Abstract
Background
Well-designed trials are of paramount importance in improving the delivery of care to patients with kidney disease. However, it remains unknown whether contemporary clinical trials within nephrology are of sufficient quality and quantity to meet this need.
Study Design
Systematic review.
Setting & Population
Studies registered with ClinicalTrials.gov.
Selection Criteria for Studies
Interventional (i.e., non-observational) studies (both randomized and nonrandomized) registered between October 2007 and September 2010 were included for analysis. Studies were independently reviewed by physicians and classified by clinical specialty.
Predictor
Nephrology versus cardiology versus other trials.
Outcomes
Select clinical trial characteristics.
Results
Of the 40,970 trials overall, 1054 (2.6%) were classified as nephrology. The majority of nephrology trials were for treatment (75.4%) or prevention (15.7%), with very few diagnostic, screening, or health services research studies. Most nephrology trials were randomized (72.3%), including 24.9% that included a single study group, 64.0% that included parallel groups, and 9.4% that were crossover trials. Nephrology trials, compared with 2264 cardiology trials (5.5% overall), were more likely to be smaller (64.5% versus 48.0% enrolling ≤100 patients), phase I-II (29.0% versus 19.7%), and unblinded (66.2% versus 53.3%; P<0.05 for all). Nephrology trials were also more likely than cardiology trials to include a drug intervention (72.4% versus 41.9%) and were less likely to report having a data monitoring committee (40.3% versus 48.5%; P<0.05 for all). Finally, there were few trials funded by the National Institutes of Health (3.3%, nephrology; 4.2%, cardiology).
Limitations
Does not include all trials performed worldwide, and frequent categorization of funding source as university may underestimate NIH support.
Conclusions
Critical differences remain between clinical trials in nephrology and other specialties. Improving care for patients with kidney disease will require a concerted effort to increase the scope, quality, and quantity of clinical trials within nephrology.
Chronic kidney disease (CKD) affects nearly 26 million US adults1 and about 10%-18% of adults worldwide.2,3 Although CKD is common, 3 observations highlight an important problem in its management. First, CKD is associated with poor outcomes—patients with CKD suffer from increased morbidity and mortality.4 Second, CKD is costly—those with CKD represent only 8% of the US Medicare population, yet their care accounts for around 22% of total Medicare expenditures.5 Finally, although substantial resources are expended for this common, high-risk condition, therapies for CKD are not well studied. Research has suggested that the quality and quantity of randomized controlled trials (RCTs) within nephrology are suboptimal and that the reporting of results from these RCTs is of low quality.6-11 There is a clear need to develop additional therapies through high-quality trials to treat this common and costly condition. Consequently, many have issued a call to improve the quality and quantity of trials in nephrology.6-8 However, little is known about the characteristics of contemporary clinical trials being performed in nephrology and whether these studies can fill existing gaps in our understanding of effective therapies for kidney disease.
To facilitate greater access to clinical trial information, the Food and Drug Administration Modernization Act of 1997 mandated that the National Institutes of Health (NIH) set up and run a public information portal on efficacy studies of drugs, including biological drug products, to treat serious or life-threatening diseases and conditions when the studies are conducted under the US Food and Drug Administration's (FDA's) investigational new drug regulations.12 The NIH developed the Clinical Trials Data Bank, also known as ClinicalTrials.gov, a web-based registry of clinical trials. In 2007, Congress expanded ClinicalTrials.gov by requiring additional trial information.13 Because it requires registration of all phase II–IV interventional trials of drugs, biologics, or devices under FDA jurisdiction that have at least one study site within the United States, ClinicalTrials.gov is considered to be the most utilized source for clinical trial information worldwide.13,14
Given the paucity of data regarding ongoing clinical trials in nephrology and whether contemporary studies are of sufficient quality to advance medical knowledge in nephrology, we used ClinicalTrials.gov to describe the characteristics of nephrology trials during the 3 years following mandatory registration. We evaluated whether clinical trials in nephrology are fewer in number but with study designs similar to cardiology trials, which are known to have greater clinical trial activity, and also compared these trials to trials in other specialties.
METHODS
Creation of the ClinicalTrials.gov Dataset
On September 27, 2010 (3 years after enactment of the FDA Amendments Act of 200713), a dataset of 96,346 studies registered in ClinicalTrials.gov was downloaded. Following XML download of the data from ClinicalTrials.gov, a relational database (Oracle RDBMS version 11.1g, Oracle Corporation, Redwood Shores, CA) was created to facilitate aggregate analysis of data.15 Details of this resource (the Database for Aggregate Analysis of ClinicalTrials.gov [AACT]), data definitions, and data dictionaries are available at the Clinical Trials Transformation Initiative website.16
Creation of the Nephrology Study Dataset
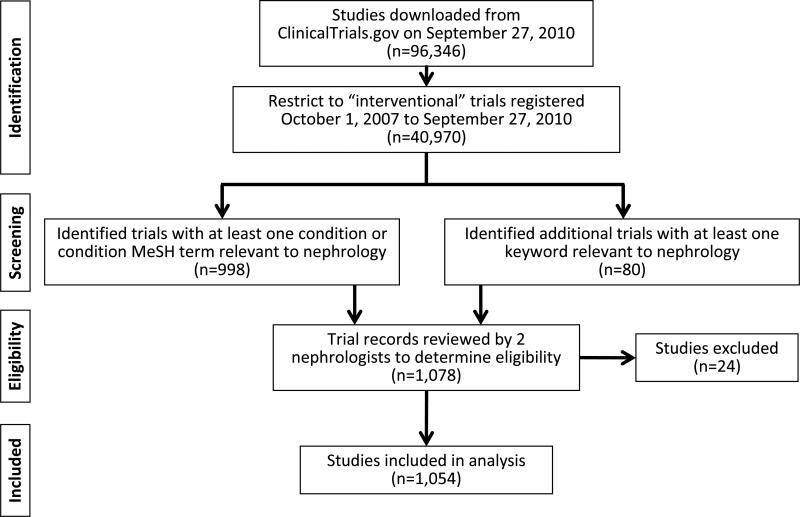
The nephrology dataset was restricted to interventional study types registered in the larger dataset between October 1, 2007 and September 27, 2010 (n=40,970 of 94,346; Figure 1). Non-interventional studies (such as observational or cohort studies) were eliminated to minimize bias related to the lack of reporting requirements for these studies. The dataset was created by using disease-specific condition terms (both Medical Subject Headings [MeSH] and non-MeSH) that were provided by the data submitters, and additional MeSH terms that were generated by a National Library of Medicine algorithm. Investigators at Duke reviewed 18,491 records from the MeSH dictionary, and terms were annotated according to their relevance to nephrology. MeSH terms with conflicting tags (i.e., multiple records in the dictionary) were adjudicated by the investigators. Of the 9031 unique MeSH terms individually adjudicated, 83 were tagged to be nephrology-specific (Item S1, available as online supplementary material). Also, investigators reviewed 1220 unique, common, free text terms and identified 31 nephrology-specific terms.
Figure 1.
Flow Diagram of nephrology-classified trials registered between October 1, 2007 and September 27, 2010. Abbreviation: MeSH, medical subject heading;
An initial dataset of 998 studies were identified as having conditions relevant to nephrology. Another 80 studies (identified by additional keywords and free text) were added. In total, 1078 studies were individually reviewed by two nephrologists (brief title, keywords, conditions, MeSH terms, and, if necessary, the full ClinicalTrials.gov record) to determine relevance to nephrology. In total, 24 studies were excluded as irrelevant to nephrology, leaving a final dataset of 1054 studies. Similar methods were used to identify 2325 cardiology studies; 61 studies that were also in the nephrology group were excluded, resulting in a subset of 2264 cardiology studies used for comparison in this analysis. The remaining group of “other” studies (n=37,652) includes those not classified as nephrology or cardiology.
Analysis and Definitions
Descriptive statistics (frequency and percentage for categorical variables, as well as mean and standard deviation, and median and interquartile range) were used to describe nephrology, cardiology, and other studies. Chi-squared tests were used to compare class variables, and the Wilcoxon rank sum test was used to compare continuous variables. A P-value <0.05 was deemed to be statistically significant, and no adjustment was made for multiple comparisons. Missing values were excluded from descriptive summaries unless the data submitter supplied a value such as “N/A” or “None.” The percentage of missing data was compared across groups and reported when differences were significant.
Variables collected in the ClinicalTrials.gov registration database are described in the Data Element Definitions document provided by the National Library of Medicine.16a For studies reporting an interventional model of “single group” and the number of arms as “one,” the value of allocation (if missing) was assigned as “non-randomized” and the value of blinding (if missing) was assigned as “open.” The reported enrollment for each study could be flagged by the data submitter as actual (for studies that had completed enrollment and updated the enrollment value before September 27, 2010) or anticipated. Summaries of enrollment include studies with anticipated or actual enrollment values. Study duration was derived from the study start date and the anticipated or actual primary completion date (the date of completion of follow-up for the primary endpoint). Study location was determined from the country provided in the address of the reported study facilities. Studies that did not report facilities were excluded from summaries of study locations. Source of funding was derived from the reported lead sponsor and collaborator information as follows: if the lead sponsor was from industry or the study had at least one collaborator from industry and no NIH involvement, then the funding source was determined to be from industry. Otherwise, if the lead sponsor was from the NIH or at least one collaborator was from the NIH and the lead sponsor was not from industry, then the funding source was determined to be from the NIH. Age inclusions and exclusions were based on age limits for eligibility. Statistical analyses were performed using SAS version 9 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Trial Design Characteristics
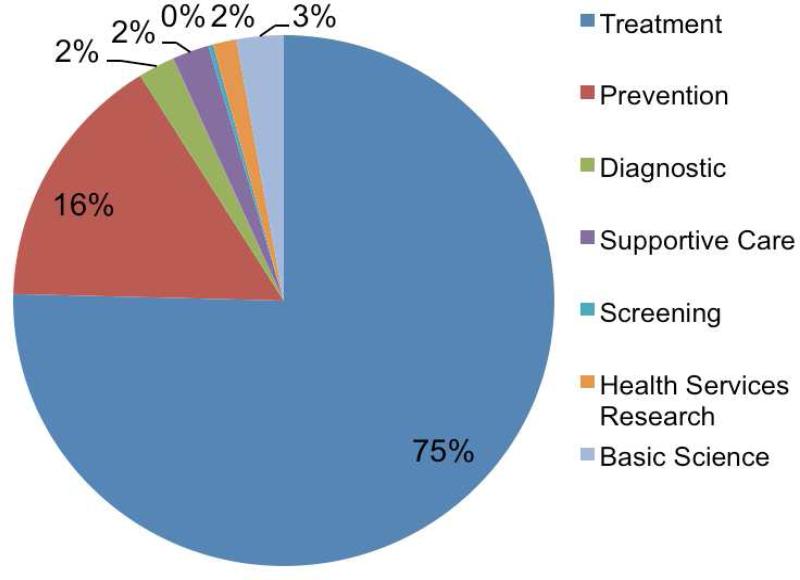
Of 40,970 overall studies selected for specialty classification from interventional studies, 1054 (2.6%) were classified as nephrology, 2264 (5.5%) were classified as cardiology, and 37,652 (91.9%) were classified as other (neither nephrology nor cardiology) (Table 1). The primary purpose of nephrology trials, compared with that of cardiology trials and of trials in other specialties, was more likely to be treatment (75.4% vs 71.4% vs 75.1%, respectively) or prevention (15.7% vs 11.5% vs 10.7%, respectively) and were significantly less likely to be diagnostic or related to supportive care, screening, or health services (Table 1, Figure 2). Nephrology trials and trials overall were also more likely to be phase I-II (29.3% and 42.6%, respectively) compared with cardiology trials (19.7%). Also, nephrology studies, compared with cardiology studies, were equally likely to be randomized (72.3% versus 74.1%, respectively) but more likely to have a crossover study design (9.4% versus 5.4%, respectively). The nephrology subset had a smaller number of single-group design studies (24.9%). Compared with cardiology (53.3%) and with other trials (55.6%), however, nephrology trials were also more likely to be open label without blinding (66.2%).
Table 1.
Attributes of interventional clinical trials from ClinicalTrials.gov: October 1, 2007-September 27, 2010
| Subset Comparison | ||||
|---|---|---|---|---|
| Trial Attribute | Nephrology (n=1054) | Cardiologya (n=2264) | P-value | Otherb (n=37,652) |
| Primary purpose | n=1004 | n=2186 | <0.001 | n=35,009 |
| Treatment | 757 (75.4) | 1561 (71.4) | 26,287 (75.1) | |
| Prevention | 158 (15.7) | 251 (11.5) | 3743 (10.7) | |
| Diagnostic | 22 (2.2) | 189 (8.6) | 1278 (3.7) | |
| Supportive care | 22 (2.2) | 60 (2.7) | 1208 (3.5) | |
| Screening | 3 (0.3) | 10 (0.5) | 182 (0.5) | |
| Health services research | 14 (1.4) | 56 (2.6) | 663 (1.9) | |
| Basic science | 28 (2.8) | 59 (2.7) | 1648 (4.7) | |
| Phase | n=1054 | n=2264 | <0.001 | n=37652 |
| 0 | 3 (0.3) | 17 (0.8) | 296 (0.8) | |
| I | 93 (8.8) | 93 (4.1) | 6036 (16.0) | |
| I/II | 39 (3.7) | 65 (2.9) | 2001 (5.3) | |
| II | 174 (16.5) | 288 (12.7) | 8022 (21.3) | |
| II/III | 44 (4.2) | 75 (3.3) | 936 (2.5) | |
| III | 177 (16.8) | 357 (15.8) | 5663 (15.0) | |
| IV | 254 (24.1) | 580 (25.6) | 4725 (12.5) | |
| Not available | 270 (25.6) | 789 (34.8) | 9973 (26.5) | |
| Allocation | n=1035 | n=2228 | 0.3 | n=35,977 |
| Randomized | 748 (72.3) | 1652 (74.1) | 24,627 (68.5) | |
| Non-randomized | 287 (27.7) | 576 (25.9) | 11,350 (31.5) | |
| Intervention model | n=1046 | n=2241 | <0.001 | n=35,682 |
| Single group | 260 (24.9) | 586 (26.1) | 11,298 (31.7) | |
| Parallel | 669 (64.0) | 1495 (66.7) | 19,618 (55.0) | |
| Crossover | 98 (9.4) | 120 (5.4) | 4133 (11.6) | |
| Factorial | 19 (1.8) | 40 (1.8) | 633 (1.8) | |
| Masking | n=1048 | n=2238 | <0.001 | n=36,585 |
| Open (unblinded) | 694 (66.2) | 1192 (53.3) | 20,348 (55.6) | |
| Single blind | 80 (7.6) | 337 (15.1) | 4040 (11.0) | |
| Double blind | 274 (26.1) | 709 (31.7) | 12,197 (33.3) | |
Note: Unless otherwise indicated, values are given as number (percentage). Differences in missing data between trials were not significant unless specified in the table. Chi-squared test used for class variables, Wilcoxon rank sum test used for continuous variables.
Excludes studies classified as both nephrology and cardiology.
Excludes studies classified as nephrology or cardiology.
Figure 2.
Primary purpose of clinical trials in nephrology based on studies registered in ClinicalTrials.gov between October 1, 2007, and September 27, 2010.
Enrollments and Endpoint Classification
Median target or actual enrollment was significantly lower among nephrology trials than cardiology trials (68 versus 112 patients, respectively; P<0.001) (Table 2). Nephrology trials tended to be smaller than cardiology trials, with 64.5% versus 48%, respectively, of studies anticipating or actually enrolling ≤100 patients (Figure S1). Less than 2% of nephrology, nearly 9% of cardiology, and 3.4% of other studies anticipated or actually enrolled >1000 patients. On average, nephrology studies reported slightly shorter actual or anticipated durations than cardiology studies (median of 21 months versus 24 months, respectively), but had durations similar to those in other studies (median of 20 months).
Table 2.
Enrollment and endpoint classification
| Subset Comparison | ||||
|---|---|---|---|---|
| Trial Attribute | Nephrology (n=1054) | Cardiologya (n=2264) | P-value | Otherb (n=37,652) |
| Enrollment | n=1041 | n=2240 | <0.001 | n=37,085 |
| Mean | 178 ±621 | 557±2143 | 451 ± 14,582 | |
| Median | 68 [30–156] | 112 [50–300] | 61 [30–172] | |
| Patient enrollment | n=1041 | n=2240 | <0.001 | n=37,085 |
| 0 | 4 (0.4) | 0 (0.0) | 82 (0.2) | |
| 1–10 | 58 (5.6) | 62 (2.8) | 1555 (4.2) | |
| 11–50 | 376 (36.1) | 564 (25.2) | 14,329 (38.6) | |
| 51–100 | 233 (22.4) | 449 (20.0) | 7760 (20.9) | |
| 101–500 | 310 (29.8) | 818 (36.5) | 10,310 (27.8) | |
| 501–1000 | 42 (4.0) | 150 (6.7) | 1796 (4.8) | |
| >1000 | 18 (1.7) | 197 (8.8) | 1253 (3.4) | |
| Study duration | n=970 | n=2129 | 0.002 | n=34,803 |
| Mean (mo) | 26 ± 20 | 28 ± 23 | 25 ± 24 | |
| Median (mo) | 21 [12–34] | 24 [13–36] | 20 [11–34] | |
| Endpoint classification | n=906 | n=1869 | <0.001 | n=30,060 |
| Safety | 54 (6.0) | 139 (7.4) | 2721 (9.1) | |
| Efficacy | 302 (33.3) | 747 (40.0) | 10,164 (33.8) | |
| Safety/efficacy | 450 (49.7) | 918 (49.1) | 13,871 (46.1) | |
| Bio-equivalence | 9 (1.0) | 3 (0.2) | 935 (3.1) | |
| Bio-availability | 3 (0.3) | 0 (0.0) | 242 (0.8) | |
| Pharmacokinetics | 58 (6.4) | 15 (0.8) | 1112 (3.7) | |
| Pharmacodynamics | 12 (1.3) | 28 (1.5) | 425 (1.4) | |
| Pharmacokinetics/dynamics | 18 (2.0) | 19 (1.0) | 590 (2.0) | |
| Missing | 148 (14.0) | 395 (17.4) | 0.01 | 7592 (20.2) |
| No. of primary outcomes measured | n=1039 | n=2230 | 0.3 | n=36,982 |
| Mean | 1.2 ± 0.75 | 1.2 ± 0.87 | 1.3 ± 1.06 | |
| Median | 1 [1.0–1.0] | 1 [1.0–1.0] | 1 [1.0–1.0] | |
| A primary outcome measures safety | 338/996 (33.9) | 785/2153 (36.5) | 0.2 | 10,106/35,421 (28.5) |
| No. of secondary outcomes measured | n=1040 | n=2232 | <0.001 | n=36989 |
| Mean | 2.2 ± 2.88 | 2.9 ± 5.33 | 2.4 ± 3.29 | |
| Median | 1 [1.0–3.0] | 1 [1.0–4.0] | 1 [1.0–3.0] | |
| A secondary outcome measures safety | 363/815 (44.5) | 836/1765 (47.4) | 0.2 | 113,07/35,646 (31.7) |
Note: Values for categorical variables are given as number (percentage) or n/N (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Differences in missing data between trials were not significant unless specified in the table. Chi-squared test used for class variables, Wilcoxon rank sum test used for continuous variables.
Abbreviations: IQR, interquartile range; SD, standard deviation.
Excludes studies classified as both nephrology and cardiology.
Excludes studies classified as nephrology or cardiology.
Most of the trials in nephrology had an endpoint classification of safety or efficacy, or both (89%). A moderate percentage of trials also had endpoints classified as pharmacokinetics, pharmacodynamics, or both (9.7% nephrology, 3.3% cardiology, and 7.1% other). A median of one primary and secondary outcome was measured for nephrology, cardiology, and other trials. Of the nephrology studies, 33.9% reported at least one primary outcome measure and 44.5% reported at least one secondary outcome measure. Bio-equivalence or bio-availability studies constituted 1.3% of studies in nephrology, 0.2% in cardiology, and 3.9% in other studies.
Study Arms, Interventions, Patient Eligibility, and Monitoring
Most nephrology trials included ≥2 treatment arms (Table 3). Trials in nephrology and cardiology were equally likely to have an active comparator arm (50.8% versus 49.6%, respectively) and include a placebo comparator arm (23.6% for both). Nephrology studies, compared with cardiology, were more likely to include some form of experimental arm (71% versus 66.3%, respectively) but less likely to report a no-intervention arm (9.7% versus 12.7%, respectively).
Table 3.
Study arms, interventions, patient eligibility, and monitoring
| Subset Comparison | ||||
|---|---|---|---|---|
| Trial Attribute | Nephrology (n=1054) | Cardiologya (n=2264) | P-value | Otherb (n=37,652) |
| No. of arms | n=1021 | n=2217 | <0.001 | n=36,005 |
| 0 | 0 (0.0) | 0 (0.0) | 11,714 (32.5) | |
| 1 | 258 (25.3) | 604 (27.2) | 17,307 (48.1) | |
| 2 | 577 (56.5) | 1321 (59.6) | 3817 (10.6) | |
| 3 | 100 (9.8) | 184 (8.3) | 1949 (5.4) | |
| 4 | 56 (5.5) | 82 (3.7) | 1218 (3.4) | |
| ≥5 | 30 (2.9) | 26 (1.2) | n=36,005 | |
| Arm typesc | n=959 | n=2017 | n=32,119 | |
| Active comparator | 487 (50.8) | 1000 (49.6) | 0.5 | 13,327 (41.5) |
| No-intervention arm | 93 (9.7) | 257 (12.7) | 0.02 | 2706 (8.4) |
| Experimental arm | 681 (71.0) | 1338 (66.3) | 0.01 | 24,716 (77.0) |
| Placebo comparator arm | 226 (23.6) | 476 (23.6) | 0.9 | 8255 (25.7) |
| Sham comparator arm | 6 (0.6) | 24 (1.2) | 0.2 | 460 (1.4) |
| Other arm | 53 (5.5) | 167 (8.3) | 0.007 | 1780 (5.5) |
| Intervention typesc | ||||
| Drug | 763 (72.4) | 949 (41.9) | <0.001 | 23,039 (61.2) |
| Procedure | 91 (8.6) | 341 (15.1) | <0.001 | 3672 (9.8) |
| Biologic | 36 (3.4) | 61 (2.7) | 0.3 | 2851 (7.6) |
| Behavior | 35 (3.3) | 143 (6.3) | <0.001 | 3129 (8.3) |
| Device | 75 (7.1) | 632 (27.9) | <0.001 | 3092 (8.2) |
| Radiation | 4 (0.4) | 14 (0.6) | 0.4 | 910 (2.4) |
| Dietary supplement | 37 (3.5) | 51 (2.3) | 0.04 | 1515 (4.0) |
| Genetic | 0 (0.0) | 13 (0.6) | 0.01 | 368 (1.0) |
| Other | 87 (8.3) | 273 (12.1) | 0.001 | 4750 (12.6) |
| Sex of participants | 0.1 | |||
| Female only | 6 (0.6) | 26 (1.1) | n=37,652 | |
| Male only | 9 (0.9) | 31 (1.4) | 3698 (9.8) | |
| Both | 1039 (98.6) | 2207 (97.5) | 2188 (5.8) | |
| Accepts healthy volunteers | 99/1043 (9.5) | 86/2249 (3.8) | <0.001 | 9271/37,376 (24.8) |
| Agec | ||||
| Max age ≤18 y | 27 (2.6) | 8 (0.4) | <0.001 | 2847 (7.6) |
| Min age ≥65 y | 5 (0.5) | 28 (1.2) | 0.04 | 439 (1.2) |
| Excludes ages >65 y | 163 (15.5) | 88 (3.9) | <0.001 | 12,796 (34.0) |
| Min age ≥75 y | 1 (0.1) | 10 (0.4) | 0.1 | 28 (0.1) |
| Excludes ages >75 y | 328 (31.1) | 398 (17.6) | <0.001 | 16,784 (44.6) |
| Has DMC | 425/931 (45.6) | 1097/2070 (53.0) | <0.001 | 12,122/37652 (32.2) |
| No report on DMC | 123 (11.7) | 194 (8.6) | 0.005 | 7038 (18.7) |
Note: Unless otherwise indicated, values are given as number (percentage) or n/N (percentage);.
Differences in missing data between trials were not significant unless specified in the table. Chi-squared test used for class variables, Wilcoxon rank sum test used for continuous variables.
Abbreviations: DMC, data monitoring committee; max, maximum; min, minimum.
Excludes studies classified as both nephrology and cardiology.
Excludes studies classified as nephrology or cardiology.
Rows are not mutually exclusive.
Drug trials were more common within nephrology than cardiology and other studies (72.4%, 41.9%, and 61.2%, respectively). Nephrology trials, compared with cardiology trials, were less likely to involve a procedure (8.6% versus 15.1%), device (7.1% versus 27.9%), or genetic intervention (0% versus 0.6%). Nearly all nephrology studies included both male and female participants. Both the nephrology and the other subsets tended to include more healthy volunteers (9.5% and 24.8%, respectively), mostly as part of pharmacokinetic or pharmacodynamic studies (44% of nephrology studies open to healthy volunteers were pharmacokinetic or pharmacodynamic studies). Perhaps because of age exclusions within nephrology and the overall studies, there was a trend toward having younger participants in these subsets compared with the cardiology subset. More studies within cardiology reported having a data monitoring committee than did nephrology or other studies (53% versus 45.6% and 32.2%, respectively).
Nephrology Trials by Funding Source, Number of Centers, and Locations
There was little difference in source of funding between nephrology and cardiology studies (Table 4). Few studies within nephrology or cardiology reported NIH funding (3.3% and 4.2%, respectively) compared with 9.1% of other studies. Nearly 45% of nephrology studies had industry funding. More than 50% of nephrology studies received funding from sources other than industry or NIH. A manual review of the lead sponsors for these studies indicated that these studies were primarily funded by institutions and academic medical centers both inside and outside of the United States. Irrespective of funding source, most nephrology and cardiology studies were single center (65.5% and 63.3%, respectively) and conducted outside of the United States (56.8% and 61.2%, respectively), whereas other studies were evenly distributed between US and foreign sites. Within all three subsets, most studies had one sponsor or collaborator.
Table 4.
Funding source, number of centers, and locations
| Subset Comparison | ||||
|---|---|---|---|---|
| Trial Attribute | Nephrology (n=1054) | Cardiologya (n=2264) | P-value | Otherb (n=37,652) |
| Fundingc | 0.3 | |||
| Industry | 465 (44.1) | 950 (42.0) | 17,422 (46.3) | |
| NIH | 35 (3.3) | 95 (4.2) | 3408 (9.1) | |
| Other | 554 (52.6) | 1219 (53.8) | 16,822 (44.7) | |
| 1 sponsor or collaborator | 711 (67.5) | 1455 (64.3) | 0.07 | 25,155 (66.8) |
| Sponsor; no. of centers classification | n=996 | n=2096 | 0.1 | n=34,428 |
| Industry funded; single center | 188 (18.9) | 350 (16.7) | 7485 (21.7) | |
| Industry funded; multicenter | 248 (24.9) | 507 (24.2) | 7896 (22.9) | |
| NIH funded; single center | 19 (1.9) | 59 (2.8) | 2050 (6.0) | |
| NIH funded; multicenter | 14 (1.4) | 35 (1.7) | 1077 (3.1) | |
| Other; single center | 445 (44.7) | 917 (43.8) | 13,275 (38.6) | |
| Other; multicenter | 82 (8.2) | 228 (10.9) | 2645 (7.7) | |
| Location | n=996 | n=2096 | 0.008 | n=34,428 |
| US only | 375 (37.7) | 673 (32.1) | 16,031 (46.6) | |
| Foreign only | 566 (56.8) | 1283 (61.2) | 16,147 (46.9) | |
| Both US and foreign | 55 (5.5) | 140 (6.7) | 2250 (6.5) | |
Note: Unless otherwise indicated, values are given as number (percentage). Each study has one lead sponsor, but a study may have several collaborators. Chi-squared test used for class variables, Wilcoxon rank sum test used for continuous variables.
Excludes studies classified as both nephrology and cardiology.
Excludes studies classified as nephrology or cardiology.
Derived from lead sponsor and collaborator fields.
Abbreviations: NIH, National Institutes of Health; US, United States.
Nephrology Trials by Kidney Disease Classification
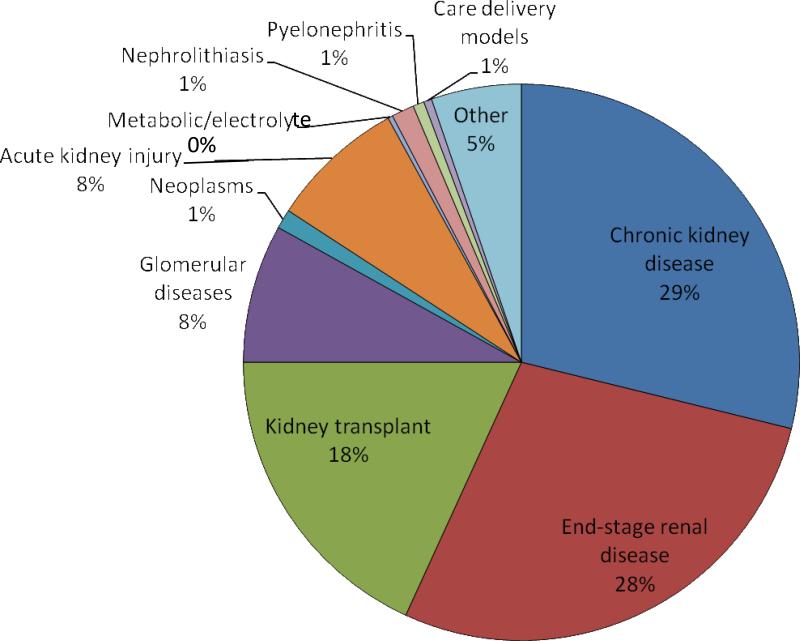
The majority of trials were performed among patients with CKD (29%), end-stage renal disease (28%), or kidney transplantation (18%) (Figure 3). Similar numbers of studies were performed among those with glomerular diseases and acute kidney injury (~8%, respectively), whereas the remainder were performed among patients with a variety of primary nephrology conditions (tables a-d of Item S2). Patterns of clinical trial attributes varied by major kidney disease classification. For example, the purpose of acute kidney injury trials was more likely to be prevention, and these trials were more likely to use randomized allocation; however, the purpose of trials of glomerular diseases was most likely to be treatment, and these trials were least likely to be randomized.
Figure 3.
Primary patient population of nephrology trials registered in ClinicalTrials.gov between October 1, 2007, and September 27, 2010. The “other” category consists of drug studies, pharmacokinetic studies, or other interventions among non-nephrology patients but with renal endpoints.
DISCUSSION
More effective therapies for CKD are desperately needed not only because the disease is increasingly common but also because it is associated with major health and economic burdens. Improving care for CKD requires well-designed phase I-IV studies to identify more effective therapies. In this systematic review, we compared critical trial characteristics essential for the proper testing and development of CKD therapies. We found that nephrology trials were few in number and small in scale, comprising only 2.6% of interventional studies in ClinicalTrials.gov between 2007 and 2010 and typically enrolling ≤100 patients. On one hand, many trial characteristics were similar between nephrology trials and all others, including rates of randomized versus nonrandomized trials. In fact, nephrology trials were sometimes more likely to have favorable study design characteristics (such as having more than one treatment arm). On the other hand, nephrology trials were also more likely to have a number of unfavorable study design characteristics, such as a large number of unblinded studies and a small number of studies with data monitoring committees. These findings raise concerns that the growing CKD patient population may still not be receiving the attention, funding, or efforts necessary to identify new therapies.
By providing a contemporary review of the landscape of registered clinical trials within nephrology, the current study corroborates prior observations but also extends them to include all investigational clinical trials and not just published RCTs. Strippoli et al8 demonstrated that between 1966 and 2002, published RCTs within nephrology were fewer in number and lower in quality than other subspecialties. Also, the proportion of trials within nephrology analyzed by Strippoli et al increased over time but remained the lowest of 12 subspecialties evaluated (1.2%).8 This previous study and a more recent study by Deo et al9 also highlighted that the reporting of key elements that are essential to a well-designed trial are more likely to be missing from nephrology trials. In general, our findings for more recently registered trials are similar but also provide a snapshot of studies that are underway and have yet to be published. We also found that nephrology studies continue to remain low in number (2.6% of all registered interventional trials from 2007-2010) and small in size and scale. Although the presence of a modest number of phase I and II studies suggests that a promising therapeutic pipeline may exist, it is unclear whether these studies will be of sufficient quality to meet the medical needs of the growing high-risk CKD population.
This study offers several insights regarding ongoing or recently completed nephrology clinical trials. The studies by Strippoli et al and Deo et al, while informative regarding published randomized trials within nephrology through 2008, did not include the full spectrum of clinical studies within nephrology and may have been limited by publication bias. Rather than restricting our sample to completed and published RCTs, we included all registered nephrology trials (both randomized and nonrandomized, as well as phase I-IV) from a contemporary time period. Through comparisons across important subspecialties, this study demonstrates that there is room for improvement in the number and quality of clinical trials in nephrology. Although it is imperative that study designs and publications are transparent, it is also important that all studies are appropriately designed to answer meaningful research questions that advance the field. Small, underpowered studies have a high risk of a type II error—failing to reject the null hypothesis and inappropriately concluding that a therapy or intervention is ineffective when, in fact, the sample size was too small to identify a significant effect. Recent initiatives that seek to improve the quality of clinical trials may be particularly relevant. First, the Clinical Trials Transformation Initiative, a public-private partnership founded by the FDA and Duke University, provides useful tools and recommendations for improving the efficiency, quality, monitoring, and reporting of clinical trials.16 Second, the recently published SPIRIT 2013 Statement (Standard Protocol Items: Recommendations for Interventional Trials) includes a 33-item checklist to improve the quality of clinical trial protocols.17 Perhaps greater attention to such resources may facilitate higher-quality clinical trials within nephrology.
One notable finding of our analysis was that more than 96% of contemporary studies within nephrology were funded by sources other than the NIH. However, a large number of studies were funded by academic medical centers, which, in the United States, likely receive support from government resources (such as through Clinical and Translational Science Awards, George M. O'Brien Kidney Research Core Centers, etc.). Given the increasingly constrained fiscal environment for publically funded medical research in the United States and the additional burdens facing academic medical centers,18 creative efforts to promote CKD studies will be critical to improving care. Two complementary approaches under way offer some hope. First, advocacy by stakeholders promoting dedicated research funding can have a dramatic impact,19 and some initiatives have been launched.20 Second, innovative consortia and collaborative efforts to conduct clinical trials more efficiently within nephrology will also be required to test therapies and translate them into practice.21,22 Over the past 10 years, few new therapies for CKD patients have received FDA approval. To alleviate this paucity of treatment options, the Kidney Health Initiative has been launched as an innovative collaboration between the American Society of Nephrology and the FDA. In addition to addressing barriers to innovation in nephrology, this initiative facilitates dialogue to inform regulatory processes and establishes expert consensus in a transparent manner around key definitions, including endpoint selection with defined regulatory pathways.21
Although observational, uncontrolled, and single-arm studies are important for generating hypotheses, properly designed RCTs are needed to substantiate effective therapies. Our findings support prior observations that such trials are less common in nephrology. In fact, a lack of such trials earlier in the development of erythropoietin-stimulating agents may have led to the delay in our understanding of their potential harms.23 Delays in obtaining such data facilitated perverse financial incentives that encouraged overuse of erythropoietin-stimulating agents. Ironically, altering these incentives led the Centers for Medicare & Medicaid Services to implement a revised financing system that bundled payment for dialysis-related services.24 It is likely that these bundled payments will create a financial disincentive for innovative therapies in end-stage renal disease and could decrease new developments and clinical trials.25,26 It is imperative that key stakeholders and leaders work to ensure that clinical trials in nephrology are funded, well-designed, appropriately powered, and properly executed.
Although our study is unique, it is not without limitations. ClinicalTrials.gov does not include all trials performed worldwide. In addition, trials may not have been included in our analysis if essential elements and/or keywords were not included upon registration. Also, misclassification may have led to some inappropriate conclusions. However, our review process attempted to minimize these potential biases; all studies were reviewed by 2 physicians independently and with agreement prior to final classification. This analysis was limited to 3 years, as ClinicalTrials.gov has only had mandatory requirements for registration since 2007. Our smaller time window likely missed some large phase III trials that may have been ongoing or registered outside this time frame, and may also have impeded meaningful comparisons of changes in trial designs over time. In addition, our analyses likely underestimate the funding received from government sources. A vast majority of the studies were performed at academic medical centers, many of which receive institutional support from government sources that may not have been recorded on ClinicalTrials.gov.
Finally, although these findings suggest that better trials are needed, they also highlight the need for improvements in monitoring. It is essential that there is transparency in trial design, execution, and results followed by rapid dissemination of findings into clinical practice. The present effort to classify contents within the public domain required substantial effort and resources,14,15 limiting the ability for more frequent evaluation. Ongoing monitoring of clinical trials and transparency of their characteristics may facilitate completion of a greater number of high-quality trials that seek to provide evidence for more effective therapies to treat and cure kidney disease.
In summary, our study identified that nephrology trials were not only few in number but also were less likely to have optimal characteristics of high-quality trial designs (e.g., blinding, data monitoring committees, etc.). A concerted effort among the NIH, industry, key stakeholders, and academia is required to increase the quality and quantity of clinical trials in nephrology with a significant goal of improving the identification and delivery of effective therapies for patients with kidney disease.
Supplementary Material
Acknowledgements
We thank Peter Hoffman and Morgan deBlecourt for their editorial assistance, supported by grant U19FD003800 from the FDA.
Support: Financial support for this work was provided by grant U19FD003800 from the FDA, awarded to Duke University for the Clinical Trials Transformation Initiative. Dr Inrig was supported by American Heart Association grant 12CRP11680033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1–e476. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Novak JE, Inrig JK, Patel UD, Califf RM, Szczech LA. Negative trials in nephrology: what can we learn? Kidney Int. 2008;74(9):1121–1127. doi: 10.1038/ki.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Zeeuw D, de Graeff PA. Clinical trial in nephrology at hard end point? J Am Soc Nephrol. 2004;15(2):506–508. doi: 10.1097/01.asn.0000113164.35698.49. [DOI] [PubMed] [Google Scholar]
- 8.Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15(2):411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- 9.Deo A, Schmid CH, Earley A, Lau J, Uhlig K. Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis. 2011;58(3):349–355. doi: 10.1053/j.ajkd.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Sciancalepore M, Strippoli G. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis. 2011;58(3):335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Samuels J, Molony DA. Randomized controlled trials in nephrology: State of the evidence and critiquing the evidence. Adv Chronic Kidney Dis. 2012;19(1):40–46. doi: 10.1053/j.ackd.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration [December 16, 2012];Food and Drug Administration Modernization Act of 1997 (FDAMA) Public Law 105-15. CFR parts 312 and 812. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm.
- 13.US Food and Drug Administration [December 16, 2012];Food and Drug Administration Amendments Act of 2007. Public Law 110-85. Available at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCos meticActFDCAct/SignificantAmendmentstotheFDCAct/FoodandDrugAdministrationAmendmentsActof2007/FullTextofFDAAALaw/default.htm.
- 14.ClinicalTrials.gov. [December 16, 2012];ClinicalTrials.gov background. Available at: http://clinicaltrials.gov/ct2/info/about.
- 15.Tasneem A, Aberle L, Ananth H, et al. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7(3):e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Trials Transformation Initiative [December 16, 2012];AACT database (Aggregate Analysis of ClinicalTrials.gov) Available at: http://www.ctti-clinicaltrials.org/project-topics/clinical-trials.gov/aact-database. [Google Scholar]; b National Library of Medicine Data Element Definitions. Available at http://prsinfo.clinicaltrials.gov/definitions.html. [Google Scholar]
- 17.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzau VJ, Cho A, Ellaissi W, et al. Transforming academic health centers for an uncertain future. N Engl J Med. 2013;369(11):991–993. doi: 10.1056/NEJMp1302374. [DOI] [PubMed] [Google Scholar]
- 19.Best RK. Disease politics and medical research funding: three ways advocacy shapes policy. Am Sociol Rev. 2012;77:780–803. [Google Scholar]
- 20.Newswise [December 16, 2012];ASN creates new foundation to prevent and cure kidney disease. Available at: http://www.newswise.com/articles/asn-creates-new-foundation-to-prevent-and-cure-kidney-disease.
- 21.Kidney Health Initiative [December 16, 2012];New partnership will advance treatment for patients with kidney disease. Available at: http://www.asn-online.org/khi/KHI_News_Release.pdf.
- 22.US Department of Health and Human Services [December 16, 2012]; Request for application RFA-DK-12-014. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-12-014.html.
- 23.Coyne DW. Use of epoetin in chronic renal failure. JAMA. 2007;297(15):1713–1716. doi: 10.1001/jama.297.15.1713. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services, Centers for Medicare & Medicaid Services . Medicare Programs; End-Stage Renal Disease Prospective Payment System, 42 CFR Parts 410, 413, and 414. US Government Printing Office; Washington, DC: 2009. [Google Scholar]
- 25.Sedor JR, Watnick S, Patel UD, et al. ASN End-Stage Renal Disease Task Force: perspective on prospective payments for renal dialysis facilities. J Am Soc Nephrol. 2011;21(8):1235–1237. doi: 10.1681/ASN.2010060656. [DOI] [PubMed] [Google Scholar]
- 26.Watnick S, Weiner DE, Shaffer R, Inrig J, Moe S, Mehrotra R. Comparing mandated health care reforms: the Affordable Care Act, accountable care organizations, and the Medicare ESRD Program. Clin J Am Soc Nephrol. 2012;7(9):1535–1543. doi: 10.2215/CJN.01220212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.