Abstract
Background:
Use of HIV prevention methods may vary for women by types of sexual partners. In a microbicide safety and effectiveness trial (HPTN 035) differences in adherence to a microbicide study gel were compared between women with new versus ongoing partnerships over time.
Methods:
1,757 women in the three HPTN 035 trial’s arms completed the Follow-up Partner Status (FPS) questionnaire at their last study visit. Women married at baseline were asked if they had the same husband, new husband or new partner. Unmarried women were asked if they had changed partners or married. Self-reported gel adherence during the last sex act was compared at each quarterly visit between women with ongoing versus new partners. High gel adherence was compared with low gel adherence (85-100% versus <85% of last vaginal sex acts reported with gel use, respectively) in multivariable models to assess associations with partner change.
Results:
Overall 7% of women (n=123) reported a new partner and 41% (51) of those reported a new husband. Median gel adherence was reported to be 100% in women with ongoing partners and 75% for women with new partners (p<0.001). In women reporting no gel use in their last sex act, only 12.5% of the women with a new partner and none of those with an ongoing partner reported using condoms (p<0.001). Fewer women with new partners reported using both the gel and condom during the last sex act as compared to women with ongoing partners (median: 50 versus 71.4%, p<0.001). After adjusting for age, site, education level, and sexual frequency, women with ongoing partners were more likely to report high gel adherence than those with new partners (AOR 2.5, 95% CI: 1.6, 3.9). (95% CI: 1.6, 3.9) more likely to report high gel adherence than those with new partners. This pattern persisted when gel use over time was compared between women with new vs. ongoing partners.
Conclusions:
In the HPTN 035 trial, women with new partners had higher HIV incidence and reported less gel use and higher condom use. Specific counseling and support are needed to help women use potential HIV prevention methods, including microbicides, when they are changing partners.
Keywords: Adherence, microbicide, HIV, partner status
INTRODUCTION
Clinical trials of HIV prevention methods often require participants alter their routine or behavior around sexual activities to incorporate the product being tested including their use of condoms or other contraceptives1. Some behaviors may be adopted without partner awareness, but many methods such as topical microbicides require insertion or application that may be observed or otherwise evident to partners during sexual activity. Topical microbicides are prophylactic agents that hold potential for HIV prevention. Several formulations are being evaluated in clinical trials and it is likely that microbicides will eventually be available in a range of forms from gels to vaginal rings and other methods2. Their successful development could critically expand the variety of HIV prevention options available to sexually active individuals with diverse needs, behavior patterns and levels of risk.
Partnership status including the stability and duration of a relationship may have important bearing on a woman’s adherence to the trial method. In Africa, findings from recent clinical trials suggest sexual partners may influence women’s patterns of adherence to HIV prevention methods. To date, the highest adherence in any study was achieved among individuals with a stable partner in The Partners PrEP Study, although these were all partners with known HIV sero-discordance3. In this study it was not possible to disentangle the difference between just having a stable partner and one who is stable and also sero-discordant. Nevertheless, these findings suggest the role partners play in facilitating adherence requires further investigation.
HIV prevention trials that enrolled women with more variability in partnership status have reported much worse adherence. A recent trial of oral prophylaxis among women in Africa found detectable drug levels in blood below 50%4. This study has not yet reported an effect of partnership status on adherence but fewer of these trial participants were in stable, ongoing partnerships than in the Partners in PrEP trial due to their recruitment approach. This phenomenon of enhanced adherence has also been observed in trials where participants and their partners hold “preventive misconceptions”, or perceptions that overstate the effectiveness of the trial method (or its placebo) and its ability to protect them from infection1. In these cases, adherence to the trial method may be higher but sometimes paired with more frequent sexual risk-taking behavior.
Some evidence for the influence on adherence from being in a new or less stable partnership comes from qualitative interviews with women in a pilot study of a microbicide vaginal gel in Africa. Women reported that they would use gel in “long-term relationships, but not for casual sex” and some women reported that they told their “permanent” partner, but not another one of their gel use. Discussions about gel use were shown to support building of trust in relationships, while condom use implied a lack of trust; although those interviewed about partners were notably self-selected5. These qualitative data suggest use of HIV prevention methods, particularly those detectable during sexual intercourse such as vaginal gels, may be particularly challenging for women with new sexual partners. Moreover, a change in a sexual partnership may reflect a change in exposure to HIV because each new partner has a different probability of being HIV infected.
Understanding how partnership dynamics affects adherence requires insight into the context of sexual partnerships in Sub-Saharan Africa. There has been considerable attention and debate regarding the effect of concurrent partnerships on the HIV epidemic in the region6,7 and much evidence that the practice of concurrent partnering is common in most countries, perhaps even recognized as a social norm in South Africa8. There is also some evidence that when women are knowingly engaged in risky partnerships such as those involving transactional sex or concurrency, they will adopt greater measures to protect themselves from HIV such as using condoms9. This would suggest greater adherence to HIV prevention methods among women who practice concurrency or who may suspect their partners are concurrent. However, the literature on sexual power dynamics in Africa concerning age differences between men and women – specifically “sugar daddies”– have been cited as possibly enhancing vulnerability of women to HIV infection10 as in such scenarios women may be less able to use protection. Economic need has also been cited as a force in power differentials and the exchange of sex for goods and support may enhance women’s HIV risk by making them less able to use protection11. Yet even when some women in South Africa report expectations of greater power, financial independence, and freedom in decision making including around sexuality they report being in relationships that have much intimate partner violence, infidelity, and low condom use12. Therefore, it must be recognized that a woman’s ability to adhere to a new method of HIV prevention will occur within these complicated dynamics of the sexual partnership that occur with new partners as well as within ongoing relationships and in the context of established gender roles13.
Understanding how adherence to different microbicide methods will be achieved by select populations is an important step in development of HIV prevention methods. For women in Africa, partnership factors may be critical in determining method use and adherence, particularly across populations where partnership status fluctuates frequently. To assess if the status of the partnership affected women’s adherence to product use in a microbicide trial, we analyzed the effect of partnership change on gel use, condom use, and sexual frequency in a microbicide safety and effectiveness trial (HPTN 035)14.
METHODS
HPTN 035 was a phase II/IIb, four-arm, randomized controlled trial conducted between February 2005 and January 2009 at multiple sites in Blantyre and Lilongwe, Malawi, Durban and Hlabisa, South Africa, Harare and Chitungwiza, Zimbabwe, Lusaka, Zambia and Philadelphia, U.S.A. HIV-negative, non-pregnant women at least 18 years of age who were sexually active (had vaginal intercourse at least once in the past 3 months) were eligible for the study. The exclusion criteria included a history of adverse reactions to latex, use of non-therapeutic injection drugs in the past 12 months, and a history of vaginal intercourse more than an average of two times per day in the past 2 weeks. Women were randomly assigned in equal proportions to one of the four study arms: BufferGel, 0.5% PRO2000 gel and two comparator arms comprising HEC placebo gel or no gel. All three study gels were similar in appearance and were packaged in identical vaginal applicators. Study participants also had quarterly HIV tests and medical and speculum-aided pelvic examinations. Further details on study procedures as well as the trials safety and effectiveness results are reported elsewhere14.
Behavioral data were collected during quarterly visits from self-reports of gel and condom use during the last coital act and during all coital acts in the last 7 days. At the last study visit, 1,757 women in the HPTN 035 gel arms completed a Follow-up Partner Status (FPS) questionnaire. The FPA asked women who reported being married at baseline if they had the same husband or a new husband or new partner; women unmarried at baseline were asked if a partner had changed or they married. Gel adherence was compared between women with ongoing partners versus new partners.
ANALYTIC APPROACH
Adherence was assessed as an average across all quarterly visits as percent of last vaginal sex with gel use comparing medians for bivariate analyses and as higher gel use (85-100% of last vaginal sex acts) and lower gel use (<85% of last vaginal sex acts). Summary measures over the entire follow-up period were computed for each woman. For a given woman, all the quarterly measures obtained during follow-up were summarized by dividing the sum of all the quarterly numerators by the sum of all the quarterly denominators. For example, the proportion of last vaginal sex acts with study gel only was calculated as: (Number of last vaginal sex acts with study gel only) / (Number of last vaginal sex acts).
Because there were no data on the timing of partner change, change in level of adherence between the first reported adherence in follow-up and the latest adherence measure in follow-up were compared. This assumed that the partnership change occurred between the two time points. The amount of this change over time was then compared for ongoing partners vs. new partners. There were 32 of 1694 participants excluded from this analysis who either: 1) did not provide data on adherence to gel at the last sex act within the first 6 months of follow-up, or 2) did not provide data on adherence to gel at the last sex act at both the early and late time point. Comparisons were tested with chi-square analysis.
The distributions of these summary measures are compared between partnership status groups using 1) non-parametric Wilcoxon Rank Sum tests for the continuous variable and 2) Cochran Mantel-Haenszel tests for the ordinal (categorical) variable, stratified by study site due to large differences in proportions of women married at baseline between sites. For the primary adherence measure of interest, study gel use at last vaginal sex act (regardless of condom use), the per-woman cumulative proportion across all quarterly visits and at early and late visit was dichotomized into low/high gel use defined as <85% and ≥85%. A multivariable logistic regression model was fit to low/high gel use, comparing partnership status groups, adjusting for age, study site, education level, and sexual frequency (at study exit).
ETHICS
All participants demonstrated adequate understanding of the trial and provided written informed consent. The trial (NCT00074425) was approved by 11 institutional review boards that oversee research conducted at the eight study sites as well as regulatory authorities in the U.S.A., South Africa and Zimbabwe. All women were provided comprehensive HIV prevention services, including HIV pre-test, risk reduction and post-test counseling, condoms and sexually transmitted infection testing and treatment as per local standards.
RESULTS
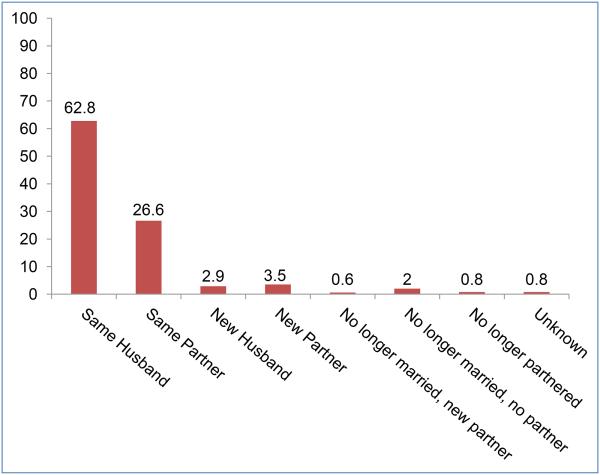
At study exit, a new partner was reported by 7% of women (n=123), and of those, 41% (51/123) had a new husband. Overall, 3% of the sample (49/1757) reported no longer having a partner and partner status was unknown for 1% (14/1757) (Figure 1). These 63 participants were excluded from further analyses, which are based on the 1694 participants reporting a husband or partner at study exit. Regarding adherence, 60% (946/1571) of women with ongoing husbands/partners were high adherers, compared to 39% (48/123) of women reporting a new husband/partner who were high adherers (p<0.0001). The average proportion of last vaginal sex reported with gel use (with or without a condom) was higher for women with ongoing partners than for women with new partners (median: 100 versus 75%; p<0.001). The proportion of last sex reported with a condom only was higher for those with a new partner than for those with an ongoing partner (median: 12.5 versus 0%; p<0.001). The proportion of women reporting using gel in combination with a condom was lower for those with a new partner (median: 50 versus 71.4%, p<0.001).
Figure 1.
Partnership Status at Last Study Visit: Women in 035 Gel Arms. n=1,757
Partner type (husband or partner) and duration of the partnership (started in the past year or began >1 year ago, but since start of the trial) were also analyzed for its association with self-reported adherence among the 123 participants reporting a new partnership during the study (120 with duration data). Overall, 43% (32/74) of those reporting a new husband/partner in the past year were considered high adherers (85-100%) compared to 35% (16/46) of those reporting a new husband/partner more than one year ago, although given the small sample size this is not a statistically significant difference.
Among those women reporting a changed partnership status, some reported entering marriage with new husbands and others reported new partners. Among the 50 participants who gained new husbands in the past year, 19% (3/16) were high adherers compared to 35% (12/34) of women with high adherence who gained new husbands more than one year ago, although these numbers were also not statistically significant. Among the 70 participants with new partners, 50% (29/58) were high adherers with new partners they met in the past year compared to 33% (4/12) with high adherence who gained new partners more than one year ago (also not statistically significant).
The multivariable analysis (Table I) shows the following factors are associated with self-reported high adherence after controlling for site: having an ongoing partner compared to having a new partner (AOR 2.51; 95% CI 1.62, 3.88), of older age (AOR 1.05; 95% CI 1.03, 1.07) and reporting greater sexual frequency in the past week (AOR 1.11; 95% CI 1.04, 1.19); education was not. Duration of time in the study was analyzed as well and was not significantly associated with either having a new partner or adherence and was included in a preliminary multivariable model but was not statistically significant so was not included in the final model.
Table I.
Characteristics and Behaviors Associated with Self Reports of High Versus Low Adherence to Vaginal Gel Use in a Microbicide Safety and Effectiveness Trial (HPTN 035): Frequencies, Univariate and Multivariable Logistic Regression (n= 1,606)*
| % or mean(SD) | OR (95% CI) | AOR (95% CI) | |
|---|---|---|---|
| Ongoing Partner vs New Partner | 89% vs 7% | 2.37 (1.63, 3.45) | 2.51 (1.62, 3.88) |
| Age | 26.0 (5.8) | 1.05 (1.03, 1.07) | 1.05 (1.03 , 1.07) |
| Primary school education or less vs Some 2ndary school education or more | 37% vs 63% | 0.74 (0.61, 0.90) | 0.78 (0.58 , 1.05) |
| Number of vaginal sex acts in week prior to study exit | 2.6 (2.3) | 1.15 (1.08, 1.23) | 1.11 (1.04 , 1.19) |
| Controlled for site – only Chitungwiza, Zimbabwe had significantly higher gel use than the reference site Philadelphia | |||
From the 1694 participants reporting a sexual partnership at study exit, 88 were excluded for missing data from a factor in the model (2 were missing the adherence outcome, 1 was missing education status, and 85 were missing the number of vaginal sex acts in the week prior to study exit.)
Acquisition of HIV was higher for those with new partners; 12 (9.8%) of the 123 participants with new partners seroconverted during the study, while 71 (4.5%) of the 1571 participants with ongoing partners seroconverted during the study (p=0.01). Therefore, participants with new partners appear to have been at higher risk for HIV, but it does not appear that acquiring HIV infection during the study was associated with high vs. low adherence (univariate p=0.77) and in the multivariable model p=0.51) so it was not included in the final model presented.
Among 1662 participants who reported on gel use at the last vaginal sex act prior to a visit in the first six months of follow-up AND at their latest follow-up visit, 1549 participants reported an ongoing partner throughout the study, and 113 reported a partnership change involving a new partner. Those with new partners self-reported less overall adherence to gel use during the last sex act at the latest follow-up visit than at the early follow-up visit (chi-squared p-value=0.007). More participants with new partners (29%) reported using gel with last vaginal sex at the early visit and not with last vaginal sex at the later visit than participants with ongoing partners (17%). Comparing participants with new partners to those with ongoing partners, fewer reported the same level of gel adherence at both time points (63% vs. 72%), indicating greater fluctuation in gel use among those experiencing partner change. A greater proportion of those with new partners also reported discontinuing gel use over the course of the trial. Only 17% of participants with ongoing partners reported using gel at the early visit, but not at the later visit compared to 29% of participants with new partners. Finally, more participants with ongoing partners reported initiating gel use later in the trial as compared to their peers with new partners (10% vs. 8%) (Table II).
Table II.
Self Reports of Adherence to Vaginal Gel Used Early and Late in a Microbicide Safety and Effectiveness Trial (HPTN 035) By Partner Change Status (n=1,662)
| New Partners (n=113) | Ongoing Partners (n=1549) | |
|---|---|---|
| Used gel at early visit, and not at late visit | 33 (29.2%) | 270 (17.4%) |
| No change (did not use gel at either early visit or late visit, or used gel at both early visit and late visit) | 71 (62.8%) | 1119 (72.2%) |
| Did not use gel at early visit, but did use gel at late visit | 9 (8.0%) | 160 (10.3%) |
Chi-squared p-value for difference between groups, p=0.007.
Within each group, there was a significantly lower proportion of participants reporting gel use at the later follow-up visit, but it appears the drop in gel usage from early to late time point was more pronounced among those participants with new partners at the end of follow-up. Mean follow-up time from the early to late time points was similar in the two groups: new partners, 1.5 person years (PY); ongoing partners, 1.4 PY. min, max were 0.25, 2.25 for each group.
DISCUSSION
These findings suggest that having a new partner affects adherence (self-reported) to a microbicide gel in a clinical trial. Women may struggle with having the kinds of discussions with partners and establishing the trust that, according to a qualitative study, may be required for women to use vaginal gels for HIV protection5. Women who experienced a change in their partnership status and acquired a new partner while being part of the 035 microbicide trial reported using a microbicide gel less than women who reported that there was no change in their partner status and kept an ongoing partner. Most of the women in this trial were from study sites in Africa and because HIV transmission within established partnerships in Africa is not uncommon, the use of gel for HIV prevention within these partnerships may offer women a viable option if a gel can be proven effective for HIV prevention. The encouraging findings from CAPRISA 004, a phase llb trial of 1% tenofovir gel, provided the first evidence that a vaginal microbicide gel may offer protection against HIV acquisition15. Results from that trial, in which women were asked to use the gel before and after sexual intercourse, revealed the critical role of adherence; HIV incidence was significantly higher for women in the trial who reported using gel less frequently than directed15. Unfortunately results from CAPRISA 004 have not been replicated with other vaginal gels including those tested in this clinical trial14. Sadly the most recent trial of a topical microbicide gel in Africa also provided disappointing results – with no evidence of effectiveness yet notably low adherence (<40%) to product use throughout the trial16. New products and formulations of microbicide gels are in development and other clinical trials of approaches to HIV prevention such as vaginal rings are in process and may also find partnership status affects decision to use and adhere to these products. Moreover, more long-acting methods may prove less susceptible to partner dynamics reducing the effect on adherence. Nevertheless, we hope our findings may encourage future studies to refine measurements of partnership status to better assess their effect on choices to use HIV prevention methods. Our findings particularly resonate because we demonstrate that in this trial women who had a new partner have higher HIV incidence than women with an ongoing partner, highlighting the importance of measuring partnership status and the change in partnership status among women in HIV prevention trials.
A major limitation of this study is that adherence to gel use was self-reported. Comparisons between self-reports of oral pill adherence and a biomarker were recently published from a small trial and demonstrated a large difference between self-reports adherence and the biomarker (94% reported adherence vs 64% detected adherence)17. Although this trial included a gel arm, detection of the tenofovir concentration was not able to be determined for gel use but it is likely differences with self-reports were similar across arms. This builds on growing evidence that self-reports of adherence to HIV prevention methods are over-reported in clinical trials. The first clinical trial of an antiviral oral prophylaxis (IPrEx) that included biomarker validation of self-reported adherence demonstrated large discrepancies with self-reported levels of pill taking18. This was followed by a trial of oral prophylaxis among women in Africa demonstrating even greater discrepancies between self-reported pill taking and drug levels detected in blood19. Self-reported medication adherence rates have been found to be inflated when compared to adherence rates demonstrated from electronic or biomarker monitoring methods20,21. The Carraguard trial found large discrepancies between self-reports of gel adherence and their dye stain assay to assess applicator insertion22. These findings illustrate the pitfalls of reliance on self-reported adherence in HIV prevention trials.
Therefore, it can be assumed that adherence was over-reported in our study, especially by those with established partnerships where very high adherence was reported. The self-reports of non-adherence maybe more accurate because trials that validated adherence reports with biomarkers have not found evidence of use among those who reported non-use. This suggests differences between self-reports and actual use may have been greater within the established partnerships yet the low adherence noted with new partners can be assumed to represent a best case scenario - as adherence may have been even less than the relatively low level reported. Validation of patterns of gel use by partner type with a biomarker would confirm the need for adherence interventions to focus on those with new partners.
Another issue with the self-reports has to do with the validity of the status of partnerships reported. In an ancillary study we conducted within this trial we learned women were much more likely to admit to not having a partner to ACASI than to an interviewer23. Because having regular sex was a requirement of study participation, women were reluctant to admit when they did not have a partner. As data reported in this analysis of partnership type and adherence were collected by interviewers, it is likely not having a partner was underreported and some of those with “ongoing” partners may not, in fact, have had a partner. It may be stigmatizing for women to admit they are now “partnerless” or “single” to a nurse in an interview especially since that means the participant is not compliant with study protocol. Having a new partner also may represent a sensitive behavior to report to an interviewer, therefore, we may have misclassified and analyzed women in ongoing partnerships who actually had new partners. This may have reduced the difference we were able to detect between adherence among those with ongoing and new partners. Such a difference may be greater and more evident in future studies that utilize ACASI to ask women about their partner status. But observed differences in dissolving partnerships could not have contributed to this because we exclude participants with dissolved partnerships in our analysis of adherence early to late in the study, unless a new partnership resulted by the end of study. Women who were married at the beginning of the trial may report higher adherence because they were likely to have informed their husbands about their participation in the trial and explained the product use required to them – therefore making product adherence easier. Finally, a further limitation is that partnership status was assessed at enrollment and not again until the last quarterly visit in the trial when it was reported as any change since trial enrollment. Ongoing assessments of partnership status would have allowed for detailed analyses of timing of adherence by status and enhanced data quality. Therefore, more precise measurement of partnership status in future studies could better untangle its’ effect.
These findings raise concern for challenges to the use of new HIV prevention methods for women with new partners. New partners may represent a heightened risk for women as the partner may have concurrent partners until the relationship becomes established. The same may be said of study participants who were not monogamous during the course of the trial. Moreover, women in this study with new partners did experience higher HIV incidence than those with ongoing partners. While condom use may be more acceptable within such new partnerships5, community education will be needed so that men are more aware of alternatives and initiate discussion with partners about them before women may be willing to introduce them into their new partnerships. Specific counseling for some HIV prevention methods may be needed for women who are not in established partnerships or experience partner change within the setting of clinical trials, as well as in the future when such methods hopefully become available for broader use. Women with new partners in particular may struggle with using novel methods of HIV prevention and efforts must be made to provide adequate counseling and support to help women introduce methods such as microbicides to a new sexual partner.
ACKNOWLEDGEMENTS
HPTN 035 was funded by the US National Institutes of Health and designed and implemented by the HPTN and the MTN. HPTN (U01AI46749) has been funded by the National Institute of Allergy and infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Drug Abuse, and National Institute of Mental Health (NIMH). MTN (U01AI068633) has been funded by NIAID, NICHD, and NIMH.
REFERENCES
- 1.Woodsong C, Alleman P, Musara P, et al. Preventive misconception as a motivation for participation and adherence in microbicide trials: evidence from female participants and male partners in Malawi and Zimbabwe. AIDS and behavior. 2012 Apr;16(3):785–790. doi: 10.1007/s10461-011-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karim QA, Baxter C, Karim SA. Topical microbicides--what’s new? Journal of acquired immune deficiency syndromes. 2013 Jul;63(Suppl 2):S144–149. doi: 10.1097/QAI.0b013e3182986f80. [DOI] [PubMed] [Google Scholar]
- 3.Baeten J, Donnell D, Ndase P, et al. ARV PrEP for HIV-1 Prevention among Heterosexual Men and Women; 19th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle. 2012. [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, et al. The FEM-PrEP Trial of Emtricitabine/Tenofovir Disoproxil Fumarate (Truvada) among African Women; Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI); Seattle. Mar 5–8, 2012. 2012. [Google Scholar]
- 5.Montgomery CM, Lees S, Stadler J, et al. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care. 2008;20(6):733–740. doi: 10.1080/09540120701693974. [DOI] [PubMed] [Google Scholar]
- 6.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010 Feb;14(1):11–16. doi: 10.1007/s10461-008-9433-x. dicussion 34-17. [DOI] [PubMed] [Google Scholar]
- 7.Sawers L, Stillwaggon E. Concurrent sexual partnerships do not explain the HIV epidemics in Africa: a systematic review of the evidence. Journal of the International AIDS Society. 2010;13:34. doi: 10.1186/1758-2652-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mah TL, Maughan-Brown B. Social and cultural contexts of concurrency in a township in Cape Town, South Africa. Cult Health Sex. 2013;15(2):135–147. doi: 10.1080/13691058.2012.745951. [DOI] [PubMed] [Google Scholar]
- 9.Onoya D, Reddy P, Sifunda S, et al. Transactional sexual relationships, sexually transmitted infection risk, and condom use among young Black Women in peri-urban areas of the Western Cape Province of South Africa. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2012 May-Jun;22(3):e277–282. doi: 10.1016/j.whi.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Wyrod R, Fritz K, Woelk G, et al. Beyond sugar daddies: intergenerational sex and AIDS in urban Zimbabwe. AIDS Behav. 2011 Aug;15(6):1275–1282. doi: 10.1007/s10461-010-9800-2. [DOI] [PubMed] [Google Scholar]
- 11.Zembe YZ, Townsend L, Thorson A, Ekstrom AM. “Money talks, bullshit walks” interrogating notions of consumption and survival sex among young women engaging in transactional sex in post-apartheid South Africa: a qualitative enquiry. Globalization and health. 2013;9:28. doi: 10.1186/1744-8603-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettifor A, Macphail C, Anderson AD, Maman S. ‘If I buy the Kellogg’s then he should [buy] the milk’: young women’s perspectives on relationship dynamics, gender power and HIV risk in Johannesburg, South Africa. Cult Health Sex. 2012;14(5):477–490. doi: 10.1080/13691058.2012.667575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery ET, Chidanyika A, Chipato T, van der Straten A. Sharing the trousers: gender roles and relationships in an HIV-prevention trial in Zimbabwe. Cult Health Sex. 2012;14(7):795–810. doi: 10.1080/13691058.2012.697191. [DOI] [PubMed] [Google Scholar]
- 14.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011 Apr 24;25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrazzo Jea. 20th Conference on Retroviruses and Opportunistic Infections. Atlanta: 2013. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003) [Google Scholar]
- 17.Minnis AM, Gandham S, Richardson BA, et al. Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2013 Feb;17(2):737–747. doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Damme LCA, Ahmed K, Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak’Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirumurthy H, Siripong N, Vreeman RC, et al. Differences between self-reported and electronically monitored adherence among patients receiving antiretroviral therapy in a resource-limited setting. Aids. 2012 Nov 28;26(18):2399–2403. doi: 10.1097/QAD.0b013e328359aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren SR, Raisch DW, Campbell HM, et al. Medication adherence assessment in a clinical trial with centralized follow-up and direct-to-patient drug shipments. Clinical trials. 2013;10(3):441–448. doi: 10.1177/1740774511410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Dec 6;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 23.Gorbach PM, Mensch BS, Husnik M, et al. Effect of computer-assisted interviewing on self-reported sexual behavior data in a microbicide clinical trial. AIDS and Behavior. 2013;17(2):790–800. doi: 10.1007/s10461-012-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]