Summary
This study investigates the mechanism of action behind the long-term responses (12–16 months) of two BRAF WT melanoma patients to the AKT inhibitor MK-2206 in combination with paclitaxel and carboplatin. Although single agent MK-2206 inhibited phospho-AKT signaling, it did not impact in vitro melanoma growth or survival. The combination of MK-2206 with paclitaxel and carboplatin was cytotoxic in long-term colony formation and 3D spheroid assays, and induced autophagy. Autophagy was initially protective with autophagy inhibitors and deletion of ATG5 found to enhance cytotoxicity. Although prolonged autophagy induction (>6 days) led to caspase-dependent apoptosis, drug resistant clones still emerged. Autophagy inhibition enhanced the cell death response through reactive oxygen species and could be reversed by anti-oxidants. We demonstrate for the first time that AKT inhibition in combination with chemotherapy may have clinical activity in BRAF WT melanoma and show that an autophagy inhibitor may prevent resistance to these drugs.
Significance
Approximately 30% of all cutaneous melanomas are wild-type for both BRAF and NRAS. As yet, no targeted therapy strategies exist for this sub-set of tumors. Constitutive signaling through the PI3K/AKT pathway is a common occurrence in cutaneous melanoma, irrespective of the driver mutation. Here we report durable responses to the AKT inhibitor MK-2206 in combination with carboplatin and paclitaxel in two patients with BRAF wild-type melanoma. Through mechanistic studies, we demonstrate a role for autophagy induction in the response to the AKT inhibitor/chemotherapy combination and suggest that autophagy inhibitors may be one strategy to enhance efficacy in the clinical setting.
Keywords: melanoma, BRAF, autophagy, chemotherapy, AKT, apoptosis
Introduction
Recent years have seen great strides in the development of targeted therapies for melanoma. This approach has been exemplified by the use of small molecule BRAF kinase inhibitors in individuals whose melanomas harbor activating BRAF mutations (Chapman et al., 2011; Flaherty et al., 2010; Hauschild et al., 2012). In randomized phase III clinical trials, treatment with the BRAF inhibitor vemurafenib is associated with significant levels of tumor shrinkage and a progression-free survival of 6.8 months (Chapman et al., 2011). Although resistance is nearly inevitable, small numbers of patients have been identified who show prolonged (>3 year) responses to single-agent BRAF inhibitor therapy (Kim et al., 2012). Resistance to BRAF inhibitors is complex, multi-factorial, and typically dependent upon reactivation of the MAPK signaling pathway (Fedorenko et al., 2011). The importance of MAPK pathway signaling recovery was demonstrated in phase II clinical trials in which the combination of a BRAF inhibitor with a MEK inhibitor significantly increased progression-free survival compared to BRAF inhibitor alone (Infante et al., 2011; Paraiso et al., 2010).
Despite the significant improvements in systemic melanoma therapy, few effective targeted therapy options are available for the 50% of melanoma patients whose tumors lack activating BRAF mutations. One significant group of BRAF WT melanoma, accounting for 15–20% of all cutaneous melanomas, are those harboring activating NRAS mutations (Devitt et al., 2011; Fedorenko et al., 2012). Highly potent allosteric inhibitors of MEK are currently being evaluated in NRAS mutant melanoma (Ascierto et al., 2013). In recent phase II clinical trials, the MEK inhibitor MEK162 was associated with a response rate of 20% in NRAS mutant melanoma with a median PFS of 3.6 months (Ascierto et al., 2013). Combination strategies for NRAS mutant melanoma are being actively explored. The remaining 30% of all melanomas are wild-type for both BRAF and NRAS, and no obvious oncogenic drivers have yet been identified for this sub-group - despite intensive whole genome and whole exome sequencing efforts (Berger et al., 2012; Hodis et al., 2012; Krauthammer et al., 2012). Novel strategies for targeting BRAF/NRAS WT melanoma are therefore urgently needed.
A large number of studies support a role for phospho-inositide-3-kinase (PI3K)/AKT signaling in the development and progression of melanoma (Madhunapantula and Robertson, 2009). Upon activation, PI3K phosphorylates phosphotidylinositol-4,5, biphosphate (PIP2) to PIP3, which in turn activates the downstream kinases PDK1 and AKT. Of these, AKT plays a critical role in survival through the phosphorylation of BAD as well as the regulation of cell cycle entry by phosphorylating and inactivating glycogen-3 synthase kinase (GSK3)-β, leading to the modulation of cyclin D1 (Diehl et al., 1998; Frame and Cohen, 2001). PI3K/AKT signaling also has important downstream effects upon protein turnover and cell glucose metabolism via the regulation of the mTOR/S6K and GSK3β signaling pathways. Despite single agent PI3K inhibition having little effect upon melanoma growth and survival, there is evidence that PI3K targeted agents enhance the efficacy of MEK inhibition in both in vitro and in vivo studies (Bedogni et al., 2004; Jaiswal et al., 2009; Posch et al., 2013; Smalley et al., 2006).
Autophagy is an adaptive response to metabolic and drug-induced stress that involves the sequestration, lysosomal degradation and recycling of organelles and proteins (Mathew et al., 2007). Although the induction of autophagy constitutes an important mechanism of cell survival, persistent or high-level autophagy can lead to the depletion of key organelles and the activation of caspase-dependent apoptosis (Lum et al., 2005; Mathew et al., 2007; Tormo et al., 2009). Autophagy thus plays a complex, context-specific, role in cancer development that is often contradictory, with studies linking autophagy to both oncogenic transformation as well as tumor suppression (Qu et al., 2003; Yue et al., 2003). In the current study, we investigated the mechanism underlying the clinical activity of the AKT inhibitor MK-2206 in combination with paclitaxel and carboplatin in BRAF WT melanoma patients. We present new data showing that autophagy induction is critical for both the cytotoxic activity of this drug combination as well as constituting an important therapeutic escape mechanism.
RESULTS
AKT inhibition enhances the efficacy of chemotherapy in melanoma patients
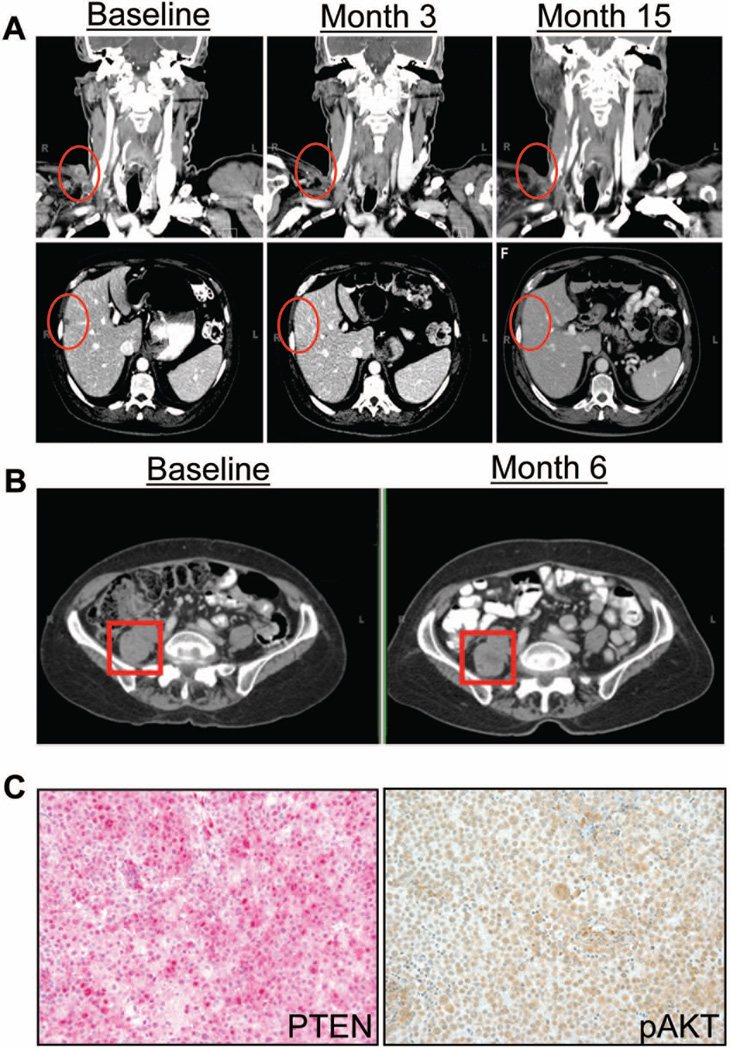
Two stage IV melanoma patients were enrolled at the Moffitt Cancer Center on a phase I clinical trial of escalating doses of MK-2206 in combination with carboplatin at an area under the curve (AUC) concentration of 6 and paclitaxel at 200 mg/m2, both administered every 3 weeks (NCT00848718). Both patients were confirmed to have BRAF WT melanoma by pyrosequencing: the NRAS status of the specimens was not available. Each patient received MK-2206 given orally prior to the administration of chemotherapy. Patient 1 was a 66-year-old male with liver, subcutaneous and hilar lymph node metastases and was treatment naïve prior to entering the trial. He received MK-2206 at 90 mg every 3 weeks for 5 cycles and then went on to receive maintenance MK-2206 at 125 mg weekly. Restaging scans demonstrated a confirmed partial response with resolution of metastases in the right trapezius and liver observed (Figure 1A). This response continued while on maintenance MK-2206 for approximately 16 months, until restaging scans demonstrated progression of disease including new sites of tumor. Patient 2 was a 68-year-old woman with an intra-abdominal mass and hilar lymph node involvement, and was also treatment naïve before trial enrollment. She received MK-2206 135 mg every 3 weeks for 4 cycles followed by maintenance MK-2206 at 135 mg weekly. This patient experienced disease stabilization for approximately 1 year (Figure 1B) until restaging imaging demonstrated progression in the brain. She thus underwent resection of the solitary brain metastasis, and IHC of the resulting melanoma brain metastasis ten days after the cessation of MK-2206 therapy showed positive staining for PTEN and phospho-AKT (Figure 1C). The intra-abdominal mass subsequently increased in size significantly after discontinuing MK-2206 and became more symptomatic. Therefore, the patient was treated with radiation to this area and has not required further therapy since that time. Both subjects were alive at the time of submission.
Figure 1. MK-2206 enhances the efficacy of carboplatin and paclitaxel in patients with BRAF-WT melanoma.
(A) Patients were treated with carboplatin, paclitaxel and MK-2206 in a phase I dose escalation study (NCT00848718). Patient 1 experienced a confirmed partial response. Upper 3 panels demonstrate resolution of a metastasis at the right trapezius. Lower 3 panels demonstrate resolution of a liver metastasis in the liver lobe. This response continued while on maintenance MK-2206 for 16 months. (B) Patient 2 experienced stable disease, which continued for 12 months while on maintenance MK-2206. (C) Immunohistochemistry conducted upon disease progression in the brain from patient 2 revealed PTEN (pink) expression and high levels of pAKT positivity (brown).
MK-2206 inhibits AKT signaling in melanoma cell lines that are BRAF WT
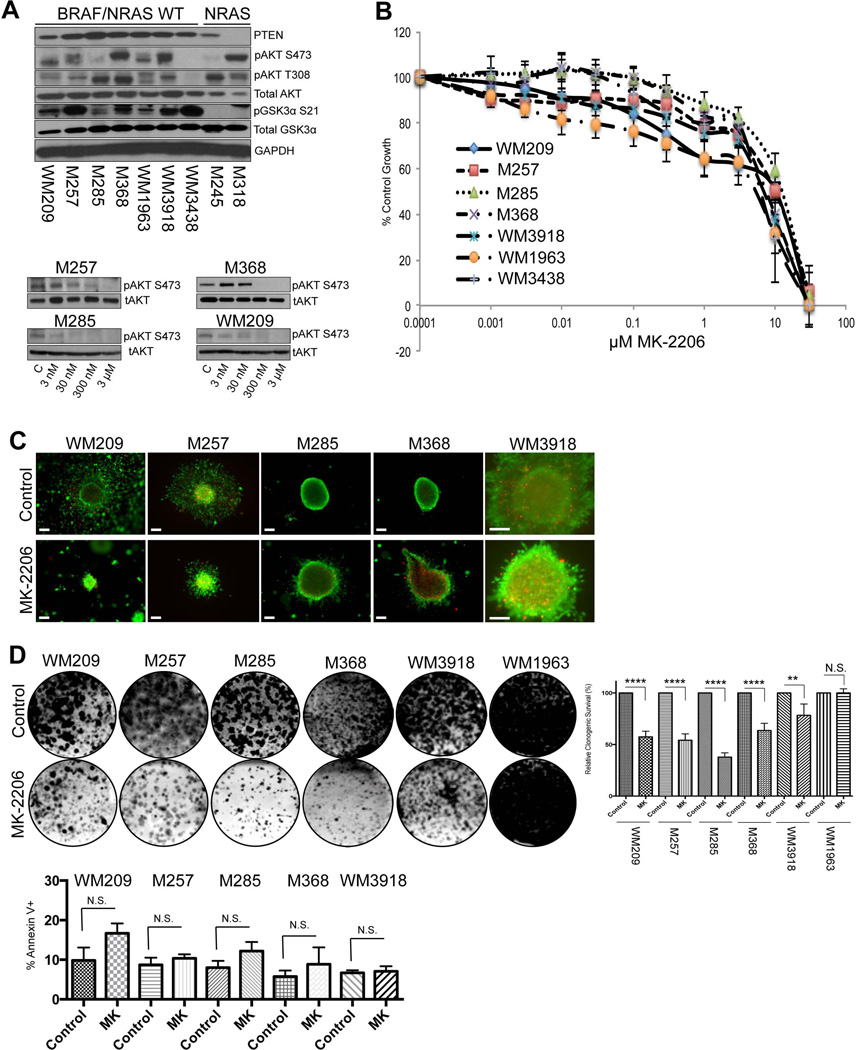
Western Blot studies showed the majority of BRAF/NRAS WT melanoma cell lines to have constitutive phosphorylation of AKT at Ser473 and Thr308 and to retain expression of the negative PI3K/AKT pathway regulator PTEN (Figure 2A). Baseline AKT signaling in the BRAF WT cell lines was indicated by the constitutive phosphorylation of GSK3α (S21) (Figure 2A). Two cell lines that were NRAS mutant and BRAF WT showed some constitutive activation of AKT, with one cell line – M318 – lacking PTEN expression (Figure 2A). Treatment of a panel of 4 BRAF WT melanoma cell lines with MK-2206 inhibited constitutive AKT signaling as shown by the reduction in AKT phosphorylation at Ser473 (Figure 2A). Despite AKT being inhibited by MK-2206, little effect was seen upon the growth, apoptosis and survival of BRAF WT melanoma cell lines under both 2D and 3D cell culture conditions at concentrations up to >1 µM (Figure 2B,C). In colony formation assays performed for 4 weeks, MK-2206 had modest, but significant growth inhibitory effects upon the long-tem survival of the WM209, M257, M285, M368 cell lines but little effect upon the WM3918 and WM1963 cell lines (Figure 2D). Correlations were not noted between basal levels of pAKT and the ability of MK-2206 to inhibit growth in long-term colony formation assays. No significant increases in apoptosis were seen in any of the cell lines following treatment with MK-2206 (Figure 2D).
Figure 2. The AKT inhibitor MK-2206 suppressed the growth of BRAF-WT melanoma cells, but did not induce cytotoxicity.
(A) (Upper) Western Blot showing basal PTEN, phospho-AKT (pAKT) (S473, T308) and phospho-GSK3α (S21) expression in BRAF/NRAS-WT (WM209, M257, M285, M368, WM1963, WM3918 and WM3438) and NRAS mutant (M245 and M318) melanoma cell lines. (Lower) MK-2206 inhibits AKT signaling in BRAF/NRAS-WT-mutated melanoma cells. Cells were treated with increasing concentrations of MK-2206 (0.003–3µM, 24h); proteins were extracted and probed for expression of phospho-AKT (pAKT) (S473). Blots were stripped once and reprobed for total-AKT to show even protein loading. (B) Increasing concentrations of MK-2206 reduced the growth of BRAF/NRAS-WT melanoma cells. Cells were treated with drug (1 nM-30 µM) for 72 h, and cell numbers were quantified using Alamar blue assay. Bars show s.e. mean. (C) MK-2206 reduces invasion, but not viability of BRAF-WT melanoma cells grown as 3D collagen-implanted spheroids. Preformed spheroids were implanted into collagen and overlayed with media. Cells were treated with MK-2206 (5 µM for 72 h) before being treated with calcein-AM and Ethidium bromide. Green, viable cells; red, dead cells. Lack of green staining also indicates a loss of cell viability. Magnification × 10. (D) (Upper) A panel of BRAF/NRAS-WT melanoma cell lines were treated with vehicle or MK-2206 (5µM) for 4 weeks. After this time, colonies were fixed and stained with crystal violet. Photographs are representative of three independent experiments and relative clonogenic survival quantitation is shown to the right. (Lower) MK-2206 induces little apoptosis at short time points. A panel of BRAF/NRAS-WT melanoma cell lines were treated with MK-2206 (5µM) for 3 days before being stained with Annexin-V and analyzed by flow cytometry. Statistical significance is indicated where ns>0.05, * P<0.05, ** P<0.01, *** P<0.001 and ****P<0.0001
Induction of cell death following chronic treatment with MK-2206 in combination with paclitaxel and carboplatin
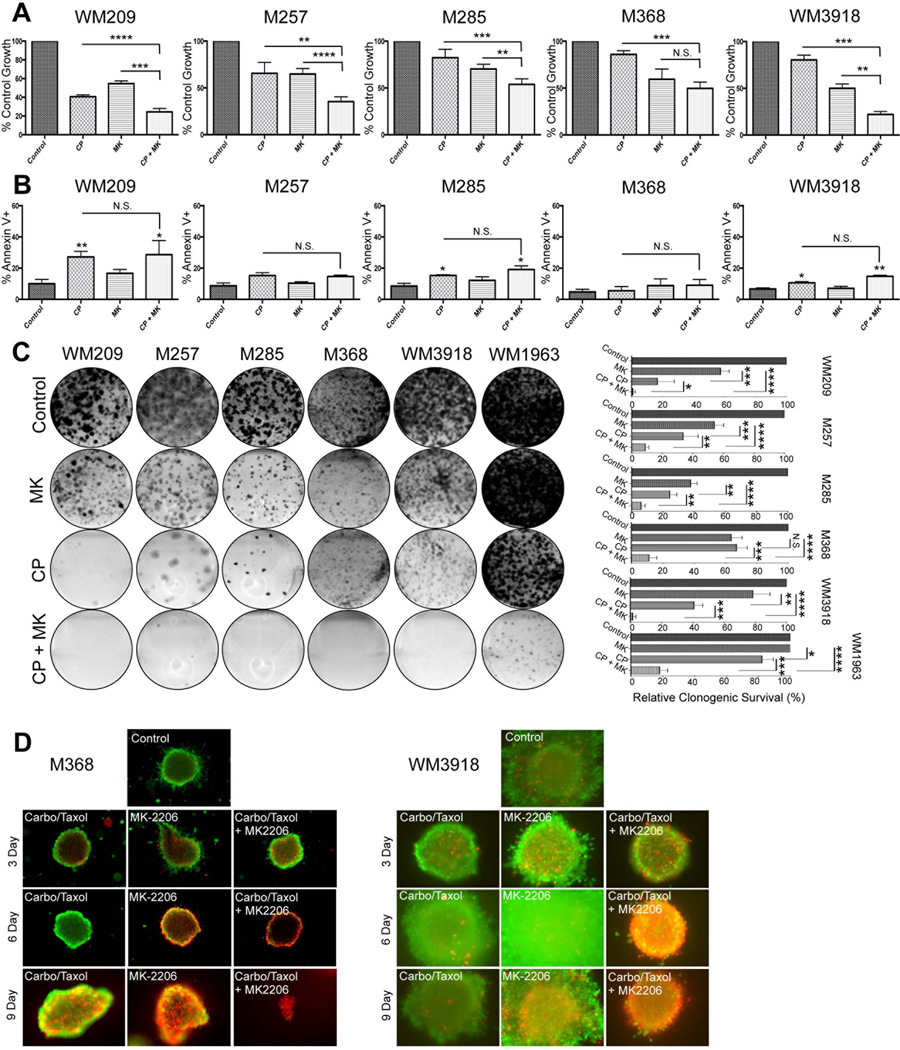
Since clinical responses were observed to the combination of MK-2206 with cytotoxic chemotherapy, we next asked whether combination with paclitaxel and carboplatin enhanced the cell death response in vitro. Although it was noted that treatment of WM209, M257, M285, M368 and WM3918 melanoma cells with the combination was associated with a significant inhibition of proliferation, these effects were less than additive (Figure 3A). Little increase in apoptotic cell death was seen to the combination of MK-2206 + chemotherapy following 72 hour treatment (Figure 3B). Instead, more dramatic effects were seen in longer-term 6 and 9 day apoptosis experiments (Supplemental Figure 1A) and in 4 week colony formation experiments with the combination of MK-2206 and chemotherapy found to almost completely prevent the formation of clones in the majority of cell lines, compared to either MK-2206 or chemotherapy alone (Figure 3C). Similar findings were also observed in 3D collagen implanted spheroid assays, with a 9-day treatment with the MK-2206 + chemotherapy combination being associated with a marked reduction in cell viability, with only a few live cells remaining (Figure 3D and Supplemental Figure 1B). Equivalent effects were seen to the drug combination in 2 NRAS mutant melanoma cell lines (data not shown).
Figure 3. The AKT inhibitor MK-2206 enhances the cytotoxic effects of carboplatin and paclitaxel after prolonged drug exposure.
(A) Acute treatment of MK-2206 enhances the anti-proliferative efficacy of carboplatin and paclitaxel treatment. Melanoma cells were either treated with vehicle, MK-2206 (5 µM), Carboplatin (1 µM), Paclitaxel (3 nM) or the combination of all three agents for 72 h, and cell numbers were quantified using Alamar blue assay. (B) Acute treatment of MK-2206 does not enhance Carboplatin and Paclitaxel-induced apoptosis. Melanoma cells were either treated with vehicle, MK-2206 (5 µM), Carboplatin (1 µM), Paclitaxel (3 nM) or the combination of all three agents for 72 h, and apoptosis levels were assessed by annexin-V staining and flow cytometry. Data show the mean of three experiments. (C) Long-term treatment of MK-2206 with Carboplatin and Paclitaxel significantly enhances the suppression of colony formation. A panel of BRAF/NRAS-WT melanoma cell lines were treated with vehicle, MK-2206 (5 µM), Carboplatin (1 µM), Paclitaxel (3 nM) or the combination of all three agents for 4 weeks. After this time, colonies were fixed and stained with crystal violet. Photographs are representative of three independent experiments. (D) Combined treatment with MK-2006, Carboplatin and Paclitaxel enhanced cytotoxicity in a panel of 3D collagen-implanted spheroids. Cells were treated with MK-2206, carboplatin and paclitaxel (3, 6 or 9 days) before being treated with calcein-AM and Ethidium bromide. Green, viable cells; red, dead cells. Lack of green staining also indicates a loss of cell viability. Magnification × 10. Statistical significance is indicated where ns>0.05, * P<0.05, ** P<0.01, *** P<0.001 and ****P<0.0001
AKT inhibitors enhance chemotherapy-induced autophagy
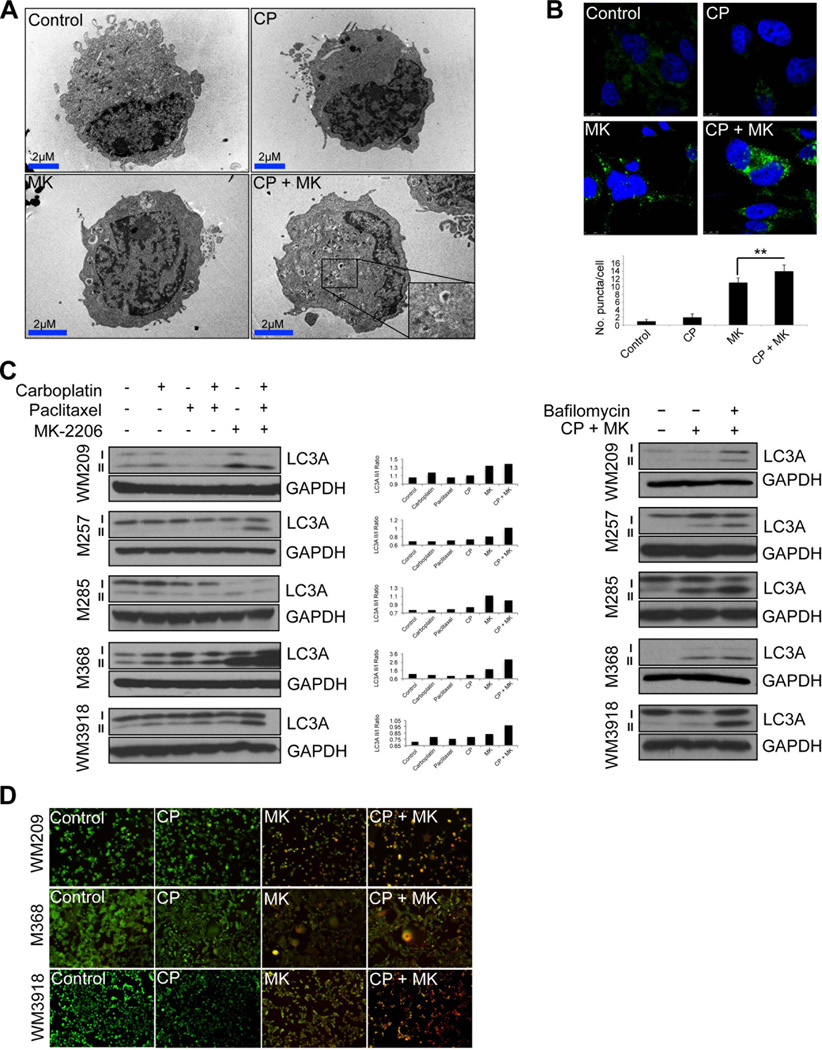
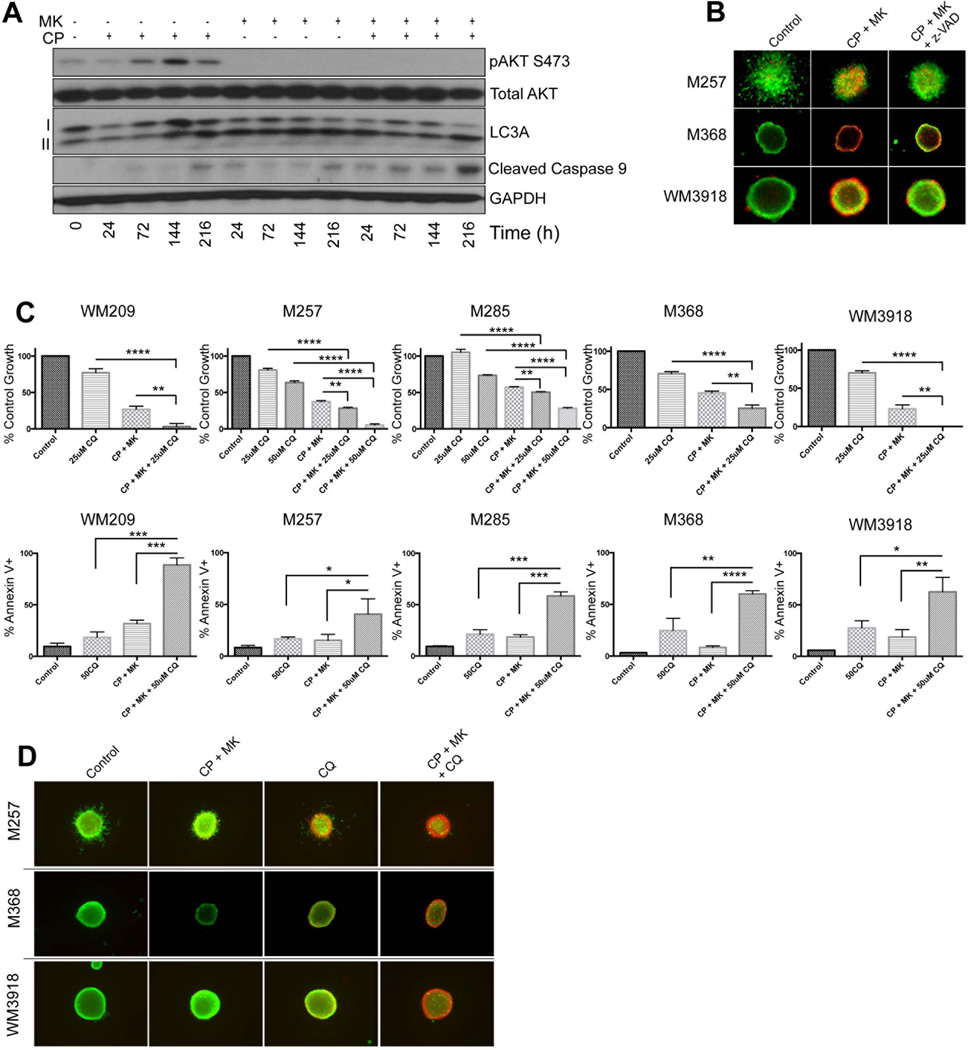
As the combination of MK-2206 with chemotherapy induced little apoptosis at time periods up to 72 hours, we next determined the potential involvement of autophagy induction in the cytotoxic response. The identification of autophagosomes through electron microscopy is the most definitive approach for demonstrating autophagy induction. It was noted that, whereas treatment with carboplatin/paclitaxel alone or single agent MK-2206 increased the numbers of autophagosomes compared to the DMSO control, the largest number of autophagosomes were seen in cells treated with the MK-2206 + chemotherapy combination (Figure 4A). In a similar vein, the AKT inhibitor + chemotherapy combination also increased the accumulation of LC3-II expression and the formation of LC3 puncta, compared to AKT inhibitor or chemotherapy alone (Figures 4B,C). Treatment of the cells with bafilomycin, an inhibitor of the lysosomal proton pump, also increased the accumulation of LC3-II in the presence of the chemotherapy/MK-2206 combination, indicating an increase in autophagic flux (Figure 4C and Supplemental Figure 2A). As the late stages of autophagy involve increased lysosome formation, we next stained drug-treated cells with acridine orange, a dye that changes from green to orange upon protonation in acidic lysosomes. It was observed that treatment with MK-2206 or chemotherapy alone only induced limited protonation of acridine orange, while intense staining for protonated acridine orange was observed when BRAF/NRAS WT and NRAS mutant cell lines were treated with the combination of MK-2206 + chemotherapy (Figure 4D, Supplemental Figure 2B and not shown).
Figure 4. Combining the AKT inhibitor MK-2206 with carboplatin and paclitaxel leads to hyperactivation of autophagy.
(A) The MK-2206, carboplatin and paclitaxel combination increases autophagosome accumulation. BRAF-WT melanoma cells (M368) were treated with either MK-2206 (5µM), Carboplatin (1µM) and Paclitaxel (3nM) or the combination of all three agents over the course of 72h. Cells were subsequently processed for EM. Shown are representative images with increasing AVs per cell (AV per cell; arrows), scale bar: 2µm. (B) Immunofluorescence staining of LC3A (green) and DAPI (blue) in M368 cells following treatment with either MK-2206, Carboplatin and Paclitaxel or the combination of all three agents for 72h. (C) (left) The MK-2206 chemotherapy combination increases the accumulation of the lipidated form of LC3. Western blot studies show the treatment of BRAF-WT melanoma cell lines (WM209, M257, M285, M368 and WM3918) with MK-2206, Carboplatin and Paclitaxel or the combination of all three agents (72 hr: as described previously) to increase expression of LC3 II. (Right) Treatment with bafilomycin A1 reveals MK-2206/chemotherapy increases autophagic flux. A panel of BRAF-WT melanoma cell lines (WM209, M257, M285, M368 and WM3918) were treated with MK-2206 (5µM), Carboplatin (1µM) and Paclitaxel (3nM) for 24 hours in the absence or presence of Bafilomycin A1 (1nM), and the levels of LC3A were examined by Western blot. GAPDH was used as a loading control. (D) Fluorescence imaging of WM209, WM3918, M368 cells treated as indicated for 72h and stained with AO. Orange: aggregated AO; green: diffuse AO. Statistical significance is indicated where ns>0.05, * P<0.05, ** P<0.01, *** P<0.001 and ****P<0.0001
Autophagy initially protects from MK-2206+chemotherapy mediated cell death
Autophagy has been paradoxically linked to both cell death and the promotion of survival. We next determined the role of autophagy induction and whether this preceded the later cell death response. In WM209, M285, M368 and WM3918 BRAF/NRAS WT melanoma cell lines, it was noted that autophagy induction as shown by accumulation of LC3-II was relatively rapid, compared to the much slower induction of apoptosis, as demonstrated by increased detection of cleaved caspase-9(Figure 5A). We next demonstrated that the cytotoxic activity of AKT inhibition + chemotherapy was dependent upon caspase-mediated cell death with pre-treatment with the pan-caspase inhibitor z-vad-FMK found to partly reverse MK-2206/carboplatin/paclitaxel-mediated cytotoxicity in a 3D spheroid assay (Figure 5B). Addition of the autophagy inhibitors bafilomycin and chloroquine significantly enhanced the anti-proliferative effects seen to the combination of MK-2206 and chemotherapy (Figure 5C) and increased the levels of apoptosis (Figures 5C and Supplemental Figures 2C–E). It was further noted that the addition of chloroquine enhanced the cell death response to MK-2206 + chemotherapy after 3 days in the spheroid assay (Figure 5D). Taken together, these results suggested that the induction of autophagy was protective from the short-term effects of MK-2206 and chemotherapy.
Figure 5. MK-2206/chemotherapy-induced autophagy is initially protective, followed by long-term cytotoxicity.
(A) Long-term treatment with the combination of MK-2206, carboplatin and paclitaxel leads to hyperactivation of autophagy and later the induction of apoptosis. Western blot showing time-dependent increases in autophagy (accumulation of LC3 II) and apoptosis (caspase 9 cleavage) following treatment with the combination of MK-2206, paclitaxel and carboplatin. (B) Inhibition of caspases partly reverses MK-2206/paclitaxel/carboplatin-induced cytotoxicity. 3D collagen-implanted spheroids of WM3918, M257 and M368 melanoma cells were treated with Carboplatin and Paclitaxel, MK-2206, MK-2206/Carboplatin/Paclitaxel, or the combination of MK-2206/Carboplatin/Paclitaxel with the caspase inhibitor z-Vad-FMK (50µM) over the course of 144 hours. Spheroids were subsequently imaged with immuno-fluorescent microscopy following treatment with calcein-AM and Ethidium bromide. Green, viable cells: red, dead cells. (C) (Upper) A panel of BRAF-WT melanoma cell lines (WM209, M257, M285, M368 and WM3918) were treated with chloroquine (12.5–50µM), MK-2206/Carboplatin/Paclitaxel or the combination of all agents for 72h. Cell numbers were quantified using Alamar blue assay and standardized against control. (Lower) Melanoma cells were treated with chloroquine alone (50µM), MK-2206/Carboplatin/Paclitaxel, or the combination of all four agents for 72h. Apoptosis was subsequently assessed by annexin-V staining and flow cytometry. (D) 3D collagen-implanted spheroids were treated with vehicle, MK-2206/Carboplatin/Paclitaxel, in the absence or presence of chloroquine (50µM) for 72h, before being treated with calcein-AM and Ethidium bromide. Green, viable cells; red, dead cells.
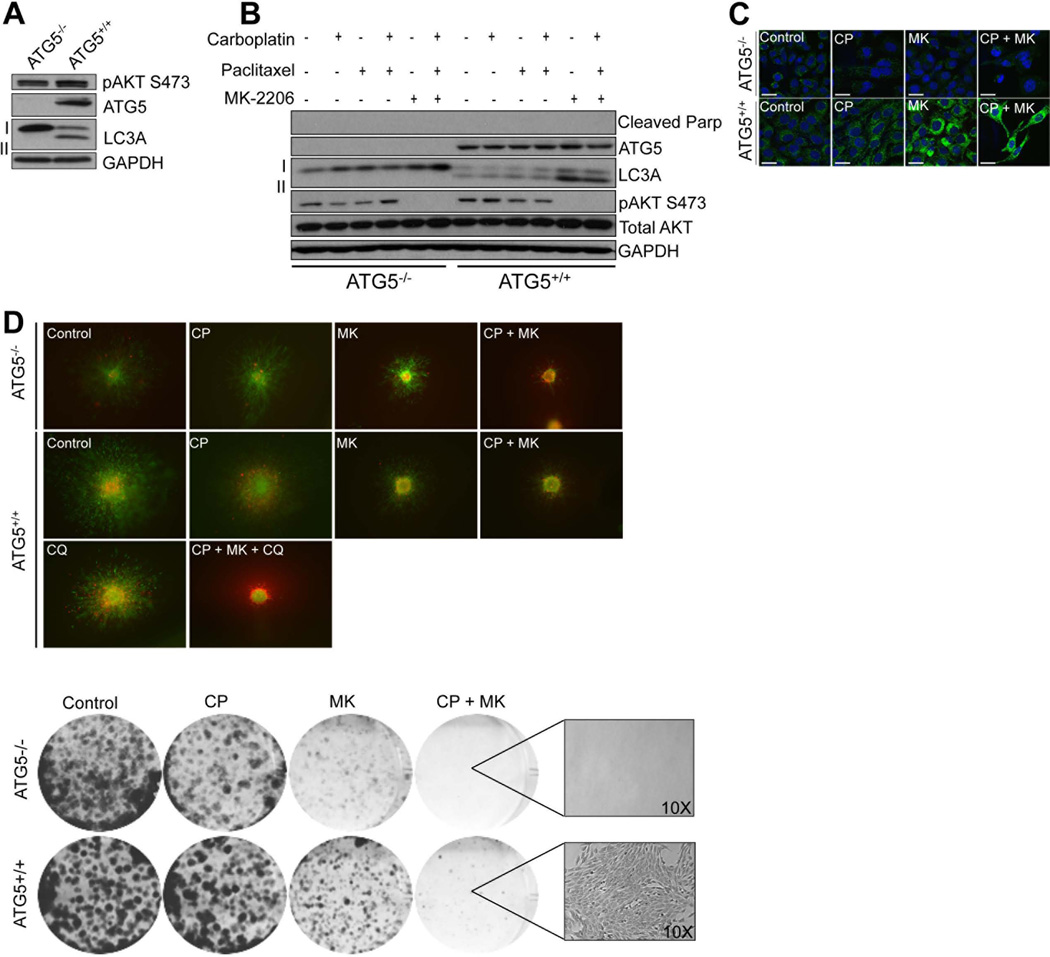
Depletion of ATG5 prevents autophagy induction and limits escape from the combination of MK-2206 + chemotherapy
We next explored the role of autophagy induction in the responses to the combination of MK-2206+chemotherapy combination using a genetic model in which autophagy was abrogated (Figure 6A). In MEFs null for ATG5 (−/−), the AKT inhibitor + chemotherapy combination was unable to induce autophagy, as demonstrated by the lack of LC3-II induction by Western blot and the absence of LC3 puncta (Figure 6B,C). In contrast, treatment of isogenic ATG5+/+ MEFs with this drug combination led to the strong induction of LC3-II and the formation of puncta (Figure 6B,C). In short-term assays, no induction of PARP cleavage was noted in either the ATG5+/+ or ATG5−/− cells (Figure 6B). It was further observed that the ATG5−/− cells exhibited more cell death following treatment with the drug combination than the ATG5+/+ MEFs in 5 day 3D spheroid assays (Figure 6D). In 4 week colony formation assays, clones were observed in the ATG5+/+ but not the ATG5−/− MEFs following MK-2206 + chemotherapy treatment, suggesting a requirement for autophagy induction in the eventual escape from therapy (Figure 6D).
Figure 6. Long-term autophagy induction is required for the escape from MK-2206/paclitaxel/carboplatin treatment.
(A) The MK-2206, paclitaxel, carboplatin combination does not induce autophagy in ATG5−/− MEFs. Western blot showing lack of ATG5 expression, phosho-AKT (S473), cleaved-Parp and LC3 II in MEFs that are ATG5−/−. (B) MK-2206 and the MK-2206/chemotherapy combination induces LC3 II expression in ATGF5+/+ but not ATG5−/− MEFs. (C) MK-2206 and the MK-2206/chemotherapy combination induces LC3 puncta in ATG5+/+ but not ATG5−/− MEFs. (D) (Left) Deletion of ATG5 prevents escape from MK-2206 and MK-2206/carboplatin/paclitaxel mediated cytotoxicity. Preformed ATG5+/+ and ATG5−/− MEF spheroids were implanted into collagen and overlayed with media. Cells were treated with carboplatin/paclitaxel (3 nM, 1 µM, 72 hr), MK-2206 (5 µM for 72 h) or carboplatin/paclitaxel/MK-2206 before being stained with calcein-AM and Ethidium bromide. Green, viable cells; red, dead cells. Lack of green staining also indicates a loss of cell viability. Magnification × 10. (Right) Deletion of ATG5 abrogates therapeutic escape. Long-term colony formation assays with ATG5−/− and ATG5+/+ MEFs following treatment with vehicle, paclitaxel/carboplatin, MK-2206 alone and paclitaxel/carboplatin/MK-2206. Cells were grown in the presence of drug for 4 weeks before being stained with crystal violet.
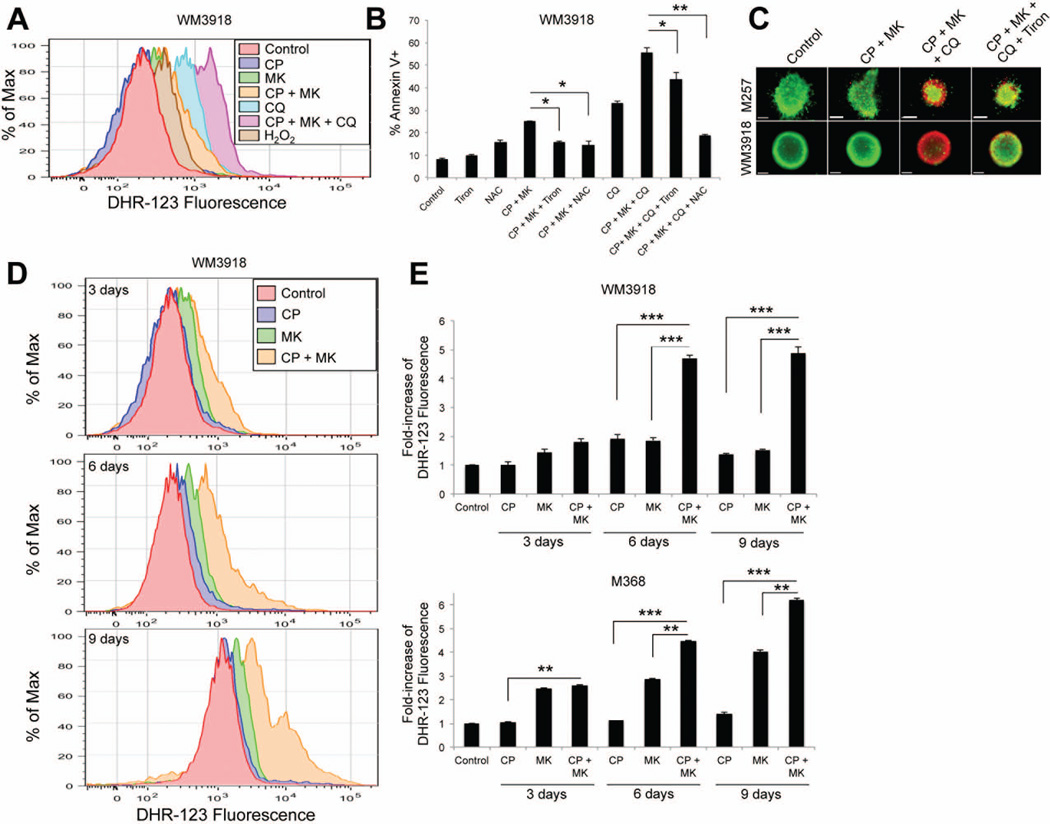
MK-2206 + chemotherapy induces ROS-mediated cytotoxicity in BRAF WT melanoma cells
The blockade of lysosomal function in cells reliant on autophagy is known to increase the levels of reactive oxygen species (ROS) (Degtyarev et al., 2008). Pre-treatment of cells with the ROS detector dihydrorhodamine-123 (DHR-123) showed the combination of MK-2206 + chemotherapy to increase intracellular levels of ROS compared to either AKT inhibitor or chemotherapy alone (Figures 7A and Supplemental Figure 3A). Even greater increases in intracellular ROS levels were seen when cells were treated with chloroquine (Figure 7A), an effect that was further enhanced following treatment with the combination of chloroquine, MK-2206 and chemotherapy (Figure 7A). The importance of ROS generation for the apoptotic effects of the combination was demonstrated by the protection conferred by the anti-oxidants Tiron and n-acetyl cysteine (NAC) (Figures 7B). It was further noted that the addition of chloroquine increased the pro-apoptotic effects of 72 hr MK-2206/chemotherapy treatment (Figure 7B). Treatment with Tiron and NAC also reversed cytotoxicity to the MK-2206, chloroquine and chemotherapy combination in 3D spheroid assays (Figure 7C and Supplemental Figure 3B). The accumulation of ROS following treatment with the AKT inhibitor and chemotherapy combination was time-dependent, and mirrored the time-course of apoptosis induction, with maximal ROS levels detected following 6 and 9 days of treatment depending upon the cell line (Figures 7D and Supplemental Figure 3C).
Figure 7. Autophagy prevents ROS-mediated cytotoxicity in BRAF WT melanoma cells treated with MK-2206 + chemotherapy.
(A) WM3918 cells were treated with MK-2206 (5µM), Chloroquine (50µM), carboplatin (1µM) and paclitaxel (3nM) or the combination of all agents over the course of 72h. H2O2 (250µM, 10 min) was used as a positive control for reactive oxygen species detection. All cells were subsequently stained with DHR-123 (10µM, 10 min) and fluorescence was measured using flow cytometry. (B) Treatment with anti-oxidants rescued cells from apoptosis. WM3918 cells were treated with either Tiron (2µM), NAC (5µM), Carboplatin (1µM), Paclitaxel (3nM), MK-2206 (5µM) and/or Chloroquine (50µM) as labeled on the graph for 72 hours. Apoptosis was assessed by annexin-V staining and flow cytometry. (C) Spheroids were formed with M257 and WM3918 cells and treated with either Tiron (2µM), Carboplatin (1µM), Paclitaxel (3nM), MK-2206 (5µM) and/or Chloroquine (50µM) as labeled, before being treated with calcein-AM and Ethidium bromide. Green, viable cells; red, dead cells. (D) (left) WM3918 and M368 were treated with MK-2206 (5µM), carboplatin (1µM) or paclitaxel (3nM) or the combination of all agents over the course of 9 days. All cells were subsequently stained with DHR-123 (10µM, 10 min) and fluorescence was measured using flow cytometry. (right) Increased ROS detection in cells treated with MK-2206 (5µM), Carboplatin (1µM) and Paclitaxel (3nM) or the combination of all agents over the course of 9 days was standardized compared to control levels. Data represents the mean of 3 independent experiments. Statistical significance is indicated where ns>0.05, * P<0.05, ** P<0.01, *** P<0.001 and ****P<0.0001
Discussion
Melanoma continues to be the poster child for targeted therapy development in cancer, with the MAPK signaling pathway representing a uniquely successful target for this disease (Chapman et al., 2011; Flaherty et al., 2012; Smalley and Sondak, 2010). Despite most melanomas showing some level of constitutive activity in the MAPK signaling pathway, other signal transduction cascades including the PI3K/AKT pathway are also important for melanoma development (Dankort et al., 2009; Madhunapantula and Robertson, 2009; Stahl et al., 2004). In melanoma, PI3K/AKT signaling can be driven through loss of PTEN, increased expression of AKT3, NF1 loss, oncogenic NRAS and rarely through AKT and PI3K mutations. Activity in this pathway plays a key role in melanoma development by allowing BRAF-transformed melanocytes to bypass oncogene-induced senescence (Davies et al., 2008; Fedorenko et al., 2011; Maertens et al., 2012; Stahl et al., 2004; Tsao et al., 2004; Vredeveld et al., 2012). Once melanoma is initiated, AKT signaling plays a role in tumor progression through effects upon survival (Dhawan et al., 2002; Liu et al., 2006). Recent work from our laboratory has further shown a role for adaptive PI3K/AKT signaling in the escape of BRAF mutant melanoma cells from the pro-apoptotic effects of BRAF inhibition (Paraiso et al., 2011). Despite these data, the potential utility of targeting AKT in BRAF/NRAS WT melanoma has been little explored. The encouraging clinical activity in the form of a partial response and prolonged stable disease in 2 BRAF WT melanoma patients treated with MK2206 and carboplatin and paclitaxel suggested that further mechanistic understanding of this regimen in preclinical models could eventually help guide the development of this and other AKT+ chemotherapy combinations for melanoma.
An initial analysis of BRAF/NRAS WT melanoma cell lines showed the majority to have constitutive AKT phosphorylation at both Ser473 and Thr308 - indicative of baseline pathway signaling activity. Despite AKT being implicated in cell survival through the direct regulation of BAD phosphorylation at Ser99, single-agent MK-2206 treatment had some, but relatively minor, effects on melanoma cell growth and induced little cell death under either 2D or 3D cell culture conditions – particularly over short time periods (up to 72 hrs) (Datta et al., 1997). Our results mirror previous studies on NRAS mutant melanoma, showing that shRNA knockdown and pharmacological inhibition of PI3K to be ineffective as a single agents, with concurrent BRAF or MEK1/2 inhibition being required for significant anti-tumor activity (Jaiswal et al., 2009; Posch et al., 2013). The reason for the limited single agent activity of MK-2206 likely results from the high level of signaling redundancy in melanoma cells. It is already known from extensive studies in cell lines harboring BRAF mutations that melanoma survival is dependent upon the MAPK pathway, which exerts its effects in part through regulation of the pro-apoptotic proteins BIM and BMF (Boisvert-Adamo and Aplin, 2008; Cartlidge et al., 2008). Given that the survival of melanoma cells is regulated through the balanced expression of both pro and anti-apoptotic BH3 family proteins, it seems likely that AKT inhibition alone is not sufficient to alter the life/death equilibrium and push the cells into apoptosis.
Despite having minor effects in the monotherapy setting, AKT inhibitors are known to synergize with many other agents including chemotherapy drugs (camptothecin, docetaxel, doxorubicin, 5-FU, carboplatin) and targeted therapy agents (erlotinib and gefitinib) (Cheng et al., 2012; Hirai et al., 2010). In BRAF/NRAS WT and NRAS mutant melanoma cell lines, the combination of MK-2206 with paclitaxel and carboplatin did not substantially enhance either growth inhibition or apoptosis induction at time points up to 72 hrs. As these small-scale effects were not in agreement with the observed prolonged anti-tumor activity of the MK-2206/paclitaxel/carboplatin combination seen in melanoma patients, we next undertook longer-term treatments in which the cultures were grown in drug for extended periods of time (up to 4 weeks), using a schedule analogous to that used in the phase I clinical trial (an initial dose of chemotherapy followed by continuous MK-2206 treatment). Under these conditions, profound levels of growth inhibition were observed, suggesting that the AKT inhibitor/chemotherapy combination had effects beyond the short-term induction of apoptosis and growth inhibition.
One of the major targets of AKT is the mammalian target of rapamycin (mTOR), a serine/threonine kinase that plays a key role in the regulation of cell growth through its activity as a sensor of amino acid and ATP levels. Inhibition of the PI3K/AKT/mTOR signaling pathway mimics nutrient starvation and leads to the induction of autophagy (Ravikumar et al., 2004; Yazbeck et al., 2008). From a signaling standpoint the ULK1 (ATG1) kinase complex is critical in integrating metabolic stress signals from the mTOR signaling pathway. Following the inhibition of mTORC1, the cytoplasmic autophagy machinery is recruited onto phospholipid membranes derived from the endoplasmic reticulum (ER) and the golgi apparatus leading to the formation of autophagosomes, which then mature with the aid of ATG5 and LC3 (Rabinowitz and White, 2010). As the final step, the autophagosomes are delivered to the lysosomes, the contents broken down by lysosomal hydrolases and the degradation products are recycled back to the cytosol (Rabinowitz and White, 2010).
Many anti-cancer drugs, including the AKT inhibitor MK-2206 and chemotherapeutic agents such as cisplatin, 5-fluorouracil and paclitaxel, are known to activate autophagy (Cheng et al., 2012; Xi et al., 2011; Xu et al., 2012). Treatment of BRAF/NRAS WT melanoma cells with MK-2206 induced autophagy as shown by an increased accumulation of the lipidated form of LC3 (LC3-II), enhanced autophagic flux (increased staining for the protonated form of acridine orange, increased LC3-II accumulation in the presence of bafilomycin) and the formation of autophagosomes as detected by electron microscopy. The addition of paclitaxel+carboplatin to MK-2206 markedly enhanced autophagy levels, and at later time points increased the extent of cell death. In cancer, autophagy is most often associated with increased cell survival, particularly under conditions of stress such as hypoxia, nutrient deprivation and drug therapy. Melanoma is a tumor with high basal levels of autophagy (typically 20–27% of specimens show evidence of LC3 puncta) and this has been linked to both chemoresistance and increased tumor aggressiveness (Ma et al., 2011; Xie et al., 2013).
Autophagy also plays a role in therapeutic escape in cancer by allowing malignant cells to remain dormant but yet viable in the presence of drug, as well as facilitating the evasion from alkylating agents by limiting the DNA damage response (Mathew et al., 2007). In BRAF WT melanoma cells, autophagy induction at the earlier time points of drug treatment was associated with therapy resistance, with enhanced cell death responses in 3D culture and increased levels of apoptosis observed following the addition of the autophagy inhibitors chloroquine and bafilomycin. A similar phenotype was also observed in melanoma cells in which the Atg5 gene, which encodes for an acceptor protein for the ubiquitin-like protein Atg12.27, was stably knocked down by shRNA. Knockdown or deletion of ATG5 prevents the autophagy process from being completed by blocking autophagosome maturation (Baerga et al., 2009). Treatment of isogenic pairs of MEFs that either expressed ATG5 or had ATG5 deleted (+/+ or −/−) also revealed a role for autophagy in resistance to the MK-2206+paclitaxel/carboplatin combination, with no ATG5−/− MEF colonies remaining after 4 weeks of drug treatment. In all cases, inhibition of autophagy was associated with an increased accumulation of ROS. A link between ROS generation and eventual cell death was demonstrated by the ability of the anti-oxidants Tiron and NAC to abrogate the extent of cell death induced by the MK-2206/paclitaxel/carboplatin combination. Our observations agree with previous studies showing that autophagy induces mitochondrial ROS and that these effects are enhanced following chloroquine treatment (Degtyarev et al., 2008). In this context, the impaired autophagosome production following chloroquine treatment contributes to ROS-mediated damage through the accumulation of deleterious oxidative products such as lipofuscin/ceroid (Degtyarev et al., 2008).
Despite being mostly linked to cells survival, persistent, high-level induction of autophagy can also be detrimental (Cheng et al., 2012; Tormo et al., 2009). Treatment of BRAF WT melanoma cells with MK-2206+chemotherapy for extended periods of time (6 and 9 days) induced high autophagy levels and an eventual switch to caspase-mediated apoptotic cell death, as shown by increased PARP cleavage and the ability of the caspase inhibitor z-vad-fmk to reverse the cytotoxic effects of the drug combination. The switch from protective autophagy to eventual cell death upon prolonged drug treatment has been reported in other tumor systems. In glioma, the combination of MK-2206 + the EGFR inhibitor gefitinib has been shown to be initially protective and sensitive to increased chloroquine-mediated cytotoxicity before eventual entry into apoptotic cell death (Cheng et al., 2012). In melanoma, a similar switch to autophagy-mediated cell death has been reported following treatment with polyinosine-polycytidylic acid (Tormo et al., 2009). As such, the inhibition of autophagy using the lysomotropic agents chloroquine and hydroxychloroquine is currently being explored clinically in combination with other anti-cancer agents in multiple solid tumors. There is already some suggestion of clinical benefit to inhibiting autophagy, with a phase III clinical trial of glioblastoma patients showing an increased response rate when hydroxychloroquine was added to carmustine and radiation (Sotelo et al., 2006). In another clinical trial, the combination of hydroxychloroquine and the mTOR inhibitor temsirolimus was associated with stable disease in 10/14 (including 4/5 individuals with melanoma) patients (Algazy et al., 2011). In summary, we have shown for the first time the potential efficacy of AKT inhibition in combination with chemotherapy in BRAF WT melanoma. Although our studies suggest this combination to be effective in the BRAF WT cell lines, it likely that BRAF and NRAS WT are genetically heterogeneous - raising the possibility that some WT tumors may not respond. Further work will be required to determine whether there is an underlying genetic basis for response to the AKT inhibitor/chemotherapy combination. Through mechanistic studies, we have demonstrated this combination to induced high, sustained levels of autophagy leading to eventual caspase-mediated cell death. Induction of autophagy was found to be critical in allowing minor populations of cells to escape from this regimen, and we suggest that the addition of an autophagy inhibitor may be one strategy of limiting the onset of resistance.
METHODS
Cell culture and MTT assay
Melanoma cell lines were a gift from Dr. Meenhard Herlyn (The Wistar Institute, Philadelphia, PA) and were grown in RPMI-1640 media (Corning) supplemented with 5% FBS (Sigma). MTT assays were performed as described in (Smalley et al., 2006). M257, M285 and M368 cells were a gift from Dr. Antoni Ribas (UCLA, Los Angeles, CA). ATG5−/− and ATG5+/+ MEFs were a kind gift from Dr Shengkan Jin (Rutgers University, Pisctaway, NJ). Cell line identity was confirmed by STR analysis (Moffitt Cancer Center, Molecular Genomics Core).
Western blotting
Proteins were blotted for as described in (Smalley et al., 2006). The antibodies to phospho-AKT (Ser473 and Thr308), total AKT, cleaved-PARP, ATG5 and LC3A were from Cell Signaling Technology.
Flow cytometry and ROS Detection
Cells were treated with 3 nM paclitaxel, 1 µM carboplatin, 5 µM MK-2206, 50 µM chloroquine and/or 3nM bafilomycin and harvested after 72 hours. Annexin-V/TMRM staining was performed as described in (Paraiso et al., 2010). ROS levels were detected in cells treated for 72 hours with paclitaxel, carboplatin, MK-2206 and or chloroquine. Cells were subsequently stained with dihydrorhodamine 123 (10 µM) for 10 minutes and fluorescence intensity was analyzed by flow cytometry. Rescue from ROS-mediated apoptosis was assessed using the antioxidants Tiron and NAC.
Immunofluorescence staining
Cells were plated onto coverslips and treated with paclitaxel, carboplatin and/or MK-2206 for 72 hours before being fixed and permeabilized with methanol and imaged with a Leica confocal at 40X magnification. LC3A punctae were visualized using a immuno-fluorescent antibody against LC3A (Cell Signaling Technology).
3D spheroid assays
Collagen implanted spheroids were prepared using the liquid overlay method and were treated with carboplatin, paclitaxel, MK-2206, chloroquine or all drugs in combination for 3, 6 and 9 days before being analyzed by fluorescence microscopy as described in (Smalley et al., 2006; Smalley et al., 2008).
Electron Microscopy
Human melanoma cells were fixed with 2% gluteraldehyde and stored at 4°C until embedding. Cells were subsequently fixed with 2% osmium tetroxide, followed by a dehydration step composed of a series of increasing ethanol dilutions and propylene oxide. Cells were then embedded in resin and sections were cut ultrathin (90 nm), placed on uncoated copper grids, and stained with 0.2% lead citrate and 1% uranyl acetate. Images were taken with a JEOL 1400 transmission electron microscope at 80kV.
Statistical analysis
Data show the mean of at least 3 independent experiments. GraphPad Prism 5 statistical software was used to perform the Student’s t test where ns>0.05, * P<0.05, ** P<0.01, *** P<0.001 and ****P<0.0001
Supplementary Material
Acknowledgement
The MK-2206 was generously provided by Merck Sharp & Dohme, Corp. and the National Cancer Institute, NIH.
Grant support: Work in the Smalley lab is supported by U54 CA143970-01 and R01 CA161107-01 from the National Institutes of Health. R.R. Massaro was supported by fellowship FAPESP #2012/05732-7; #2010/50300-2
References
- Algazy KM, Schuchter L, Demichele AM, David VJ, Torigian DA, Chang CY, Redlinger M, Davis LE, O'dwyer PJ, Amaravadi R. Combined mTOR and autophagy inhibition: Phase I trial of temsirolimus and hydroxychloroquine in patients with advanced solid tumors. AACR Annual Meeting Abstracts, 4500. 2011 [Google Scholar]
- Ascierto PA, Schadendorf D, Berking C, Agarwala SS, Van Herpen CM, Queirolo P, Blank CU, Hauschild A, Beck JT, St-Pierre A, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. The lancet oncology. 2013;14:249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- Baerga R, Zhang Y, Chen PH, Goldman S, Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, O'neill MS, Welford SM, Bouley DM, Giaccia AJ, Denko NC, Powell MB. Topical treatment with inhibitors of the phosphatidylinositol 3'-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–2560. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E, Ghosh P, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–3312. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, Mcmahon M. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, Zhou L, Liao J, Keihack H, Yan L, et al. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Molecular Cancer Therapeutics. 2012;11:154–164. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, Depinho RA, Mcmahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, Lazar AJ, Gershenwald JE, Mills GB. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, Van Dijk S, Wu H, Gray DC, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. The Journal of cell biology. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt B, Liu W, Salemi R, Wolfe R, Kelly J, Tzen CY, Dobrovic A, Mcarthur G. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigm Cell Melanoma R. 2011;24:666–672. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Research. 2002;62:7335–7342. [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko IV, Gibney GT, Smalley KS. NRAS mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene. 2012 doi: 10.1038/onc.2012.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, Mcarthur GA, Sosman JA, O'dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular Cancer Therapeutics. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li LR, Place C, et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante JR, Falchook GS, Lawrence DA, Weber J, Kefford R, Bendell J, Kurzrock R, Shapiro GI, Kudchadkar R, Long GV, et al. Phase I/II Study of the oral MEK1/2 inhibitor GSK1120212 dosed in combination with the oral BRAF inhibitor GSK2118436. J Clin Oncol. 2011;29:CRA8503. [Google Scholar]
- Jaiswal BS, Janakiraman V, Kljavin NM, Eastham-Anderson J, Cupp JE, Liang Y, Davis DP, Hoeflich KP, Seshagiri S. Combined targeting of BRAF and CRAF or BRAF and PI3K effector pathways is required for efficacy in NRAS mutant tumors. PLoS One. 2009;4:e5717. doi: 10.1371/journal.pone.0005717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Amavaradi RK, Flaherty K, Chapman PB, Ribas A, Mcarthur G, Sosman J, Nolop K, Puzanov I. Significant long-term survival benefit demonstrated with vemurafenib in ongoing phase I study. Pigm Cell Melanoma R. 2012;25:866. [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, Mccusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature genetics. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Ma XH, Piao S, Wang D, Mcafee QW, Nathanson KL, Lum JJ, Li LZ, Amaravadi RK. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhunapantula SV, Robertson GP. The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma. Pigment Cell Melanoma Res. 2009;22:400–419. doi: 10.1111/j.1755-148X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens O, Johnson B, Hollstein P, Frederick DT, Cooper ZA, Messaien L, Bronson RT, Mcmahon M, Granter S, Flaherty KT, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2012 doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature reviews. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, Messina JL, Flaherty KT, Smalley KS. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Research. 2011;71:2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, Chong K, Peng L, Dimon MT, Phillips T, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. The Journal of clinical investigation. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nature genetics. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Noma K, Haass NK, Herlyn M. In vitro three-dimensional tumor microenvironment models for anticancer drug discovery. Expert Opin Drug Discov. 2008;3:1–10. doi: 10.1517/17460441.3.1.1. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010;363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- Tormo D, Checinska A, Alonso-Curbelo D, Perez-Guijarro E, Canon E, Riveiro-Falkenbach E, Calvo TG, Larribere L, Megias D, Mulero F, et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell. 2009;16:103–114. doi: 10.1016/j.ccr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D, Mcmahon M, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Gene Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, Yuan H. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Letters. 2011;307:141–148. doi: 10.1016/j.canlet.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Xie X, White EP, Mehnert JM. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PLoS ONE. 2013;8:e55096. doi: 10.1371/journal.pone.0055096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yu H, Qin H, Kang J, Yu C, Zhong J, Su J, Li H, Sun L. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Letters. 2012;314:232–243. doi: 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- Yazbeck VY, Buglio D, Georgakis GV, Li Y, Iwado E, Romaguera JE, Kondo S, Younes A. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Experimental hematology. 2008;36:443–450. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.