humoral and cell-mediated responses,52 and a similar approach has been used to eliminate solid tumors in preclinical models using PLG nanoparticle scaffolds that encapsulate GM-CSF while immobilizing tumor lysate and CpG oligonucleotides on its surface.53 The pulsed release of GM-CSF from the PLG scaffold led to the sustained recruitment, activation and trafficking of DCs bearing antigen from the scaffold to the draining lymph node, leading to tumor immunity that significantly delayed tumor growth and cured over 20% of the tumor-bearing recipients.53
The NP platform chosen for vaccine delivery can also possess inherent immunostimulatory properties. Since their initial description as adjuvants 4 decades ago,54 liposomes have been extensively studied for their versatility in vaccine delivery.55 Depending on the type of lipid used to fabricate the NP, liposomes can significantly enhance the immunogenicity of the associated antigen, and in some cases are sufficient enough to elicit immune activation on their own. This appears to be particularly true of cationic liposomes which can promote DC activation, CD80/CD86 expression, and chemokine release.56 Cationic adjuvant formulations (CAF01) are a class of liposomal adjuvants composed of trehalose 6,6′-dibehenate (TDB), a synthetic analogue of a mycobacterial glycolipid antigen, and dimethyldioctadecylammonium (DDA), a lipid with positively charged head groups.57 CAF01 has been reported to induce high levels of humoral and cellular immune activation when administered with antigen,58 and this response could be further modified when TDB/DDA liposomes were complexed with poly(I:C) and adsorbed OVA protein, leading to the induction of a long-term, multifunctional memory CD8+ T cell response to OVA.59 The adjuvanticity of the liposome formulation may also be affected by the structure of the liposome. While thiol conjugation of antigen to unilamellar vesicles induced high titers of antibody production,60 multilamellar vesicles (MLVs) induced both high antibody titers and TH1 responses when compared to unilamellar vesicles.61 Moon et al. reported enhanced adjuvanticity of MLVs by embedding Monophosphoryl lipid A, an immunomodulator and TLR4 agonist that is derived from the cell wall of gram-negative bacteria, into the walls of interbilayer-crosslinked multilamellar vesicles (ICMVs).62,63 Another NP formulation that has been demonstrated to be innately immunogenic are NPs composed of α-Al2O3, an alum derivative. When α-Al2O3 NPs were conjugated with OVA protein (α-Al2O3-OVA) and administered subcutaneously to B16-OVA tumor bearing mice, these recipients rejected the tumors while mice that were immunized with alum and OVA succumbed from tumor burden.64 Lastly, 25 nm antigen-conjugated Pluronic-stabilized PPS particles were reported to induce DC activation and expression of CD80, CD86, and CD40 through activation of the alternative complement cascade.65 While various synthetic molecules can be used as adjuvants to direct DC activation, the use of adjuvants that elicit multiple pathways of immune activation (such as complement cascade activation and lymph node remodeling by mast cell granules66) may represent a more promising alternative to synthetic molecular adjuvants by recapitulating additional mechanisms that are involved in the generation of immunity to natural pathogens.
Virus-Like Particles (VLPs)
The issue of biocompatibility can often be addressed by biologically derived, rather than synthetic, nanoparticles. VLPs are one of the most successful examples of such a platform for antigenic display. VLPs are comprised of viral capsid proteins and have the intrinsic ability to self-assemble into particles that range in size between 20 and 100 nm, reflecting the diversity of insect, plant, mammalian, or bacteriophage viruses from which they are derived. Although they resemble live virus in their particulate nature and multivalent structure, VLPs lack a viral genome, making them noninfectious. The small size and repetitive, multivalent structure of VLPs are examples of how size and surface geometry can affect the uptake of NPs by immune cells. Because VLPs are antigenically indistinguishable from infectious virus, they are highly immunogenic, and are able to elicit strong cellular and humoral immune responses, particularly through their interactions with APCs and B cells. Due to their inherent ability to provoke or enhance immune responses, VLPs are often used as vaccines against the virus from which they are derived, (notable examples of this are the FDA-approved human papilloma virus (HPV) and hepatitis B virus (HBV) vaccines) or as platforms to target heterologous molecules that are poorly immunogenic, such as self-antigens, lipids and polysaccharides, small molecules, and non-exposed antigens. Thus, peptides displayed on the surface of a VLP have the potential to present both B- and T-cell epitopes, eliciting a comprehensive humoral and cellular immune response. There are approximately 30 VLP-based therapies undergoing clinical trials today that target a wide range of medical conditions, including both autoimmune and infectious disease, cancer, and addiction.67,68
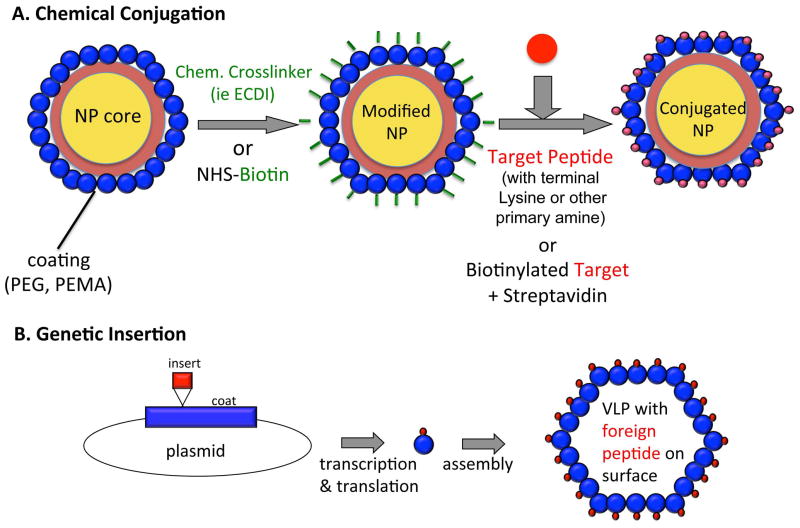
Common methods for attaching antigen to the particle surface include simple chemical conjugation and genetic insertion (Figure 2). Genetic insertion involves site-directed mutagenesis to incorporate a foreign amino acid sequence into the viral coat protein to create multivalent surface presentation of peptides. The limiting factors determining whether a site is amenable to genetic insertion are 1) the inserted peptide must not interfere with protein folding and VLP assembly, and 2) the inserted peptide must be displayed on the outside surface of the VLP. Assembly is further limited by the size of peptide chosen for insertion - insertions of less than 20–30 amino acids are typically well tolerated. Although plant, bacteriophage, and animal viruses such as papillomavirus, rhinovirus, and parvovirus have all been used for genetic display, the most well-characterized are HBV VLPs.69,70 HBV core antigen (HBcAg) is well suited for genetic insertion of foreign epitopes into a site in the protein’s immunodominant loop, or at the N- and C- termini of the protein. Peptides as large as 55 amino acids have been successfully inserted into particles, and the resultant recombinant HBcAg VLPs have been shown to induce strong cellular and humoral immune responses against a variety of targets.70,71 Two recombinant HBcAg-based vaccines have been tested in humans: the influenza vaccine ACAM-FLU-A,72 and the malaria vaccine Malariavax (ICC-1132), which displays sequences from the circumsporozoite protein from Plasmodium falciparum.73
FIGURE 2.
Methods of displaying antigen on the particle surface. A) Chemical conjugation uses chemical crosslinkers or biotin-streptavidin cross-bridges to link the desired peptide to the NP surface. B) Genetic insertion results in recombinant particles in which the peptide of interest is inserted into a coat protein and displayed on the surface of a VLP following successful translation.
Common methods of chemical conjugation include using bi-functional chemical cross-linkers such as ECDI and SMPH, or creating biotin-streptavidin bridges. As with genetic insertion, the efficiency of chemical conjugation is dependent to a degree on the size of the target antigen. One advantage of targeting peptide epitopes is that they allow for precise targeting of critical epitopes involved in infection or other aspects of the disease pathogenesis. Smaller peptides can also be attached at a very high density to the NP surface, as steric hindrance from secondary and tertiary structures is not a consideration. However, chemical conjugation also opens the door for coupling larger targets, including whole proteins or non-protein molecules, to the NP surface. These conjugates have the potential to induce a broad range of antibodies capable of recognizing both linear and conformational epitopes on the target molecule. For example, as many as 240 molecules of a 12-amino acid peptide can be linked to VLPs composed of the RNA bacteriophage Qβ,74,75 while only approximately 18 copies of a 34 kDa IL-17 homodimer can be linked to Qβ VLPs.76 Regardless, the Qβ VLP platform has been widely used for the development of VLP-based vaccines currently in human clinical trials, and many more are in development. These include vaccines targeting amyloid-β,74,77 angiotensin II78,79 and nicotine,80,81 for treatment of Alzheimer’s disease, hypertension, and nicotine dependence, respectively.
Filamentous Nanostructures
In addition to serving as natural NP platforms, VLPs have also helped inform the development of synthetic NPs. For example, the filamentous shape of several viruses led to the development of likewise filamentous nanostructures including nanorods, nanowires and nanotubes, which are gaining momentum for their application in the fields of molecular imaging82, biomarker detection83, drug and gene delivery84, tissue engineering85 and radiation therapies,86 among others. While much of this work is beyond the scope of this review, we can highlight some observations with regards to nanorod and carbon nanotube (CNT) interactions with the immune system. A recent study showed that displaying the major protective antigen, fusion (F) protein, of respiratory syncytial virus (RSV) on the surface of gold nanorods induced potent human T cell responses when co-cultured with DCs treated with the vaccine. Notably, the vaccine not only took into consideration the importance of the NP core’s filamentous shape in securing its immunogenicity, but also the conformation of the F protein epitopes on its surface. Successful conjugation of the full-length antigenic protein and therefore maintenance of its secondary structure was likely essential in mimicking free protein and delivering protective viral antigens to human APCs.87 While this study highlights the importance of NP shape on the immune response, it also calls attention to a common deficit in CNT research for biomedical application: much of the research is conducted in vitro and, accordingly, there is a limited amount of in vivo data on CNT immunotoxicity. A more comprehensive review on the topic has been published by Andersen, et al. 88 As with nanoparticle composition in general, the wide array of CNT formulations makes it difficult to compare results across experiments. For example, Sanjiv S Gambhir, et al. used cyclic RGD peptide-conjugated single-walled CNTs as photoacoustic molecular imaging agents in tumor-bearing nude mice. While the experiments were performed using live mice, no toxicity studies were performed, and the mice were sacrificed within 4 hours of the intravenous injection of particles82. The same group recently showed that undecorated gold nanorods could also be used to image subcutaneous xenografts of ovarian cancer cell tumors in nude mice. Again, no immune assays were performed, though the imaging was carried out up to 2 days following injection89. A more immunology-focused in vitro study by Son, et al. showed that nanorods functionalized with immune cell ligands such as mannose and RGD peptides formed nanobridges that could increase immune cell proximity, thereby facilitating antigen presentation and cytokine release. When co-cultured with dendritic cells and T cells, longer (4μm as compared to 1–2 μm) nanorods enhanced pro-inflammatory IL-2 and IFN-γ secretion. This work, although strictly in vitro, provides an intriguing alternative to direct delivery of antigens and adjuvants to APCs as a means of modulating immune responses90. In contrast, Aldinucci et al. recently reported that multi-walled CNT scaffolds incorporated into DC cultures resulted in a lower immunogenic profile (transcripts for proinflammatory cytokines such as IL-12, IL-23 were undetectable) and did not induce cell death85. Other examples of synthetic nanoparticles that have been used as vaccine carriers include co-polymer hydrogels or ‘nanogels’, cationic liposomes (discussed previously) and biodegradable poly(lactide-co-glycolide) (PLGA or PLG) nanoparticles.
NANOPARTICLES AND T CELL ACTIVATION
Although current vaccines are able to elicit potent humoral immunity, the ability to induce a long-lasting, protective memory CD8+ T cell response remains a significant challenge in the design of rational vaccines. Chronic viral infections such as HIV that may persist in cellular reservoirs during antiviral therapy, and proliferating tumor cells that are resistant to chemotherapy necessitate elimination by cytotoxic T cells. While intracellular antigens and viral proteins present in the cytosol are subsequently degraded by the proteasome and directed for presentation in the context of MHC class I to CD8+ T cells, antigens that are acquired exogenously by DCs typically do not access the cytosol, but are degraded in the endo-lysosome and enter the MHC class II loading pathway.91 This presents a significant barrier to the generation of cytotoxic CD8+ T cell responses to soluble or endocytosed antigens, since the ability to cross-present exogenously acquired antigen to CD8+ T cells is limited to specialized populations of DCs, such as the population of splenic and lymph node resident CD8α+DEC205+ DCs and the mucosal population of CD103+ expressing DCs.92 Indeed, the priming of CD4+ and CD8+ T cells may be preferentially mediated by divergent subsets of DCs in vivo.93 To overcome this obstacle, one PLG nanoparticle vaccine strategy incorporated the use of the antibodies targeting the DEC205 receptor that is expressed by CD8α+ DCs to promote cross-presentation of the vaccine to CD8+ T cells.48 However, a study that immobilized anti-DEC205 antibodies to the surface of OVA-bearing PLG particles by avidin-conjugation reported that crosslinking of the DEC205 receptor led to increased production of IL-10 by DCs and T cells, even when the nanoparticle was used to boost immunity induced by an OVA-CFA immunization.94 This observation is not entirely surprising since a number of studies exist that have targeted chimeric antibody-antigen complexes to DEC205 for the purpose of immunoregulation.95–98 The discrepancy between the two nanovaccine studies may be attributed to the incorporation of TLR ligands with regards to the former study,48 and a high density of anti-DEC205 antibody in the latter study,94 which purportedly led to increased DEC205 cross-linking.
Other strategies that have been used to enhance the cross-presentation of nanovaccines to CD8+ T cells involve optimizing parameters of the NP design such as size, material and charge. In agreement with the work published by Hubbell and colleagues,65 Li et al. observed that smaller (60 nm) α-Al2O3-OVA NPs showed better accumulation in the lymph nodes draining the site of injection and induced more robust OT-I proliferation than larger (200 nm) α-Al2O3-OVA NPs.64 Interestingly however, 25 nm α-Fe2O3-OVA NP did not induce similar levels of OT-I proliferation, suggesting that the ability of α-Al2O3-OVA NP to induce optimal cross-presentation was a consequence of the inherent functional properties of α-Al2O3 NP. Li and colleagues observed that the superior potential of the α-Al2O3-OVA NP to cross-prime CD8+ T cells could be attributed to the ability of the α-Al2O3-OVA NPs to access the autophagosome, a subcellular compartment that degrades damaged or decaying organelles and is consequently rich with endogenous antigens that can be presented via the MHC class I pathway.99 To this extent, the authors found that cross-presentation of α-Al2O3-OVA could be inhibited by treatment of α-Al2O3-OVA-bearing DCs with Brefeldin A, an inhibitor the non-canonical autophagy pathway.100 Cationic nanoparticles bearing protein antigens have also been reported to promote cross-priming of CD8+ T cells due to their ability to rupture the endo-lysosome and release its contents into the cytosol where the antigens can be directed towards the MHC class I pathway. OVA protein adsorbed onto cationic DDA/TDB NPs incorporating poly(I:C) were demonstrated to generate long-term central and effector memory CD8+ T cell responses to SIINFEKL, an MHC class I-restricted peptide derived from OVA, and that this response was further enhanced if the DDA/TDB-OVA NPs were fabricated by the double emulsion method to form MLVs.59 The observation that MLVs were more efficient at cross-priming a CD8+ T cell response is in agreement with the study by Moon et al. in which MLVs and ICMVs induced a greater CD8+ T cell response to OVA than did monolayer liposomes encapsulating OVA.62 MLVs may better promote cross-presentation due to their stability in the extracellular environment, persistence in the lymph nodes, and prolonged antigen delivery. Reduction-sensitive antigen-conjugation to NPs has been reported to better facilitate the cross-priming of cytotoxic T cells when compared to antigen delivery by encapsulation or reduction-insensitive conjugation,101 and this has been suggested to be the result of conjugated antigens rapidly accessing the endosome in their native form.
Artificial Antigen Presenting Cells
One strategy that obviates the necessity of targeting nanoparticle vaccines to the appropriate APC population for the development of protective immunity is the use of artificial APCs (aAPCs). This approach involves the use of nanoparticles that express TCR agonists, with or without antibodies that stimulate costimulatory receptors to directly activate T cells. In one study, 60-160 OVA323-339/I-Ad molecules were incorporated into 60 nm liposomes through biotin-avidin conjugation, leading to the production of IL-2 by OVA323-339 peptide-specific CD4+ T cell hybridomas.102 Whether or not this approach led to the induction of a functional T cell response was not investigated, although the likely outcome of TCR stimulation in the absence of costimulation is anergy.103 In another study, aAPCs were manufactured by coupling HLA-Ig and anti-CD28 antibodies to the surface of polystyrene particles pulsed with viral antigen. The culture of CD8+ T cells with these aAPCs promoted the generation and expansion of both low and high-affinity virus-specific CTLs that were capable of mediating cytotoxicity against cells endogenously presenting CMV and MART-1 viral antigens.104 Interestingly, one study reported that the administration of aminated iron-oxide nanoparticles coated with peptide/MHC complexes bearing diabetogenic epitopes from islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), a pancreatic β cell antigen, induced the expansion of low-avidity IGRP reactive CD8+ T cells in vivo.105 However, rather than enhancing autoimmunity diabetes, this treatment led to the induction of a regulatory CD8+ T cell population that suppressed the development of type 1 diabetes in the NOD mouse. This strategy was also effective in preventing diabetes onset and restoring euglycemia in a humanized NOD model of diabetes if IGRP and Insulin-HLA complexes were conjugated to the nanoparticle surface. The ability of this platform to induce tolerance was dependent upon perforin-mediated killing of APCs, and the induction of indoleamine-2,3-dioxygenase (IDO) since treatment with 1-methyl-[D]-tryptophan, an IDO inhibitor, prevented the therapeutic effect of the nanoparticle administration.105 Since this study did not incorporate costimulatory agonists into the nanoparticle platform, it remains to be determined whether or not the administration of aAPCs can also be effective at boosting anti-tumor or anti-viral responses in vivo.
Nanoparticles and Tolerance
The induction of antigen-specific immunological tolerance has vast potential for the treatment of autoimmunity, allergy, and transplant rejection. Central to each of these disorders is the dysregulation of adaptive immunity caused by the breakdown of peripheral tolerance,106 and the subsequent induction of T cell-mediated responses that target tissue and environmental antigens. Treatment of these disorders are often limited to broad immunosuppression, and as such, the induction of immunological tolerance has occupied an area of intense interest for the better part of a century and remains the holy grail of basic and translational scientific research for the regulation of immune-mediated diseases.107 While nanoparticles as platforms for immunotherapy have most often been described in the context of vaccination delivery, the immunomodulatory potential of nanoparticles has warranted their investigation as a tool for immunoregulation. To this extent, strategies have been developed with the purpose of inhibiting T cell function at the level of the APC-T cell interaction or by targeting pathogenic T cells directly and indirectly via the activation of Tregs.108 Moreover, many of the same principles critical for promoting T cell activation in vaccination strategies are important for directing approaches for the induction of T cell tolerance including the route of administration, properties of the nanoparticle platform – size and surface charge, and the use of adjuvants are all parameters that can dictate the fate of a T cell during antigen-recognition.
The intravenous route of administration has long been associated with the induction of immunological unresponsiveness. The Sulzberger-Chase studies dating back to the late 1920’s reported that the intravenous administration of haptens led to the induction of hapten-specific, immunological unresponsiveness,109 and intravenous soluble protein or peptide therapy has also been a potent strategy for inducing peripheral tolerance in experimental settings.110 Particulate substances injected intravenously are rapidly engulfed and degraded by specialized phagocytes in the spleen and liver, limiting the duration of antigen exposure which is critical for mounting an effective immune response. Furthermore, marginal zone macrophages (MZ MΦ) of the spleen clear the body of apoptotic cells and debris, while concomitantly suppressing adaptive immune responses to the re-presented self-antigens.111 This second point is exemplified by the observation that disruption of the MZ MΦs by treatment with low-dose clodronate liposomes has been observed to enhance the development of autoimmune systemic lupus erythematosus in genetically susceptible mice.111 Although the exact mechanism behind the unresponsiveness induced by the intravenous administration of antigen is not known, it may be due in part to the short availability of antigens presented intravenously,112 and the immature or tolerogenic phenotype of the APCs that encounter, acquire, and present the antigens to T cells.
One approach that was born out of the Sulzberger-Chase phenomenon was a form of coupled cell tolerance using ECDI-fixed, antigen-coupled syngeneic splenocytes (Ag-SP).113 Ag-SP is a powerful strategy for the induction of peripheral immunological tolerance, and has been demonstrated to both prevent and treat aberrant T cell responses in experimental models of autoimmunity, allergy, and transplant rejection.107,113,114 The use of ECDI chemistry to conjugate antigens to the surface of splenocytes also causes rapid apoptosis in the fixed cells following intravenous infusion,115,116 thereby leading to their subsequent uptake by MZ MΦs via scavenger receptors.117 The downstream effect of Ag-SP internalization is the induction of a regulatory phenotype by the MZ MΦ; these cells upregulate the production of immunosuppressive cytokines IL-10 and TGF-β, exhibit increased expression of the regulatory costimulatory ligand PD-L1, and fail to enhance the expression of positive costimulatory ligands such as CD80 and CD86.117 In the absence of positive costimulation, these inhibitory signals converge upon a T cell during antigen-recognition to constrain the production of IL-2, culminating in a state of T cell anergy.103 In addition, antigen presentation in the presence of IL-10 and TGF-β is a potent immunoregulatory milieu leading to the activation of antigen-specific Tregs which synergizes with anergy to mediate Ag-SP tolerance.114,117 This approach can be used to selectively inhibit responses to either antigenic peptides or whole proteins, and has significant potential for limiting the progression of autoimmune disease by epitope spreading.118
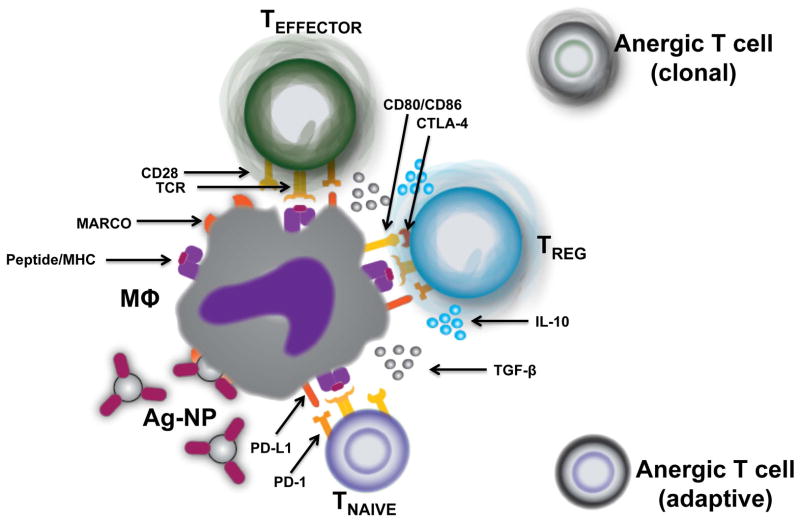
We have recently demonstrated that the intravenous infusion of antigen-coupled, highly carboxylated 500 nm polystyrene and PLG microparticles can function effectively as surrogates for the Ag-SP to promote the establishment of peripheral T cell tolerance (Figure 3).118 While biodegradable PLG NPs are well-characterized and frequent candidates for encapsulated antigen delivery, studies showing their applicability as antigen platforms were limited. Tolerance induced by ECDI-fixed, antigen-coupled microparticles (Ag-MP) led to a defect in in the proliferation of antigen-specific T cells, both in vitro and in vivo; additionally, proliferation could be rescued in vitro if exogenous IL-2 was present during antigen-specific restimulation, and these observations are consistent with the induction of an anergic state.119 T cell differentiation and cytokine production was also largely inhibited by Ag-MP treatment, since antigen-specific T cells isolated from mice that were challenged with Ag-MP failed to produce IFN-γ and IL-17 when they were restimulated ex vivo with specific antigen or by PMA and ionomycin. Tolerance induction mediated by treatment with Ag-MP was dependent upon the route of administration, since intravenous injection of Ag-MP led to the establishment of tolerance while subcutaneous administration did not. Size was also a determining factor in the induction of tolerance by Ag-MP in that 500 nm Ag-MP were efficient at inducing tolerance for the prevention of experimental autoimmune encephalomyelitis (EAE), a mouse model of the Th1/17 autoimmune diseases multiple sclerosis, while 100 nm Ag-MP failed to induce tolerance and protect mice from developing disease and particles larger than 1 μm were not as efficient. Ag-MPs were found in the spleen, liver, and lungs of mice following intravenous infusion, and analyses of the spleen via immunohistochemistry at 3 hours post-injection showed localization of the fluorescent Ag-MP with the macrophage scavenger receptors MARCO and SIGNR-1, suggesting a possible role for scavenger receptor mediated endocytosis of the Ag-MP. To this extent, mice genetically deficient in MARCO could not be rendered tolerant by Ag-MP but were still susceptible to tolerance induction by Ag-SP, demonstrating a non-redundant role for MARCO in the uptake of Ag-MP. MARCO has been shown to play a role in the clearance of polyanionic substances such as LPS, and the crosslinking of antigen onto the carboxylated particles may provide repeated motifs that solicit engagement by MARCO. In turn, MARCO-mediated endocytosis has been reported to affect innate immune deactivation and tolerance120.
FIGURE 3.
The uptake of Ag-NP by splenic marginal zone macrophages leads to the induction of T cell tolerance. A) Ag-NP in the spleen are phagocytosed by marginal zone macrophages (MΦ) through the scavenger receptor MARCO, leading to a tolerogenic phenotype and an increase in PD-L1 and immunoregulatory cytokine expression. B) The immunoregulatory milieu supports the induction and/or activation of Foxp3+ Tregs that may limit the ability of splenic APCs to prime naïve T cells by producing immunosuppressive cytokines and by CTLA-4-mediated trans-endocytosis of CD80/CD86 molecules. Cross-presentation of the antigen to a T cell (signal 1) in the absence of costimulation (signal 2) causes abortive activation and defective IL-2 production, leading to the induction of adaptive or clonal T cell anergy in naïve and differentiated effector T cells, respectively.
The incorporation of tolerogenic ligands into nanoparticle platforms has also been described. The cationic polymer polyamine polyethelenimine (PEI) can be used to form conjugates with DNA (DNA/PEI) as a delivery platform for plasmid DNA transduction. However, due to the presence of CpG oligonucleotides present in DNA this approach has been demonstrated to promote significant immune activation and pro-inflammatory cytokine production.121,122 Interestingly, a recent study by Huang et al. reported that DNA/PEI nanoparticles could promote the induction of tolerance in vivo. When modified DNA/PEI nanoparticles lacking CpG motifs were administered to mice with rheumatoid arthritis, a significant therapeutic effect was observed. Treatment with DNA/PEI inhibited joint inflammation, suppressed antigen-specific T cells responses, and led to the induction of Tregs through IFN-αβ-mediated IDO expression by DCs.123 Alternatively, activation of the aryl hydrocarbon receptor (AHR) by the ligand 2-(1′H -indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) promotes a tolerogenic phenotype in DCs that leads to the differentiation of Tregs.124 A recent study reported that the conjugation of CNS myelin peptides and ITE to 60 nm gold particles (NP-ITE) was found to suppress the development of EAE, and therapeutically ameliorated ongoing EAE disease following repeated administration.125 Furthermore, the transfer of CD4+ T cells from NP-ITE treated mice suppressed the induction of EAE in naïve recipients, but only if Tregs were present in the transferred CD4+ T cell population since depletion of FoxP3+ cells expressing a GFP reporter abrogated the suppressive effect of the transferred CD4+ T cells.125 Together, these data illustrate the usefulness of incorporating tolerogenic ligands into nanoparticles as adjuvants to promote regulatory outcomes.
CONCLUSIONS
The use of antigen-conjugated nanoparticles for vaccine delivery is a rapidly expanding method of immunotherapy. For the investigator, a significant level of attention must be invested in the design of the nanoparticle platform as the nanoparticle material, size, shape, charge, route, and the incorporation of ligands can be manipulated to affect nanoparticle interaction with the immune system to achieve the desired outcome. Evidence from recent publications that have reported the use of nanoparticles for the induction of immunological tolerance has suggested that these same parameters can be used to effectively target immunoregulatory pathways for the treatment of aberrant T cell responses and experimental models of autoimmunity. While the use of antigen-conjugated nanoparticles appears promising as an immunotherapy under experimental settings, its viability as a clinical application remains to be determined. Future studies will need to examine dosing and toxicology associated with the infusion antigen-conjugated nanoparticles in preclinical models in order for the methodology to progress to the clinic.
Acknowledgments
Work discussed in this review was funded by grants from the National Institutes of Health, the National Multiple Sclerosis Society, the Juvenile Diabetes Research Foundation and the Myelin Repair Foundation
References
- 1.Zhang L, Webster TJ. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 2.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Ann Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Ann Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001;14:495–498. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 7.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 8.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Ann Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 11.Cubas R, Zhang S, Kwon S, Sevick-Muraca EM, Li M, Chen C, Yao Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32:118–128. doi: 10.1097/CJI.0b013e31818f13c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 13.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 14.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci USA. 2011;108:989–997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stano A, van der Vlies AJ, Martino MM, Swartz MA, Hubbell JA, Simeoni E. PPS nanoparticles as versatile delivery system to induce systemic and broad mucosal immunity after intranasal administration. Vaccine. 2011;29:804–812. doi: 10.1016/j.vaccine.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 17.Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, Pasquier M, Simeoni E, van der Vlies AJ, McKinney JD, Hubbell JA, Swartz MA. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29:6959–6966. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 19.Makino K, Yamamoto N, Higuchi K, Harada N, Ohshima H, Terada H. Phagocytic uptake of polystyrene microspheres by alveolar macrophages: effects of the size and surface properties of the microspheres. Colloids and Surfaces B: Biointerfaces. 2003;27:33–39. [Google Scholar]
- 20.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 21.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma A, Uzun O, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 24.Campbell RB, Balasubramanian SV, Straubinger RM. Influence of cationic lipids on the stability and membrane properties of paclitaxel-containing liposomes. J Pharm Sci. 2001;90:1091–1105. doi: 10.1002/jps.1063. [DOI] [PubMed] [Google Scholar]
- 25.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 26.Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Cont Rel. 2003;93:271–282. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 30.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Cont Rel. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 31.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Cont Rel. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 32.Kwon YJ, Standley SM, Goh SL, Frechet JM. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J Cont Rel. 2005;105:199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hagan DT, Singh M, Dong C, Ugozzoli M, Berger K, Glazer E, Selby M, Wininger M, Ng P, Crawford K, Paliard X, Coates S, Houghton M. Cationic microparticles are a potent delivery system for a HCV DNA vaccine. Vaccine. 2004;23:672–680. doi: 10.1016/j.vaccine.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc Natl Acad Sci USA. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 36.Keegan ME, Falcone JL, Leung TC, Saltzman M. Biodegradable microspheres with enhanced capacity for covalently bound surface ligands. Macromol. 2004;37:9979–9784. [Google Scholar]
- 37.Lewis JS, Zaveri TD, Crooks CP, 2nd, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33:7221–7232. doi: 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle--peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem. 2008;19:145–152. doi: 10.1021/bc700227z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 40.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK, Stauber RH. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 42.Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 44.Yan Y, Gause KT, Kamphuis MM, Ang CS, O’Brien-Simpson NM, Lenzo JC, Reynolds EC, Nice EC, Caruso F. Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines. ACS nano. 2013;7:10960–10970. doi: 10.1021/nn404481f. [DOI] [PubMed] [Google Scholar]
- 45.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 46.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 48.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 49.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Ann Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Broos S, Sandin LC, Apel J, Totterman TH, Akagi T, Akashi M, Borrebaeck CA, Ellmark P, Lindstedt M. Synergistic augmentation of CD40-mediated activation of antigen-presenting cells by amphiphilic poly(gamma-glutamic acid) nanoparticles. Biomaterials. 2012;33:6230–6239. doi: 10.1016/j.biomaterials.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv Mater. 2012;24:3981–3987. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou HY, Lin XZ, Pan WY, Wu PY, Chang CM, Lin TY, Shen HH, Tao MH. Hydrogel-delivered GM-CSF overcomes nonresponsiveness to hepatitis B vaccine through the recruitment and activation of dendritic cells. J Immunol. 2010;185:5468–5475. doi: 10.4049/jimmunol.1001875. [DOI] [PubMed] [Google Scholar]
- 53.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Recent Results Cancer Res. 1976:58–64. doi: 10.1007/978-3-642-81049-7_8. [DOI] [PubMed] [Google Scholar]
- 55.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Jr, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol. 2006;23:385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 57.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, Agger EM, Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PloS one. 2008;3:e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, Foged C. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Cont Rel. 2011;150:307–317. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Shek PN, Heath TD. Immune response mediated by liposome-associated protein antigens. III. Immunogenicity of bovine serum albumin covalently coupled to vesicle surface. Immunology. 1983;50:101–106. [PMC free article] [PubMed] [Google Scholar]
- 61.Bhowmick S, Mazumdar T, Sinha R, Ali N. Comparison of liposome based antigen delivery systems for protection against Leishmania donovani. J Cont Rel. 2010;141:199–207. doi: 10.1016/j.jconrel.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 66.St John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–257. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev Vaccines. 2011;10:1569–1583. doi: 10.1586/erv.11.135. [DOI] [PubMed] [Google Scholar]
- 68.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, Highfield PE, Rowlands DJ, Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature. 1987;330:381–384. doi: 10.1038/330381a0. [DOI] [PubMed] [Google Scholar]
- 70.Morrow J. Vaccinology: principles and practice. Wiley-Blackwell; Chichester, West Sussex: 2012. [Google Scholar]
- 71.Whitacre DC, Lee BO, Milich DR. Use of hepadnavirus core proteins as vaccine platforms. Expert Rev Vaccines. 2009;8:1565–1573. doi: 10.1586/erv.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 73.Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, Maier C, Andrews L, Andersen RF, Gilbert S, Poulton I, Webster D, Dubovsky F, Tierney E, Sarpotdar P, Correa S, Huntcooke A, Butcher G, Williams J, Sinden RE, Thornton GB, Hill AV. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 74.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006;24:6321–6331. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 75.Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T, Bachi T, Storni T, Jennings G, Pumpens P, Renner WA, Bachmann MF. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20:3104–3112. doi: 10.1016/s0264-410x(02)00266-9. [DOI] [PubMed] [Google Scholar]
- 76.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 77.Li QY, Gordon MN, Chackerian B, Alamed J, Ugen KE, Morgan D. Virus-like peptide vaccines against Abeta N-terminal or C-terminal domains reduce amyloid deposition in APP transgenic mice without addition of adjuvant. J Neuroimmune Pharmacol. 2010;5:133–142. doi: 10.1007/s11481-009-9183-1. [DOI] [PubMed] [Google Scholar]
- 78.Maurer P, Bachmann MF. Immunization against angiotensins for the treatment of hypertension. Clin Immunol. 2010;134:89–95. doi: 10.1016/j.clim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk HD, Stocker H, Muller P, Jennings GT, Wagner F, Bachmann MF. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 80.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PloS one. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 82.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kierny MR, Cunningham TD, Kay BK. Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano Rev. 2012:3. doi: 10.3402/nano.v3i0.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JH, Choi YJ, Lim YB. Self-assembled filamentous nanostructures for drug/gene delivery applications. Expert Opin Drug Deliv. 2010;7:341–351. doi: 10.1517/17425240903559841. [DOI] [PubMed] [Google Scholar]
- 85.Aldinucci A, Turco A, Biagioli T, Toma FM, Bani D, Guasti D, Manuelli C, Rizzetto L, Cavalieri D, Massacesi L, Mello T, Scaini D, Bianco A, Ballerini L, Prato M, Ballerini C. Carbon nanotube scaffolds instruct human dendritic cells: modulating immune responses by contacts at the nanoscale. Nano Lett. 2013;13:6098–6105. doi: 10.1021/nl403396e. [DOI] [PubMed] [Google Scholar]
- 86.Ji SR, Liu C, Zhang B, Yang F, Xu J, Long J, Jin C, Fu DL, Ni QX, Yu XJ. Carbon nanotubes in cancer diagnosis and therapy. Biochim et Biophys acta. 2010;1806:29–35. doi: 10.1016/j.bbcan.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Stone JW, Thornburg NJ, Blum DL, Kuhn SJ, Wright DW, Crowe JE., Jr Gold nanorod vaccine for respiratory syncytial virus. Nanotechnol. 2013;24:295102. doi: 10.1088/0957-4484/24/29/295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andersen AJ, Wibroe PP, Moghimi SM. Perspectives on carbon nanotube-mediated adverse immune effects. Adv Drug Deliv Rev. 2012;64:1700–1705. doi: 10.1016/j.addr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS nano. 2012;6:10366–10377. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Son YJ, Kim H, Leong KW, Yoo HS. Multifunctional nanorods serving as nanobridges to modulate T cell-mediated immunity. ACS nano. 2013;7:9771–9779. doi: 10.1021/nn403275p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nair-Gupta P, Blander JM. An Updated View of the Intracellular Mechanisms Regulating Cross-Presentation. Front Immunol. 2013;4:401. doi: 10.3389/fimmu.2013.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 93.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 94.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32:3094–3105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci U S A. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petzold C, Riewaldt J, Koenig T, Schallenberg S, Kretschmer K. Dendritic cell-targeted pancreatic beta-cell antigen leads to conversion of self-reactive CD4(+) T cells into regulatory T cells and promotes immunotolerance in NOD mice. Rev Diabet Stud. 2010;7:47–61. doi: 10.1900/RDS.2010.7.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukherjee G, Geliebter A, Babad J, Santamaria P, Serreze DV, Freeman GJ, Tarbell KV, Sharpe A, DiLorenzo TP. DEC-205-mediated antigen targeting to steady-state dendritic cells induces deletion of diabetogenic CD8(+) T cells independently of PD-1 and PD-L1. Int Immunol. 2013;25:651–660. doi: 10.1093/intimm/dxt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–2947. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- 99.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 100.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 101.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine. 2010;28:7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 102.Prakken B, Wauben M, Genini D, Samodal R, Barnett J, Mendivil A, Leoni L, Albani S. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat Med. 2000;6:1406–1410. doi: 10.1038/82231. [DOI] [PubMed] [Google Scholar]
- 103.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 105.Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 106.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 107.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 108.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells -- ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Sulzberger MB. Hypersensitivities to arsphenamine in guinea pigs: Experiments in prevention and in desensitization. Arch Dermatol Syphilol. 1929;20:669–697. [Google Scholar]
- 110.Chiller J, Weigle W. Cellular events during induction of immunologic unresponsiveness in adult mice. J Immunol. 1971;106:1647–1653. [PubMed] [Google Scholar]
- 111.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 112.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 113.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol. 2013;191:5341–5346. doi: 10.4049/jimmunol.1302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaneko K, Morelli AE, Wang Z, Thomson AW. Alloantigen presentation by ethylcarbodiimide-treated dendritic cells induces T cell hyporesponsiveness, and prolongs organ graft survival. Clin Immunol. 2003;108:190–198. doi: 10.1016/s1521-6616(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 116.Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 117.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwartz RH. T cell anergy. Ann Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 120.Jing J, Yang IV, Hui L, Patel JA, Evans CM, Prikeris R, Kobzik L, O’Connor BP, Schwartz DA. Role of macrophage receptor with collagenous structure in innate immune tolerance. J Immunol. 2013;190:6360–6367. doi: 10.4049/jimmunol.1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawakami S, Ito Y, Charoensit P, Yamashita F, Hashida M. Evaluation of proinflammatory cytokine production induced by linear and branched polyethylenimine/plasmid DNA complexes in mice. J Pharmacol Exp Ther. 2006;317:1382–1390. doi: 10.1124/jpet.105.100669. [DOI] [PubMed] [Google Scholar]
- 122.Rodrigo-Garzon M, Berraondo P, Ochoa L, Zulueta JJ, Gonzalez-Aseguinolaza G. Antitumoral efficacy of DNA nanoparticles in murine models of lung cancer and pulmonary metastasis. Cancer Gene Ther. 2010;17:20–27. doi: 10.1038/cgt.2009.45. [DOI] [PubMed] [Google Scholar]
- 123.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, McGaha TL, Ravishankar B, Lee JR, Munn DH, Mellor AL. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188:4913–4920. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109:11270–11275. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]