Abstract
The recent approval in the United States of the first rapid home test to diagnose HIV raises questions about its potential use and impact. We reviewed the existing literature on the unassisted use of home tests involving self-collection and testing of biological samples by untrained users – including existing HIV self-testing studies – to shed some light on what can be expected from the availability of the HIV home test. The studies reviewed showed that most participants could properly perform home tests, obtain accurate results, and interpret them – yielding high correlations with laboratory and health-professional performed tests. Users often had trouble performing blood-based tests. Participants generally understood the need to confirm positive test results. Materials accompanying HIV home tests should emphasize symptoms of acute infection and the need for additional testing when recent infection is suspected. Different home-test-based screening modalities, personalized HIV-counseling resources and HIV home test impact evaluation methods should be studied.
Keywords: HIV, home testing, self-test, home test, rapid test
INTRODUCTION
Home tests – also called self-tests or home-use tests – are typically sold over the counter (OTC) and allow users to test self-collected specimens and interpret the results on their own without the help of trained health professionals. These types of tests differ from home-collection tests, which require users to collect samples at home, mail them to a laboratory or clinic for analysis, and obtain the results by telephone a few days later. Although US Food and Drug Administration (FDA)-approved HIV home-collection tests have been on the market since 1996 [1], various concerns have prevented the approval of a home test until recently [2–8].
In July 2012, the FDA approved the first ever rapid HIV home test [9]. The OraQuick™ In-Home HIV Test, which allows users to test themselves for HIV infection in the privacy of their homes and obtain the results in as little as 20 minutes, went on sale to the general public in the United States (US) in October 2012. The test is seen as a possible method for increasing HIV status awareness in the US [10], where almost 20% of HIV-infected individuals are unaware of their seropositivity [11]. The use of similar rapid HIV tests in US clinical settings since 2002 already has led to increases in HIV testing rates and in the number of people who receive their test results [12–14].
Despite FDA-approval, many remain skeptical about the potential impact of the test, and several important questions remain unanswered [10]. For instance, some have questioned whether the most-at-risk populations will be able to afford the test, whether people may be coerced to use the test, whether home testing will actually be private, and whether high-risk individuals will be motivated to seek out more sensitive testing after receiving negative home tests results [10].
Yet, home testing is not a new phenomenon. FDA-approved home tests have existed in the US for the detection, diagnosis or management of various health conditions for years [15, 16]. While these home tests are either designed to detect curable, arguably more benign conditions than HIV or to monitor chronic diseases, important lessons applicable to HIV home tests can be garnered from their performance with untrained users.
We reviewed the extant literature on past and existing home tests to compile evidence of their performance, ease of use, diagnostic accuracy, and the consequences of their use by non-clinically trained personnel. Our aim was to identify testing experiences that might inform the potential impact of the newly licensed HIV home test (and any other similar tests that may be approved in the future). We also examined the few existing studies on rapid HIV self-testing by untrained users in research settings. Specifically, we sought to answer the following questions: What lessons have we learned from the use of existing home tests, and how may they be applied to the new HIV home tests?
METHODS
For the purpose of this review, “home tests” were defined as those performed by individuals with no medical or laboratory training and without assistance from trained professionals. As a result, we also reviewed studies on self-tests or point-of-care tests that might not necessarily have been performed at home. To ensure the inclusion of literature covering both home tests and self tests, we searched Medline (through OVID: 1948- March Week 4, 2011), PsycINFO (through OVID: 1806- March Week 4, 2011), PubMed, and Scopus using the following search terms: “home test,” “home testing,” “self test,” “self testing,” “home self test,” “home self testing,” “home-use test,” “home-use testing,” “home-based test,” “home-based testing,” “home-based self test” OR, “home-based self testing.” Searches were limited to literature in English and involving humans. We also searched the bibliographies of included articles for additional relevant studies.
Studies were included in the review if they:
Involved the self-collection of a biological sample such as blood, oral fluid, or urine; self-testing of the sample and the subsequent interpretation of the test results by the individual following written or visual instructions and without assistance or instruction from any trained personnel, AND
Involved a discussion of factors pertaining to the technical steps involved in using the tests and users’ ability to perform them; a discussion of the tests’ performance under different conditions (or among different populations); or a comparison of the efficacy of the home tests to that of clinic or laboratory-based tests.
Therefore, we excluded studies involving home-collection tests and testing of laboratory-prepared specimens. This was done to limit our inquiry to those tests whose use mimic the conditions under which the new HIV home tests will be used. We also excluded the use of home tests by laboratory-trained individuals as their performance with the tests might differ from that of the general populace.
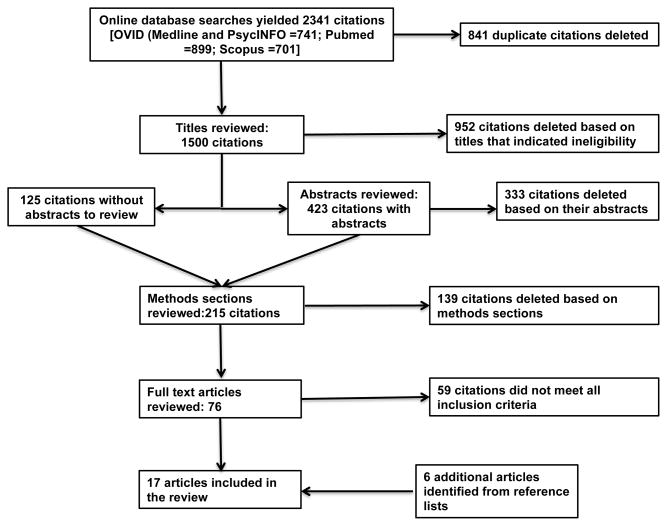
The results of our systematic literature search are shown in Figure 1. Our search yielded 2341 citations, of which 841 were duplicates. Once duplicates had been eliminated, we reviewed the remaining citations and excluded those that did not meet inclusion criteria based on their titles, abstracts or methods sections. Examples of citations eliminated included knowledge self-tests for continuing education credit, studies of self-collection tests, or tests that do not involve the collection of biospecimens (e.g., Alzheimer’s olfactory tests), as well as commentaries and letters to the editor with no empirical data on the use of the tests they addressed. The remaining articles (n=76) were read in full by at least two of the authors, who then independently determined their eligibility for inclusion in the review. Discrepancies were resolved in consultation.
Figure 1.
Article selection process
We also identified various articles and conference abstracts through this and other literature searches on studies that had been conducted using HIV rapid tests for self-testing and included them in the review. All included articles were analyzed and summarized with respect to our study question.
RESULTS
Our search of the literature yielded 23 non-HIV studies that met all inclusion criteria. Of these, five involved home fertility tests (to detect ovulation or sperm levels) [17–21]. Other studies involved tests to detect or facilitate diagnosis of vaginal infections (n=4) [22–25], tests for diabetes and its complications (e.g. blood glucose or microalbuminuria; n=4) [26–29], malaria tests (n=3) [30–32], pregnancy tests (n=3) [33–35], cholesterol tests (n=1) [36], fecal occult blood tests (n=1) [37], urinary tract disease screening tests (n=1) [38], and oral anticoagulation monitoring tests (n=1) [39]. Table I provides a brief summary of the studies of non-HIV home tests included in the review.
Table I.
Articles on non-HIV home testing reviewed
| Author s (year published) | Participants sample size/gender (age range) country [if specified] | Type of test kit used name (manufacturer) | Result determination method | Participant result verification method | Outcomes relevant to study questions |
|---|---|---|---|---|---|
| Urine tests to predict ovulation (by detecting luteinizing hormone (LH) surge) | |||||
| Anderson et al. (1996) [17] | 64 F (Mean=32yrs) | Conceive (Quidel, San Diego, CA), Clearplan (Unipath, Bedford, UK), Predictor (Schefaro International BP, Rotterdam, Netherlands) | Color change compared to reference color | Laboratory assay of tested urine sample | 1) 72% of participants (ppts) correctly detected an LH surge and were inseminated on the right day; 2) 86% thought kits were easy to use; 3) 75% thought results were easy to interpret; 4) 81% were confident in their use of the test kits; 5) 33% would have liked some assistance with the tests; 6) 48% would prefer home testing in the future. |
| Nielsen et al. (2001) [21] | 63 F (22–43) USA | OvuQuick® (Quidel, Inc., San Diego, CA), followed by the following 1-step tests: OvuQuick One-Step [formerly Conceive] (Quidel, Inc., San Diego, CA), SureStep (Applied Biotech, Inc., San Diego, CA), and ClearPlan Easy (Unipath, Ltd, Bedford, UK) | Presence and intensity of color in the test area compared to the reference area of the test | None | 1) The one-step tests detected 68%–84% (95%CI: 56–93) of LH surges detected by OvuQuick (considered the gold standard test for the study); 2) More ppts were comfortable with OvuQuick than the 1-step tests (37% vs. 8%–25%) because they thought it was more accurate; 3) Most ppts found the 1-step tests easier and less time consuming to use and thought their instructions were simpler; 4) Some ppts disliked needing a cup and dropper for the tests; 5) Some ppts found it easier to read the results of tests with large round test areas than those with thin lines. |
| Robinson et al. (1992) [20] | 27 F (younger than 38) UK | OPT (LH Color, Organon, Holland) | Color change on the test device | Insler score (an indicator of ovulation based on cervical mucus) | 1) 80% of ppts were awarded Insler scores ≥11 at their clinic visit indicating good correlation with LH surge detected by the home tests. |
| Semen tests to determine sperm levels | |||||
| Coppola et al. (2010) [18] | a) 61 M b) 32M USA | SpermChek Fertility | Presence of one or 2 test lines (one for low and 2 for normal sperm counts) in addition to the red control line | i) Compared with same test results interpreted by laboratory personnel (LP) for sample a; ii) questionnaire on test performance (all ppts) | 1) 95.1% agreement between ppt and LP interpretation of results with only 1 invalid test; 2) ppts correctly responded over 93% of the time (range: 93–100%) to questions regarding performance of the test, and reading and understanding test results; 3) ppts agreed over 97% of the time (range: 97–100%) with questionnaire statements about the ease of use of the test and clarity of test instructions. |
| Klotz et al. (2008) [19] | 50 M (18 - older than 65) | SpermChek Vasectomy® | Presence or absence of a positive (blue) line in addition to control (red) line on lateral flow test strip device | SpermChek test and hemacytometer counts performed by LP | 1) 100% agreement between ppt and LP SpermChek tests; 2) 99% of ppts correctly responded to questions about performing the test; 3) 95% of ppts correctly answered questions about reading and understanding test results; 4) 98% of ppts agreed that the test was easy to use and instructions were clear. |
| Vaginal secretion tests for Trichomoniasis diagnosis | |||||
| Jones et al. (2007) [23] | 626 F [313 home, 313 clinic] (14–25) South Africa | XenoStrip TV test (Xenotope Diagnostics, San Antonio, TX) | Presence or absence of a positive line in addition to control line on test strip | Laboratory performed polymerase chain reaction (PCR) test on patient-collected vaginal swab and observation of clinic ppts by a healthcare professional (HP) | 1) Ppt interpretation of test results matched that of HP 91.7% of the time; 2) based on observations clinic staff believed 99.2% of clinic ppts self tested with ease; 3) most ppts thought it was easy to collect the test sample (85.8% vs. 96.2%), follow the instructions on performing the test (94.7% vs.97%), and interpret the results (80.5 vs. 90.1%) home vs. clinic ppts respectively; 4) 85% of ppts would purchase self-sampling and self-testing kits if commercially available. |
| Lippman et al. (2007) [24] | 818 F [410 home, 408 clinic] (18–40) Brazil | XenoStrip TV test (Xenotope Diagnostics, San Antonio, TX) | Presence or absence of a positive line in addition to control line on test strip | Laboratory performed PCR test on patient-collected vaginal swab and observation of clinic ppts by HP | 1) Home ppts’ interpretations of test results matched that of HP 98% of the time; 2) most ppts (94% home and 98% clinic) successfully performed the TV test on their first attempt; 3) ppts who were not successful on their first attempt failed due to “poor understanding of instructions or small mishaps”; 4) based on HP observation most clinic ppts collected the test sample (98%), performed the test (99%) and read the results (94%) with ease; 5) 96% of ppts found self-sampling easy and comfortable; 6) most ppts found self test easy to perform (93 vs. 91%), results easy to read (91% vs. 87%), and trusted the results they obtained (93 vs. 97%) in the home and clinic testing ppts respectively. |
| Vaginal pH tests (testing vaginal secretions) to facilitate diagnosis of vaginal infections | |||||
| Geva et al. (2006) [22] | 516 F (18–60) Israel | VI-SENSE diagnostic panty liner (Common Sense, Caesarea, Israel) | Color change on indicator in panty liner | i) Laboratory results; ii) physician diagnosis; iii) amine test; iv) nitrazine paper | 1) Compared to clinic and lab diagnoses VI-SENSE yielded 90.8% true positive (TP) and 9.2% false negative (FN) results in ppts with bacterial vaginosis, trichomoniasis or both; 2) in ppts with mixed vaginal infections the self-test was superior to other methods, yielding positive results for 92.4% of the ppts compared to 60.8, 70, and 71.7% for other test methods; 3) 86% of ppts said reading test results was clear. |
| Roy et al. (2003) [25] | 151 F (17–73) USA | pHEM-ALERT (GYNEX, Redmond, WA) | Color change on the vaginal swab is compared to an included pH test color chart | HP performed pH test | 1) High concordance between pH readings by ppt and HP with κ >0.870 in all analyses; 2) 97% of ppts found the test easy to use; 3) 96% of ppts found the instructions easy to follow. |
| Blood tests to monitor glucose levels in diabetics | |||||
| Kristensen et al. (2004) [26] | 101 F, 92 M (22–75) Norway | Glucometer Dex (Bayer Diagnostics, Tarrytown, NY) and GlucoMen Glyco (Menarini, Firenzi, Italy) | Not specified | Tests performed by laboratory technicians on both types of study meters as well as using the reference tests for both meters | 1) 6% of ppts who used GlucoMen Glyco used too little blood, and 16% did not code the meter properly; 2) Of the Glycometer Dex measurements done by ppts, 24% were done with too little blood; 3) ppts who did not receive training on how to perform the tests were more likely to use too little blood compared to those who were trained (31% vs. 18%); 4) in general, ppts who received training had more precise measurements on both meters than those who were not trained; 5) untrained users were more likely to read the manual accompanying the home test kit than those who received training on how to use the test. |
| Müller et al. (2006) [27] | 249 F, 213 M (27–89) Germany | Not specified (participants used their own glucose meters) | Not specified | Steps in performing the test were assessed using a standardized checklist at baseline and after participants underwent training on how to properly perform the test | 1) At the beginning of the study only 17% of participants performed the test without making any errors, this increased to 59% after training; 2) The most common error (49%) was pressing the finger while extracting the blood sample; 3) at study entry 61.3% made errors which made the obtained results useless, this was reduced to 24% after training; 4) 58% of insulin users in the study reported adjusting their dosages based on home testing results, of these, 53% made errors which rendered results useless at baseline; this decreased to 22% after training; 5) 26.6% made errors which led to false readings at baseline, this decreased to 9.1% after training; 6) only a minority of ppts (13%) had never received instruction on self-monitoring of blood glucose prior to entering the study. |
| Urine tests to detect (micro) albuminuria (indicative of diabetes-related complications such as nephropathy) | |||||
| Nielen et al. (2009) [28] | 38654 F, 33060 M (18 - older than 70) Netherlands | 3 semi-quantitative dipstick tests (Machery-Nagel, Düren, Germany) | Color change of dipstick compared to color chart | No verification done. | 1) 0.5% of ppts with negative results and 25% of ppts with positive test results visited a physician for follow-up, with another 0.5% negative and 31% positive ppts planning on seeking follow-up medical attention at a later date; 2) Of 3983 ppts who sought follow up medical attention, 152 cases of hypertension, 31 cases of diabetes, and 25 cases of kidney disease were newly detected; 3) Of 280 ppts with negative results who sought follow up care, 8 new cases of disease were detected. |
| Piehlmeier et al. (1998) [29] | 59 F, 49 M (Mean=31yrs for type 1 diabetics and 59yrs for type 2 diabetics) Germany | Micral-Test® S | Color change compared to comparison colors on the label of the test tube, which correspond to different levels of albuminuria. Diagnosis was based on either 1 out 3 positive self-tests or 2 out of 3. | Laboratory immunoturbidimetry on same urine samples | 1) 79.6% and 89.8% of ppts correctly classified themselves as either positive or negative for microalbuminuria when 1 out of 3 and 2 out of 3 positive tests were used to determine positive test results respectively; 2) compared to lab tests, home tests yielded 90% sensitivity (SE), 77% specificity (SP), positive predictive value (PPV) of 49%, and negative predictive value (NPV) of 97% when only 1 positive home test was considered as a positive test result; 3) compared to lab tests, home tests yielded SE of 81%, SP of 92%, PPV of 71%, and NPV of 95% when 2 positive home tests were required for a positive test result. |
| Blood tests for malaria self-diagnosis | |||||
| Jelinek et al. (1999) [30] | 46 F, 52 M Kenya | ICT Malaria Pf (ICT Diagnostics, Sydney, Australia) | Not specified | i) Steps in performing the test were assessed by a HP using a standardized questionnaire; ii) microscopy | 1) 68% of ppts were able to perform the test; 2) Of the ppts who were unable to properly perform the test 87% could not interpret the test results, 71% could not obtain a blood sample, 58% could not identify the bands that indicate the test results, 39% did not wait the indicated amount of time, and 26% could not properly place the blood specimen on the test; 3) Only 1 ppt with microscopy diagnosed malaria was able to perform the test. |
| Trachsler et al. (1999) [31] | 67 F, 93 M (18–67) Switzerland | ParaSight F (Becton Dickinson Diagnostics, Cockeysville, MD) | Presence of a red test line in addition to control line | Not specified | 1) 82.5% of ppts obtained valid test results (90% among those who received oral and written instructions, 75% among those who only received written instructions); 2) 25% of those with only written instructions considered the test impossible to perform compared to 8% of those with oral and written instructions; 3) 66.8% had trouble obtaining the blood sample, but only 7.5% of ppts couldn’t perform the test because of this; 4) 3.1% had difficulty interpreting their test results; 5) 91.9% of ppts would use the test if it were available. |
| Whitty et al. (2000) [32] | 50 F, 103 M (16–60) UK | ICT Malaria P.F. cards (ICT Diagnostics, Sydney, Australia) | Presence of test line in addition to control line | Malaria microscopy (thick and thin films) | 1) 89% of participants obtained valid test results; 2) compared to the gold standard the valid self-tests yielded 95% SE and 97% SP; 3) 75% of participants thought the instructions were easy to follow, and 84% thought results were easy to read; 4) of the 11% with invalid tests, failures were often due to inability to use the lancet to collect a blood sample (25%), insufficient blood (18.8%), and inability to read the test card (25%); 5) 87% said they would purchase the test kits if commercially available |
| Urine tests for pregnancy detection | |||||
| Doshi (1986) [33] | 109 F (Childbearing age) | Daisy 2™ Home Pregnancy Test Kit (Ortho Pharmaceutical Corp., Raritan, NJ); e.p.t. ® In-Home Early Pregnancy Test Kit (Warner/Chilcott, Morris Plains, NJ); Answer® At-Home Early Pregnancy Test Kit (Carter Products, New York, NY) | Not specified | i) HP performed in-home test (same brand as participant); ii) Sensi-Tex™ (hospital diagnostic kit; Roche Diagnostics, Nutley, NJ) | 1) Results obtained by participants < 21 years of age (95% CI: 21.1–78.9%), and those with < high school education (95% CI: 23.4–83.3%) deviated the most from manufacturer claims of accuracy (72–99%). |
| Grob et al. (1973) [34] | 86 F UK | Predictor (Chefaro Proprietaries, Ltd) | Presence of a ring in the bottom of the tube of the test after 2 hours | i) HP-performed Predictor; ii) standard Pregnosticon test | 1) 96% performed the test correctly and obtained the right results; 2) 2 of 3 ppts who failed to obtain correct results moved the test before the 2 hours were up |
| Lindstedt et al. (1982) [35] | 201 F [with only 196 samples returned for testing] Sweden | Predictor pregnancy test (Chefaro International BV, Holland) | Presence or absence of a dark ring at the bottom of the test’s tube after 2h. | i) Pharmacy-performed Pregnosticon All-In (Organon Teknika BV, Holland) [a pregnancy test similar to that used by participants]; ii) radioimmunoassay | 1) 98% of ppts had same test results as pharmacists; 2) Compared to the immunoassay the home test had an overall accuracy of 98%, 100% SE, 98% PPV, and 100% NPV |
| Blood tests to determine cholesterol levels | |||||
| McNamara et al. (1996) [36] | 303 F, 183 M (18–81) USA | AccuMeter Cholesterol Test (ChemTrak, Inc., Sunnyvale, CA) | Peak height of the test’s reaction bar read from a thermometer like display and converted to mg/dl using test result chart | i) HP interpretation of participant performed test; ii) 2 HP performed AccuMeter tests (at 3 of the 4 study sites); iii) serum cholesterol laboratory test using the Abell-Kendall reference method (all sites) | 1) For ppts with both self and HP test, 91% obtained results within 1mm of each other; 2) 52 ppts excluded themselves from the study due to lack of confidence in their performance of the test (27 of them were unable to obtain a result); 19 other ppts were excluded because their test readings were outside the calibration range and thus could not obtain a result; 3) 75% of ppts gave a “medically responsible” response to a question on what they would do if the home test showed a high cholesterol concentration (32% would see a doctor, 32% would alter their diet and exercise regimen, 9% would do both, and 2% would get re-tested). |
| Fecal occult blood tests to screen for colorectal cancer | |||||
| Tate et al. (1989) [37] | 404 patients (17–89) | EZ-Detect™ (NMS Pharmaceutical Inc., USA) and Haemoccult™ (Röhm Pharma, FRG) | For EZ-Detect (EZD) change of the color of the reagent cross to blue. Not specified for Haemoccult (HO) | Double-contrast barium enema or colonoscopy | 1) 10% false positive (FP) with EZD, and 10.2% FP with HO; 2) 12% recorded the positive EZD control test result as negative; 3) In detecting colorectal cancer EZD had 36.4% SE, 89.3% SP, and 16.3% PPV, while HO had 80% SE, 88.5% SP, and 28.6% PPV; 4) In detecting all neoplasia EZD had 25.9% SE and 28.6% PPV, while HO had 46.2% SE and 42.9% PPV). |
| Urine tests to screen for hematuria (indicative of urinary tract diseases) | |||||
| Messing et al. (1989) [38] | 253 M (≥50 years) USA | Ames Hemastix (Ames Co., Miles Inc., Elkhart, IN) | Not specified | Not specified | 1) Most participants (>90.6%) used correct testing techniques, returned test results cards on time and performed most of the scheduled tests; 2) Of 41 ppts randomly visited by study staff, 100% did the expected number of tests correctly; 3) 18.7% of ppts (44 men) had at least 1 positive test result, of which 15 men had conditions that warranted immediate medical attention. |
| Blood anticoagulation monitoring tests to determine international normalized ratio (INR) | |||||
| Ryan et al. (2008) [39] | 93 M, 57 F (19–91) Ireland | CoaguChek S® [replaced with CoaguChek XS® during the course of the study due to a recall of Coaguchek S® test strips] (Roche Diagnostics, East Sussex, UK) | INR reading on meter | Laboratory INR performed on venous blood using Sysmex® CA-7000 or CA-1500 coagulometers | 1) 87% of ppt and laboratory INR measurements were within 0.5 units of each other; 2) agreement was highest for lab INR values ≤1.9 (97.8%), and lowest for lab INR values ≥3.6 (67%); 3) 3 ppts discontinued home testing due to differences > 0.5 units between reading on at least 2 occasions. |
Note: CI=confidence interval, F=female, FN=false negative, FP=false positive, HP=healthcare professional, LP=laboratory personnel, M=male, NPV=negative predictive value, ppt=participant, PPV=positive predictive value, SE=sensitivity, SP=specificity, TP=true positive.
We also identified six studies on HIV self-testing that met all inclusion criteria [40–45]. A seventh HIV study [46] was included in the review although the participants did not collect their own specimens but instead used prepared blood samples. This exception was made due to the dearth of articles on HIV self-testing at the time and the applicability of the study findings. Table II contains a summary of the HIV self-testing studies. There were two types of HIV tests used – oral fluid tests and blood tests. The oral fluid tests required participants to swab each gum once and then place the test device in the developer fluid for a specified amount of time [47]. For blood tests, users obtained a blood sample via finger prick, either transferred or directly applied it to the testing device, then either added test solution to the device or placed the device directly in the solution. The test was then set aside for a specified amount of time before the results were read [47–50].
Table II.
Articles on HIV self-testing reviewed
| Author(s) (year published) | Participants sample size/gender (age range) country | Name & Type of test kit used (manufacturer) | Participant result verification | Outcomes |
|---|---|---|---|---|
| Carballo-Diéguez et al. (2012) [40] | 57 M (18–62) USA | OraQuick Advance HIV-1/2 oral fluid test (OraSure Technologies Inc., Bethlehem, PA) | Interviewer observation of test performance | 1) 74% of participants (ppts) opted to self-test; 2) most ppts who self-tested performed the test correctly; 3) several ppts paid more attention to visual card than to written instructions; 4) common mistakes included touching the test pads with fingers, swabbing gums more than once, and eating or drinking right before testing; 5) several ppts said they would seek confirmatory testing followed by treatment as a next step after testing positive. |
| Choko et al. (2011) [41] | 283 ppts (Median=27yrs) Malawi | OraQuick Advance HIV-1/2 oral fluid test (OraSure Technologies Inc., Bethlehem, PA) | Health professional (HP) performed Determine (Abbott Laboratories, Abbott Park, IL) and Unigold (Trinity, Berkeley, CA) [both blood-based rapid tests] [SD Bioline HIV I/II (Standard Diagnostics, Inc.) was also used in the event of discordant confirmatory tests] | 1) 91.9% opted to self-test; 2) among those who tested, there was a 99.2% concordance between self- and HP-performed tests (95% CI: 97.0–100); 3) Self-tests had 97.9% sensitivity (95% CI: 87.9–100) and 100% specificity (95% CI: 97.8–100); 4) 98.5% of ppts thought the test was “very easy” to perform; 5) 10% of ppts requested additional help in performing the tests, mostly for clarification on how to perform the mouth swab; 6) 10% of ppts erred in performing the tests. Mistakes included removing the test from the developer fluid too early or spilling the fluid; 7) 2 ppts were unable to read the results of their test due to “a very faint test line”; 8) All ppts would recommend OraQuick for self-testing to friends and family; 9) 58.2% of women and 64.8% of men ppts would prefer home testing for next HIV test; 10) most ppts thought HIV counseling was still necessary. |
| Gaydos et al. (2009) [43] | 218 HIV-negative ppts (18–64) USA | OraQuick Advance oral fluid test (OraSure Technologies Inc., Bethlehem, PA) or Unigold blood test (Trinity, Berkeley, CA) | HP performed OraQuick test | 1) 92% chose an oral test, 8% chose a blood test; 2) 99% of ppts had same result as HP; 3) ppts who used the blood test “very much” trusted their own results more than HP’s (94% vs. 78%) while those who used the oral test had a similar trust level for both results (86% vs. 88%); 4) Most ppts found it “not at all hard to collect” the sample they tested (96% oral, 78% blood); 5) 97% (oral) and 100% (blood) of ppts would “definitely” or “probably recommend” self-testing to others; 6) Over 94% would probably or definitely perform home tests if available. |
| Gaydos et al. (2011) [42] | 478 ppts (18–64) USA | OraQuick Advance HIV-1/2 oral fluid test (OraSure Technologies Inc., Bethlehem, PA) or Unigold blood test (Trinity, Berkeley, CA) | HP performed OraQuick oral fluid test | 1) 91% chose oral test, 9% chose blood test; 2) Concordance with HP test results was 99.6% (weighted κ=0.75); 3) 97.2% (oral) and 84.4% (blood) thought it was “not hard at all” to collect the specimen; 4) 96.3% (oral) and 80% (blood) thought it was “not hard at all” to perform the test; 5) 94% (oral) and 86.7% (blood) thought their test results were “definitely correct”; 6) if available OTC, 87.5% (oral) and 82.2% (blood) would “definitely test at home”; 7) 94% (oral) and 84.4% (blood) would definitely recommend self-testing to a friend; 8) Based on HP observations, 5–10% of ppts had difficulties in interpreting results, reading result charts, reading instruction chart, opening test kit, following instructions, and swabbing/finger-pricking correctly. |
| Granade et al. (2004) [46] | 99 ppts USA | OraQuick rapid HIV-1 antibody test (OraSure Technologies Inc., Bethlehem, PA) and Hema-Strip (Chem-Bio Diagnostic Systems Inc., Medford, NY) | Each ppt tested 6 prepared blood specimen of known HIV status | 1) With OraQuick test, 95.1% of ppts who were given a demonstration (demo) of how the test works and 90.2% of ppts who received no demo obtained correct test results; 2) with OraQuick 1.6% of ppts had invalid results; 3) false results with OraQuick were due to transcription errors, sample mix-ups and improper test performance and actual test failures; 4) with Hema-Strip, 87.5% of ppt who were given a demo and 70.6% of those who received no demo obtained correct test results; 5) 16.8% received invalid results with Hema-Strip; 6) Ppts had trouble with the proper insertion of the Hema-Strip device into the test’s buffer vial. |
| Lee et al. (2007) [44] | 350 ppts [88 known HIV-positive; 262 at-risk] Singapore | Determine HIV 1/2 rapid blood test (Abbott Laboratories, Abbott Park, IL) | Trained personnel confirmed results of ppt self-tests, and performed a second test | 1) 88% of ppts thought test was easy to use; 2) 91% thought instructions were easy to understand; 3) 61% of HIV+ ppts, and 92% of at-risk ppts did not perform all test steps correctly (based on trained personnel observations); 4) 56% of ppts had invalid test results; 5) Inter-rater agreement between ppt tests and trained personnel tests was low (κ=0.28); 6) 12% of ppts could not correctly identify all possible test results (i.e., positive, negative, invalid); 7) 67% thought blood sample collection and transfer was the most difficult part of testing; 8) 89% of ppts preferred testing in private; 9) 87% thought pre-test counseling by trained professional is needed; 10) 79% thought post-test counseling was needed; 11) 94% and 89% of HIV+ and at-risk ppts respectively thought confirmatory testing was necessary. |
| Spielberg et al. (2003) [45] | 240 HIV-positive ppts USA | OraQuick oral fluid and blood tests (OraSure Technologies Inc., Bethlehem, PA) | Staff performed test | 1) Ppts had less difficulty performing oral test vs. blood test (95% vs. 89% concordance with staff performance); 2) ppts had more difficulty interpreting oral vs. blood tests (95% vs. 97% concordance); 3) the number of invalid or negative test results obtained were reduced (4.3% to 4% oral; 14% to 9% blood) by making changes to instructions and labeling; 4) 61% would prefer to use home test if they were unaware of their HIV status. |
Note: CI=confidence interval, HP=healthcare professional, ppt=participant
Untrained users can generally perform home tests correctly and obtain accurate results; however, they often have difficulty performing blood-based tests
With few exceptions [27, 30, 44], most participants in the studies reviewed were able to properly perform home tests and obtain accurate results, yielding high correlations with laboratory and health professional-performed tests. While this is certainly expected for FDA-approved home tests, several foreign studies we reviewed used tests that are available abroad but not approved for OTC sale in the US.
Results of the studies that involved direct observation of participants showed that they were able to perform the tests correctly and easily [23, 24, 40]. Other studies included questionnaires on various aspects of performing the home tests and found that most participants indicated by their responses that they knew how to perform the tests [18, 19].
In general, blood tests were the most difficult to perform, and participants typically struggled with obtaining adequate blood samples or applied too little blood to the test strips [26, 27, 30, 31]. For instance, in a study of blood glucose testing, at baseline only 17% of participants were able to perform the test without errors based on assessments by health professionals using a standardized checklist [27]. The most common mistake was squeezing the finger while trying to extract the blood sample to be tested.
In the HIV studies that involved the use of oral fluid or blood tests, more participants obtained invalid results with blood tests compared to oral tests [45, 46]. In fact, the only HIV study in which most participants could not accurately perform the tests exclusively used a blood test [44]. In this study performed in Singapore, 61% of known HIV-positive participants and 92% of at-risk (i.e. HIV-negative) participants failed to perform all the steps in the test correctly. Furthermore, more than half of the participants (56%) had invalid test results, mostly due to errors with blood sampling and transfer [44].
End-users can usually interpret the results of home tests correctly although some kinds of tests prove tricky to interpret
Users of home tests were generally able to interpret the results of their tests correctly. Several studies compared participants’ test result interpretations to those of trained health professionals and found excellent agreement [18, 19, 23, 24]. When asked about their test results, participants often expressed confidence in their interpretations [24, 42, 43] or thought the results were easy to interpret [17, 22–24, 32]. In addition, Traschler et al., found that only 3.1% (n=5) of their study participants who used a malaria home test had difficulty interpreting test results [31]. However, in a different malaria home test study, 32% of participants could not properly perform the test – 87% due to problems interpreting the results, and 58% due to problems identifying the bands that indicated the test results [30]. Similarly, in a study of fecal occult blood tests, 12% of participants interpreted a positive control test as negative [37].
Participants also had trouble interpreting results for tests that involved complex calculations and conversions of obtained results. This was the case with a blood-based cholesterol test that required users to convert the reading obtained on a thermometer-like display using a results chart. About one-tenth (10.7%) of participants excluded themselves from the study because they were not confident in their performance of the test. Over half (51.9%) of these participants were unable to obtain any results at all [36]. Participants in one study noted that they preferred tests with large round test areas to those with thin lines, suggesting that bigger result windows may facilitate easier interpretation [21].
Users usually understand that the results of diagnostic home tests are preliminary and require follow-up tests to confirm diagnoses
Few of the reviewed studies directly assessed users’ understandings of the need for follow-up testing to confirm positive results from home tests. In an HIV study in which this was addressed, most (94% of HIV-positive and 89% of at-risk) participants thought confirmatory testing was necessary [44]. In another HIV study involving in-depth interviews, several participants stated that their next step after receiving a positive home test result would be “to seek confirmatory testing followed by treatment” [40]. However, neither of these studies provided information on whether or not participants who received positive test results did indeed seek confirmatory testing.
In a study that used home urine tests for population screening for albuminuria (indicative of chronic renal failure or its risk factors), 25% of participants who reported positive home test results had attended a clinic for further evaluation at the time of study follow-up, and an additional 31% were planning to seek medical attention [28].
Home tests can prove useful in supporting public health efforts through population-based or peer-driven screening initiatives
One of the studies reviewed involved the use of a home test for population-based screening for albuminuria [28]. The study yielded 152 cases of hypertension, 31 cases of diabetes, and 25 cases of kidney disease that were previously undiagnosed, thus suggesting the utility of home tests for public health screening efforts. An additional eight new cases of disease were detected among participants who had tested negative at home but chose to seek care. In addition, several other studies reported that participants stated they were very likely to use the home tests again in the future [23, 31, 32] – or in the case of HIV, to use such tests if available in the future since these studies were all conducted prior to the approval of the OraQuick™ In-Home test [40, 42, 43]. Participants also reported being very likely to recommend the tests to others [41–43].
Although users could perform home tests unassisted, they often expressed a preference for having clinically trained personnel present for the testing process
Participants sometimes indicated a desire for assistance or reassurance from clinically trained personnel while performing home tests. For instance, while 86% of participants in an ovulation study found the test easy to use, and 81% expressed confidence in their home test use, 33% indicated that they would have liked some assistance in performing the test [17]. In other instances, participants’ responses suggested increased confidence in test performance when being observed by a health professional. For example, while users of a trichomoniasis test did not specifically state a need for assistance with the tests, those who performed the tests in the clinic while being observed by study staff were more likely to think the test was easy to perform and interpret [23] and trusted their test results more [24] than those who performed them at home without medical personnel present.
While users of HIV “home tests” liked home testing, they often still felt a need for counseling
Most participants in the study by Lee and colleagues [44], which was conducted in Singapore, thought pre-test (87%) and post-test (79%) counseling were necessary for HIV testing. However, most of them (88%) also thought HIV home testing should be made available. Similarly, in a study conducted in Malawi, although more than half (58.2% of women and 64.8% of men) of the participants would prefer to use a home test for their next HIV test, most thought that HIV counseling was still necessary [41].
DISCUSSION
It is certainly not surprising that lay users generally can use and interpret home tests accurately given the rigorous levels of evaluation most tests must undergo prior to licensing by the FDA. However, not all the tests used in the studies we examined were subject to FDA regulatory requirements. For instance, several of the studies reviewed were performed outside the US using home tests that are only available in those countries. In addition, some home tests available in the US, such as pregnancy tests, predate FDA regulations for home testing devices and are therefore not subject to the same approval processes as newer home tests [33].
Unexpected, however, was the finding that users often had great difficulty performing blood-based home tests compared to tests that use other biological specimens. This finding is particularly surprising given the widespread use of blood-based tests for the management of potentially life-threatening conditions such as diabetes and coagulation disorders. The finding also has important implications for HIV home testing. While the only HIV home test currently approved by the FDA is an oral fluid test, other tests, including blood-based ones, are likely to come on the market in the future. In fact, manufacturers of blood-based test kits are either seeking or have stated intentions to seek FDA approval for their products [7, 51]. Therefore, further studies are required to identify the factors that make blood-based tests more difficult to implement, factors that could include psychological ones such as the aversion that many people experience at the sight of blood.
On the other hand, a recent meta-analysis of rapid HIV tests showed that oral fluid tests have a lower positive predictive value than blood-based tests despite the high sensitivity and specificity of the tests, thus yielding a greater number of false positive results particularly in low prevalence populations [52]. This is of great concern given the possible psychological effects of falsely thinking one is HIV positive. Also, high rates of false results have the potential to erode the general public’s confidence in the test. This might lead users to gravitate towards and public health officials to encourage the use of blood-based HIV home tests instead of oral-fluid tests if/when the former become available. According to the aforementioned meta-analysis, both blood-based and oral-fluid tests perform similarly in high-prevalence populations [52]. Therefore, HIV home tests – particularly oral-fluid tests – may be more appropriate for use by populations with high HIV prevalence. As such, public health initiatives should make HIV home tests more easily accessible within populations with high HIV-prevalence to maximize their impact. In addition, instructions accompanying any future blood-based HIV home tests should emphasize the proper steps involved in collecting and transferring the blood sample to the testing device. This can be combined with resources for additional education on how to properly use the test kits.
We found that users generally understood the need for confirmatory testing or medical follow-up after testing positive with a home test. However, rapid HIV antibody tests would yield false negative results in persons recently or acutely infected with HIV due to the window period of the tests – the period before the body generates antibodies. This limitation is currently emphasized in the packaging material of the OraQuick In-Home test kit. It is advisable that accompanying materials additionally list common symptoms of acute HIV infection and urge users to seek confirmatory testing even in the event of negative test results if they have reason to believe they might have been recently exposed to HIV and/or are experiencing any symptoms of acute HIV infection. This could lead to the detection of new cases of disease that would have otherwise gone undetected by the home test as was the case in the population-based screening for albuminuria study we reviewed [28] in which eight new cases of disease were detected among participants with negative home test results who sought follow-up testing.
Participants in about half of the HIV self-testing studies we reviewed reported a preference for or likelihood of using an HIV home test in the future [41–43, 45]. Furthermore, participants in two of the studies reported that they would recommend HIV home testing to others [41, 42]. These finding suggest that HIV home testing could prove useful as a population-based or peer-driven screening strategy; however, this should be limited to high-prevalence populations given the fact that HIV home tests (particularly oral tests) would yield high levels of false positive results in populations with low HIV-prevalence. Future studies on HIV home testing should explore the utility of home tests for this purpose.
Several important questions remain unanswered by our review. For instance, how will the lack of pre- and post-test counseling affect HIV home test users? While parallels can be drawn between HIV and some of the health conditions for which the reviewed home tests exist, HIV/AIDS has long occupied a category of its own [53]. For example, counseling is not required for any of the non-HIV conditions for which home testing studies were reviewed whereas even with the revised (and somewhat relaxed) HIV testing guidelines in the US [54], counseling is still recommended for HIV testing in non-healthcare settings and for high-risk persons [55]. Moreover, most participants in the two HIV self-testing studies we reviewed that addressed the issue of counseling expressed a need for counseling [41, 44]. While the manufacturer of the only currently available FDA-approved HIV home test has a 24-hour customer support center staffed by bilingual (English and Spanish) individuals trained specifically to address questions related to the use of the tests, they provide neither pre- nor post-test counseling. Instead, users of the test who call the center are given referrals to counseling services within their communities. Given that this process might prove burdensome to some, especially users faced with HIV-positive home test results, more streamlined and expeditious modes of linking users to local HIV counseling services are necessary and should be explored. For instance, public health agencies could work with home test manufacturers to develop protocols that directly link users to existing local HIV hotlines.
Prior to the approval of the home test kit, several studies had explored alternatives to the traditional voluntary counseling and testing model and found that users were receptive to modes of counseling other than in-person meetings [12, 56, 57]. These alternatives, such as the use of written counseling material, phone-based counseling and computer-assisted, personalized, risk assessment and risk-reduction counseling could easily be adapted for use with home tests. For instance, a computer-assisted counseling system could provide pre- and post-test HIV counseling and expedite the process of linking available counselors to those users who require more assistance. Further research is needed to determine how these counseling options can be incorporated into the HIV home testing experience.
Furthermore, unlike several of the conditions for which the reviewed home tests exist, surveillance and partner notification are crucial for preventing the spread of HIV. The fact that most participants in the HIV testing studies we reviewed expressed an understanding of the need for confirmatory testing after a positive home test is promising. The confirmatory testing visit could serve as a critical step in linking users to necessary medical services and providing the information needed for surveillance purposes. In fact, the confirmatory visit could also play a key role in determining the effect of home tests on HIV testing rates and diagnoses. Patient intake forms at local HIV testing sites should be updated to include questions on home test use to assess whether the HIV home test leads to increased testing and results in the request for further confirmatory testing. The visit also would allow health professionals to initiate partner notification efforts. On the other hand, user-initiated partner notification systems could also be used by home test users who receive a positive HIV home test result to send anonymous notifications via text message or email to anyone with whom they may have had sexual contact [58].
Although none of the reviewed studies addressed misuse of home tests, potential abuse is a valid concern with the HIV home tests as with pregnancy and genetic tests. Yet, these tests are widely available OTC and have strong anti-abuse laws guiding their use. Existing HIV confidentiality laws should be expanded to include legislation to protect against HIV home test abuse. The laws guiding the use of other home tests could provide a road map for achieving this.
For populations of low socioeconomic status, who have the highest HIV diagnoses and prevalence rates in the US [59, 60], the cost of HIV home tests may be an important barrier to their use. Several studies conducted prior to the FDA approval of the HIV home test showed that in some cases participants were not willing to pay more than $20 per kit [44, 45, 61]. The current price of the HIV home test – $40 per kit – therefore might render it less accessible to those most likely to benefit from it. Nonetheless, the sale price of HIV home tests is likely to decrease in the future through competition from other manufacturers and greater demand as has occurred with other home tests. For example, pregnancy tests originally cost $10 when first introduced in 1978 [62], but now sell for half that amount. Also, the benefits of HIV home testing could be maximized by establishing programs for providing the kits to persons desiring to use them at reduced or no cost, similar to strategies used to make antiretroviral medications available for the indigent.
Although it is still too early to determine the public health impact of the new home test, market analysis reports from the manufacturer of the only HIV home test currently approved for sale in the US are encouraging. Based on publicly available data from the manufacturer, sales of the home test kits have been concentrated in areas with high HIV prevalence, and the top-selling retailers have been those serving large numbers of men who have sex with men (MSM) and African Americans. In a recent report, the company indicated that about 50% of their sales occurred in the top 15 highest HIV prevalence markets in the US [63]. In addition, based on a public awareness study the company conducted, they reported high levels of interest in the home tests among several key populations including MSM, African Americans, Latinos and sexually active adults between the ages of 18 and 34 [63]. While these data are promising, it is important that test manufacturers and public health officials actively collect data on home testing use and utilize these data in a concerted manner to determine the public health impact of the tests.
A limitation of this study is that only published articles were reviewed. This could have introduced publication bias into our results, as studies with negative results are less likely to be published. Nonetheless, the studies we reviewed were not limited to positive findings. Furthermore, not all tests used in the reviewed studies were subject to the same regulatory requirements prior to becoming available, and most of the home tests used in the reviewed studies were for conditions more benign than HIV, thus limiting the extent to which direct comparisons to the HIV home test could be made.
CONCLUSIONS
It is encouraging that most studies of self-administered tests show that participants generally can properly perform home tests, obtain accurate results, and correctly interpret the results. Materials accompanying HIV home test kits should emphasize the signs of acute HIV infection along with the importance of confirmatory testing following negative results in cases where recent infection is suspected. It is imperative that future blood-based HIV test kit manufacturers address the difficulties users have in obtaining and applying adequate blood samples to the test kits. Research is needed to explore ways by which HIV home test users can gain more direct access to counseling services. The possibility of using HIV home tests for population-based and peer-driven screening initiatives, particularly in populations at high-risk for HIV acquisition should also be explored. In addition, public health agencies should update their community surveys and other surveillance instruments to include measures to capture home test use and users’ perceptions of the test. It is imperative that public health agencies explore ways in which the data they collect can be used in concert with data collected by test manufacturers to determine the impact (both positive and negative) of HIV home test use on consumers.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R01 MH79692) to Alex Carballo-Diéguez, Ph.D., Principal Investigator. Additional support came from NIMH to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator: Anke A. Ehrhardt, Ph.D.). The authors thank William Brown, III, Farnaz Kaighobadi, Susie Hoffman, Tsitsi Masvawure, Raymond Smith, and Juan Valladares for their helpful comments in the early stages of development of this manuscript.
References
- 1.Home use tests: Human immunodeficiency virus (HIV) US Food and Drug Administration; [Accessed December 1, 2011]. Web site. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/HomeUseTests/ucm125797.htm. Updated March 17, 2010. [Google Scholar]
- 2.Campbell S, Klein R. Home testing to detect human immunodeficiency virus: Boon or bane? J Clin Microbiol. 2006;44(10):3473–6. doi: 10.1128/JCM.01511-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frith L. HIV self-testing: A time to revise current policy. Lancet. 2007;369(9557):243–5. doi: 10.1016/S0140-6736(07)60113-5. [DOI] [PubMed] [Google Scholar]
- 4.Pai NP, Klein MB. Are we ready for home-based, self-testing for HIV? Future HIV Therapy. 2008;2(6):515–20. [Google Scholar]
- 5.Paltiel AD, Pollack HA. Price, performance, and the FDA approval process: The example of home HIV testing. Med Decis Making. 2010;30(2):217–23. doi: 10.1177/0272989X09334420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walensky RP, Paltiel AD. Rapid HIV testing at home: Does it solve a problem or create one? Ann Intern Med. 2006;145(6):459–62. doi: 10.7326/0003-4819-145-6-200609190-00010. [DOI] [PubMed] [Google Scholar]
- 7.Whellams M. The approval of over-the-counter HIV tests: Playing fair when making the rules. J Bus Ethics. 2008;77(1):5–15. [Google Scholar]
- 8.Wright AA, Katz IT. Home testing for HIV. N Engl J Med. 2006;354(5):437–40. doi: 10.1056/NEJMp058302. [DOI] [PubMed] [Google Scholar]
- 9.FDA approves first over-the-counter home-use rapid HIV test. US Food and Drug Administration; [Accessed November 19, 2012]. [Press Release]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310542.htm. Updated July 13, 2012. [Google Scholar]
- 10.Koval CE. Home testing for HIV: Hopefully, a step forward. Cleve Clin J Med. 2012;79(10):713–6. doi: 10.3949/ccjm.79a.12128. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2010. HIV Surveillance Supplemental Report. 2012;17(3 part A) [Google Scholar]
- 12.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20(12):1597–604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- 13.Roberts KJ, Grusky O, Swanson A-N. Outcomes of blood and oral fluid rapid HIV testing: A literature review, 2000–2006. AIDS Patient Care STDs. 2007;21(9):621–37. doi: 10.1089/apc.2006.0196. [DOI] [PubMed] [Google Scholar]
- 14.San Antonio-Gaddy M, Richardson-Moore A, Burstein GR, Newman DR, Branson BM, Birkhead GS. Rapid HIV antibody testing in the New York State anonymous HIV counseling and testing program: Experience from the field. J Acquir Immune Defic Syndr. 2006;43(4):446–50. doi: 10.1097/01.qai.0000243055.65698.51. [DOI] [PubMed] [Google Scholar]
- 15.Home use tests. US Food and Drug Administration; [Accessed December 1, 2011]. Web site. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/HomeUseTests/default.htm. Updated March 17, 2010. [Google Scholar]
- 16.Drugs of abuse home use test. US Food and Drug Administration; [Accessed December 1, 2011]. Web site. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/DrugsofAbuseTests/ucm125722.htm. Updated June 1, 2011. [Google Scholar]
- 17.Anderson RA, Eccles SM, Irvine DS. Home ovulation testing in a donor insemination service. Hum Reprod. 1996;11(8):1674–7. doi: 10.1093/oxfordjournals.humrep.a019468. [DOI] [PubMed] [Google Scholar]
- 18.Coppola MA, Klotz KL, Kim KA, et al. Spermcheck fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25(4):853–61. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klotz KL, Coppola MA, Labrecque M, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180(6):2569–76. doi: 10.1016/j.juro.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson JN, Lockwood GM, Dalton JDE, Franklin PA, Farr MMC, Barlow DH. A randomized prospective study to assess the effect of the use of home urinary luteinizing hormone detection on the efficiency of donor insemination. Hum Reprod. 1992;7(1):63–5. doi: 10.1093/oxfordjournals.humrep.a137560. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen MS, Barton SD, Hatasaka HH, Stanford JB. Comparison of several onestep home urinary luteinizing hormone detection test kits to OvuQuick. Fertil Steril. 2001;76(2):384–7. doi: 10.1016/s0015-0282(01)01881-7. [DOI] [PubMed] [Google Scholar]
- 22.Geva A, Bornstein J, Dan M, Shoham HK, Sobel JD. The VI-SENSE-vaginal discharge self-test to facilitate management of vaginal symptoms. Am J Obstet Gynecol. 2006;195(5):1351–6. doi: 10.1016/j.ajog.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Jones HE, Altini L, de Kock A, Young T, van de Wijgert JH. Home-based versus clinic-based self-sampling and testing for sexually transmitted infections in Gugulethu, South Africa: Randomised controlled trial. Sex Transm Infect. 2007;83(7):552–7. doi: 10.1136/sti.2007.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippman SA, Jones HE, Luppi CG, Pinho AA, Veras MA, van de Wijgert JH. Home-based self-sampling and self-testing for sexually transmitted infections: Acceptable and feasible alternatives to provider-based screening in low-income women in Sao Paulo, Brazil. Sex Transm Dis. 2007;34(7):421–8. doi: 10.1097/01.olq.0000245958.34961.27. [DOI] [PubMed] [Google Scholar]
- 25.Roy S, Caillouette JC, Faden JS, Roy T, Ramos DE. Improving appropriate use of antifungal medications: The role of an over-the-counter vaginal pH self-test device. Infect Dis Obstet Gynecol. 2003;11(4):209–16. doi: 10.1080/10647440300025523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristensen GBB, Nerhus K, Thue G, Sandberg S. Standardized evaluation of instruments for self-monitoring of blood glucose by patients and a technologist. Clin Chem. 2004;50(6):1068–71. doi: 10.1373/clinchem.2004.031575. [DOI] [PubMed] [Google Scholar]
- 27.Müller U, Hämmerlein A, Casper A, Schulz M. Community pharmacy-based intervention to improve self-monitoring of blood glucose in type 2 diabetic patients. Pharmacy Practice. 2006;4(4):195–203. doi: 10.4321/s1885-642x2006000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielen MM, Schellevis FG, Verheij RA. The usefulness of a free self-test for screening albuminuria in the general population: A cross-sectional survey. BMC Public Health. 2009;9:381. doi: 10.1186/1471-2458-9-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piehlmeier W, Renner R, Kimmerling T, et al. Evaluation of the Micral-Test S, a qualitative immunologic patient self-test for microalbuminuria: The PROSIT project. Proteinuria Screening and Intervention. Diabet Med. 1998;15(10):883–5. doi: 10.1002/(SICI)1096-9136(199810)15:10<883::AID-DIA684>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Jelinek T, Amsler L, Grobusch MP, Nothdurft HD. Self-use of rapid tests for malaria diagnosis by tourists. Lancet. 1999;354(9190):1609. doi: 10.1016/s0140-6736(99)01969-8. [DOI] [PubMed] [Google Scholar]
- 31.Trachsler M, Schlagenhauf P, Steffen R. Feasibility of a rapid dipstick antigen-capture assay for self-testing of travellers’ malaria. Trop Med Int Health. 1999;4(6):442–7. doi: 10.1046/j.1365-3156.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitty CJM, Armstrong M, Behrens RH. Self-testing for falciparum malaria with antigen-capture cards by travelers with symptoms of malaria. Am J Trop Med Hyg. 2000;63(5–6):295–7. [PubMed] [Google Scholar]
- 33.Doshi ML. Accuracy of consumer performed in-home tests for early pregnancy detection. Am J Public Health. 1986;76(5):512–4. doi: 10.2105/ajph.76.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grob PR, Manners BTB, Beynon GPJ, Gibbs FJ. Do-it-yourself pregnancy test. BMJ. 1973;1(5845):112–3. [Google Scholar]
- 35.Lindstedt G, Himmelmann CE, Salsmans R, Valentin K. Home testing for pregnancy--can it be recommended? Scand J Clin Lab Invest. 1982;42(4):371–6. [PubMed] [Google Scholar]
- 36.McNamara JR, Warnick GR, Leary ET, et al. Multicenter evaluation of a patient-administered test for blood cholesterol measurement. Prev Med. 1996;25(5):583–92. doi: 10.1006/pmed.1996.0093. [DOI] [PubMed] [Google Scholar]
- 37.Tate JJ, Northway J, Royle GT, Taylor I. Evaluation of a ‘DIY’ test for the detection of colorectal cancer. J R Soc Med. 1989 Jul;82(7):388–90. doi: 10.1177/014107688908200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messing EM, Young TB, Hunt VB, Wehbie JM, Rust P. Urinary tract cancers found by homescreening with hematuria dipsticks in healthy men over 50 years of age. Cancer. 1989;64(11):2361–7. doi: 10.1002/1097-0142(19891201)64:11<2361::aid-cncr2820641128>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Ryan F, O’Shea S, Byrne S. The reliability of point-of-care prothrombin time testing. A comparison of CoaguChek S and XS INR measurements with hospital laboratory monitoring. Int J Lab Hematol. 2008;32(1 Pt 1):e26–33. doi: 10.1111/j.1751-553X.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 40.Carballo-Diéguez A, Frasca T, Dolezal C, Balan I. Will gay and bisexually active men at high risk of infection use over-the-counter rapid HIV tests to screen sexual partners? J Sex Res. 2012;49(4):379–87. doi: 10.1080/00224499.2011.647117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choko AT, Desmond N, Webb EL, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: A cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8(10):e1001102. doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaydos CA, Hsieh Y-H, Harvey L, et al. Will patients “opt in” to perform their own rapid HIV test in the emergency department? Ann Emerg Med. 2011;58(1 Suppl 1):S74–S8. doi: 10.1016/j.annemergmed.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaydos CA, Hsieh YH, Bura A, et al. Can we ever expect to have individuals perform their own HIV rapid tests?. 47th Annual Meeting of the Infectious Diseases Society of America; Philadelphia. 2009. [Google Scholar]
- 44.Lee VJ, Tan SC, Earnest A, Seong PS, Tan HH, Leo YS. User acceptability and feasibility of self-testing with HIV rapid tests. J Acquir Immune Defic Syndr. 2007;45(4):449–53. doi: 10.1097/QAI.0b013e318095a3f3. [DOI] [PubMed] [Google Scholar]
- 45.Spielberg F, Camp S, Ramachandra E. HIV home self-testing: Can it work?. 2003 National HIV Prevention Conference; Atlanta. 2003. [Google Scholar]
- 46.Granade TC, Parekh BS, Phillips SK, McDougal JS. Performance of the OraQuick and Hema-Strip rapid HIV antibody detection assays by non-laboratorians. J Clin Virol. 2004;30(3):229–32. doi: 10.1016/j.jcv.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.The rapid HIV-1/2 antibody test: step-by-step instructions. OraSure Technologies, Inc; [Accessed February 18, 2013]. Available at: http://www.orasure.com/docs/pdfs/products/oraquick_advance/OraQuick-Advance-Procedure-English.pdf. [Google Scholar]
- 48.Hema-Strip HIV rapid test. World Health Organization; [Accessed February 18, 2013]. Web site. Available at: http://www.who.int/diagnostics_laboratory/documents/guidance/hemastrip.pdf. [Google Scholar]
- 49.Determine HIV rapid test. World Health Organization; [Accessed February 18, 2013]. Web site. Available at: http://www.who.int/diagnostics_laboratory/documents/guidance/determine.pdf. [Google Scholar]
- 50.Uni-Gold HIV rapid test. World Health Organization; [Accessed February 18, 2013]. Web site. Available at: http://www.who.int/diagnostics_laboratory/documents/guidance/uni_gold.pdf. [Google Scholar]
- 51.Willyard C. Recommendation of HIV test brings diagnostic dilemma home. Nat Med. 2012;18(6):841. doi: 10.1038/nm0612-841. [DOI] [PubMed] [Google Scholar]
- 52.Pai NP, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12(5):373–80. doi: 10.1016/S1473-3099(11)70368-1. [DOI] [PubMed] [Google Scholar]
- 53.Smith JH, Whiteside A. The history of AIDS exceptionalism. J Int AIDS Soc. 2010;13:47. doi: 10.1186/1758-2652-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 55.HIV counseling with rapid tests. Centers for Disease Control and Prevention; [Accesed May 29, 2012]. Web site. Available at: http://www.cdc.gov/hiv/topics/testing/resources/factsheets/rt_counseling.htm. Updated March 28, 2007. [Google Scholar]
- 56.Mackenzie SL, Kurth AE, Spielberg F, et al. Patient and staff perspectives on the use of a computer counseling tool for HIV and sexually transmitted infection risk reduction. J Adolesc Health. 2007;40(6):572.e9–16. doi: 10.1016/j.jadohealth.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Spielberg F, Kurth A, Gorbach PM, Goldbaum G. Moving from apprehension to action: HIV counseling and testing preferences in three at-risk populations. AIDS Educ Prev. 2001;13(6):524–40. doi: 10.1521/aeap.13.6.524.21436. [DOI] [PubMed] [Google Scholar]
- 58.inSPOT: Tell Them. Internet Sexuality Information Services (I.S.I.S.), Inc; 2012. [Accessed May 4, 2012]. Available at: http://www.inspot.org/TellThem/tabid/58/language/en-US/Default.aspx. [Google Scholar]
- 59.An Q, Prejean J, McDavid Harrison K, Fang X. Association between community socioeconomic position and HIV diagnosis rate among adults and adolescents in the United States, 2005 to 2009. Am J Public Health. 2013;103(1):120–6. doi: 10.2105/AJPH.2012.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denning P, DiNenno E. [Accessed 15 July 2013];Communities in crisis: Is there a generalized HIV epidemic in impoverished urban areas in the United States? Updated April 23, 2013. Available at: http://www.cdc.gov/hiv/risk/other/poverty.html.
- 61.Katz D, Golden M, Hughes J, Farquhar C, Stekler J. Acceptability and ease of use of home self-testing for HIV among men who have sex with men. 19th Conference on Retroviruses and Opportunistic Infections; Seattle. 2012. [Google Scholar]
- 62.The Office of NIH History. [Accessed 22 July 2013];A thin blue line: The history of the pregnancy test kit. Available at: http://history.nih.gov/exhibits/thinblueline/index.html.
- 63.Thomson Reuters StreetEvents. Edited Transcript OSUR – Q2 2013 OraSure Technologies, Inc. Earnings Conference Call; August 7, 2013; [Accessed 16 September, 2013]. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=99740&p=irol-presentations. [Google Scholar]