Abstract
Hair follicles are skin appendages of the mammalian skin that have the ability to periodically and stereotypically regenerate in order to continuously produce new hair over our lifetime. The ability of the hair follicle to regenerate is due to the presence of stem cells that along with other cell populations and non-cellular components, including molecular signals and extracellular material, make up a niche microenvironment. Mounting evidence suggests that the niche is critical for regulating stem cell behavior and thus the process of regeneration. Here we review the literature concerning past and current studies that have utilized mouse genetic models, combined with other approaches to dissect the molecular and cellular composition of the hair follicle niche. We also discuss our current understanding of how stem cells operate within the niche during the process of tissue regeneration and the factors that regulate their behavior.
Keywords: Stem Cells, Niche, Hair Follicle, Regeneration, Fate
1. Introduction
Adult stem cells have the unique capacity to generate all the differentiated cell types within the tissue, and to self-renew in order to replenish their pool. Due to these features, stem cells can maintain and regenerate tissues both in homeostasis and after injury. Research in various tissues suggests that regeneration largely depends on the interactions between stem cells and the environment where they reside and operate [1,2]. The importance of the microenvironment is highlighted by the fact that stem cells reside in topographically and molecularly well-defined locations across different organs in the adult body [3–10]. These specialized tissue compartments that host the stem cells and every other component necessary for their function, including neighboring cell populations, molecular signals and other extracellular components are commonly referred to as “niches” [11–13].
The skin is the largest organ in the mammalian body and a tissue that is continuously replenished throughout our lifetime. In the interfollicular epidermis (IFE), which constitutes the outermost part of the skin, cells in contact with the basement membrane constantly proliferate and differentiate while gradually being displaced to more superficial layers to replace cells that are naturally being shed [14,15]. Thus, IFE is maintained uniformly through a seemingly continuous regeneration process fueled by stem cells dispersed throughout the basal IFE but the presence and the defined characteristics of an IFE niche is subject to further research [11,16,17]. In contrast, skin appendages and other accessory organs, including hair, nails, sebaceous and sweat glands have distinct niches and stem cells that serve to maintain each respective skin compartment [10,18–21]. In particular, hair follicles constitute an anatomically and molecularly well-defined stem cell niche. Typically hair follicles regenerate in cyclic bouts consisting of growth, regression and rest phases that collectively result in the production of a mature, fully-grown hair shaft that extends over the surface of the skin [22]. The process of hair growth is stereotypical and elegantly choreographed so that stem cells and differentiated cell types occupy distinct locations within the niche, consistent with their particular role in hair growth. Taken together these features make the hair follicle niche one of the most attractive and intensely studied systems in mammalian stem cell biology.
While the molecular and cellular composition of the hair follicle stem cell niche is very well characterized, understanding how all the niche elements work together to stereotypically and physiologically regenerate a new hair is still a work in progress. Some of the lingering questions include: 1) how stem cells are activated to commence a new regeneration cycle, 2) how stem-cell fate and respective contribution to the regeneration program is determined and 3) what are the dynamic cellular behaviors that lead to a properly regenerated hair? In this report we will review the current literature and discuss our present understanding of these fundamental questions.
2. Molecular and cellular composition of the hair follicle niche
The hair follicle undergoes dramatic morphological and molecular changes during the different phases of the hair cycle, as stem cells in the niche become activated and proliferate to generate a transiently amplified population that ultimately follows a differentiation process to build the new hair shaft. In the following segments of this review and for reasons of clarity we refer to the hair follicle niche at its minimal state during the resting phase of the hair cycle (Telogen; Fig. 1), unless otherwise stated.
Figure 1. Anatomy and molecular heterogeneity of the hair follicle niche.
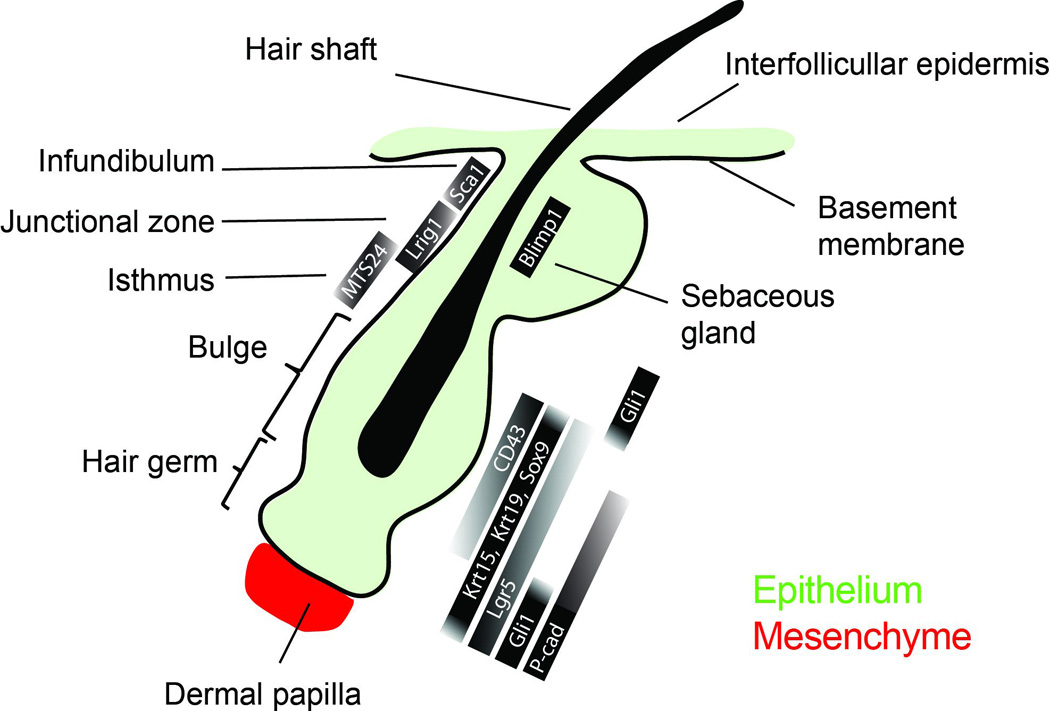
Scheme of the mouse hair follicle at the resting phase of the hair regeneration cycle. Hair follicle stem cells reside in the bulge, while a pool of progenitor cells, which forms the hair germ, is situated directly below and in contact with the mesenchymal dermal papilla. Other epithelial cell populations exist in defined anatomical tissue compartments located above the bulge. The hair follicle niche displays a pronounced molecular heterogeneity, which is evident by the distribution and level of expression of various genes.
2.1 Bulge and hair germ
Within the hair follicle niche the first stem cell compartment to be identified was the bulge (Fig. 1). Specifically, pulse-chase experiments using nucleotide analogs in young mice revealed slow-cycling cell populations residing within the non-cycling, permanent portion of the follicle [10]. These findings were later expanded with the use of a mouse genetic system to pharmacologically control the expression of histone H2B linked to green fluorescent protein (GFP), which allowed further molecular characterization of bulge stem cells [18]. Although the term “bulge” was first introduced to describe certain anatomical characteristics in the developing human hair, the bulge is not always morphologically well-defined as a distinct cell compartment throughout the lifecycle of the hair follicle [23,24]. A more simple definition of the bulge simply refers to the location within the hair follicle where stem cells reside [25].
Since a universal marker that can indisputably identify stem cells in adult tissues is still elusive, researchers have utilized an arsenal of molecular and cell-based assays to purify and characterize bulge stem cells. Early experiments using micro-dissected rat whiskers showed that bulge cells display high clonogenic capacity in vitro and multipotency in ex vivo transplantation and grafting experiments [26,27]. Subsequently, the identification of bulge-specific molecular markers led to a more detailed characterization of bulge stem cells [18,28–30]. In vivo lineage tracing experiments showed that the progeny of Keratin 15 (K15) positive stem cells participated in all the epithelial lineages of the hair follicle in full growth [31]. Furthermore, purification and subsequent transplantation of K15+/integrin α6+ or CD34+/ integrin α6+ bulge cells, showed a contribution to all the epithelial skin layers confirming their identity as hair follicle stem cells [31,32]. In addition to these markers, bulge stem cells in the mouse hair follicle can also be identified based on the expression of Krt19 [33,34], Lgr5 [9,35] as well as the transcriptional factors Gli1 [36], Hopx [8], Lhx2 [37], Nfatc1 [38], Sox9 [39] and Tcf3 [40] (Fig 1). Therefore, bulge stem cells can be reliably identified and studied by their specific location within the hair follicle niche, their slow cycling nature and their specific molecular profile.
For many years the bulge was considered the single most important epithelial cell pool, required for hair regeneration [10,27,41]. The hair germ, which represents an anatomically recognizable epithelial population situated below the bulge and in direct contact with a specialized mesenchymal compartment called dermal papilla (DP) [42], was not considered as a functionally distinct niche compartment (Fig. 1). However, it was later demonstrated that the hair germ was indeed biochemically different from the bulge [30,43–45]. More recent studies radically changed the bulge-centric view of the hair follicle niche based on the discovery that the hair follicle niche has a molecular and functional bi-compartmental organization [13,46,47]. These studies demonstrated that cells in the hair germ are the first to express genes indicative of stem cell activation and the first to proliferate at the onset of a new hair regeneration cycle, before the subsequent bulge proliferation at later growth stages [46,47], thus establishing the hair germ as a distinct niche population. In contrast to the bulge, hair germ cells do not express CD34 or Nfatc1 but instead display high levels of P-cadherin [46] (Fig1).
2.2 Isthmus, infundibulum and sebaceous gland
The isthmus is the epithelial compartment that is situated between the bulge and the base of the sebaceous gland (Fig1). Cells in the isthmus are Krt15- and CD34- but instead express high levels of Gli1, MTS24 and Lgr6 [36,48–50]. However, these markers are only partially overlapping within the isthmus suggesting a functional heterogeneity of cells that occupy this compartment. Isthmus cells display stem cell characteristics and can generate hair follicle lineages either in homeostasis or after grafting in skin reconstitution assays. [36,49]. Another marker specific for stem cells situated in the isthmus is Lrig1 [51,52]. Lrig1+ cells which occupy the same space as Lgr6+ cells in the isthmus do not participate in hair follicle regeneration under physiological conditions [52,53]. Instead, long-term lineage tracing showed that Lrig1+ cells contribute to the maintenance of the infundibulum [53]. However, in contrast to the infundibulum different stem cell populations appear to contribute to sebaceous gland maintenance including Lrig1+ cells and a different population expressing Blimp1, situated at the base of the sebaceous glands in the junctional zone [19,31,32,52]. Recent studies suggest that Blimp1 marks mature sebocytes that are situated suprabasally and originate from Lrg1+ stem cells distinct from those contributing to the infundibulum [53,54]. Finally the upper portion of the infundibulum is occupied by another stem cell population that expresses Sca-1 and can regenerate the IFE but not the hair follicle [49].
3. Defining the hair follicle niche microenvironment
3.1 The mesenchymal niche
The mesenchymal niche is primarily composed by a dense group of dermal fibroblasts known as the dermal papilla (DP), which are in direct contact with the epithelium at the tip of the hair follicle (Fig. 1, 2a). Lineage analysis suggests that DP cells derive from the neural crest at least in the cranial region of the skin but may have a diverse origin in other parts of the body [55]. The ability of the DP to induce hair growth and its fundamental role as a signaling center in hair regeneration were demonstrated by pioneering transplantation experiments using microdissected DPs [56,57]. Subsequently, molecular characterization and purification of DP cells verified these results by showing their inductive capacity when co-transplanted with keratinocytes [58,59]. More recently, using laser-induced or genetic ablation of dermal papilla cells, it was unequivocally demonstrated that the mesenchymal niche is required for hair growth but not for epithelial hair follicle maintenance at the quiescent state [47,60]. The origin of the DP, its size, organization and level of interaction with the epithelium are all factors that significantly affect different aspects of the hair follicle physiology, including its regenerative capacity, cycling characteristics and hair type specification [57,60–62]. In addition to the DP, a layer of mesenchymal fibroblasts called the dermal sheath (DS), lines the outer surface of the hair follicle continuously from the base of the DP to the upper bulge. The DS displays some similarities with the DP in terms of origin and gene expression, which has led to the hypothesis of a dynamic cell trafficking between the two compartments [62–65]. However, the exact role of the DS on stem cell physiology and hair regeneration is not well understood.
Figure 2. The hair follicle niche microenvironment and stem cell fate.
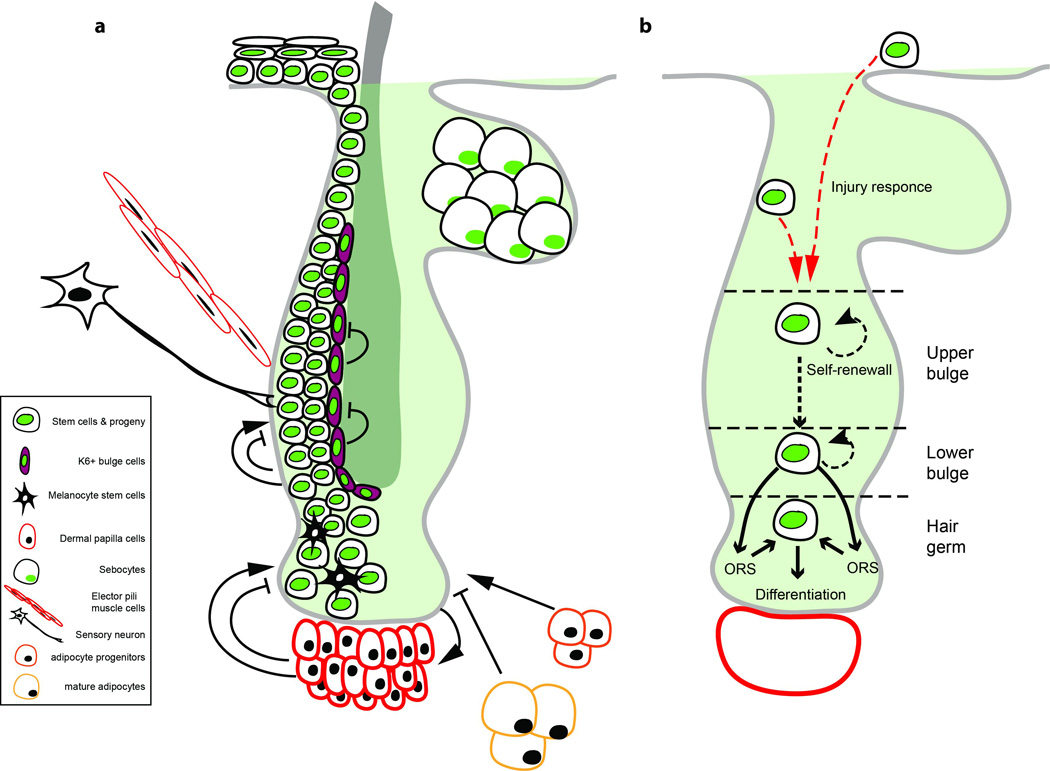
a) Scheme of the hair follicle depicting the various factors that constitute the niche microenvironment. b) Scheme of stem cell behavior in homeostasis and after injury. Under physiological conditions stem cells in the upper portion of the bulge do not participate directly to hair regeneration and are more likely to self-renew. Cells in the lower bulge generate precursors that populate the ORS during hair growth, while those that survive the regressing phase return to the niche to form, at least in part, the new hair germ before the onset of a new regeneration cycle. Cells in the hair germ directly contribute to hair growth producing all the differentiated cell types. Following niche injury, non-hair epithelial cells above the bulge may enter the niche changing their fate to actively participate in hair regeneration.
3.2 Molecular signals
Hair follicle physiology is subject to several signaling pathways that converge to regulate stem cell quiescence, proliferation and differentiation, including Wnt, BMP and FGF18 [18,66–70] (Fig. 2a). BMP, TGF-β and FGF ligands as well as Wnt inhibitors, emanating from the mesenchymal dermal papilla or from the bulge act collectively to maintain quiescence in the niche during the resting phase (telogen) [70–73]. Despite the relative cellular inactivity these signals constantly compete to maintain a delicate balance that when is tipped primes cells in the hair follicle to commence a new growth phase [46,70]. Activation of canonical Wnt signaling and subsequent stabilization and nuclear localization of β-catenin is critical for entry into anagen [68,70,74,75]. Proliferation in the matrix also depends on Wnt signaling [69,76]. Conversely, BMP ligands and receptors regulate the terminal differentiation program of the IRS and hair shaft precursors [58]. Notch signaling is also critical for differentiation in the mature hair follicle [77].
An open question is how these molecular signals act upon individual cells in the hair follicle niche to induce divergent behaviors at different stages of the hair cycle. In general, BMP signals repress cell proliferation while Wnt and other signals promote stem cell activation and growth. Bulge stem cells maintain a slow-cycling behavior due to an overabundance of BMP and FGF18 signals that are secreted from various sources including the DP, the inner K6+ bulge layer but also from the bulge stem cells themselves. When BMP suppression is overcome by activating Wnt signals, proliferation begins first in the hair germ possibly due the proximity of these cells to the DP which is the likely source of these signals [46]. Hair follicle growth is then sustained either from continuous signaling activity from the DP or alternatively due to a cascade of Wnt signals propagated from within the epithelium by a non-cell autonomous mechanism. All the signaling pathways that act upon the hair follicle are also subject to systemic regulation. A recent finding that circadian clock genes may be differentially active in distinct populations of hair follicle stem cells suggests that the response of the individual niche cells to activating stimuli may also be regulated systemically[78].
3.3 Other niche factors
Neighboring cell populations with diverse origin and function are also critical for defining the microenvironment of the niche (Fig. 2a). For example, melanocyte stem cells (MSCs) are also resident within the hair follicle niche and are tasked with generating the mature melanocytes that pigment the new hair during growth [79]. The physiology of MSCs and hair follicle stem cells is tightly linked since the survival and growth of MSCs depends on signaling from epithelial cells in the hair niche [80,81]. Furthermore, it was recently shown that the transcription factor NFIB is essential for linking the behavior of both hair follicle and MSCs [82].
Other cell populations outside the niche are also important for the normal physiology of the hair follicle (Fig. 2a). Adipocytes constitute a large population situated on the other side of the basement membrane in the deeper layers of the dermis. Subcutaneous adipose tissue follows a parallel growth pattern as the hair follicle, becoming thicker during anagen due to an increased adipocyte proliferation [70,83,84]. Evidence suggests that adipose cells play a role in regulating different stages of the hair cycle. Mature adipocytes act to keep the hair follicle niche in quiescence during the rest phase (telogen) by expressing BMP2, while immature adipocytes induce hair growth [70,84].
Each individual hair follicle is innervated by specialized sensory neurons. Hair follicle innervation is important for Hh signaling in the niche and the maintenance of a stem cell population situated above the bulge that expresses Gli1 [36]. This Gli1+ population, although multipotent, preferentially contributes to the IFE repair following injury but their role in hair regeneration is not clear. Furthermore, the arrector pili muscle attaches to the side of the hair follicle at the height of the bulge region and erects the hair shaft based on signals from the sympathetic nervous system. Bulge stem cells interact with the arrector pili muscle through integrins and this communication is essential for proper attachment of the muscle to the hair follicle [85]. The immune system may also play an important yet not a well-understood role in hair physiology. A recent finding that dermal γδ T-cells modulate hair follicle regeneration after skin injury provides a hint to that direction [86].
Non-cellular components of the hair follicle niche microenvironment such as the extracellular matrix are critical for regulating stem cell behavior [87]. The extracellular matrix acts on the niche directly by establishing the basal lamina, with which stem cells establish direct contacts. Integrins are transmembrane receptors that modulate these contacts, but also mediate critical signal transduction molecules that confer information from the environment to regulate fundamental cell functions [88–90]. There is increasing evidence that the physical forces acting on the niche microenvironment may regulate stem cell fate [91,92]. The extracellular matrix also provides critical polarity cues to niche stem cells, which affects their division patterns and their fate [93–95]. All these factors act in complex and often intersecting patterns to form a diverse niche microenvironment.
4. Stem cell behavior in hair follicle regeneration
Post-natal hair follicles have the ability to undergo spontaneous cycles of regeneration throughout the lifetime of the organism. This process, which is commonly referred to as the hair cycle, is highly stereotypic and consists of three distinct phases of growth (anagen), regression (catagen) and rest (telogen) [22] (Fig. 3). The hair cycle depends on stem cells, which provide the necessary number and type of specialized cells that are needed to construct and support the new hair. Understanding how stem cells behave within the niche is critical for elucidating the mechanism of hair follicle regeneration.
Figure 3. Cell dynamics in the hair cycle.
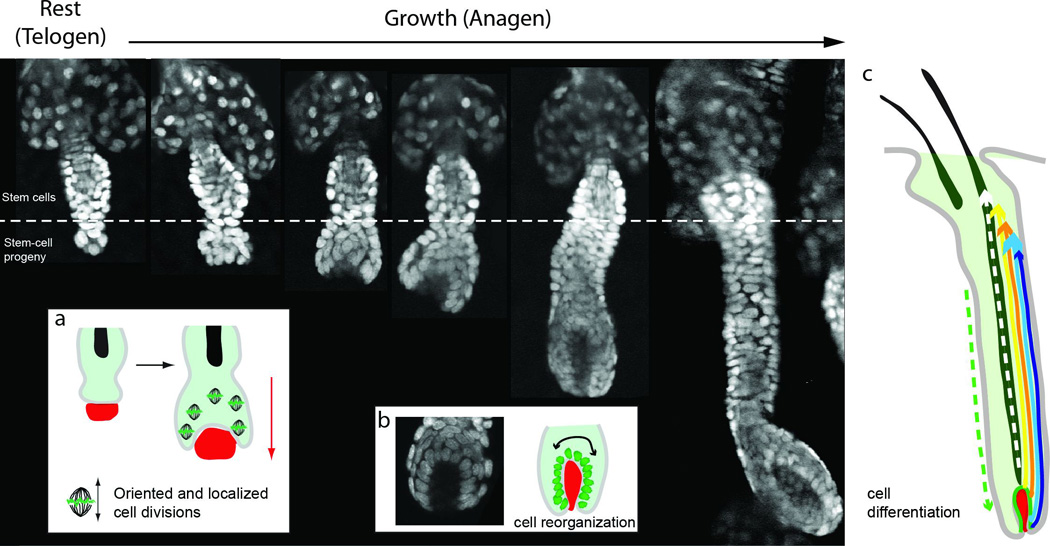
The figure depicts side views of live hair follicles, at rest (telogen) and sequential stages of hair growth (anagen), as visualized by multiphoton microscopy using a K14-H2BGFP reporter. At the onset of a new regeneration cycle the lower portion of the hair follicle begins to rapidly proliferate and expands downwards into the dermis. a) At the initial stages of hair growth cell proliferation is spatially regulated, occurring predominately within the hair germ and with an axis of division parallel to the long axis of hair follicle growth. b) Epithelial nuclei actively re-organize around the mesenchymal dermal papilla progressively surrounding it while the matrix is formed. c) Epithelial precursor cells in the matrix generate all the differentiated cell types that form the seven concentric layers of the hair shaft and inner root sheath (IRS), which expand upwards and toward the surface of the skin. At the same time the basal outer root sheath (ORS) layer also expands through localized proliferation and downward cell migration.
4.1 Stem cell activation and initiation of hair follicle growth
A typical hair cycle begins when after a seemingly uneventful period of quiescence cells within the niche become activated and begin to proliferate. The molecular characteristics of stem cell activation and the conditions by which it is triggered are complex and still not well understood. The first cells to respond and begin proliferation at the onset of a new regeneration cycle are those situated in the hair germ at the interface with the DP [45–47] (Fig. 3). It is unclear what prompts cells in the germ to start dividing first. One possibility is that activating signals from the DP reach this population first due to its close proximity to the mesenchymal niche [46] (Fig. 2a). It is also possible that hair germ cells are “primed” for activation, which can be triggered by changes in the niche microenvironment. This “primed” state of the hair germ may be reflected in the molecular signature of this population and can be linked to the origin of this compartment as will be discussed in a later segment [46,72]. Alternatively, it has also been proposed that stem cell activation may occur first in the bulge during the rest phase of the hair cycle (telogen), prompting bulge cells to migrate to the hair germ before the onset of hair growth [96].
The precise sequence of cellular events within the niche following stem cell activation was until recently not well understood. However, recent technological advances have allowed us to visualize stem cell behavior non-invasively, in live mice [47]. Thus, at these early stages of hair growth cell divisions within the niche are spatially regulated (Fig. 3a). Specifically, divisions which occur predominantly in close proximity with the DP are oriented so that the axis on which the two daughter cells separate is parallel to the long axis of growth [47]. In addition, cells in the niche dynamically change their shape to collectively cause a rapid elongation of the hair follicle [47]. At these early stages there is still no evidence of cell differentiation and the expanding lower follicle appears molecularly homogeneous [46]. Therefore, regulated cell proliferation and extension are the main drivers of initial hair follicle growth into the dermis.
A critical component of these initial stages of hair growth is the interplay between the mesenchymal DP and the epithelial cells within the niche. The DP is both necessary and sufficient to induce hair growth; it is therefore plausible that the first initiating signals emanate from the mesenchymal niche [46,47,56] (Fig. 2a). Such signals appear to have a direct effect on the adjacent epithelial population in the lower hair germ as these cells begin to proliferate and start to gradually engulf the DP (Fig. 3a, b). As the epithelial population expands so does the DP as a result of a two-way communication between the two compartments [62,97,98]. During this process, epithelial cells actively organize in the space around and in contact with the DP [47] (Fig. 3b). Such re-organization may be setting up the stage for cell-fate specification and differentiation that is to follow in the subsequent growth stages [99] (Fig. 3c).
4.2 Cell differentiation and hair production
During the initial stages of hair growth (anagen) intense cell proliferation leads to the volumetric expansion of the epithelial lower portion of the hair follicle followed by extensive dispersion of cells within this compartment that progressively engulf the DP (Fig. 3). After this initial relatively uniform growth spur, epithelial cells in contact with the DP form a layer of highly proliferative precursors that will generate the differentiated cell types of the new mature hair [99–104] (Fig. 3c). It is currently unclear how these precursors emerge, but their relative position around the DP is significant, as each of those precursors is restricted to generating a single differentiated lineage of cells that are expanded as a sole column parallel to the long axis of growth [99,104]. It is possible that exposure of these precursors to diverse signals emanating from different parts of the DP preferentially restricts their potential to a specific inner lineage [40,58,76,105]. The differentiated cells produced collectively form seven concentric inner root sheath (IRS) and hair shaft layers of the mature hair follicle [27,106]. At this stage of advanced hair growth, proliferation in the inner follicle is confined in the area surrounding the DP as the restricted precursors self-renew and generate transient progenitors that progressively become differentiated post-mitotic cells [99]. This area of cell proliferation and differentiation is commonly referred to as the matrix. At this stage of hair growth a column of post-mitotic cells generated in the matrix spearheads an upward expansion that ultimately reaches the canal from which the new hair shaft exits from the epidermis. In this gradual process the hair follicle continues to elongate and expand into the dermis.
The upward expansion of the inner differentiated layers results in the outward displacement of the proliferative epithelial cells, which populated the expanding lower follicle at the initial stages of growth. These relatively undifferentiated basal cells become the outer root sheath (ORS), which envelops the growing hair follicle [35,96,107]. Lineage tracing experiments and more recent live imaging data showed that the ORS follows the growth of the inner layers by expanding downwards and towards the bulb through regionally restricted cell proliferation and migration [35,72,108,134]. Specifically, as the hair follicle elongates, proliferation in the upper ORS gradually stops and is only maintained in a narrow zone which gradually moves downward but at a relatively constant distance from the DP [108,109,134]. The increasing distance of the upper ORS population from the inductive influence of the DP may explain their return to quiescence [46,110]. The space in the basal layer, beyond the proliferative zone of the ORS, is populated by cells that rapidly migrate downwards to reach the distal tip of the follicle. At the same time, during these advanced stages of anagen, bulge stem cells begin to divide in order to self-renew their pool within the niche [46,96,134].
4.3 Hair follicle regression and transition to quiescence
After a period of continuous active hair growth (anagen) which determines the total length of the new hair, proliferation ceases and the hair follicle begins to rapidly regress (catagen) due to massive epithelial apoptosis [111]. It is currently not well understood how the transition from active hair growth to regression is regulated. It is possible that matrix cells stop fueling the hair growth due to exhaustion or by an unknown internal clock mechanism [112]. Alternatively signals from the DP or other sources may actively regulate the entry into the regression phase [113–115]. As regression proceeds, the bulb looses its volume presumably due to the increased rate of apoptosis of the lower ORS, forming a narrow epithelial strand that retracts upward and at the end of which trails the DP. It is currently unknown if the differentiated IRS and hair shaft layers also undergo apoptosis in the same process. Cells from the ORS that survive the regression phase home back to the niche forming, at least in part, the new hair germ [45,72,134]. Next the hair follicle enters a rest phase (telogen), which is characterized by an apparent inactivity at least at the cellular level. At this stage the hair shaft becomes the club hair which is anchored by the inner bulge cell layer expressing Keratin 6 [8,72] (Fig. 2a). These K6+ bulge cells lack multipotency but may play a critical role in maintaining stem cell quiescence in the niche during the rest phase [72].
5. Modulation of stem cell fate in the hair follicle niche
5.1 Niche heterogeneity and fate during homeostasis
The hair follicle stem cell niche begins to establish already during embryonic life. Nucleotide pulse-chase experiments showed that slow-cycling cells find resident in the bulge even before birth [39]. Moreover, these cells display a molecular signature consistent with a stem cell identity [39]. Sox9, which is one of the early stem cell markers of bulge cells, is required for niche maintenance and hair regeneration; thus, highlighting the functional importance of this cell pool [116]. In adult life however, the niche displays a remarkable level of molecular heterogeneity (Fig. 1, 2). During the rest phase, when the hair follicle is at its minimal state, many genes are differentially expressed within the niche cell pool. Cells in the bulge display different levels of integrin α6 depending on their proximity to the basement membrane [32]. In addition CD34 marks exclusively the bulge, while expression of Krt15, Krt19, Sox9 and Lgr5 spans across both the bulge and hair germ [29,30,34,35,39]. Conversely, P-cadherin shows a pronounced enrichment in the hair germ, while the transcription factor Gli1 is preferentially expressed in the hair germ and upper bulge but not in the bulge itself [36,46]. The picture becomes even more complex due to the fact that most of these markers variably overlap between the different regions of the hair follicle niche (Fig. 1). Moreover, the distribution of many niche markers changes dramatically during active hair growth [32,35,36,46]. An intensely debated question is whether the molecular heterogeneity of the hair follicle niche signifies a functional compartmentalization of the niche with the presence of distinct subpopulations that fulfill different fates during regeneration [13]. Furthermore, it is not clear if the expression of many of these genes reflects the intrinsic potential and therefore fate of a cell or merely its current physiological state at the particular position, which the cell occupies within the niche.
Previous attempts to address these questions relied in genetic lineage tracing approaches with the use of stem-cell specific promoters [31,35,36,39,72,96,109]. These studies have provided great insight on the niche dynamics during hair regeneration; however, a single-cell resolution of the molecular heterogeneity of the niche is critical in directly answering how cell fate is determined in the hair follicle niche. A combination of conventional genetic lineage tracing with multiphoton microscopy using live mice, now allows us to visualize and monitor individual cells and their respective lineages by re-visiting the same undisturbed niche at various timepoints during full hair regeneration cycles [134]. These experiments showed that the exact location of Krt19+ or Lgr5+ cells within the niche could reliably predict their fate and contribution to hair growth [134]. Specifically, marked cells located in the lower bulge generated ORS lineages while those situated further down in the hair germ contributed to the IRS and hair shaft layers (Fig. 2b). Cells in the mid and upper bulge often self-renewed but did not contribute directly to the regenerating portion of the follicle. These data support the hypothesis that stem cell fate is niche-imposed rather than an intrinsic property of particular cell populations. Based on this model direct participation in the regenerating portion of the hair follicle is established anew in every hair cycle during the transition from quiescence to growth based on their location in the niche and independently from the self-renewal of the stem cell pool [96,104,134] (Fig. 2b). Consistent with this is the observation that bulge stem cells can be dispensable for hair growth following partial or complete ablation of their pool, since neighboring epithelial populations, even those that do not normally contribute to hair regeneration, can repopulate the lost stem cell pool and maintain the capacity of the niche to fuel future regeneration programs by dynamically altering their fate [45,134] Fig. 2b).
After the growth phase of the hair cycle is complete, the niche undergoes reorganization before it settles to a minimal resting state (telogen). Specifically during hair follicle regression (catagen), the vast majority of ORS cells undergo apoptosis while those that survive contribute to establishing the new hair germ compartment [72,134] (Fig. 2b). It has been proposed that cells in the upper portion of the ORS that have undergone a limited number of cell divisions can home back and establish a “new” bulge compartment while the “old” bulge may serve as a reserve for injury response, but the functional distinction between a “new” and “old” remains to be tested [72]. In addition, it is not clear if cells that have contributed to hair regeneration after departing from the bulge can return to a quiescent state or if these cells have crossed a point of not return and are bound to contribute in subsequent hair cycle.
5.2 Stem cell plasticity in pathophysiology
It is well established that injury or other pathological conditions such as cancer can alter the environment of the tissue inducing stem cells to change their normal behavior [117,118]. Lineage tracing of hair follicle populations showed that following injury cells readily leave the niche to re-epithelialize the epidermis [36,53,119–121]. After leaving the niche these cells no longer express niche markers but rather switch to an epidermal profile. Evidence suggest that even though different hair follicle populations respond to repairing the epidermis, the extent and timing of their contribution varies. Bulge stem cells respond rapidly to fuel the early stages of wound re-epithelialization but their progeny does not persist long term [41,120]. In contrast, niche cells situated above the bulge, in the isthmus and junctional region not only contribute to wound repair but their progeny is able to sustain epidermal maintenance in stem cell fashion [16,36,53]. Hair neogenesis after extensive skin wounding raised the possibility of functional compensation by epithelial cells that do not normally participate in hair regeneration; however, until recently this had not been directly demonstrated [122,123]. Laser ablation of the entire bulge populations unequivocally demonstrated that non-hair epithelial cells cross the boundaries between the different niche compartments to functionally reconstitute the lost cell pool, highlighting the fact that cell behavior is dictated by the niche microenvironment rather than cellular origin [134] (Fig. 2b).
Stem cells in the hair follicle niche may be mobilized in another kind of pathological condition, that of cancer. One of the most common types of skin tumors is basal cell carcinoma (BCC), which is the result of defective Hedgehog signaling [124]. It has long been hypothesized that BCCs originate from hair follicle stem cells, however the conditions under which bulge cells can induce a BCC tumor and the extent of their contribution to the growing tumors are not fully understood [34,125,126]. The finding that wounding stimulates hair follicle stem cells to participate and promote BCC tumor growth may be a further indication that changes in the niche microenvironment are critical in modulating stem cell behavior in pathophysiology as well as in homeostasis [127,128]. Keratoacanthomas are another class of squamous skin tumors that have the unique property of spontaneous regression [129,130]. This self-regression feature which recapitulates physiologic hair follicle regression has led to the hypothesis that Keratoacanthomas may originate from hair follicle stem cells; however this remains to be tested [131–133]. A still unanswered question is whether stem cells from the hair follicle or other epithelial niches are initiating skin tumors or if their participation in the tumoral growth is secondary, caused by changes in the microenvironment that the tumor is instigating on the niche.
6. Concluding remarks
The process of tissue regeneration relies on stem cells and is modulated through their interactions with various elements of their microenvironment. The hair follicle in the mammalian skin is a self-contained mini-organ that periodically and stereotypically undergoes cycles of physiological regeneration. The compartmentalized anatomy of the hair follicle, where different cell types including stem cells occupy distinct locations within the niche, combined with a molecularly complex microenvironment make the hair follicle an ideal system to study stem cell biology and the mechanisms that govern tissue regeneration. While great progress has been made in the molecular characterization of the hair follicle and the identification of the different cell populations that occupy the niche, we still lack a critical understanding of the cellular mechanisms that govern hair regeneration. Recent advances have started to unravel the complex sequence of cellular behaviors that stem cells adopt during the hair cycle. These studies suggest that the molecular heterogeneity of the hair follicle niche reflects, at least in part, a functional diversity between the individual cells. One of the fundamental questions is whether stem cell behavior and therefore fate are regulated by a cell intrinsic mechanism or if the microenvironment influences different behaviors in different parts of the niche. Evidence so far point to the latter, however further studies are needed to fully address this problem. Towards that direction it is imperative that we identify the precise molecular characteristics of important milestones in the life of a stem cell, including the transition from quiescence to activation and fate decisions that commit a stem cell and its progeny to a particular lineage. In addition, it is critical that we dissect the niche microenvironment to identify all those different factors that act upon a stem cell to potentially determine its fate. Precise manipulation of the relevant niche factors will elucidate their role in regulating stem cell behavior. To effectively address all these problems it is important that we can increase the resolution by which we can study and alter the molecular heterogeneity of the hair follicle niche, down to a single cell level in vivo. Answers, to these questions will have far reaching implications that stretch beyond the confines of skin biology, to understanding the general mechanisms of stem cell biology and devise effective strategies for future medical applications.
Highlights.
Hair follicles are mammalian skin appendances that have the capacity to periodically and stereotypically regenerate.
Hair follicle regeneration is fueled by adult epithelial stem cells that reside in a specialized tissue niche.
The hair follicle niche constitutes a complex tissue microenvironment, consisting of neighboring cells, molecular signals and extracellular material.
Stem cell behavior and contribution to regeneration is influenced by the niche microenvironment.
Changes in the niche microenvironment due to injury or other pathological conditions may alter stem-cell fate.
Acknowledgments
P.R. is a New York Stem Cell Foundation - Druckenmiller Fellow. This work was supported by The New York Stem Cell Foundation and grants to V.G. from the American Cancer Society (RGS-12-059-01-DCC) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1RO1AR063663-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 2.Spradling AC, Nystul T, Lighthouse D, Morris L, Fox D, Cox R, Tootle T, Frederick R, Skora A. Stem cells and their niches: integrated units that maintain Drosophila tissues. Cold Spring Harb. Symp. Quant. Biol. 2008;73:49–57. doi: 10.1101/sqb.2008.73.023. [DOI] [PubMed] [Google Scholar]
- 3.Kopp H-G, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 4.Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celso LoC, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda N, Jain R, Leboeuf MR, Padmanabhan A, Wang Q, Li L, Lu MM, Millar SE, Epstein JA. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013;140:1655–1664. doi: 10.1242/dev.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 10.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker H, Kligman AM. Technique for estimating turnover time of human stratum corneum. Arch Dermatol. 1967;95:408–411. [PubMed] [Google Scholar]
- 15.Pinkus H. Examination of the epidermis by the strip method. II. Biometric data on regeneration of the human epidermis. J. Invest. Dermatol. 1952;19:431–447. doi: 10.1038/jid.1952.119. [DOI] [PubMed] [Google Scholar]
- 16.Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 17.Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 18.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu CP, Polak L, Rocha AS, Pasolli HA, Chen S-C, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499:228–232. doi: 10.1038/nature12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Unna P. Beiträge zur Histologie und Entwiekelungsgeschichte der menschlichen Oberhaut und ihrer Anhangsgebilde. Arch mikr Anat. 1876;12:665–741. [Google Scholar]
- 24.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J. Invest. Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 25.Myung P, Ito M. Dissecting the bulge in hair regeneration. J. Clin. Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 28.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell. Sci. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Invest. Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 30.Trempus C, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 31.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 32.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 35.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 36.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mardaryev AN, Meier N, Poterlowicz K, Sharov AA, Sharova TY, Ahmed MI, Rapisarda V, Lewis C, Fessing MY, Ruenger TM, et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development. 2011;138:4843–4852. doi: 10.1242/dev.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. [Internet] Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 42.Chase HB, Rauch R, Smith VW. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Kizawa K, Toyoda M, Morohashi M. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J. Invest. Dermatol. 2002;119:1310–1316. doi: 10.1046/j.1523-1747.2002.19644.x. [DOI] [PubMed] [Google Scholar]
- 44.Ito M, Kizawa K. Expression of calcium-binding S100 proteins A4 and A6 in regions of the epithelial sac associated with the onset of hair follicle regeneration. J. Invest. Dermatol. 2001;116:956–963. doi: 10.1046/j.0022-202x.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 46.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Cruz-Racelis Dela J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nijhof JGW, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LHF, Watt FM, de Gruijl FR, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 49.Jensen UB, Yan X, Triel C, Woo S-H, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell. Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 51.Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. The Epidermis Comprises Autonomous Compartments Maintained by Distinct Stem Cell Populations. [Internet] Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cottle DL, Kretzschmar K, Schweiger PJ, Quist SR, Gollnick HP, Natsuga K, Aoyagi S, Watt FM. c-MYC-induced sebaceous gland differentiation is controlled by an androgen receptor/p53 axis. Cell Rep. 2013;3:427–441. doi: 10.1016/j.celrep.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandes KJL, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 56.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–593. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 58.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Driskell RR, Juneja VR, Connelly JT, Kretzschmar K, Tan DWM, Watt FM. Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J. Invest. Dermatol. 2012;132:1084–1093. doi: 10.1038/jid.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J. Invest. Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 63.Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development. 1992;116:563–571. doi: 10.1242/dev.116.3.563. [DOI] [PubMed] [Google Scholar]
- 64.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Invest. Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 65.Jahoda CAB. Cell movement in the hair follicle dermis - more than a two-way street? J. Invest. Dermatol. 2003;121:ix–xi. doi: 10.1111/j.1523-1747.2003.12585.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, He XC, Tong W-G, Johnson T, Wiedemann LM, Mishina Y, Feng JQ, Li L. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 68.Kobielak K, Stokes N, la Cruz de J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 70.Plikus MV, Mayer JA, la Cruz de D, Baker RE, Maini PK, Maxson R, Chuong C-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plikus MV, Baker RE, Chen C-C, Fare C, la Cruz de D, Andl T, Maini PK, Millar SE, Widelitz R, Chuong C-M. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Invest. Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 77.Demehri S, Kopan R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development. 2009;136:891–896. doi: 10.1242/dev.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng H-YM, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 79.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S-I. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 80.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 81.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang C-Y, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat. Rec. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- 84.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 88.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 89.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J. Cell. Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 92.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niessen MT, Iden S, Niessen CM. The in vivo function of mammalian cell and tissue polarity regulators--how to shape and maintain the epidermal barrier. J. Cell. Sci. 2012;125:3501–3510. doi: 10.1242/jcs.092890. [DOI] [PubMed] [Google Scholar]
- 95.Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, Niessen CM. aPKCλ controls epidermal homeostasis and stem cell fate through regulation of division orientation. J. Cell Biol. 2013 doi: 10.1083/jcb.201307001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collins CA, Jensen KB, MacRae EJ, Mansfield W, Watt FM. Polyclonal origin and hair induction ability of dermal papillae in neonatal and adult mouse back skin. Dev. Biol. 2012;366:290–297. doi: 10.1016/j.ydbio.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Legué E, Nicolas J-F. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- 100.Kopan R, Lee J, Lin M-H, Syder AJ, Kesterson J, Crutchfield N, Li CR, Wu W, Books J, Gordon JI. Genetic mosaic analysis indicates that the bulb region of coat hair follicles contains a resident population of several active multipotent epithelial lineage progenitors. Dev. Biol. 2002;242:44–57. doi: 10.1006/dbio.2001.0516. [DOI] [PubMed] [Google Scholar]
- 101.Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamimura J, Lee D, Baden HP, Brissette J, Dotto GP. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J. Invest. Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- 103.Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Legué E, Sequeira I, Nicolas J-F. Hair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineages. Development. 2010;137:569–577. doi: 10.1242/dev.044123. [DOI] [PubMed] [Google Scholar]
- 105.Kiso M, Tanaka S, Saba R, Matsuda S, Shimizu A, Ohyama M, Okano HJ, Shiroishi T, Okano H, Saga Y. The disruption of Sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9292–9297. doi: 10.1073/pnas.0808324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hardy MH. The secret life of the hair follicle. [Internet] Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 107.Pinkus H, Iwasaki T, Mishima Y. Outer root sheath keratinization in anagen and catagen of the mammalian hair follicle. A seventh distinct type of keratinization in the hair follicle: trichilemmal keratinization. J. Anat. 1981;133:19–35. [PMC free article] [PubMed] [Google Scholar]
- 108.Sequeira I, Nicolas J-F. Redefining the structure of the hair follicle by 3D clonal analysis. Development. 2012;139:3741–3751. doi: 10.1242/dev.081091. [DOI] [PubMed] [Google Scholar]
- 109.Hsu Y-C, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. Journal of cell. 2001 doi: 10.1242/jcs.114.19.3419. [no volume]. [DOI] [PubMed] [Google Scholar]
- 111.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) Am. J. Pathol. 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- 112.Paus R, Cotsarelis G. The biology of hair follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 113.Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- 114.Hébert JM, Rosenquist T, Götz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 115.Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 116.Vidal VPI, Chaboissier M-C, Lützkendorf S, Cotsarelis G, Mill P, Hui C-C, Ortonne N, Ortonne J-P, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 117.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 118.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin. Cell Dev. Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis - Nature Medicine. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 121.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 122.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 123.Chuong C-M. Regenerative biology: new hair from healing wounds. Nature. 2007;447:265–266. doi: 10.1038/447265a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 125.Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, Wilbert D, Patel RM, Ferris J, Diener J, et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J. Clin. Invest. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang GY, Wang J, Mancianti M-L, Epstein EH. Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/−) mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kasper M, Jaks V, Are A, Bergström Å, Schwäger A, Svärd J, Teglund S, Barker N, Toftgård R. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blessing K, Nafussi al A, Gordon PM. The regressing keratoacanthoma. Histopathology. 1994;24:381–384. doi: 10.1111/j.1365-2559.1994.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 130.Ko CJ, McNiff JM, Bosenberg M, Choate KA. Keratoacanthoma: clinical and histopathologic features of regression. J. Am. Acad. Dermatol. 2012;67:1008–1012. doi: 10.1016/j.jaad.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 131.Ghadially FN. The role of the hair follicle in the origin and evolution of some cutaneous neoplasms of man and experimental animals. Cancer. 1961;14:801–816. doi: 10.1002/1097-0142(199007/08)14:4<801::aid-cncr2820140417>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 132.Kossard S, Tan K-B, Choy C. Keratoacanthoma and infundibulocystic squamous cell carcinoma. Am J Dermatopathol. 2008;30:127–134. doi: 10.1097/DAD.0b013e318161310c. [DOI] [PubMed] [Google Scholar]
- 133.Ramselaar CG, van der Meer JB. Non-immunological regression of dimethylbenz(A) anthracene-induced experimental keratoacanthomas in the rabbit. Dermatologica. 1979;158:142–151. doi: 10.1159/000250755. [DOI] [PubMed] [Google Scholar]
- 134.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]


