Abstract
Background
Cytotoxic T lymphocytes (CTLs) modified with chimeric antigen receptors (CARs) for adoptive immunotherapy of hematologic malignancies are effective in pre-clinical models, and this efficacy has translated to success in several clinical trials. Many early trials were disappointing, due in large part to the lack of proliferation and subsequent persistence of transferred cells. Recent investigations have pointed to the importance of delivering highly proliferative cells, whether of naïve or early memory phenotypes.
Methods
We investigated the influence of two common cell culturing methods used in early trials and their relationship to T cell phenotype and pre-clinical efficacy.
Results
We observed that stimulation with soluble OKT3 and high-dose interleukin-2 (IL-2) produces more effector memory type T cells with shorter average telomeres when compared to cells generated with CD3/CD28 beads. When used in xenograft models of leukemia, bead-stimulated cells proliferated earlier and to a higher degree than those generated with OKT3/IL2 and resulted in better disease control despite no difference in distribution or migration throughout the mouse. Inclusion of the known successful clinical 4-1BB endodomain in the CAR could not rescue the function of OKT3/IL-2 cultured cells. T cells isolated from animals that survived long-term (>120 days) retained a central memory-like phenotype, and demonstrated a memory response to a large re-challenge of CD19 positive leukemia.
Discussion
In summary, we confirm that cells with a younger phenotype or higher proliferative capacity perform better in pre-clinical models and that cell culturing influences cell phenotype seemingly independent of the 4-1BB endodomain in the CAR structure.
Keywords: CD19, chimeric antigen receptor, leukemia, T cell memory, T lymphocytes
Introduction
One of the great barriers to the success of cell therapies for cancer has been poor in vivo expansion and persistence of transferred cells. Several studies have demonstrated that objective clinical responses correlate with both parameters,1–3 and modest persistence directly correlates with modest clinical response.4 In contrast, a recent phase 1 clinical trial for chronic lymphocytic leukemia (CLL) demonstrated that significant in vivo expansion and long-term persistence correlated with a remarkable reduction in disease burden.5, 6 This study also demonstrated that the number of cells transferred may not determine clinical efficacy, as one patient received an effector:target ratio of ~1:93,000 and experienced a complete remission, suggesting that a small number of highly proliferative T cells is better than a large number of T cells of more limited expansion potential.
T lymphocytes can be classified into the following subgroups: (1) antigen-inexperienced naïve T cells, (2) central memory T cells (TCM), which migrate to the lymph nodes and exhibit rapid proliferation upon re-exposure to antigen, (3) effector memory T cells (TEM), which circulate in the peripheral blood and have immediate effector function, and (4) terminally-differentiated effector T cells (TEff).7 Several lines of evidence suggest that the T cell populations historically used for cellular therapy clinical trials have been TEM or TEff.3, 8, 9 While these cells have potent in vitro cytotoxicity against target cells, in vivo data suggest that they may have exhausted the expansion and proliferation potential of younger (or less-differentiated) T cell populations.1, 10, 11 It is reasonable to speculate that trials employing these cell types may thus been biased against demonstrating maximal clinical activity. The cells may not need to persist to work, but may in fact persist because they worked.
Several theories exist to explain the lineage relationship between TCM and TEM. While it remains unclear if one population derives from the other or if they are two distinct lineages, it does seem that TCM possess greater self-renewal capability and are functionally less-differentiated cells than TEM.12, 13 Delineating this relationship has proven challenging, as murine T cell differentiation differs from human, presenting difficulties in experiment design and the ability to extrapolate results seen in adoptive therapy models using murine T cells to T cell biology in humans. Nevertheless, TCM represent a promising population of cells for use in adoptive therapy where this self-renewal could be highly advantageous, because, when compared head-to-head, cells derived from TCM persist and expand to a greater degree in vivo than those derived from TEM.14 In functional studies using a mouse model of infection, superior protective immunity is observed upon transfer of TCM as compared to TEM.15 Using murine models of spontaneous melanoma it has been shown that TCM exhibit enhanced expansion, mediate an enhanced anti-tumor response, and improve overall survival.16 Translating these findings to the clinic, the phase 1 study of adoptive therapy for CLL discussed above found that T cells harvested from patients who experienced a complete remission were phenotypically and functionally TCM.5 This finding raises the question of whether the cells that persisted after eradication of tumor were TCM, or whether there was enrichment for TCM cells prior to adoptive transfer.
Unfortunately, selective isolation of these cells from the peripheral blood to investigate their potential in a clinical trial is limited by the number of antigen-specific TCM in the peripheral blood, thus necessitating ex vivo expansion to achieve sufficient anti-tumor dose.17
Several ex vivo cell manufacturing platforms exist that can produce clinical-grade products with large numbers of T cells for use in adoptive therapy trials. One of the first methods described involved culture of harvested lymphocytes with soluble anti-CD3 antibody (OKT-3) in the presence of IL-2.18 This method has been used broadly and continues to be employed in cell therapy trials today. Preliminary studies suggest that this method of expansion produces cells that are largely TEM and TEff in phenotype.9, 10 Potential improvements have been tried, including the addition of feeder cells and the use of supplemental cytokines19, 20.
T cells require two signals to become fully activated: signal one, which is delivered via antigen engagement of the TCR and mediated through the associated CD3 molecule, and signal two, which is antigen-independent and mediated via CD28 engagement with cognate ligands B7.H1 or B7.H2.21 Previous studies using immobilized monoclonal antibodies (mAb) directed against CD3 and soluble mAbs against CD28 demonstrated modest T cell activation22, however when these antibodies were both immobilized on magnetic beads the stimulation was greatly enhanced.23 These beads seem to mimic physiologic stimulation via natural antigen presenting cells (APCs) and serve as artificial APCs that can be used for large-scale expansion of cells for adoptive therapy.
Our group has experienced significant clinical activity using T cells engineered to express chimeric antigen receptors (CARs) that target tumor-associated antigens, such as CD19 in acute lymphoblastic leukemia (ALL) and CLL.5, 24, 25 In an effort to determine what role the cell manufacturing process utilizing CD3/CD28 beads itself may have in producing these outcomes, we set out to compare expansion using OKT3 and IL-2 with our CD3/CD28 bead-based approach in an in vivo xenograft model of cell therapy for ALL using CD19 CAR expressing T cells (CART19). Here we demonstrate that cells expanded using CD3/CD28 beads display and maintain a central memory phenotype, mediate a greater anti-tumor response, and are able to respond to a large re-challenge of leukemia. As all other variables including structure of the CAR and the use of the 4-1BB endodomain are held constant, we confirm that the youth and proliferative capacity of early memory T cells is the critical condition modulated by the cell culturing technique. Preferentially generating T cell subsets of high proliferative capacity for adoptive therapy may not only lead to improved efficacy, but in turn also reduce the number of cells needed to elicit a clinical response, thus expanding the number of patients eligible for this powerful therapeutic modality.
Methods
T cell stimulation and phenotype assessment
Human T cells were procured from the Human Immunology Core at the University of Pennsylvania. CD4+ and CD8+ cells were combined at a 1:1 ratio and stimulated with magnetic beads bearing anti-CD3 and CD28 antibodies (ratio of 3:1 beads:cells, Life Technologies, Grand Island, NY) or OKT3 (50ng/mL, Ortho Biotech, Bridgewater, NJ) in the presence of IL-2 (300IU/mL). Surface antigen expression was detected by staining with appropriate fluorescently-conjugated monoclonal antibodies (BD Biosciences).
Generation of CAR constructs and RNA electroporation
CARs containing scFv domains directed against CD19 or mesothelin (meso) linked to CD3ζ and 4-1BB (BBz) intracellular signaling domains were produced as previously described.26, 27 Second generation CARs containing the CD28 signaling domain were produced similarly to create pGEM-ss1.28bbz.64A and pGEM-CD19.28bbz.64A plasmids. CAR cDNA products were confirmed by direct sequencing and linearized by SpeI prior to RNA in vitro transcription (IVT). mScript RNA System (Epicentre, Madison, WI) was utilized to generated capped IVT RNA, which was purified using an RNeasy Mini Kit (Qiagen, Inc., Valencia, CA). RNA transfection resulted in 95–100% CAR+ T-cells 24 hours after transfection.
Human T cells were isolated as described above, and expanded with either CD3/CD28 beads or OKT-3 and IL-2. When cell growth kinetics and size suggested they had rested down from activation, they were cryopreserved. 24h prior to electroporation, cells were thawed and washed three times with Opti-MEM and resuspended in Opti-MEM at a final concentration of 1–3 × 108 cells/mL. T cells were then mixed with transcribed mRNA at a concentration of 10μg mRNA/0.1mL T cells and electroporated in a 2mm cuvette using an ECM830 Electro Square Wave Porator (both from Harvard Apparatus BTX, Holliston, MA). Viability post-transfection ranged from 50 – 80% and in all cases viable T cells for injection had >99% CAR expression. For trafficking experiments, T cells were stably transduced with a click beetle red luciferase lentiviral construct prior to mRNA transfection.
Production of lentiviral vectors and T cell transduction
High-titer, replication-defective lentiviral vectors were produced using 293T human embryonic kidney cells.28 HEK293T cells were seeded at 10 × 106 cells per T150 tissue culture flask 24h hours before transfection with 7μg of pMDG.1, 18μg of pRSV.rev, 18μg of pMDLg/p.RRE packaging plasmids and 15μg of transfer plasmid in the presence of Express-In Transfection Reagent (Open Biosystems, Lafayette, CO). Transfer plasmid containing CAR constructs were modified so that expression of the CAR was under control of the EF-1α promoter as previously described.27 Viral supernatants were harvested 24h and 48h after transfection and concentrated by ultracentrifugation either overnight at 8,500 rpm or for 2 hours at 28,000 rpm with a Beckman SW28 rotor (Beckman Coulter, Fullerton, CA). Lentiviral vectors that encode the CAR constructs and eGFP separated by the 2A ribosomal skipping sequence from FMDV were also generated. These vectors permit dual expression of eGFP and the CAR from a single RNA transcript. Sequences encoding the luciferase were reverse engineered into the self-inactivating lentiviral vector and codon optimized to emit in the green (λpeak 550nm) or red (λpeak 630nm) spectrum. Human T cells were isolated and stimulated using either CD3/CD28 beads or OKT-3 and IL-2 as described above. 24h after initial stimulation, cells were exposed to lentiviral vector at a concentration of 5 viral particles per T cell. Cells were then counted and fed every 2 days and were cryopreserved when their growth kinetics and cell size suggested they had rested down from activation.
CAR detection on modified cells
Cells were washed and resuspended in FACS buffer (PBS + 1% bovine serum albumin), then incubated with biotin-labeled polyclonal goat anti-mouse F(ab)2 antibody (anti-Fab, Jackson Immunoresearch, West Grove, PA) at 4°C for 25 minutes, followed by two washes with FACS buffer. Cells were then incubated with R-phycoerythrin (PE) conjugated streptavidin for 10 minutes at 4°C and washed twice. Flow cytometry acquisition was performed on either a BD FacsCalibur or Accuri C6 Cytometer (BD Biosciences). Analysis was performed using FlowJo or Accuri software (Treestar, Inc, Ashland, OR and BD Biosciences).
Telomere Flow-FISH assay
Relative telomere length analysis was performed according to the method of Baerlocher and Lansdorp with some modifications.29, 30 Bovine thymocytes were obtained from a fresh calf thymus and fixed according to the method described. Briefly, 2–5×105 sample cells were washed and stained with antibodies to surface markers and cross-linked with 1 mM bis-sulfosuccinimidyl suberate (BS3, Thermoscientific, Rockford, IL) as previously described.31, 32 Antibodies were conjugated to quantum dot fluorophores from Invitrogen including CD3, CD4, CD8 or CD45 conjugated to QD605, 655 or 705 depending on application (Invitrogen, Grand Island, NY). QD fluorophores were required as they resist the heat inactivation required during the Flow-FISH hybridization. After surface antibody crosslinking, cells were washed and placed into polypropylene flow tubes (Fisher Scientific, Pittsburgh, PA) with the addition of 2×105 fixed bovine thymocytes. Cells were incubated with the fluorescent telomere probe (FITC-OO-CCC TAA CCC TAA CCC TAA) obtained from Biosynthesis (Lewisville, TX) for 10 minutes, then heated to 87°C for 15 minutes. Tub es were removed from heat and allowed to cool to ambient temperature for 2 hours in the dark. After washing, cells were counterstained with LDS751 (Exciton, Dayton, OH) and samples run on an Accuri C6 cytometer. Inter and intra-experimental control was completed by the use of bovine thymocytes from the same lot in every sample and in repeat experiments as well as by quality control using Quantum FITC-5 MESF beads (Bangs Laboratory, Fisher, IN) as described by Baerlocher et al.29
Mouse Xenograft studies
We performed these studies as previously reported33–35. Briefly, 6–10 week old NOD-SCID-γc−/− (NSG) were obtained from the Jackson Laboratory (Bar Harbor, ME) or bred in house under an approved institutional animal care and use committee (IACUC) protocol and maintained in pathogen-free conditions. Animals were injected I.V. via tail vein with 106 Nalm-6 or a primary pediatric human leukemia. T cells were injected 5–7 days after injection of leukemia. Animals were monitored for signs of high tumor burden as well as graft-versus-host disease, as evidenced by >10% loss in body weight, loss of fur, diarrhea, conjunctivitis and leukemia-related hind limb paralysis. Peripheral blood was obtained by retro-orbital bleeding and blood was examined for evidence of leukemia and T cell engraftment by flow cytometry using BD Trucount (BD Biosciences) tubes as described by the manufacturer’s instructions. Surface antigen and CAR expression was performed as described above.
Bioluminescent imaging
Mice were anesthetized using inhaled isoflourane (Baxter Healthcare, Deerfield, IL) and imaged on the Xenogen Spectrum imaging system using Living Image v4.2 software. Full methods description is provided in Barrett et al 201336.
Cell Line Identity Testing
Parent cell lines and primary leukemia samples were genotyped by short tandem repeat (SNP) analysis. Cell lines and samples were verified every six months, or after any genetic modification such as CD19, meso or luciferase transduction to ensure identity.
Statistical considerations
Analysis was performed with Prism 6 (Graphpad Software, La Jolla, CA). In vitro data represent means of duplicates, and comparisons of means were made via Mann-Whitney test. For comparison among multiple groups, Kruskal-Wallis analysis was performed with Dunn Multiple Comparison tests to compare individual groups. Survival curves were compared using the log-rank test with Bonferroni correction for multiple comparisons.
Results
Stimulation of peripheral T cells using CD3/CD28 beads creates a large population of Tcm cells
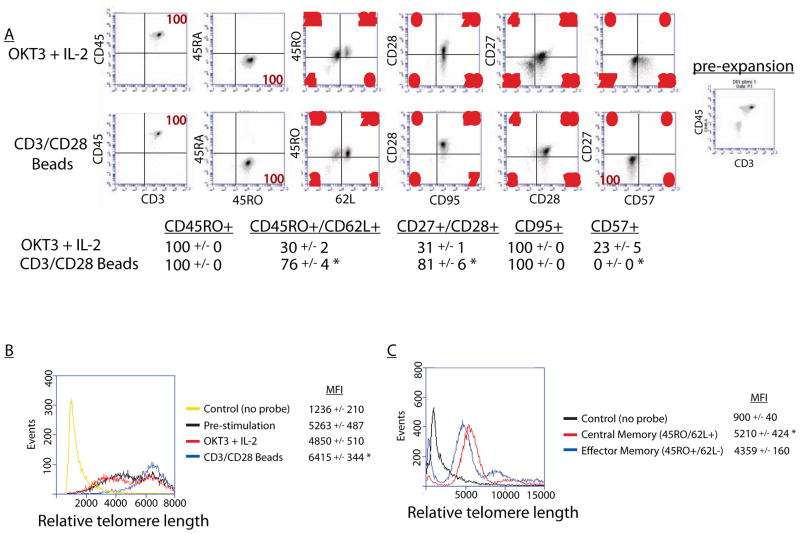
We began by evaluating the phenotype of human T cells resulting from ex vivo expansion using these two methods in permanently modified cells using lentiviral vectors. Of the many surface markers used to assess T cell functional and developmental status, there are several that differentiate TEM from TCM, namely CD27, CD28, CD62L and CCR7, which are more highly expressed by TCM37. Human T cells were stimulated with either CD3/CD28 beads or OKT-3 and IL-2 and compared to unstimulated PBMCs. Both stimulated populations demonstrated no CD45RA expression and high CD45RO, reflecting engagement of their antigen receptor (Figure 1A). Bead stimulated cells did not exhibit a significantly higher expression of the lymphoid homing molecule CCR7as compared to those stimulated with OKT-3 and IL-2. However, a large population of CD27+CD28+ double positive cells was seen with bead stimulation (Figure 1A). Bead stimulation appeared to increase or selectively preserve expression of both CD27 and CD28, while the CD27−CD28− double negative population was largely preserved in OKT-3 stimulated CD8s. Loss of CD27 expression is a hallmark of effector differentiation, and its functional loss may directly contribute to impaired expansion and survival in vivo.10, 15 CD57 expression has been used a marker of in vitro T cell senescence.10 Comparing these two stimulation methods, we found that bead stimulation produced no CD57+ cells, while OKT-3 produced a notable population of potentially senescent cells (Figure 1A).
Figure 1. Characterization of T cell phenotypes after stimulation.
CD4 and CD8 T cells were mixed 1:1 and stimulated with either OKT-3 in the presence of IL-2 or with CD3/CD28 beads. A. Both methods of expansion produced cells that were predominantly CD45RO+, however bead stimulation resulted in a larger CD62L+/CD27+/CD28+ population with essentially no CD57 expression (*p<0.01). B. Characterization of telomere lengths. Using a flow-based assay, we compared telomere lengths of various populations of T cells. Cells stimulated with OKT-3 had a similar telomere length distribution to unstimulated cells, while cells stimulated with CD3/CD28 beads were enriched for longer telomeres (*p<0.05). C. Cells from two different donors were sorted into central and effector populations based on CD45RO/CD62L expression pre-expansion. Central memory cells had longer telomeres (*p<0.05), consistent with their enhanced proliferative potential.
CD3/CD28 bead expansion promotes longer telomere length
To extend our phenotypic analysis of the cell populations produced by these two stimulation methods we examined telomere lengths. Longer telomere length has been correlated to enhanced proliferative potential, while shorter telomeres are associated with replicative senescence.38, 39 We found that peripheral T cells stimulated with OKT-3 had a similar telomere length distribution to pre-stimulation cells, while bead stimulation appeared to enrich for cells with longer telomeres (Figure 1B). We then sorted peripheral T cells from the 2 normal donors used in the phenotyping experiments into central memory and effector memory populations based on CD45RO and CD62L expression. Using a flow-based telomere length assay, we confirmed that central memory T cells had longer telomeres than effector memory cells (Figure 1C). These findings further support that cells stimulated with CD3/CD28 beads have enhanced proliferative potential similar in nature to TCM cells.
Cells expanded with CD3/CD28 beads demonstrate enhanced early anti-tumor responses in vivo
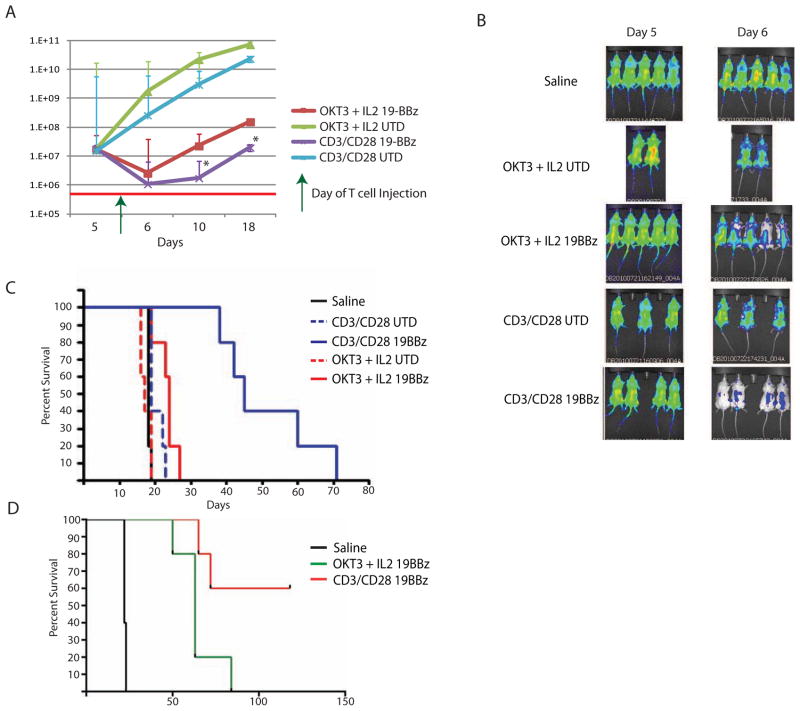
Next, we took cells expanded by both methods and assessed their in vivo function using our leukemia xenograft model. Hypothesizing that memory cells with high proliferative capacity might mediate effective anti-tumor responses, we investigated the in vivo activity of T cells using bioluminescent imaging. We have previously demonstrated that T cells engineered to transiently express CARs targeting the B-cell antigen CD19 can significantly impact established disease in a xenograft mouse model of human leukemia.40 As we needed to permanently transduce the T cells with the luciferase vector for the correlative migration studies to follow, we did not want to influence T cell function by performing a second transduction of a lentiviral vector containing the CAR which would require a second stimulation. We also wanted to be sure that every cell that was luciferase positive was also CAR positive (thus making a simultaneous double lentiviral vector transduction undesirable), and RNA transfection results in 100% CAR expression in our model system. Mice were injected intravenously with Nalm-6, a CD19+ human leukemia cell line, which we engineered to express click beetle green (CBG) luciferase. Mice were given 5 days to establish disease prior to T cell administration. Untargeted T cells (UTD) expanded by either method did not have an effect on leukemia burden, as measured by bioluminescent signal (Figure 2A). OKT-3 expanded CART19 cells demonstrated a transient disease response, while CART19 cells expressing the same signaling domains (19BBz) which had been expanded with beads demonstrated a more sustained reduction of disease burden. This response can be observed visually (Figure 2B), highlighting the transient response to OKT-3 stimulated CART19 cells, and the rapidity of disease reduction in mice given bead stimulated CART19 cells. This is consistent with our previous report that transient CAR expression can mediate a significant anti-tumor response.40 Despite this short-term (6 day) expression of the CAR, mice treated with cells expanded with beads experienced a significant long-term survival benefit (Figure 2C), as compared to mice treated with OKT-3 expanded cells. Given that no mice cleared their disease, this dramatic survival benefit seems to correlate with the magnitude of initial disease response seen with bead stimulated CART19 cells.
Figure 2. Cells expanded with CD3/CD28 beads mediate potent tumor responses in vivo.
Cells were expanded as indicated and injected into NSG mice with established ALL (bioluminescent human leukemia line Nalm-6, *p<0.05). A. Disease burden as measured by bioluminescent signal after administration of engineered T cells. B. Visual representation of disease burden in mice with Nalm-6 treated with expanded T cells. There was greater reduction in disease burden noted in mice given T cells expanded with beads and expressing CD19 CAR as compared to all other groups. C. Kaplan-Meier survival curves of mice treated in Figure 2A and 2B (p<0.01 for CD3/CD28 CD19BBz compared to all other groups). D. Kaplan-Meier survival curves of animals treated with permanently-modified CAR T-cells produced using the two cell culture methods. Survival of stable, lentiviral vector transduced T cells expanded with OKT3 and IL-2 is inferior to CD3/CD28 bead expanded T cells despite using the same 19-41BB-z CAR (p<0.001, 5 million CAR+ T cells given at Day 7 as a single treatment).
In a distinct experiment in which animals with established leukemia were treated with permanently-modified CAR T-cells produced using either OKT-3 and IL-2 or CD3/CD28 beads, significantly enhanced survival was observed in mice receiving bead-expanded T-cells. As we have noted previously, many of these mice cleared their disease (Figure 2D).27
CD3/CD28 bead stimulated cells demonstrate similar migration but greater expansion in vivo
We investigated whether the difference in disease clearance and survival seen above was to the result of a difference in migratory capacity of these two populations. Using a similar bioluminescent imaging strategy, we modified our T cells to express click beetle red (CBR) luciferase, which emits at a distinct wavelength and can be easily separated using spectral unmixing from the CBG signal from Nalm-6 cells. As opposed to the previous experiment where all mice were given leukemia, in this experiment only half of the mice were given leukemia to act as targets. After six days, mice were given T cells expanded with either beads or OKT-3 that expressed either the CD19 CAR or an irrelevant CAR (targeting the human antigen mesothelin, not expressed by Nalm-6). At 24 hours after T cell administration, it seemed presence or absence of disease had no impact on migration of T cells, nor did method of expansion (Supplemental Figure 1A). Cells were observed in potential sites of disease, including liver, spleen and bone marrow. This observation held true at 7 days after transfer, as well (data not shown). The bioluminescent signal was able to highlight that there is no difference in localization of human cells within the mouse after transfer.
We then turned to assessment of cell expansion in vivo. While variability in CBR expression did not allow for inter-group comparison, it did allow us to compare signal amplification within the same group. We found that, within 48 hours of transfer, cells expanded with beads began to expand in vivo in the presence of target (Supplemental Figure 1B). The expansion observed was also long-lived, with CART19 cells continuing to expand between days 8–15, well after CAR expression had been lost. By contrast, the cells expanded with OKT-3 demonstrated modest expansion during the first 15 days after transfer. In fact, this expansion was no greater than that of cells targeted to an irrelevant antigen when transferred into mice with leukemia (Supplemental Figure 1B), suggesting that OKT-3 expanded cells with an effector memory phenotype lack significant proliferative capacity even when exposed to the antigen which the CAR recognizes.
T cells permanently modified with CARs clear their leukemia and have a TCM phenotype
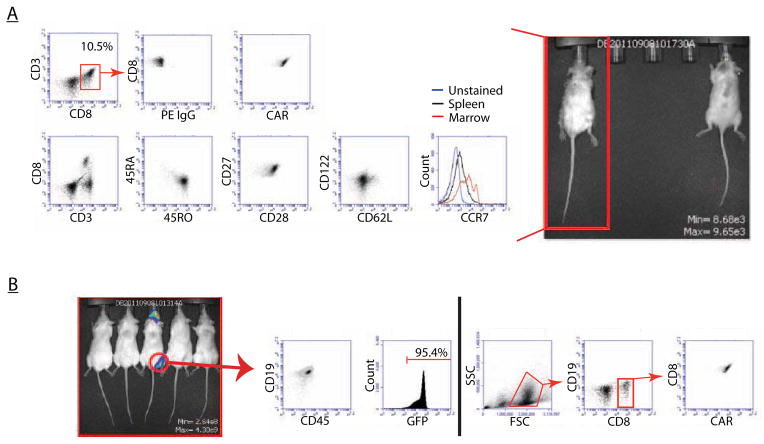
After determining that expanding cells with CD3/CD28 beads produced a larger proportion of TCM cells, and that these cells mediated rapid anti-tumor responses in vivo even with a transiently-expressed CAR, we examined the phenotype of transferred T cells after exposure to leukemia in vivo. Preliminary data using a limited sample set suggested that T cells remaining in the periphery of mice that had successfully eradicated an established human leukemia had a central memory phenotype (data not shown), so we investigated this more thoroughly. Mice with established primary human leukemia were given T cells permanently modified to express CD19 CARs using a lentiviral vector system. Spleens of mice that successfully cleared their leukemia and survived long-term were examined and phenotypic analysis demonstrated residual CAR T cells with a predominantly CAR+CCR7+CD27+CD28+ phenotype, consistent with TCM (Figure 3A). Interestingly, these cells were CD62L-/dim. It is unclear if this reflects a change in memory status, or if CD62L expression fluctuates in vivo.15 No mice given OKT3 and IL2 stimulated cells were able to clear their leukemia or survive long term, leaving no cells to analyze or animals to rechallenge.
Figure 3. Phenotypes of permanently-modified T cells that mediate leukemia clearance.
Mice with established human leukemia were administered T cells permanently modified to express CD19 CARs. A. Mice that demonstrate eradication of leukemia (at Day +120) have T cells that are phenotypically TCM (CD45RO+/CD27+/CD28+/CD62Lsubset+) and maintain CAR expression. B. Mice that initially eradicated their disease but developed loco-regional relapse were sacrificed, their sites of relapse were examined and show to be ~95.4% CD19+GFP+ leukemia, with a complete absence of T cells. Examination of the bone marrow (right of black line) from the same mouse demonstrates presence of CAR+ T cells and a lack of leukemia.
While many of the mice treated with CART19 cells remained disease-free, occasionally we observed recurrence in the form of loco-regional disease, often occurring in the jaw, shoulder or groin (Figure 3B). Upon examination of these mice after sacrifice, cells at the sites of disease recurrence were almost exclusively CD19+ leukemia and markedly lacking in human T cells. However, examination of the bone marrow from the same mouse revealed persistence of CAR+ T cells (Figure 4B). This suggests that the recurrence observed was not disseminated leukemia, but rather deposition of disease in xenograft sites that cannot be accessed by human T cells. The degree to which this is specific to the NSG xenograft model remains unclear, as does the relevance of this recurrence in clinical disease, as has been previously suggested by other groups who have observed loco-regional relapses of leukemia cell lines in the jaw or tooth root that may be unique to the mouse jaw marrow environment.41
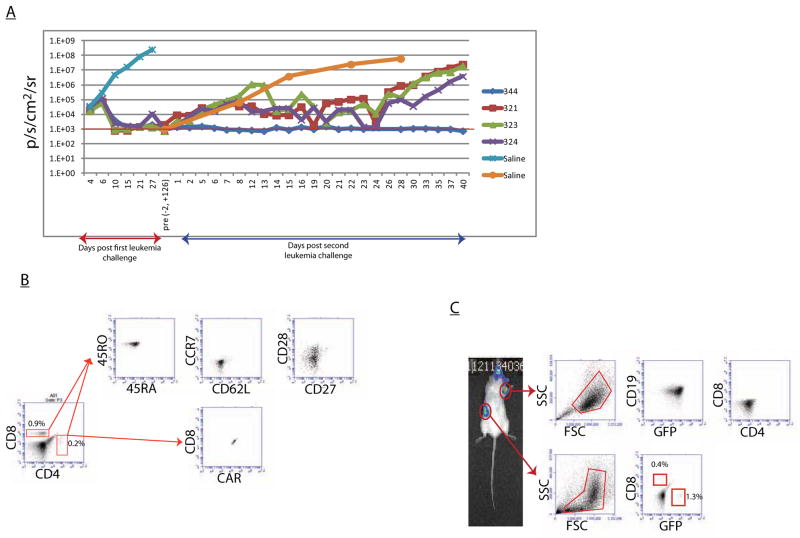
Figure 4. Engrafted T cells can impact a second challenge of leukemia with change in T cell phenotype.
Mice that cleared an initial challenge of primary patient leukemia were given a second challenge of the same leukemia. A. Disease burden as measured by bioluminescent signal demonstrating delayed control of second ALL challenge by mice 321, 323, 324 with recurrence in the form of loco-regional disease. Mouse 344 successfully cleared a second challenge of leukemia without recurrence. B. Mouse 344 had effector-memory like cells in the periphery 90 days after second antigen challenge, whereas mice in the same group had TCM after clearance of initial leukemia(Figure 4). C. Mice that developed loco-regional disease after initial clearance of their second leukemia have GFP+CD19+ leukemia within these sites of relapse without the presence of T cells, and have trace amounts of leukemia and T cells in their spleens.
Engrafted TCM cells are able to impact a second large burden of leukemia
We next investigated the functional status of this predominant TCM phenotype. Mice from the previous study that cleared their disease and had no evidence of loco-regional recurrence were then given a second large dose (106 cells) of the same leukemia. One mouse quickly cleared the re-challenge, while remaining mice had delayed responses with approximately 8–10 days elapsing before disease control was seen, consistent with expansion kinetics of CD19 CAR T cells. This was followed by an increase in bioluminescent signal resulting from loco-regional disease, as previously observed (Figure 4A). Age-matched control mice demonstrated progressive disease with the same kinetics as seen in the initial cohort of saline control mice. Previous experiments with mice engrafted with untransduced T cells demonstrated no difference in leukemia progression compared to mice without human T cells, indicating the disease control seen here was CD19 CAR specific and not dependent on an allogeneic or xenogeneic effect of the T cells (data not shown). The mouse that remained disease-free after this second challenge (mouse 344) was found to have CAR+CCR7−CD62L−CD27−CD28subset+ T cells. Though the pre-rechallenge phenotype for this individual mouse cannot be known, and a single data point is not sufficient to draw conclusions, it is worth noting this more effector memory phenotype would be expected after repeated antigen challenge. Those mice that developed loco-regional relapse were found to have GFP+CD19+ leukemia and no T cells at sites of relapse, as previously observed. Interestingly we did observe a small amount of both leukemia and T cells in the spleens of these animals (Figure 4C). This may reflect a process of disease control in which cells shed into the circulation from potentially protected loco-regional sites were eradicated in the spleen, as these mice did not develop disseminated disease.
Discussion
The results of recent clinical trials have demonstrated that chimeric antigen receptor-based T cell engineering has great potential as cancer therapy.5, 24, 42 Optimization of this emerging technique is necessary before it can be brought into the realm of clinical practice. As one of the most easily tunable aspects of this therapy, the internal signaling domains and biophysical structure of the CARs are under active exploration by many groups. Several studies have identified receptors belonging to the TNF family, such as OX40 (CD134) and 4-1BB (CD137), as key elements in promoting a proliferative phenotype,43, 44 and indeed our group has observed enhanced anti-tumor efficacy of T cells with CARs bearing the costimulatory 4-1BB signaling domain in vivo.27 The ideal internal structure for chimeric antigen receptors remains undetermined, and may in fact need to be tailored for different diseases.
A second area of active investigation is cell culturing technique. Decades of research on T cell activation and proliferation have produced several methods to expand T cells ex vivo. Many recent studies have suggested that cells with higher proliferative capacity, as opposed to greater effector function, demonstrate greater anti-tumor efficacy in adoptive transfer.1, 10, 11, 14, 16 As such, many groups are investigating the T cell expansion methods that can produce large quantities of these cells. The number and complexity of variables involved in adoptive cell therapy makes it difficult to compare – as CAR endodomains, cell manufacture, scFv structure, transmembrane linkers and the use of in vivo cytokine support make comparing even superficially similar techniques difficult. With this in mind, we sought to simplify and control the comparisons to the CD3/CD28 bead approach that produced significant clinical efficacy in both adults with CLL and children with ALL.45, 46 In this study we compared ex vivo expansion using CD3/CD28 stimulatory beads to expansion using soluble CD3 antibody in the presence of IL-2. We found that cells expanded with CD3/CD28 beads produce large numbers of T cells with a central-memory phenotype that have high proliferative potential and anti-tumor efficacy in vivo. This expansion process is already GMP and GCP optimized and has been used to prepare products for clinical trials for over a decade.
Importantly, the responses seen using cells produced with CD3/CD28 beads were observed using both permanently and transiently-modified T cells. By definition, cells that transiently express CARs do not retain a persistent antigen response, suggesting that the proliferation and response observed after infusion of these cells results from their initial expansion. Impressively, permanently-modified cells, which persist in the periphery largely as TCM after clearing disease, exhibit the ability to respond to a second challenge, and in one animal demonstrated the ability to completely clear that second challenge. These results point to the potential of TCM cells in adoptive cell therapy, and highlight a well-known mechanism (CD3/CD28 beads) for their large-scale production. Furthermore these data suggest that stimulation with CD3/CD28 beads produces a larger population of CD45RO+CD62L+CD27+CD28+ CD57− cells, consistent with a large population of TCM with high proliferative capacity, than is seen with OKT-3 and IL-2.
Previous investigations have also compared CD3/CD28 beads and plate-bound OKT-3 and IL-2, using lentiviral vector transfer of tumor specific T cell receptors (TCRs)19. These methods vary in that high dose IL-2 was added to the CD3/CD28 beads for the specific expansion of CD8+ T cells alone, and requires the addition of irradiated feeder cells to the plate-bound OKT-3 to complete expansion. The T cell response is also mediated by engagement of the TCR as opposed to through a CAR. Our methodology uses no feeder cells and no supplemental IL-2, as the combination of CD4 and CD8 cells results in efficient expansion of both populations. Recent reports also suggest that CD4 cells, in particular Th17 cells, may be exerting critical anti-tumor effects and thus necessary for clinical efficacy47. Use of cytokine cocktails (IL-7, IL-15, IL-12, and IL-21) added to the plate-bound OKT-3 and feeder cell method has also been investigated and found to generate TCM phenotypes, though our report highlights the benefit of the CD3/CD28 bead methodology in producing similar results without the need for supplemental cytokines or feeder cells20, 48. These differences help to shed light on which aspects of ex vivo culture are truly critical to generating long lived anti-tumor T cells. Taken together, it appears likely that CD3/CD28 costimulation, the combination of CD4 and CD8 T cells present during the ex vivo expansion culture, and the mechanical support provided by the small polystyrene beads reproduce effects reportedly provided by plate-bound OKT-3, feeder cells and supplemental cytokines. A known clinically efficacious CAR structure, the 19-41BBz, did not provide great proliferative capacity to the more terminally differentiated T cells generated by OKT-3 and IL-2. Taken together, the data presented here highlight the importance of maintaining and enhancing proliferative capacity of engineered T cells during the ex vivo expansion phase of manufacturing.
Administration of bead-expanded T cells to NSG mice engrafted with human leukemia resulted in expansion beginning within two days and resulting in several log higher expansion over the first two weeks, as opposed to the modest in vivo expansion of the OKT-3 expanded cells in this lymphopenic environment. Lymphopenia has been repeatedly shown to be important for the homeostatic expansion of adoptively transferred cells49, but lymphopenia alone is not sufficient to equalize the efficacy of cells produced by the two manufacturing methods. As all other conditions were kept similar between these two populations, including structure of the chimeric receptors, cell dose and degree of lymphopenia: cell manufacturing technique alone appears to play an essential and independent role in the success of in vivo expansion by influencing the T cell phenotype. When examining the phenotype of human T cells in mice that had successfully eradicated their leukemia, we found that they were central-memory like, except for their low CD62L. CD62L expression can vary, especially as an artifact of cryopreservation, and thus the impact of this finding is unclear.
Mice that were able to clear an initial burden of leukemia and then were given a second large dose of the same disease demonstrated the ability to control the rechallenge without more CAR T cells, although the disease control was incomplete in some animals. The mice that developed recurrent disease did so at discreet sites, namely jaw, shoulder and groin, as did the mice that relapsed after clearing their first leukemia. This would suggest that the xenograft model allows deposition of human leukemia in privileged sites that are not accessible to or protected from human T cells. As this does not recapitulate the mechanism of recurrent disease in humans, which is a disseminated recurrence of leukemia, it is unclear if this xenograft relapse mechanism truly bears physiologic relevance for this therapy. As syngeneic models of CAR therapy are developed, this phenomenon can be more fully investigated. Those mice that did develop loco-regional disease after clearing their second leukemia were found to have both leukemia and T cells in their spleen. This may be a “snap shot” of the struggle for these observed TEM cells, which have lost some of their expansion and persistence potential, to control this disease.
Identification of the optimal cell type for use in adoptive therapy is highly relevant to the field. First, improving overall and complete response rates is desirable for all novel cancer therapeutics. Recent improved results with CD19-directed cell therapies show that we are reaching a threshold of real clinical activity. Second, identification of the cell type best equipped to mediate an anti-tumor response potentially allows for infusion of fewer CAR-modified cells. In a patient population that suffers from profound lymphopenia as a result of previous chemotherapy, limited availability of T cells for ex vivo expansion and engineering to produce sufficient product can often prevent patients from being eligible for this therapy. If fewer cells are needed, this approach can be broadened to a much larger patient population, thus creating another option for those most in need of innovative therapies.
Supplementary Material
Acknowledgments
Supported in part by research grants R01CA120409 and P01CA066726 (CHJ), and R01CA102646 and Weinberg, Cookies for Kids and W.W. Smith Foundation grants (SAG).
Footnotes
Authorship
D.B. designed the research, performed the experiments, interpreted the data, and wrote the paper.
N.S. interpreted the data and wrote the paper.
X.L., S.J. performed research.
S.A.G, Y.Z., C.H.J. designed the research, and wrote the paper.
Disclosure/Conflict of Interest Statements
The University of Pennsylvania and Novartis have entered into an alliance for development of chimeric antigen receptors. This alliance is managed in accordance with the University of Pennsylvania conflict of interest policy. All authors are in compliance with this policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced Leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–90. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stem cell-like plasticity of naive and distinct memory CD8+ T cell subsets. Semin Immunol. 2009;21:62–8. doi: 10.1016/j.smim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195:317–26. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Dudley ME, Rosenberg SA, Morgan RA. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T-cell receptors. J Immunother. 33:648–58. doi: 10.1097/CJI.0b013e3181e311cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Ji Y, Gattinoni L, et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother. 62:727–36. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 22.Levine BL, Ueda Y, Craighead N, Huang ML, June CH. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 23.Levine BL, Bernstein WB, Connors M, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–30. [PubMed] [Google Scholar]
- 24.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grupp S, Kalos M, Barrett D, et al. Remission of ALL by chimeric antigen receptor-expressing T cells. New England Journal of Medicine. 2013 in press. [Google Scholar]
- 26.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–74. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 29.Baerlocher GM, Lansdorp PM. Telomere length measurements in leukocyte subsets by automated multicolor flow-FISH. Cytometry A. 2003;55:1–6. doi: 10.1002/cyto.a.10064. [DOI] [PubMed] [Google Scholar]
- 30.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–76. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 31.Batliwalla FM, Damle RN, Metz C, Chiorazzi N, Gregersen PK. Simultaneous flow cytometric analysis of cell surface markers and telomere length: analysis of human tonsilar B cells. J Immunol Methods. 2001;247:103–9. doi: 10.1016/s0022-1759(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 32.Spyridopoulos I, Hoffmann J, Aicher A, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–72. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- 33.Barrett DM, Seif AE, Carpenito C, et al. Noninvasive bioluminescent imaging of primary patient acute lymphoblastic leukemia: a strategy for preclinical modeling. Blood. 2011;118:e112–7. doi: 10.1182/blood-2011-04-346528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett DM, Zhao Y, Liu X, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011;22:1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-Specific Effects of RNA-Modified Chimeric Antigen Receptor T Cells in Mice with Advanced Leukemia. Hum Gene Ther. 24:717–27. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 40.Barrett DM, Zhao Y, Liu X, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011;22:1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 42.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 2013;368 doi: 10.1056/NEJMoa1215134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 44.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 45.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulos CM, Carpenito C, Plesa G, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med. 2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother. 35:689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.