Abstract
Rationale
Large conductance calcium-activated potassium (BKCa or KCa1.1) channels are well-known molecular targets for the action of alcohol and therefore may play an important role in the pathogenesis of alcohol withdrawal syndrome.
Objectives
We evaluate the modifications of total outward K+ currents and protein expression of BKCa channels α-subunit in inferior colliculus (IC) neurons obtained from controls and rats subjected to alcohol withdrawal associated with enhanced susceptibility to seizures.
Methods
Outward K+ currents and BKCa channel proteins were measured using the whole cell configuration of patch clamp techniques and western blot analysis, respectively.
Results
Total outward K+ current density was significantly reduced in IC neurons at 24 h and 48 h during the alcohol withdrawal period when the susceptibility to seizures was maximal and absent, respectively. The iberiotoxin-sensitive (BKCa) current density and conductance also were significantly reduced at 24 h following alcohol withdrawal. Consistent with functional data, the levels of protein expression of α-subunit associated with BKCa channels also was significantly reduced in IC neurons at 24 h and 48 h following alcohol withdrawal.
Conclusions
The downregulation of BKCa channels that outlasts the finite period of elevated susceptibility to alcohol withdrawal seizures. These findings indicate that BKCa channels, per se, may not be fundamentally important for the generation of alcohol withdrawal seizures.
Keywords: alcohol withdrawal, BKCa channels, iberiotoxin, outward current density, protein, seizures
Introduction
Alcohol withdrawal-induced neuronal hyperexcitability can lead to generalized tonic-clonic seizures in many species including rodent and humans. In rodents, the susceptibility to acoustically evoked generalized seizures (audiogenic seizures) occurs during a finite period following abrupt cessation of chronic alcohol intoxication. Multiple lines of evidence indicate that inferior colliculus (IC) neurons, known for their role in the processing of auditory information are critical in the initiation of audiogenic seizures following alcohol withdrawal (Frye et al. 1983; Eckardt et al. 1986; McCown and Breese 1990; Caird 1991; Faingold and Riaz 1995; Chakravarty and Faingold 1998). Electrophysiological studies reported increases in IC neuronal firing immediately before the onset of and during audiogenic seizures in rats subjected to alcohol withdrawal (Faingold and Riaz 1995; Chakravarty and Faingold 1998). Similarly, in vitro studies have reported that IC neurons exhibited elevated bursting activity following alcohol withdrawal associated with enhanced seizure susceptibility (Evans et al. 2000). The mechanisms underlying alcohol withdrawal-induced increases in IC neuronal excitability that lead to seizures are not fully understood. We have previously reported increases in the current density of the L- and P-type of the high-threshold voltage-gated Ca2+ (CaV) channels in IC neurons of rats subjected to alcohol withdrawal and exhibiting enhanced susceptibility to audiogenic seizures (N’Gouemo and Morad 2003). The enhanced CaV channel currents should lead to higher intracellular Ca2+ activity, providing the mechanism to activate the large (KCa1 or BKCa) and small (KCa2 or SKCa) conductance Ca2+-activated K+ (KCa) channels that initiate repolarization and the ensuing afterhyperpolarization (Lancaster and Adams 1986; Storm 1987). Activation of KCa channels may therefore serve as an intrinsic inhibitory mechanism of IC neurons. Loss of KCa channel activity would therefore contribute to neuronal hyperexcitability that can lead to seizures. Although IC neurons exhibit KCa channel conductances (Sivaramakrishnan and Oliver 2001), their role in the pathogenesis of alcohol withdrawal seizures remains unknown. Nevertheless, evidence suggests that neuronal BKCa channels may be important molecular targets of alcohol-action in various brain sites (Davies et al. 2003; Cowmeadow et al. 2005). Alcohol exposure potentiates BKCa channel open probability in the hypothalamic-neurohypophysial system and nucleus accumbens (Dopico et al. 1996; Martin et al. 2004; Pietrzykowski et al. 2004), while its chronic exposure decreased BKCa current density (Knott et al. 2002; Pietrzykowski et al 2004). Thus, the effect of alcohol on BKCa channels may have potential consequences on the excitability of IC neurons. Here, we report on the functional and molecular expression of neuronal BKCa channels, and find that alcohol withdrawal is accompanied by downregulation of BKCa channels in IC neurons.
Materials and methods
Ethanol administration and seizure testing
We used a convenient model of ethanol dependence that mimics alcohol withdrawal seizures seen in humans (Faingold 2008). Ethanol (95%) was given by intragastric intubation to male Sprague-Dawley rats (180–230 g) in 30% v/v solution of diluted (1:1 with water) ISOMIL Soy Protein Infant Formula (Abbott Laboratories, Abbott Park, IL). Ethanol administration protocol consisted of three daily doses every 8 h for 4 days, and the spectrum of intoxication, which is broken down into six stages (neutrality, sedation, moderate ataxia, severe ataxia, loss of righting reflex, and coma) was evaluated based on the standard behavior rating scale (Faingold 2008). The priming dose of ethanol was 5 g/kg, resulting in blood alcohol levels of 0.3±0.01g/dL (n=4). Subsequent ethanol doses were adjusted for each rat, to maintain a moderate but not severe degree of ataxia. The daily amount of administered ethanol varied from 9 to 15 g/kg for each rat, which allowed for intoxication to be maintained at a consistent moderate degree of ataxia. Ethanol was withdrawn on the fourth day after the second daily dose. Rats were sacrificed in two groups at about 24 h and 48 h, respectively, after the last ethanol administration on the basis of previous study indicating that blood ethanol levels at that time were negligible, while audiogenic seizure susceptibility was higher at 24 h but absent at 48 h (N’Gouemo et al. 1996; N’Gouemo and Morad 2003). Control animals were maintained under similar conditions and schedule but fed only with the ISOMIL diet without ethanol. To determine the incidence of audiogenic seizures, rats were subjected to acoustic stimulation every hour (until seizures occurred) for three hours, starting at the 23rd hour for the 24 h group and at the 47th hour for the 48 h group, during the ethanol withdrawal period. Two out of four rats per group, when no spontaneous seizure activity was observed, were subjected to acoustic stimulation (bell, 100 dB), which was presented until wild running seizures were elicited or for 60 sec (Riaz and Faingold 1994). The remaining rats, those not exposed to acoustic stimulation that did not exhibit seizures were used for electrophysiological and molecular studies. The seizure severity score was evaluated according to the scale of audiogenic seizures (Jobe et al. 1973). Weight loss and the incidence of mortality were negligible in this study
Cell preparation
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.). The rats’ brains were then perfused with an oxygenated (95% O2 and 5% CO2) solution containing (in mM): 110 choline chloride, 2.5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 2 sodium/pyruvate, 1 L-ascorbic acid, 20 dextrose, 0.5 CaCl2, and 1.5 MgCl2 (290–300 mOsm with sucrose). The brains were removed and immersed in a cutting sucrose solution containing (in mM): 205 sucrose, 5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 2 sodium/pyruvate, 1 L-ascorbic acid, 20 dextrose, 0.2 CaCl2, and 1 MgSO4, (osmolarity 290–300 mOsm with sucrose), and bubbled with 95% O2 and 5% CO2. Coronal brainstem slices (300 μM thick) at the level of the IC were sectioned using a Leica Vibratome 1000S. The IC external cortex was microdissected and placed in an oxygenated (100% O2; 30–32°C) Leibowitz (L-15) medium (Life Technologies, Grand Island, NY) containing papain (20 U/ml; Worthington Biochemicals, Lakewood, NJ) for 60 min. The enzyme was washed with L-15 medium containing trypsin inhibitor (1mg/ml) and albumin bovine serum (1mg/ml). IC neurons were then dissociated by gentle trituration with a fire-polished Pasteur pipette in Neurobasal-A (Life Technologies, Grand Island, NY) medium supplemented with 2% B27 (Life Technologies, Grand Island, NY); 1% horse serum; 1% fetal bovine serum; and penicillin (100 U/ml)-streptomycin (0.1 mg/ml). Dissociated IC neurons were then plated onto concavalin (50 μg/ml) coated-glass coverslip for at least 1 hr before whole cell recordings.
Electrophysiology
Total outward K+ currents were recorded at room temperature from the soma of IC neurons using the whole cell configuration of the patch clamp techniques (Hamill et al 1981). The patch electrodes were made from borosilicate glass capillaries and had 2–4 ΩM resistances when filled with solution containing (in mM): 0.5 EGTA (100 nM free Ca2+) 140 KCl, 5 MgCl2, 4 Na-ATP, 0.4 GTP, 14 phosphocreatine, 10 HEPES, and 10 glucose (pH 7.3 with KOH). After establishment of the whole cell configuration, currents were recorded using extracellular solution containing (in mM): 2 CaCl2, 140 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 10 glucose, and 0.001 tetrodotoxin (pH 7.4 with NaOH; 305 mOsm with sucrose). Voltage clamp experiments were performed with a Dagan 8900 patch clamp amplifier (Dagan Corporation, Minneapolis, MN). Currents were filtered at 10 kHz, and whole cell capacitance and series resistance were compensated with the amplifier circuitry. Leak and residual capacitance currents were not subtracted for any voltage protocols. Outward currents were evoked by voltage steps (50 ms duration), from the holding potential of −50 mV to +60 mV by 10 mV increments, and measured at 15 ms (sustained currents). For pharmacological studies, outward currents were evoked by test potential to +20 mV and monitored until a steady state was reached before applying BKCa channel blocker. To isolated BKCa channel currents, iberiotoxin (100 nM), a potent blocker of these channels, was used. Outward currents were recorded before and during iberiotoxin exposure. The iberiotoxin-sensitive current was obtained as difference current in the presence and absence of iberiotoxin.
Western immunoblotting
Rats were deeply anesthetized with pentobarbital (100 mg/kg; i.p.). Their brains were removed, and IC tissues were quickly dissected (the area corresponding to the periaqueductal grey was discarded) and stored at −70°C until processed. Tissue homogenates from each rat were prepared in a TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.4). Protein concentrations in the supernatant were determined using bicinchoninic acid assay reagent (Thermo Fisher Scientific, Rockford, IL), and each sample was diluted to a protein concentration of 5 mg/ml in the TE buffer. Homogenate proteins were separated using a 7.5% sodium dodecyl sulfate-polyacrylamide gel (Life Technologies, Grand Island, NY) electrophoresis, and transferred to a polyscreen polyvinylidene fluoride membrane (PerkinElmer, Waltham MA) in the blotting buffer (25 mM Tris-HCl, 192 mM Glycine, 20% methanol). The blots were processed using methods described by Towbin et al. (1979). Briefly, the blots were incubated in a TBST buffer (20 mM Tris-HCl, pH 7.4; 140 mM NaCl and 0.1% v/v Tween 20) containing 5% nonfat dry milk (BLOTTO buffer) with BKCa channel α-subunit antibody (Alomone labs, Jerusalem, Israel) at concentrations of 1.0 μg/ml, respectively overnight at 4°C. The blots were then washed (30 min) with a TBST buffer and incubated with a donkey anti-rabbit antibody (GE HealthCare Biosciences, Piscataway, NJ) at a dilution of 1:2000 in a BLOTTO buffer for 30 min at room temperature. After several washes of the membrane, individual bands were visualized on film using the enhanced chemiluminescence Super Signal West Pico reagents (Thermo Fisher Scientific, Rockford, IL), and analyzed.
Data analysis
Data were analyzed using Clampfit 10 software (Molecular Devices, Sunnyvale, CA) and Origin 8.0 (Originlab, Northampton, MA). Membrane potentials were corrected for the junction potential, and outward currents were normalized relative to the membrane capacitance as an estimate of current density. The ethanol intoxication/withdrawal paradigm did not alter IC neurons cell capacitance. To generate steady-state activation curves, the conductance (G=I/V-Vrev) was determine at each potential (V) and the reversal potential (Vrev) of the current was measured for the I-V curves (Fig. 3C). Normalized conductances were plotted as a function of test pulse and was best fitted with a Boltzmann equation, G/Gmax=1/(1+e[(V1/2-V)k]), where Gmax is the maximum conductance, generating values for half-maximum conductance (G) at potential (V1/2) and the slope factor (k). To quantify the abundance of BKCa channel α-subunit protein, individual bands were quantified by computer-assisted densitometry using the Bio Image Analysis Intelligent Quantifier software (Bio Image System, Inc, Jackson, MI), which calculated the integrated intensity of a band using both the density and the area of the band. The integrated intensity of the BKCa channel α-subunit protein band was compared to a standard curve of known protein concentration from rat hippocampus, as done previously in our laboratory (N’Gouemo et al. 2009). The integrated intensities of the bands from IC tissues determined at the same time and on the same piece of film were compared with the interpolated standard curve to yield a value of subunit expression relative to the standard hippocampus tissue. The utilized integrated intensities were always in the linear part of the standard curve. For total protein quantification, β-actin was used as loading control. To determine differences in current density and protein expression, one-way or two-way analysis of variance was performed. Differences were considered significant if P<0.05. The illustrated current traces represent the average of 3–5 consecutive traces. All data presented were mean ± S.E.M.
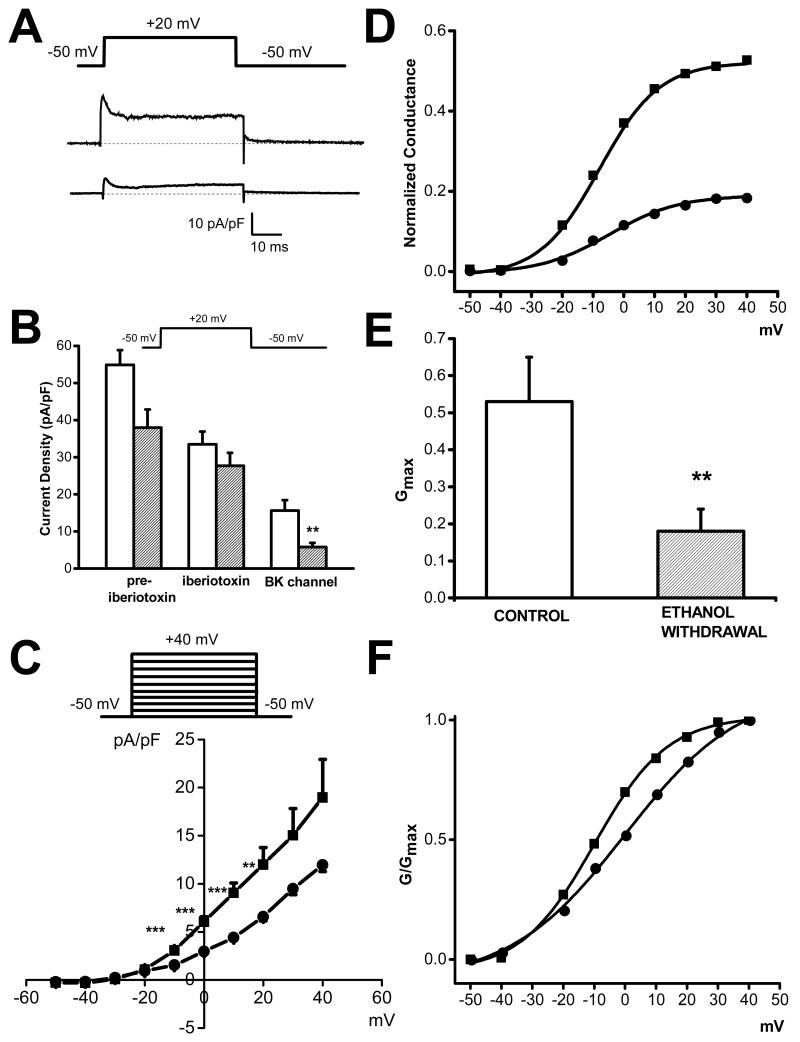
Figure 3.
Downregulation of BKCa channel currents in IC neurons following ethanol withdrawal. A. Representative iberiotoxin-sensitive (BKCa) current traces in control (upper trace) and 24 h following ethanol withdrawal (lower trace). Outward K+ currents were activated by +20 mV voltage steps from a holding potential of −50 mV using 2 mM Ca2+ as a charge carrier. B. Iberiotoxin-sensitive (BKCa) channel currents were significantly decreased at 24 h following ethanol withdrawal (filled bar graph, n=8) compared to controls (opened bar graph, n=8). C. Voltage dependence shows that iberiotoxin-sensitive currents were decreased in IC neurons at 24 h following ethanol withdrawal (filled circle, n=6) compared to controls (filled square, n=6). D. Plots of normalized conductance as function of voltage revealed that ethanol withdrawal (24 h, filled circles, n=6) is associated with ~2.5 fold decreased conductance compared to controls (filled squares, n=6). E. The maximal conductance Gmax is markedly reduced in IC neurons following 24 h ethanol withdrawal (open bar, n=6) compared to controls (filled bar, n=6). F. Ethanol withdrawal (24 h, filled circles) did not significantly alter the voltage dependence of BKCa channel activation (G/Gmax curves fitted with a Boltzmann equation), as compared to controls (filled squares). Each point represents mean ± S.E.M. **P<0.01, ***P<0.001 (one-way ANOVA).
Results
Total Outward K+ currents and enhanced susceptibility to seizures
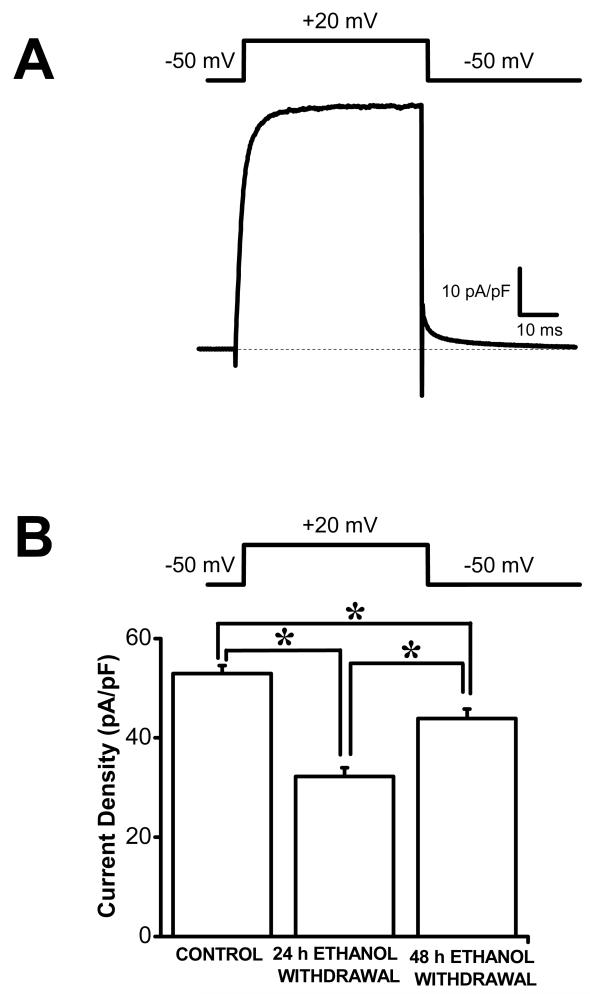
Audiogenic seizures following ethanol withdrawal consisted of wild running seizures that evolved into tonic-clonic seizures; this seizure phenotype was observed in all rats tested at 24 h following ethanol withdrawal. The susceptibility to seizures was transient such that the susceptibility to audiogenic seizures was present at 24 h but absent at 48 h following ethanol withdrawal. Figure 1A shows a representative trace of total outward K+ current evoked at +20 mV in IC neurons obtained from a control animal. Quantification reveals that the mean peak current density evoked at +20 mV was significantly decreased in IC neurons (32.2±1.8 pA/pF, n=23) obtained from rats subjected to 24 h ethanol withdrawal and exhibiting seizure susceptibility, as compared to the control group (53.0±1.6 pA/pF, n=21) and to the 48 h group (43.9±1.9, n=12, F=41, P<0.001, Fig. 1b). The decreases in outward peak current density persisted, surprisingly, at 48 h following ethanol withdrawal (43.9±1.9, n=12, compared to 53.0±1.6 pA/pF, n=21, F=13, P<0.001) even when the seizure susceptibility was no longer present (Fig. 1).
Figure 1.
Downregulation total outward K+ currents in IC neurons following ethanol withdrawal. A. Representative total outward K+ currents in IC neuron obtained from naive SD rat. Whole cell outward K+ currents were activated by voltage steps to +20 mV from the holding potential of −50 mV. B. The current density of total outward K+ channels was significantly reduced in IC neurons of SD rats subjected to 24 h (n=23) and 48 h (n=12) ethanol withdrawal when the seizure susceptibility is present and absent, respectively, compared to controls (n=23). Additional decreases in current density were found in IC neurons of SD rats at 24 h (n=23) compared to 48 h (n=12) following ethanol withdrawal. *P<0.05 (two-way ANOVA). Data represents mean ± S.E.M.
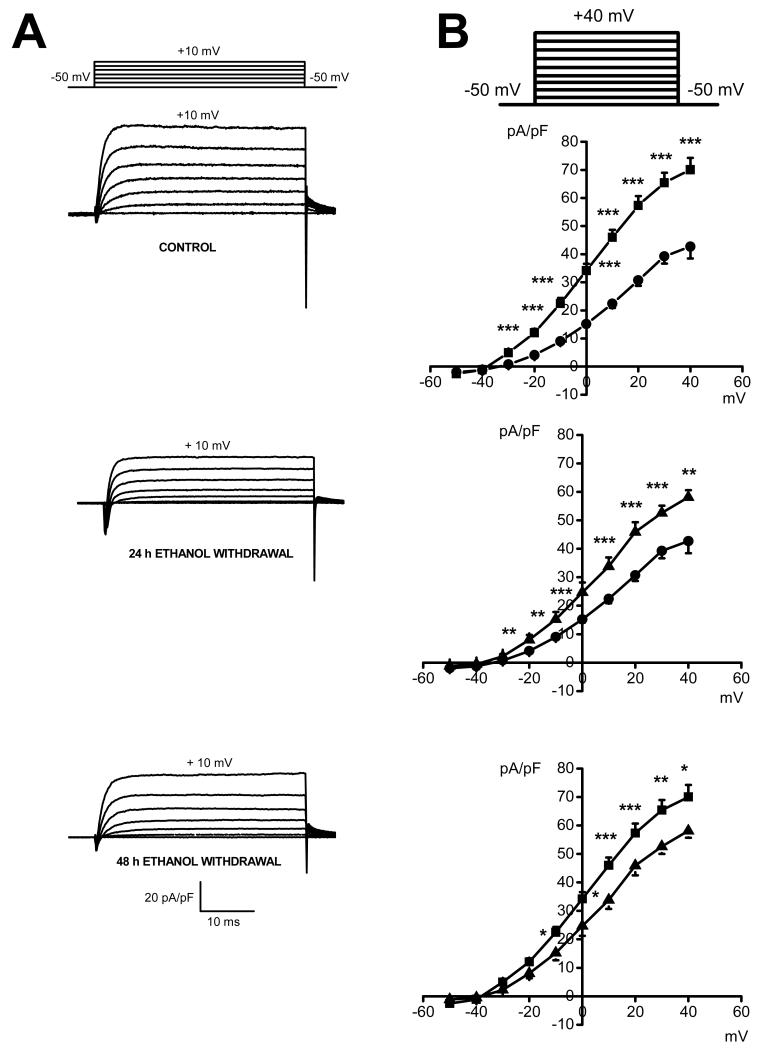
Figure 2 quantifies the voltage-dependence of total outward K+ currents in the control group and in rats subjected to ethanol withdrawal. The original traces of total outward K+ currents from three representative neurons from each group showed significant reduction of the current density 24 h (middle traces, Fig. 2a) and 48 h (lower traces, Fig. 2a) following ethanol withdrawal as compared to the control group (upper traces, Fig. 2a). Figure 2b quantifies the current density at larger range of voltages (−50 mV to +40 mV). Activation of K+ channels occurs around −35±2 mV (n=12) in the control group (filled squares), but shifts to less negative potentials of −27±2 mV (n=13) and −29±2 mV (n=9) in the 24 h group (filled circles) and 48 h group (filled triangles) following ethanol withdrawal, respectively. Comparison of the I-V relations of total outward K+ currents between the control group (filled squares) and 24 h following ethanol withdrawal (filled circles) showed a reduction of the current density, on the average, by 48±4% at voltages positive to −40 mV without significant alterations in their voltage dependence (Fig. 2b, upper curves). The current density at 24 h following ethanol withdrawal (filled circles) was also decreased compared to 48 h following ethanol withdrawal (filled triangles); this decrease, on the average, by 39±4%, was seen at voltages positive to −30 mV (Fig. 2b, middle curves). Quantification also shows that the current density at voltage positive to −20 mV was significantly decreased, on the average, by 34±5%, at 48 h following alcohol withdrawal (filled triangles) compared to the control group (filled squares; Fig. 2, lower curves). However, this decreased outward K+ current was not accompanied by ethanol withdrawal seizure susceptibility.
Figure 2.
Voltage dependence of total outward K+ currents in IC neurons following ethanol withdrawal. A. Representative whole cell outward K+ current traces in IC neuron obtained in a control (upper traces) and rats subjected to 24 h (middle traces) and 48 h (lower traces) following ethanol withdrawal. In these examples, the peak current density was decreased by about 2-fold and 1.5-fold in IC neurons 24 h and 48 h following ethanol withdrawal, respectively, compared to controls. B. Voltage dependence of total outward K+ currents in IC neurons obtained from controls (filled square, n=12), as well as 24 h (filled circle, n=13) and 48 h (filled triangles, n=9) following ethanol withdrawal. The current density was significantly decreased 24 h following ethanol withdrawal (filled circles) at all voltages compared to the control group (filled squares; upper curves). Decreases in the current density was also found in IC neurons 24 h (filled circles) compared to 48 h (filled triangles) following ethanol withdrawal (middle curves). Quantification shows (lower curves) that the current density was significantly reduced at 48 h (filled triangles) following ethanol withdrawal, compared to controls (filled squares). Each point represents mean ± S.E.M. *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA).
Contribution of BKCa channels to reduced total outward K+ currents
The possible contribution of BKCa channels to the reduced total outward K+ currents in IC neurons at 24 h following ethanol withdrawal was determined using iberiotoxin (100 nM), a potent blocker of BKCa channels. Figure 3a shows representative iberiotoxin-sensitive currents (BKCa channel currents) in IC neurons obtained from the control (upper traces) and following ethanol withdrawal (lower traces). Quantification of this effect shows that iberiotoxin-sensitive current density was significantly decreased in IC neurons (5.8±1.1, n=8, F=10.6, P<0.01, Fig. 3b) of rats subjected to ethanol withdrawal and exhibiting seizure susceptibility, as compared to the control group (15.6±2.8, n=8, Fig. 3b). Figure 3c quantifies the voltage-dependence of iberiotoxin-sensitive currents in controls (n=6) and rats subjected to ethanol withdrawal (n=6). The iberiotoxin-sensitive current was significantly reduced following ethanol withdrawal at voltages between −10 mV and +20 mV, on average by 50±3%, compared to controls. Because of the long lasting decrease in the levels of protein expression BKCa channel (see below), no attempt was made to evaluate the iberiotoxin current density in IC neurons at 48 h following ethanol withdrawal. Figure 3d shows that the mean conductance was markedly reduced, on average by 2.5 fold, in IC neurons following ethanol withdrawal (filled circles) compared to controls (square circles). Ethanol withdrawal also significantly reduced the mean value of Gmax (0.2±0.1 pS/pF, n=6 compared to controls: 0.5±0.1 pS/pF, n=6; F=8.4, P<0.01; Fig. 3e) without affecting activation parameters V1/2 (ethanol withdrawal:−0.5±5 mV, n=6; controls: −9.8±4 mV, n=6; Fig. 3f) and k values (ethanol withdrawal: 11.3±2 mV/e-fold, n=6; controls: 9.7±2 mV/e-fold, n=6; Fig. 3f).
Downregulation of BKCa channels
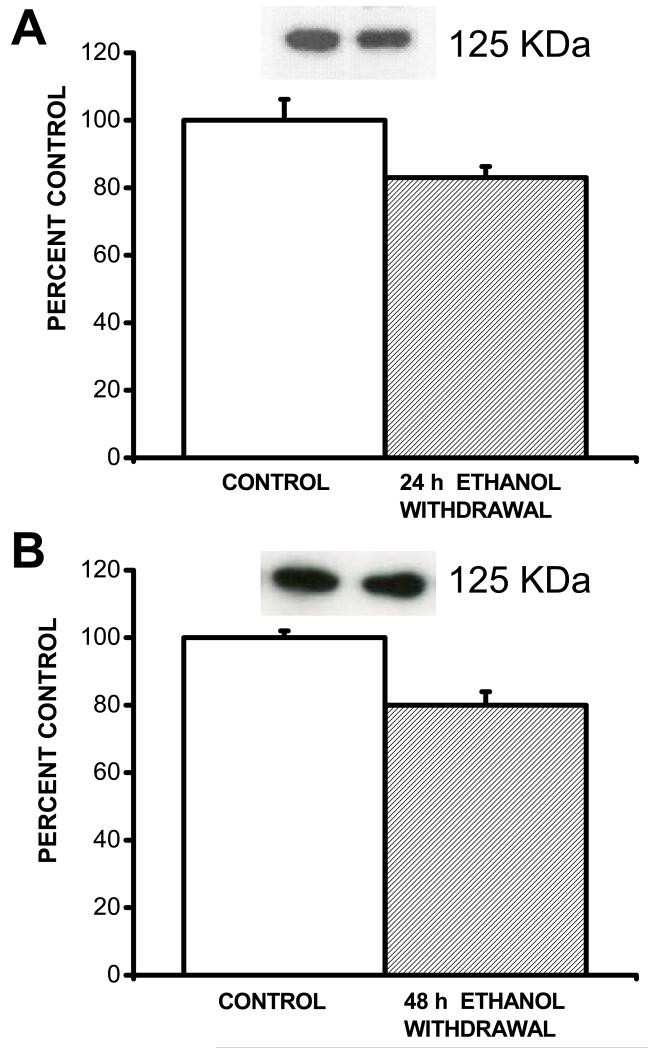
Figure 4 shows plots of total BKCa channel α-subunit proteins and their immunoreactive bands in IC tissues obtained from the control group, following ethanol withdrawal. Total BKCa channel α-subunit proteins were recognized by the antibody as 125-kD (bands in IC neurons. Quantification shows that total protein levels of BKCa channel α subunits were significantly (F=5.5, P<0.05) decreased in IC neurons at 24 h following ethanol withdrawal (83±3%, n=9) compared to the control group (100±6%, n=9; Fig. 4a). Significant (F=11, P<0.01) downregulation of total BKCa channel α subunit proteins was also seen at 48 h following ethanol withdrawal (80±4%, n=8) compared to controls (100±2%, n=8; Fig. 4b).
Figure 4.
Downregulation of BKCa channel α-subunit in IC neurons following ethanol withdrawal. Representative immunoblots of BKCa channel α-subunit obtained from control IC neurons and following ethanol withdrawal are showed in insets. The density of the 125 kDa immunoreactive band was significantly reduced in IC neurons 24 h (filled bar, n=9, panel A) compared to controls (opened bar, n=9). Similarly, the density of the 125 kDa immunoreactive band was significantly reduced in IC neurons at 48 h (filled bar, n=8, panel B) following ethanol withdrawal as compared to controls (opened bar, n=8). Data represents mean ± S.E.M. *P<0.05 (two-way ANOVA).
Discussion
The major finding of this report is that total outward K+ currents were reduced in IC neurons following alcohol withdrawal. The fraction of outward K+ currents carried by BKCa channels was also significantly reduced in IC neurons following alcohol withdrawal. BKCa channels conductance was also markedly reduced in IC neurons following ethanol withdrawal. Consistent with functional data, total protein abundance of BKCa channel α-subunit was reduced in IC neurons at 24 h and 48 h following alcohol withdrawal when the seizure susceptibility is present and absent, respectively. These findings provide direct evidence that BKCa channels are downregulated in IC neurons during alcohol withdrawal, and this effect outlasts the finite period of enhanced susceptibility to seizures. Decrease in Ca2+-activated K+ currents following ethanol withdrawal Acute alcohol application has been reported to increase BKCa channel open probability and the Ca2+-dependent afterhyperpolarization potentials, resulting in the inhibition of neuronal activity (Carlen et al. 1982; Niesen et al. 1988; Dopico et al. 1996). Consistent with these findings, spontaneous and acoustically evoked firing in IC neurons were suppressed following acute administration of alcohol in awake animals (Faingold and Riaz 1995; Chakravarty and Faingold 1998). In our study, even though the CaV channel currents were found to be enhanced in IC neurons following alcohol withdrawal, a significant decrease in total outward K+ (including BKCa) current density was observed. These findings are consistent with the observed decrease in afterhyperpolarization of dentate gyrus neurons following alcohol withdrawal (Carlen et al 1990), since both the large and small conductance KCa currents are known to contribute respectively, to the generation of fast and slow afterhyperpolarization potentials (Sah 1996; Vergara et al 1998). Activation of KCa currents are also known to terminate bursting activity, and reduction of these current should increase the propensity for bursting (Alger and Nicoll 1980). In this respect, a greater incidence of bursting activity has been reported in IC neurons during the alcohol withdrawal period, consistent with the downregulation of BKCa channels found in the present study (Faingold and Riaz 1995; Evans et al. 2000; Faingold et al., 2000). The mechanisms underlying the decrease in total outward K+ currents following alcohol withdrawal is not yet fully understood. In our study, IC neurons also exhibited large outward K+ channel currents despite the presence of Ca2+ chelator (EGTA) in the patch pipette (data not shown). Since EGTA is known to suppress the diffusion of free Ca2+ into the dendritic shaft and the cell soma, Ca2+ influx is likely to be restricted to the immediate vicinity of the Ca2+ entry sites (Stern 1992; Deisseroth et al 1996). KCa channels and the Ca2+ transporting proteins must therefore be colocalized in the same microdomain. In this respect, we have previously reported that the levels of expression of CaV2.2-α1 pore-forming subunit protein, in particular, were significantly downregulated in IC neurons following alcohol withdrawal (N’Gouemo et al. 2006). Thus, CaV2.2 channels may be preferentially colocalized in the same microdomain containing BKCa channels. This idea is supported by recent findings that BKCa channels are activated by Ca2+ influx via CaV2.2 channels in brain and spinal cord neurons (Loane et al. 2007; Ye et al. 2012). It would be interesting to characterize the functional coupling of BKCa channels and CaV2.2 channels in IC neurons, and evaluate how alcohol intoxication/withdrawal alters this coupling.
Remodeling of BKCa channels distribution may also account for the downregulation of BKCa channels in IC neurons following alcohol withdrawal. BKCa channels are mainly expressed in clusters and chronic ethanol exposure causes a declustering (and internalization) of membrane BKCa channels and also reduced the density of BKCa channels in the remaining clusters in hypothalamic-neurohypophysial explants (Pietrzkowski et al 2004). Change in the expression of specific BKCa channel splice variant may also contribute to the downregulation of the channels. Multiple neuronal BKCa channels splice variants have been described including ALCOREX (Pietrzykowski et al 2008). Interestingly, alcohol exposure dramatically reduced the expression of mRNA levels of ALCOREXBKCa channels splice variant in hypothalamic-neurohypophysial explants via a microRNA-miR-9-dependent postranscriptional mechanism (Pietrzykowski et al 2008). Whether alcohol withdrawal alters the expression of ALCOREX-BKCa channel splice variant in IC neurons remain unknown.
We have reported that alcohol withdrawal was associated with an increased current density of L-and P-type of CaV channels (N’Gouemo and Morad 2003). Preliminary studies demonstrated increases in intracellular Ca2+ transients in IC neurons following alcohol withdrawal (N’Gouemo, unpublished data). An increase in intracellular Ca2+ transient would be expected to activate KCa channels; however, in the present study, we found that BKCa current density and conductance are reduced during alcohol withdrawal. This effect was not accompanied by changes in V1/2 and k values suggesting that alcohol withdrawal did not alter the voltage sensing properties of BKCa channels. Thus, the downregulation of BKCa channels may have differential sensitivity to Ca2+ during alcohol withdrawal. However, in this study, no attempt to characterize the sensitivity of BKCa channels to Ca2+ was made. It has been reported that H+ and Ca2+ compete for the same binding site on BKCa channels (Laurido et al. 1991). Interestingly, acidification reduces the activity of BKCa channels under high levels of intracellular Ca2+ conditions such as following alcohol withdrawal (Hou et al. 2008; N’Gouemo, unpublished data; Song et al. 2010; Su et al. 2010). Thus, if H+ binding to BKCa channel during alcohol withdrawal was to occur, then the BKCa currents would decrease despite the increase in intracellular Ca2+ seen in IC neurons following alcohol withdrawal (N’Gouemo, unpublished data).
Downregulation of BKCa channels and alcohol withdrawal seizure susceptibility
The IC neurons play an important role in the pathogenesis of alcohol withdrawal seizures (Frey et al. 1983; Riaz and Faingold 1994; Faingold and Riaz 1995; N’Gouemo et al. 1996). L-type Ca2+ channel blockers are known to suppress audiogenic seizures following alcohol withdrawal (Little et al. 1986), and our previous data suggests that alcohol withdrawal associated with seizure susceptibility is accompanied by increases in high threshold CaV channel currents in IC neurons (N’Gouemo and Morad 2003). The precise mechanisms of how enhanced CaV channel current density contributes to alcohol withdrawal seizures are not fully understood. The enhancement of CaV channel currents should provide sufficient Ca2+ for Ca2+-dependent inactivation of Ca2+ currents and concomitant activation of KCa channels, leading to termination of bursting activity that accompany seizure activity. These mechanisms, however, appear to be significantly altered, as both Ca2+ channels inactivation is suppressed (N’Gouemo and Morad 2003), and BKCa α-subunit channel expression is downregulated (present study) in IC neurons following alcohol withdrawal. In this study, we found no seizure susceptibility at 48 h following alcohol withdrawal, despite reduced total outward K+ current density and dowregulation of α-subunit associated with BKCa channels in IC neurons. Interestingly, no enhancement of total CaV channel current density was reported in IC neurons at 48 h following alcohol withdrawal (N’Gouemo and Morad 2003). These findings suggest that i) downregulation of BKCa channels, per se, may not play a critical role in the generation of alcohol withdrawal seizures, ii) potential remodeling of other ion channels expression and/or neuronal networks may occur and contribute to suppress membrane hyperexcitability related to the reduction of BKCa channel function in IC neurons, and iii) the coupling between upregulation of CaV channels and downregulation of BKCa channels may play an important role in IC neuronal hyperexcitability that leads to alcohol withdrawal seizures. In this study, we found that BKCa current density and channel protein was decreased by approximately 50% and 20% following alcohol withdrawal, respectively. These findings suggest that in addition to BKCa channels, iberiotoxin suppresses other outward K+ currents in IC neurons.
The role of BKCa channels in the pathogenesis of seizures and epilepsies is complex (N’Gouemo 2011). BKCa channels are thought to provide an intrinsic inhibitory mechanism of neuronal hyperexcitability that could otherwise lead to seizures. Surprisingly, activation of BKCa channels can facilitate burst firing in hippocampus neurons (Gu et al. 2006), and cause generalized epilepsy in humans (Du et al. 2005; Diez-Sampedro et al. 2006). In the same line, blockade of BKCa channels suppressed tonic-clonic generalized seizures in presenzitized animals (Shruti et al. 2008; Sheehan et al. 2009). Genetic deletion of BKCa channel β4 subunits that results in elevated cell surface expression of BKCa channels and larger currents triggered the occurrence of limbic seizures (Brenner et al 2005; Shruti et al 2012). Interestingly, BKCa channel β4 subunit knockout mice consumed significantly more alcohol than their wild type counterparts and therefore are likely to experience alcohol withdrawal episodes (Martin et al 2008). The role of BKCa channel β4 subunits in the etiology of alcohol withdrawal seizures remains to be determined. In a fly model of ethanol intoxication/withdrawal, a blockade of slo gene (encoding for BKCa channel) neural promoter prevented the occurrence of ethanol-induced enhancement of electrographical seizure susceptibility (Ghezzi et al. 2010, 2012). However, the present study shows that downregulation of BKCa channels outlasted the finite period of alcohol withdrawal seizures suggesting that these channels, per se, may not play a critical role in the generation of alcohol withdrawal seizures.
Conclusions
We report in this study that alcohol withdrawal causes a downregulation of BKCa channels that outlast the finite period of enhanced susceptibility to audiogenic seizures following alcohol withdrawal. Thus, downregulation of BKCa channels, per se, may not be fundamentally important for the generation of alcohol withdrawal seizures. Nevertheless, the imbalance between upregulation of CaV channels and downregulation of BKCa channels may provide a novel cellular mechanism for the occurrence of alcohol withdrawal seizures.
Acknowledgments
This publication was made possible by Public Health Service grants (NS047193 and AA020073 to P.N., and HL62525 to M.M.) from the National Institutes of Health (NIH), and its contents are the responsibility of the authors and do not necessary represent the official views of NIH. All the experimental procedures used in this study were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals and approved by the Georgetown University Animal Care and Use Committee.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Alger BE, Nicoll RA. Epileptiform burst afterhyperpolarization: calcium-dependent potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Caird D. Processing in the colliculli. In: Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW, editors. Neurobiology of hearing, the central auditory system. Raven Press; New York: 1991. pp. 253–292. [Google Scholar]
- Carlen PL, Gurevich N, Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982;215:306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Carlen P, Rougier-Naquet I, Reynolds JN. Alterations of neuronal calcium and potassium currents during alcohol administration and withdrawal. In: Porter RJ, Mattson RH, Cramer JA, Diamond I, Schoenberg DG, editors. Alcohol and seizures: basic mechanisms and clinical concepts. Davis; Philadelphia: 1990. pp. 68–78. [Google Scholar]
- Chakravarty DN, Faingold CL. Comparison of neuronal response patterns in the external and central nuclei of inferior colliculus during ethanol administration and ethanol withdrawal. Brain Res. 1998;783:102–108. doi: 10.1016/s0006-8993(97)01193-1. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Diez-Sampedro A, Silverman WR, Bautista JF, Richerson GB. Mechanism of increased open probability by a mutation of the BK channel. J Neurophysiol. 2006;96:1507–1516. doi: 10.1152/jn.00461.2006. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca2+-activated K+ channels in isolated neurophypophysial terminals. Mol Pharmacol. 1996;49:40–48. [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, ez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, Campbell GA, Marietta CA, Majchrowicz E, Wixon HN, Weight FF. Cerebral 2-deoxyglucose uptake in rats during ethanol withdrawal and post-withdrawal. Brain Res. 1986;366:1–9. doi: 10.1016/0006-8993(86)91276-x. [DOI] [PubMed] [Google Scholar]
- Evans MS, Li Y, Faingold CF. Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcohol Clin Exp Res. 2000;24:1180–1186. [PubMed] [Google Scholar]
- Faingold CL. The Majochrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0928s44. Chapter 9:Unit 9.28. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Riaz A. Ethanol withdrawal induces increased firing in the inferior colliculus neurons associated with audiogenic seizure susceptibility. Exp Neurol. 1995;132:91–98. doi: 10.1016/0014-4886(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Li Y, Evans MS. Decreased GABA and increase glutamate-mediated activity on inferior colliculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain Res. 2000;868:287–295. doi: 10.1016/s0006-8993(00)02342-8. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–670. [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Pohl JB, Wang Y, Atkinson NS. BK channels play a counter-adaptive role in drug tolerance and dependence. Proc Natl Acad Sci USA. 2010;107:16360–16365. doi: 10.1073/pnas.1005439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict Biol. 20122012 doi: 10.1111/j.1369-1600.2012.00465.x. doi:10.1111/j.1369-1600.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüger Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hou S, Xu R, Heinemann SH, Hoshi T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol. 2008;15:403–410. doi: 10.1038/nsmb.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe PC, Picchioni AL, Chin L. Role of brain 5-hydroxytryptamine in audiogenic seizures in the rat. J Pharmacol Exp Ther. 1973;184:1–10. [PubMed] [Google Scholar]
- Knott TK, Dopico AM, Dayanithi G, Lemos J, Triestman Integrated channel plasticity contributes to alcohol tolerance in neurophypophysial terminals. Mol Pharmacol. 2002;62:135–142. doi: 10.1124/mol.62.1.135. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Laurido C, Candia S, Wolff D, Latorre R. Proton modulation a Ca2+-activated K+ channel from skeletal muscle incorporated into planar bilayer. J Gen Physiol. 1991;98:1025–1043. doi: 10.1085/jgp.98.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Dolin SJ, Halsey MJ. Calcium channel antagonists decrease the ethanol withdrawal syndrome. Life Sci. 1986;39:2059–2065. doi: 10.1016/0024-3205(86)90356-5. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Lima PA, Marrion NV. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci. 2007;120:985–995. doi: 10.1242/jcs.03399. [DOI] [PubMed] [Google Scholar]
- Martin GE, Puig SI, Pietrzykowski AZ, Paula Zadek, Emery P, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Hendrickson LM, Penta KL, Friesen RM, Pietrzykowski AZ, Tapper AR, Treistman SN. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proc Natl Acad Sci USA. 2008;105:17543–17548. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Niesen CE, Baskys A, Carlen PL. Reversed ethanol effects on potassium conductances in aged hippocampal dentate granule neurons. Brain Res. 1988;445:137–141. doi: 10.1016/0006-8993(88)91082-7. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P. Targeting BK (big potassium) channels in epilepsy. Expert Opin Ther Targets. 2011;15:1283–1295. doi: 10.1517/14728222.2011.620607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Morad M. Ethanol withdrawal seizure susceptibility is associated with upregulation of L-and P-type Ca2+ channels currents in rat inferior colliculus neurons. Neuropharmacology. 2003;45:429–437. doi: 10.1016/s0028-3908(03)00191-6. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Caspary DM, Faingold CL. Decreased GABA effectiveness in the inferior colliculus neurons during ethanol withdrawal in rat susceptible to audiogenic seizures. Brain Res. 1996;724:200–204. doi: 10.1016/0006-8993(96)00304-6. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Yasuda RP, Morad M. Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Res. 2006;1108:216–220. doi: 10.1016/j.brainres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Yasuda RP, Faingold CL. Protein expression of small conductance calcium-activated potassium channels is altered in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res. 2009;1270:107–111. doi: 10.1016/j.brainres.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel spice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz A, Faingold CL. Seizures during ethanol withdrawal are blocked by focal microinjection of excitant amino acid antagonists in the inferior colliculus and pontine reticular formation. Alcohol Clin Exp Res. 1994;18:1456–1462. doi: 10.1111/j.1530-0277.1994.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Benedetti BL, Barth Al. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia. 2009;50:711–720. doi: 10.1111/j.1528-1167.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem LR, Barth AL. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol Dis. 2008;30:323–330. doi: 10.1016/j.nbd.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruti S, Urban-Ciecko, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific beta4 subunit downregulates BK channel cell surface expression. PLoS ONE. 2012;7:e33429. doi: 10.1371/journal.pone.0033429. doi:10.1371/journal.pone.0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Distinct K+ currents result in physiological distinct cell types in inferior colliculus of the rat. J Neurosci. 2001;21:2861–2877. doi: 10.1523/JNEUROSCI.21-08-02861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Su W, Chen L, Ji JJ. Functional expression of large-conductance Ca2+-activated potassium channels in lateral globus pallidus neurons. Neuroscience. 2010;169:1548–1556. doi: 10.1016/j.neuroscience.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Stern MD. Buffering of calcium in the vicinity of channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fst afterhyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Song X, Ji JJ. Functional expression of a large-conductance Ca2+-activated K+ channel in mouse substantia nigra pars compacta dopaminergic neurons. Neurosci Lett. 2010;471:1–5. doi: 10.1016/j.neulet.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein form polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraga C, Latorre R, Marrion NV, Andelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Ye H, Buttigieg J, Wan Y, Wang J, Figley S, Fehlings MG. Expression and functional role of BK channels in chronically injured spinal cord white matter. Neurobiol Dis. 2012;47:225–236. doi: 10.1016/j.nbd.2012.04.006. [DOI] [PubMed] [Google Scholar]