Abstract
Background
Mitochondrial dysfunction has been closely related to many pathological processes, such as cellular apoptosis. Alterations in organelle membrane potential are associated with mitochondrial dysfunction. A fluorine -18 labeled phosphonium compound: 18F-triphenylphosphonium (18F-TPP) was prepared to determine its potential use as a mitochondria-targeting radiopharmaceutical to evaluate cellular apoptosis.
Methods
Studies were conducted in both ex vivo cell lines and in vivo using a burned animal model. Uptake of 18F-TPP was assessed in PC-3 cells by gamma counting under the following conditions: graded levels of extra-cellular potassium concentrations, incubation with carbonyl cyanide m-chlorophenylhydrazone (CCCP) and staurosporine. Apoptosis was studied in a burn animal model using TUNEL staining and simultaneous assessment of 18F-TPP uptake by biodistribution.
Results
We found that stepwise membrane depolarization by potassium (K) resulted in a linear decrease in 18F-TPP uptake, with a slope of 0.62+/−0.08 and a correlation coefficient of 0.936+/−0.11. Gradually increased concentrations of CCCP lead to decreased uptakes of 18F-TPP. Staurosporine significantly decreased the uptake of 18F-TPP in PC-3 cells from 14.2+/−3.8% to 5.6+/−1.3% (P<0.001). Burn induced significant apoptosis (sham: 4.4 +/−1.8% vs. burn: 24.6+/− 6.7 %; p<0.005) and a reduced uptake of tracer in the spleens of burn injured animals as compared to sham burn controls (burn: 1.13+/−0.24% vs. sham: 3.28+/−0.67%; p<0.005). Biodistribution studies demonstrated that burn induced significant reduction in 18F-TPP uptake in spleen, heart, lung, and liver, which were associated with significantly increased apoptosis.
Conclusions
18F-TPP is a promising new voltage sensor for detecting mitochondrial dysfunction and apoptosis in various tissues.
Keywords: 18F-TPP, Mitochondrion, Membrane Potential, Apoptosis
INTRODUCTION
Mitochondrion plays a central role in cellular function and dysfunctional mitochondria are involved in many pathologic processes, such as inflammation, degenerative diseases, aging, cancer, and arthrosclerosis [1–4]. Membrane potential (Δψm) is a reliable index of the status of mitochondrial function.
Cellular apoptosis is closely associated with the potential of the mitochondrial membrane [5, 6]. The depolarization of the inner mitochondrial membrane triggers the apoptosis pathway. Therefore, assessment of mitochondrial potential could be used to detect cellular apoptosis and the apoptosis related organ functional status. Monitoring the mitochondrial potential in vivo could be of clinical importance in monitoring disease process and assessing the efficiency of clinical treatment. Changes of mitochondrial membrane potential Δψm can be reflected by the uptake of lipophilic cations [7, 8].
A phosphonium cation, 18 Fluorine-triphenylphosphonium (18F-TPP) has been developed as a new imaging agent tracer which might be used to non-invasively assess apoptosis-related mitochondrial dysfunction. This cation is strongly lipophilic, penetrates cellular membranes and accumulates in mitochondria, which is a most electro-negative organelle. Thus, the uptake of this tracer could serve as an indicator of the transmembrane voltage gradient. In the present study, we investigated the uptake of 18F-TPP in an ex vivo cell culture system and in vivo distribution in burned and sham-burned mice, to evaluate its potential applications in monitoring disease status of the patients, using positron emission tomography (PET).
MATERIAL AND METHODS
Materials
18F-TPP was synthesized as described by Shoup et al in our laboratory 9. For ex vivo studies in cell culture system, the composition of the incubation medium is as follows (g/L): NaCl (150), KCl (5.3), CaCl2 (1.16), MgSO4 (0.67), NaH2PO4 (0.76), dextrose (5.8) and Hepes (5.2), with FBS 1.5% (v/v). The loading buffer solution is prepared as a standard solution except for the substitution of NaCl by equal molar K-asparate and lowering the Ca2+ concentration to 0.1mM. F-12K medium, fetal calf serum and Penicillin/streptomycin were obtained from ATCC (NY). Cell Death Detection kit (TUNEL) was obtained from Roche Molecular Biochemicals (Mannheim, Germany).
Animal
Wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) weighing 20 to 25 gm were randomly divided into groups (5 per group). The extent of apoptosis in spleen was evaluated by TUNEL staining. The study was approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, Harvard University, and was in compliance with the “Guide for the Care and Use of Laboratory Animals” (Publication No. NIH 78-23, 1996).
PC-3 Cell Line Culture
The studies were conducted using prostate cancer cell line PC-3. The cells were cultured in 75-cm2 tissue culture flasks in F-12K medium which was supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and streptomycin, at 37°C in a humidified 5% CO2 incubator. Viability of the cells was checked by trypan blue exclusion staining and routinely was greater than 95%. Cells were harvested by treatment with 0.02% trypsin.
Time Course of 18F-TPP Uptake by PC-3 cells
Cells were harvested by trypsinization, followed by washing three times with PBS and suspension in loading buffer at a concentration of 1×106 cells/ml. then, the cells were incubated in a water bath for one hour at 37°C, and then mixed with an equal volume of loading buffer containing 2.0 nM of 18F-TPP. The cells were harvested at: 5 min, 30 min, 1.0 hour, 1.5 hours and 2.0 hours, and tracer accumulation in the cells were determined. At each time point, 18F-TPP uptake was terminated by centrifugation at 1000rpm for 6 minutes. Radioactivity of the supernatant and the cell pellets were measured with a gamma counter (Beckman). Radiotracer uptake was expressed as the accumulation ratio (%) by dividing the radioactivity in the pellet by the total radioactivity in both supernatant and pellet per million cells.
The Effects of Proapoptotic Factors on 18F-TPP Uptake by PC-3 cells
We explored the effect of TPP concentration on 18F-TPP uptake by the PC-3 cells. Cells were incubated with 18F-TPP tracer at graded concentrations of TPP: 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0 nM. The most effective concentration of TPP was determined for subsequent studies.
The effects of potassium, carbonyl cyanide m-chlorophenylhydrazone (CCCP), staurosporine and valinomycin on 18F-TPP uptake were further investigated by incubating the cells at different concentrations of individual compounds. PC-3 Cells were harvested and washed three times before being incubated with the compounds. Additive effects on uptake were further evaluated by studying different combinations of these factors.
Burn Injury Model
All animals received general anesthesia (Ketamine 40mg/kg body weight and Xylazine 5 mg/kg body weight, IP) prior to burn injury. The dorsal surface of the animals was shaved with animal hair clippers. A full-thickness thermal injury of 30% total body surface area (TBSA) was produced by placing the animals in a mold followed by exposure of the dorsal area to a 90°C water bath for 9 seconds. The mice were immediately resuscitated by intraperitoneal injection of 1.5 ml saline. Sham burned controls received the same treatment except with the water bath set in room temperature. After the procedure, the mice were caged individually.
Twenty-four hours after the burn injury, euthanasia was performed by over dose of Phenobarbital. Organs were harvested for analyses of apoptosis and biodistribution.
Histopathological Examination
The spleen specimens were fixed in 10% formaldehyde and embedded in paraffin. Four μm tissue sections were stained with hematoxylin and eosin and examined with light microscopy by experienced pathologists that were blinded from the treatment conditions.
Evaluation of Cellular Apoptosis
Four μm tissue sections were deparaffinized in xylene and dehydrated in graded ethanol. Apoptotic cells were identified by using a Terminal Deoxynucleotidyl Transferase dUTP Nick-end Labeling (TUNEL) kit. Ten random fields from 5 slides per group were examined and the TUNEL-positive brown nuclei within the cells were scored as previously described 10. Data were expressed as percentage of TUNEL-positive cells/100 cells. Digitized images were analyzed by two investigators.
Biodistribution Study
Pilot studies were conducted to determine the optimal time for assessing 18F-TPP biodistribution in spleen. 18F-TPP was injected to animals 24 hours after burn or sham burn injury, spleen was harvested at different time points after the injection and 18F-TPP accumulation in the organ was measured. The radioactivity is expressed as the percentage of injected dose per gram tissue (%DPG).
Statistical Analysis
Data was presented as mean ± SEM. Statistical evaluations were performed using ANOVA and Student t-tests wherever indicated. P value less than 0.05 was considered significant and n represents the number of animals per group.
RESULTS
1. Time-dependent Accumulation of 18F-TPP in PC-3 Cells and the Effects of Tracer Concentration on Uptake
Cells were incubated at normal potassium concentration (5.4 μM) and harvested at different time points. The results showed time-dependent uptake kinetics of 18F-TPP. The cellular radioactivity increased gradually and reached a plateau at 60 minutes of incubation (Fig. 1, A). Therefore one hour was chosen as optimal incubation time to be used in the definitive studies.
Fig. 1. Time course study and concentration study on 18F-TPP uptake by PC-3 cells.
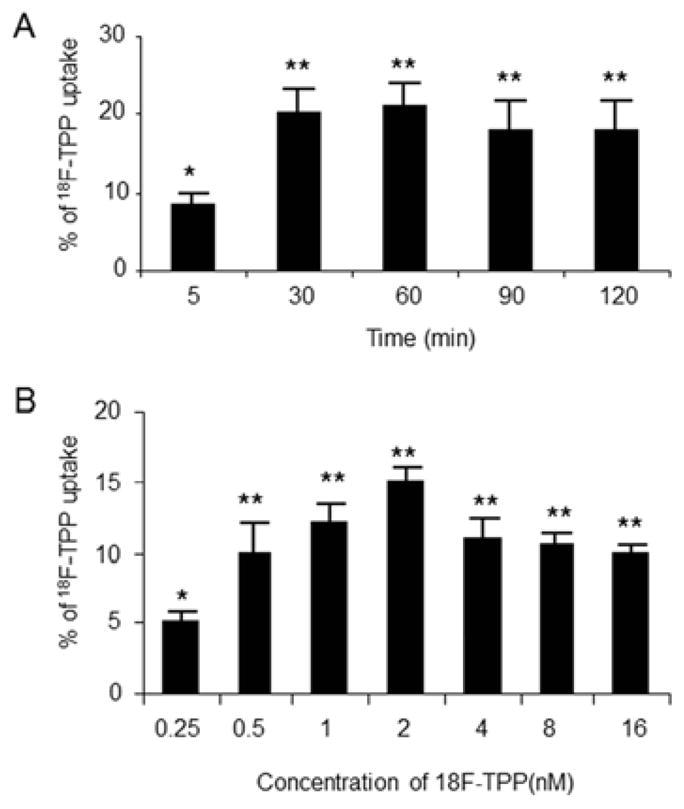
PC-3 cells were incubated with normal K concentration (5.4 μM). Time course study demonstrated time-dependent uptake kinetics of 18F-TPP by PC-3 cells. The cellular radioactivity increased gradually and reached the plateau at 60 minutes post incubation (Fig. 1 A: ANOVA, * vs. **: P<0.05; ** P>0.05). Cells incubated with 18F-TPP at different levels of extracellular concentration. The maximum uptake was shown at the concentration of 2 nM (Fig. 1 B: ANOVA, * vs. **: P<0.05; ** P>0.05).
Cells incubated with 18F-TPP at graded concentrations demonstrated that the tracer uptake remained at a relatively stable level over the extracellular concentration range of 0.5 to 16 nM. The concentration of 2 nM demonstrated the peak uptake, which was chosen for further studies (Fig. 1, B).
2. Effects of Potassium Concentration on 18F-TPP Uptake
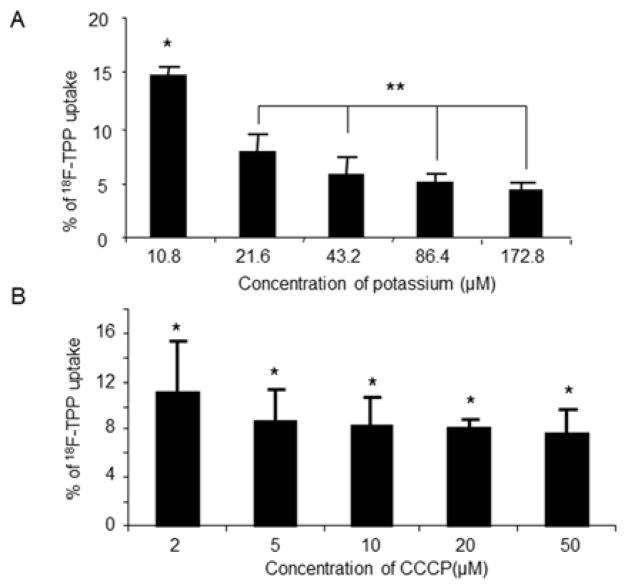
Following the increased extracellular concentration of potassium, the uptake of 18F-TPP showed a step-wise decrease. At the highest potassium concentration of 172.8 μM, the uptake of 18F-TPP reached its lowest level. The lowest potassium concentration of 10.8 μM corresponded with the highest level of 18F-TPP uptake. Overall, there is an inverse linear correlation between the 18F-TPP uptake and extracellular potassium concentrations (slope: 0.62+/− 0.78; correlation coefficient: r=0.936+/− 0.11. Fig. 2 A).
Fig. 2. Effects of potassium and CCCP concentrations on 18F-TPP uptake by PC-3 cells.
The uptake of 18F-TPP was decreased as the extracellular potassium concentration increased. At the highest potassium concentration of 172.8 μM, the uptake was the lowest, while at the lowest potassium concentration of 10.8 μM, the uptake was at the highest. An inverse linear correlation between the uptake and extracellular potassium concentration (slope: 0.62+/− 0.78; correlation coefficient: r=0.936+/− 0.11) was found (Fig. 2 A: ANOVA, * vs. **: P<0.05; ** P>0.05). Gradually increased concentrations of CCCP lead to decreased uptakes of 18F-TPP, which quickly reached a trough at CCCP concentration of 5μm (Fig. 2 B: ANOVA, * P>0.05).
3. Effects of CCCP Concentration on 18F-TPP Uptake
CCCP is a chemical inhibitor of oxidative phosphorylation which induces the uncoupling of protons in the electron transport chain11. As seen in figure 2B, increment of CCCP concentrations in the incubation medium from 2 μm to 5 μm resulted in a rapid reduction of 18F-TPP uptake into the cells, although there was no further statistically significant decrease at higher concentrations. (Fig. 2 B).
4. Effects of Staurosporine, and the Combination of K, CCCP, Valionmycin and Staurosporine on 18F-TPP Uptake
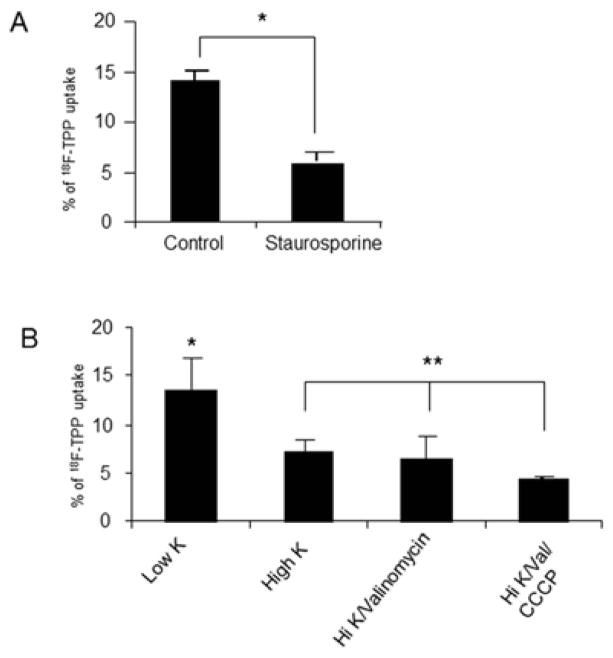
Significant reduction of 18F-TPP uptake was found at staurosporine concentration of 8μM. (Fig. 3 A, control vs. staurosporine: P<0.001). Combination of the proapoptotic factors, such as high potassium/valionmycin and High potassium/valionmycin/CCCP, demonstrated similar effects on 18F-TPP uptake as shown in Fig. 3 B.
Fig. 3. Effects of staurosporine and combined proapoptotic factors on 18F-TPP uptake.
Staurosporine at 8μM lead to a significantly decreased uptake of 18F-TPP in PC-3 cells compared with the sham treated cells (Fig. 3 A: t test: P<0.001). Combination of proapoptotic factors clearly demonstrated similar effects on the uptake (Fig. 3 B: ANOVA: * vs. **p<0.05; **p>0.05). Note: CCCP: 50 μM/L; Val: 1 μg/ml; Low K= 2 μM/L; high K= 172.8 μM/L.
5. Burn Induced Apoptosis in Spleen
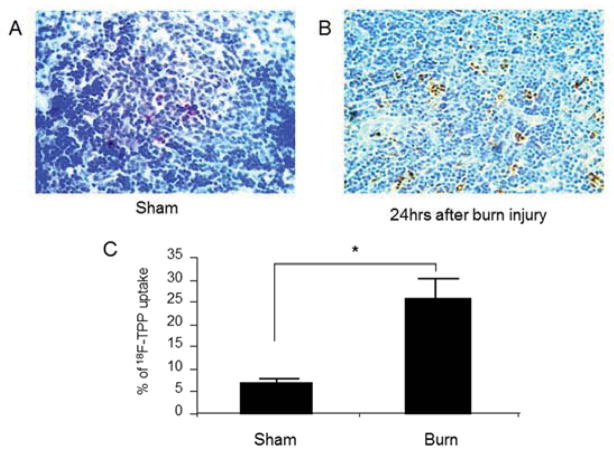
We chose 24 hours after burn as the time point for evaluation of post burn apoptosis basing on our previous experience 9. TUNEL stain on the sections of the spleens from the sham mice demonstrated diffusely scattered apoptotic cells at a rate of 4.4 +/−1.8% (Fig. 4 A). In contrast, burn induced significant cellular apoptosis in the spleen presenting an apoptotic index of as high as 24.6+/− 6.7 % (Fig. 4 B, C. sham vs. burn: P<0.005). The majority of the apoptotic cells were present in the white medulla of the spleen.
Fig. 4. Burn induced apoptosis in mouse spleen.
TUNEL staining on the sections of the spleens from the sham mice demonstrated diffusely scattered apoptotic cells at a rate of 4.4 +/−1.8% in the white medulla (Fig. 4 A). By contrast, burn induced significant cellular apoptosis in the spleen presented an apoptotic index of as high as 24.6+/− 6.7 % (Fig. 4 B, C: sham vs. burn: t test, p<0.005). The majority of the apoptotic cells were present in the white medulla area.
6. 18F-TPP Uptake by Spleen and 18F-TPP Biodistribution Changes after Burn Injury
The time course study on the uptake of 18F-TPP by the spleen in mice showed the maximum accumulation at 20 minutes after a tail vein injection (Fig. 5. A). This time point was chosen for assessing the biodistribution in organs. 18F-TPP uptake by spleens was reduced from 3.28 ±0.67% in sham burn to 1.13±0.24% in burned animals (Fig. 5 B, p<0.005).
Fig. 5. Effects of apoptosis on 18F-TPP uptake in spleen and 18F-TPP biodistribution.
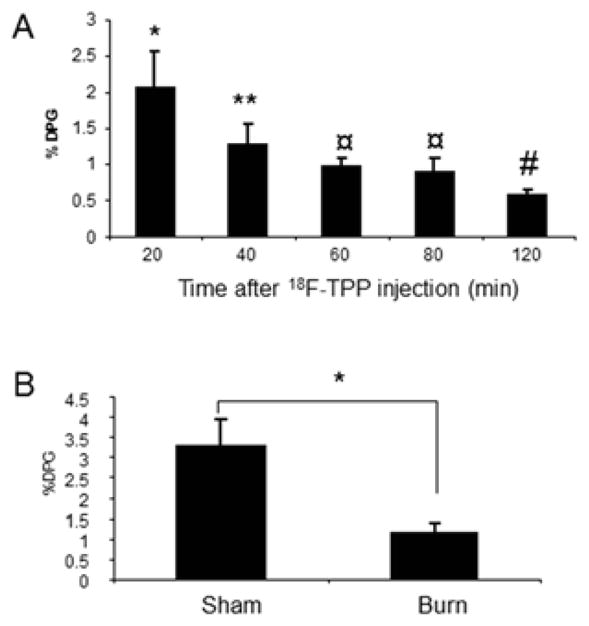
Time course study on the uptake of 18F-TPP by spleen in mice showed the maximum accumulation in 20 minutes after the injection of 18F-TPP via tail vein. This time point was chosen for biodistribution studies (Fig. 5 A: ANOVA, * vs. the rest, ** vs. #: P<0.05; ¤, ** vs. ¤, and ¤ vs. #: P>0.05). Tracer uptake in burned mouse spleens was reduces to 1.13±0.24% compared with 3.28 ±0.67% (t test: p<0.005) in spleens of sham treated mice (Fig. 5 B).
The biodistribution studies also demonstrated a significant reduction of 18F-TPP uptake in heart, lung, liver (sham v.s burn: P<0.05, Fig. 6). The results were consistent with the well-documented findings: burn induced a significant apoptosis status in multiple organs and tissues [9].
Fig. 6. 18F-TPP biodistribution.
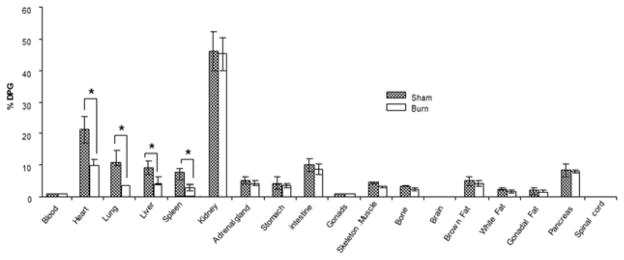
The biodistribution study showed significant reduction of 18F-TPP uptake in heart, lung, spleen, and liver in burned animals compared with sham treated ones(* burn vs. sham: t test, P< 0.05). No evident uptake differences were presented in other examined organs of burned animals by contrast with the sham treated ones (burn vs. sham: t test, P> 0.05).
DISCUSSION
The change of mitochondria membrane potential is closely related to cell function and apoptosis. The present study aimed at evaluating the changes of mitochodrial potential in relation to 18F-TPP uptake using an ex vivo cell culture system and an in vivo burn model. The ultimate goal of our study is to determine if this tracer can be used to evaluate the changes of mitochondrial membrane potential and the extent of apoptosis in multiple organ/tissues in vivo using PET imaging techniques. Thus, it may serve as a clinical index to monitor the organ function and disease status of patients, especially those suffering from critical illness.
Apoptosis, or programmed cell death, is involved in the etiology and pathogenesis following the progress of many disease conditions. The intrinsic pathway of apoptosis is mediated by permeabilization of the mitochondrial membrane [10], followed by release of proapoptotic signaling molecules, such as cytochrome c, and finally activation of the caspase family, such as caspase 6, 7 and 3. It has been proven that initiation of the complete post-mitochondrial cascade is accompanied with a decreased potential across the mitochondrial inner membrane. The extrinsic apoptotic pathway is triggered by interaction of death receptors, such as CD95, DR5, on the cellular membrane, which can activate caspase 8 as an initiator. Then caspase 8 cleaves the bcl-2 protein family and also increase the permeability of the mitochondrial membrane resulting in decreased membrane potential [11]. Furthermore, there are many interactions between the intrinsic and the extrinsic pathway which are closely associated with changes of the mitochondrial membrane potential. Thus the initiation of apoptosis can be monitored by alterations in membrane potential.
Membrane potential is maintained principally by the concentration gradient and membrane permeability to potassium and sodium. Increase of the extracellular potassium levels results in depolarization of the membrane potentials. The present study demonstrated that increase of extracellular potassium concentration resulted in significant decrease of 18F-TPP uptake and a strong inverse linear correlation between potassium concentration in the incubation medium and 18F-TPP uptake with a correlation coefficient of 0.936. The results indicated the sensitivity of this tracer uptake in relation to the alterations of mitodchondrial membrane potential. The sensitivity was further verified in conditions of altered membrane potential induced by multiple proapoptotic compounds. CCCP is a chemical inhibitor of oxidative phosphorylation which can cause an uncoupling of the proton gradient that is established during the activity of electron carriers in the electron transport chain [12]. CCCP causes the gradual destruction of living cells and as a result, leads to apoptosis [13]. Staurosporine is a broad-band protein kinase inhibitor, which can initiate cellular apoptosis through the intrinsic pathway. It has been well-documented that staurosporine leads to the loss of cross-membrane potential gradients. Valinomycin is a dodecadepsipeptide antibiotic. It functions as a potassium-specific transporter and facilitates the movement of potassium ions through lipid membranes “down” an electrochemical potential gradient [14]. Valinomycin triggers a rapid loss of the mitochondrial membrane potential and cytoplasmic acidification, leading to cysteine-active-site protease activation, DNA fragmentation and apoptosis [15]. The present study demonstrated that individual addition of these compounds into the incubation medium resulted in significant decrease of 18F-TPP uptake by PC-3 cells (Figures 3, and 4). Furthermore, combinations of these compounds resulted in similar effects in reducing 18F-TPP uptake (Figure 5). These findings suggested a high sensitivity of 18F-TPP as an indicator to detect the alterations of mitochondrial membrane potential of the cells.
The present biodistribution study demonstrated significant reductions of 18F-TPP uptake in the spleen, as well as in the heart, liver and lung of the burned mice. It is documented that burn might enhance evident apoptosis in these organs [9, 16–18]. In our present study, we focused on investigating burn induced spleen apoptosis and 18F-TPP uptake to verify the correlation between apoptosis and 18F-TPP uptake. It also indicates the sensitivity of 18F-TPP as a tracer to detect the changes of mitochondrial potential in both ex vivo system and living animals. Therefore 18F-TPP is potentially a sensitive tracer which can be used as a PET imaging agent to non-invasively monitor apoptosis and functional status of multiple organs and tissues following various disease processes. It can also be used to evaluate the efficacy of treatment in clinical patients, especially those with critical illness with high risk of multiple organ failures.
Currently, the only available imaging agent that can be used to detect apoptosis is Annexin V [19], but it has inherent disadvantages. Apoptosis is not a one-time event but rather a continual process. Annexin V scan can only get a snapshot of the apoptosis status. The unique advantage of 18F-TPP is to allow multiple “real-time” measurements. Also, 18F-TPP targets the changes of membrane function, which provides a better understanding of the apoptosis, and apoptosis related functional status of the tissue.
In conclusion, the present study suggests the potential power and unique advantages of 18F-TPP as a sensitive and accurate voltage sensor for detecting membrane potential alterations using PET. The findings established that 18F-TPP is a promising imaging agent to detect apoptosis and apoptosis related organ function in clinical practice.
Acknowledgments
This study was supported by grants from Shriners Hospitals for Children (Grant 87100) and the National Institute of Health (P50 21700).
Footnotes
Author’s Contribution: G.Z, YM.Y, DR.E, AA.B and TM.S: Conception and design; G.Z and YM.Y: Analysis and interpretation; G.Z, YM.Y and TM.S: Data collection; G.Z, YM.Y and AJ.F: Writing the article; RG.T, AJ.F and AA.B: Critical revision; RG.T and AJ.F: Obtaining funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng M, Zhang Q, Joe Y, et al. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int Immunopharmacol. 2013;15:517. doi: 10.1016/j.intimp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Scheibye-Knudsen M, Scheibye-Alsing K, Canugovi C, et al. A novel diagnostic tool reveals mitochondrial pathology in human diseases and aging. Aging (Albany NY) 2013;5:192. doi: 10.18632/aging.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang KE, Park C, Kwon SJ, et al. Synergistic induction of apoptosis by sulindac and simvastatin in A549 human lung cancer cells via reactive oxygen species-dependent mitochondrial dysfunction. Int J Oncol. 2013;43:262. doi: 10.3892/ijo.2013.1933. [DOI] [PubMed] [Google Scholar]
- 4.Gaggini M, Morelli M, Buzzigoli E, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) and Its Connection with Insulin Resistance, Dyslipidemia, Atherosclerosis and Coronary Heart Disease. Nutrients. 2013;5:1544. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilgendorf KI, Leshchiner ES, Nedelcu S, et al. The retinoblastoma protein induces apoptosis directly at the mitochondria. Genes Dev. 2013;27:1003. doi: 10.1101/gad.211326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang XY, Yao YG. Mitochondrial dysfunction and nuclear-mitochondrial shuttling of TERT are involved in cell proliferation arrest induced by G-quadruplex ligands. FEBS Lett. 2013;587:1656. doi: 10.1016/j.febslet.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Madar I, Ravert H, Nelkin B, et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging. 2007;34:2057. doi: 10.1007/s00259-007-0500-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Kim HS, Le UN, et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J Nucl Med. 2012;53:1779. doi: 10.2967/jnumed.111.102657. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Yu YM, Kaneki M, et al. Simvastatin protects hepatocytes from apoptosis by suppressing the TNF-α/caspase-3 signaling pathway in mice with burn injury. Ann Surg. 2013;257:1129. doi: 10.1097/SLA.0b013e318273fdca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Li Y, Jin J, et al. Lx2-32c, a novel taxane derivative, exerts anti-resistance activity by initiating intrinsic apoptosis pathway in vitro and inhibits the growth of resistant tumor in vivo. Biol Pharm Bull. 2012;35:2170. doi: 10.1248/bpb.b12-00513. [DOI] [PubMed] [Google Scholar]
- 11.Elumalai P, Gunadharini DN, Senthilkumar K, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215:131. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhari AA, Seol JW, Kang SJ, et al. Mitochondrial transmembrane potential and reactive oxygen species generation regulate the enhanced effect of CCCP on TRAIL-induced SNU-638 cell apoptosis. J Vet Med Sci. 2008;70:537. doi: 10.1292/jvms.70.537. [DOI] [PubMed] [Google Scholar]
- 13.Lim ML, Minamikawa T, Nagley P. The protonophore CCCP induces mitochondrial permeability transition without cytochrome c release in human osteosarcoma cells. FEBS Lett. 2001;503:69. doi: 10.1016/s0014-5793(01)02693-x. [DOI] [PubMed] [Google Scholar]
- 14.Lofrumento DD, La Piana G, Abbrescia DI, et al. Valinomycin induced energy-dependent mitochondrial swelling, cytochrome c release, cytosolic NADH/cytochrome c oxidation and apoptosis. Apoptosis. 2011;16:1004. doi: 10.1007/s10495-011-0628-7. [DOI] [PubMed] [Google Scholar]
- 15.Abdalah R, Wei L, Francis K, et al. Valinomycin-induced apoptosis in Chinese hamster ovary cells. Neurosci Lett. 2006;405:68. doi: 10.1016/j.neulet.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Costantini T, Lopez NE, et al. Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J Cell Mol Med. 2013;17:664. doi: 10.1111/jcmm.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Öksüz S, Ülkür E, Öncül O, et al. The effect of subcutaneous mesenchymal stem cell injection on statis zone and apoptosis in an experimental burn model. Plast Reconstr Surg. 2013;131:463. doi: 10.1097/PRS.0b013e31827c6d6f. [DOI] [PubMed] [Google Scholar]
- 18.Marshall AH, Brooks NC, Hiyama Y, et al. Hepatic apoptosis postburn is mediated by c-Jun N-terminal kinase 2. Shock. 2013;39:183. doi: 10.1097/SHK.0b013e31827f40ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vangestel C, Van de Wiele C, Mees G, et al. Single-photon emission computed tomographic imaging of the early time course of therapy-induced cell death using technetium 99m tricarbonyl His-annexin A5 in a colorectal cancer xenograft model. Mol Imaging. 2012;11:135. [PubMed] [Google Scholar]