Abstract
Objective
To assess the ability of reflex UroVysion fluorescence in situ hybridization (FISH) testing to predict recurrence and progression in non-muscle invasive bladder cancer (NMIBC) patients with suspicious cytology but negative cystoscopy.
Patients and methods
Patients on NMIBC surveillance were followed with office cystoscopy and urinary cytology every three-to-six months.
Between March 2007 and February 2012, 500 consecutive patients with suspicious cytology underwent reflexive FISH analysis.
Clinical and pathologic data were reviewed retrospectively.
Predictors for recurrence, progression, and findings on subsequent cystoscopy (within two-to-six months after FISH) were evaluated using univariate and multivariate Cox regression.
Results
243 patients with suspicious cytology also had negative surveillance cystoscopy.
Positive FISH was a significant predictor for recurrence (hazard ratio 2.35, 95% confidence interval [CI] 1.42, 3.90, p=0.001) in multivariate analysis and for progression (hazard ratio 3.01, 95% CI 1.10, 8.21, p=0.03) in univariate analysis, compared to negative FISH.
However, positive FISH was not significantly associated with evidence of tumor on subsequent surveillance cystoscopy compared to negative FISH (odds ratio 0.8, 95% CI 0.26, 2.74, p=1).
Conclusion
Positive FISH predicts for recurrence and progression in NMIBC surveillance patients with suspicious cytology but negative cystoscopy.
However, an association was not found between FISH result and tumor recurrence in the immediate follow-up period.
Reflex FISH testing for suspicious cytology may have limited ability to modify surveillance strategies in NMIBC.
Keywords: Non-muscle invasive bladder cancer, fluorescence in situ hybridization, cystoscopy, cytology, surveillance
Introduction
Approximately 75%–85% of patients diagnosed with bladder urothelial carcinoma present with non-muscle-invasive disease (Ta, Tis, T1). (1) Despite initial transurethral resection, 70% of patients will recur, and up to 15% will progress to muscle-invasive disease requiring radical cystectomy. (2) Therefore, NMIBC patients require frequent, long-term surveillance with cystoscopy and urinary cytology. Urinary cytology is limited by its variable sensitivity for detecting tumors. Its sensitivity ranges from 80% to 90% for high-grade lesions and carcinoma in situ, but is much poorer for low-grade papillary lesions. (3) Cytology also suffers from interobserver and inter-institutional variability. (4–6) A suspicious cytology is particularly challenging because it raises the possibility of tumor even when cystoscopy is normal, and there are no specific recommendations to guide clinicians in deciding whether to perform further testing or alter surveillance in this scenario. (1, 7, 8)
Urinary tumor markers have been proposed as an adjunct to standard surveillance to improve tumor detection. The UroVysion Bladder Cancer Kit (Abbott Laboratories, Abbott Park, Illinois, USA) is a fluorescence in situ hybridization (FISH) assay that reveals aneuploidy for chromosomes 3, 7, and 17 as well as focal loss of 9p21 from exfoliated urothelial cells in urine specimens. (9) Compared to cytology, UroVysion has improved sensitivity but lower specificity for diagnosing urothelial carcinoma, and has been studied specifically in the setting of equivocal cytology results. (10–18) Because the assay detects molecular evidence of tumor, a positive FISH in the absence of grossly visible tumor on cystoscopy has been shown to identify patients who may recur with the passage of time.(11–13) A negative FISH, on the other hand, may identify patients at low risk for disease who may then avoid unnecessary further evaluation. (17–19)
In this study, we reviewed a cohort of patients at our institution with UroVysion FISH reflexively ordered for suspicious cytology. We analyzed FISH results to determine if the assay predicts for future recurrence and progression in NMIBC patients with suspicious urinary cytology but negative surveillance cystoscopy. To determine whether assay results could be used to modify surveillance in these patients, we also analyzed its ability to predict cystoscopically evident tumor recurrence in the immediate follow-up period.
Patients and Methods
Review of clinical data for this study was approved by our Institutional Review Board. From March 2007 through February 2012, 500 patients with a history of NMIBC were found to have suspicious urinary cytology during surveillance evaluation by two high-volume urologists (G.D. and H.W.H.). Each cytology specimen was sent reflexively FISH testing. At our institution, a suspicious diagnosis is given to cases in which the degree of atypia is highly suspicious for malignancy but cell number and/or associated morphologic changes preclude a definitively positive diagnosis. (16) Specimens with unequivocally benign or malignant cytologic findings did not trigger reflex testing. An atypical diagnosis is reserved for cases in which atypical cytologic findings are strongly favored to be associated with benign, reactive, or post-treatment processes; these cases also did not trigger reflex FISH testing. Our standard surveillance regimen includes in-office flexible cystoscopy as well as urinary cytology at three-to-six month intervals. Positive cystoscopy was defined as evidence of a visible papillary tumor. Visualization of erythematous lesions suspicious for CIS was also considered a positive cystoscopy upon pathologic confirmation at TUR. Cystoscopy was recorded as negative when bladder mucosa appeared normal. Patients with positive cystoscopies in conjunction with the reflex FISH assay were excluded from the study, leaving 243 patients eligible for analysis.
All cytology slides were stained according to the Papanicolaou technique. UroVysion assays were performed on cellular material remaining after cytologic evaluation. In each case, a minimum of 25 morphologically abnormal cells were analyzed. The signal distribution for morphologically abnormal cells showing either a gain of multiple chromosomes (i.e. ≥3 signals for more than one of the probes for chromosomes 3, 7, or 17; or a homozygous loss of 9p21) was recorded. Positive FISH was defined as either ≥4 cells with gains of chromosomes or ≥12 cells with homozygous loss of 9p21. Cases were defined as indeterminate when specimens yielded insufficient numbers of cells for FISH analysis.
Our primary aim was to determine the association between FISH analysis and disease recurrence and progression. Recurrence was defined as a positive cystoscopy at any time during subsequent follow-up. Solitary, low-grade appearing lesions were generally fulgurated during cystoscopy; all other lesions underwent transurethral resection. Monitoring for upper tract recurrence was initiated when cytology was unequivocally positive but cystoscopy was negative. All visualized upper tract lesions were biopsied or resected. Pathologic specimens were reviewed by board-certified genitourinary pathologists. All tumors were staged according to the 2010 TNM classification and graded by the 2004 WHO classification.(20, 21) Treatment for recurrent non-muscle-invasive disease was left to the discretion of the treating physician and included observation or intravesical therapy (induction or maintenance). Progression was defined by pathologic upstaging to muscle-invasive (≥ pT2) disease in the bladder and/or upper tract or evidence of disease refractory to intravesical therapy requiring radical cystectomy.
As an additional outcome measure, we also assessed the ability of FISH to predict results of subsequent surveillance cystoscopy, potentially distinguishing patients who may need more aggressive surveillance from those who could undergo cystoscopy less frequently. We analyzed a subgroup of patients with documented cystoscopy performed at our institution between 2 and 6 months after reflex FISH.
Statistical analysis
Multivariable Cox regression was used to determine the association between a positive FISH result and eventual recurrence, adjusting for tumor stage (Ta v. T1 v. Tis), tumor grade (1–2 v. 3–4), presence of carcinoma in situ, and prior intravesical therapy. Univariate Cox models were used to assess predictors of progression because of the limited number of events in our cohort. Recurrence-free (RFS) and progression-free survival (PFS) rates after reflex FISH were estimated using Kaplan Meier methods. Differences in survival outcomes according to FISH result were assessed by the log-rank test.
A Cox regression model assumes that censoring is independent of any of the covariates. We were concerned that certain tumor or patient characteristics would result in more intensive follow-up for higher-risk patients and earlier detection of recurrence and progression. To avoid censoring biases, we tested for an association between each covariate and frequency of follow-up. None of the predictors were significantly associated with follow-up frequency (all p>0.1), suggesting that bias due to informative censoring is unlikely.
Logistic regression was used to assess whether FISH could predict cystoscopy results during early follow-up. Other variables analyzed included tumor stage, tumor grade, presence of carcinoma in situ, and prior intravesical therapy. Because of the limited number of positive cystoscopies in our cohort, each predictor was analyzed univariately. All analyses were conducted using Stata 12.0 (Stata Corp., College Station, Texas, USA).
Results
Median age at time of reflex FISH was 71 (interquartile range 64, 78) and 202 patients (83%) were male. FISH results were negative in 75 (31%), uninformative in 65 (27%), and positive in 103 (42%) patients. Median followup time for patients without recurrence or progression was 27 months. Complete cohort characteristics are found in Table 1.
Table 1.
Characteristics of patients included in regression and progression models (n = 243).
| Variable | No. (IQR or %) |
|---|---|
|
| |
| Age at reflex FISH, yr, median | 71 (64, 78) |
|
| |
| Male | 202 (83) |
|
| |
| T stage | |
| Ta | 106 (44) |
| T1 | 91 (37) |
| Tis | 46 (19) |
|
| |
| Carcinoma in situ | 126 (52) |
|
| |
| History of UTUC | 10 (4) |
|
| |
| Tumor grade | |
| Low | 47 (19) |
| High | 196 (81) |
|
| |
| IVT prior to FISH | 155 (64) |
| BCG onlya | 127 (52) |
| BCG+otherb | 18 (8) |
| Mitomycin-C only | 10 (4) |
|
| |
| FISH result | |
| Negative | 76 (31) |
| Uninformative | 67 (28) |
| Positive | 100 (41) |
IQR = interquartile range; FISH = fluorescence in situ hybridization; UTUC = upper tract urothelial carcinoma; IVT = intravesical therapy; BCG = bacillus Calmette-Guérin.
Includes patients receiving induction BCG only or induction plus maintenance BCG.
Other agents include interferon (10 patients), thiotepa (4 patients), gemcitabine (3 patients), Adriamycin (1 patient)
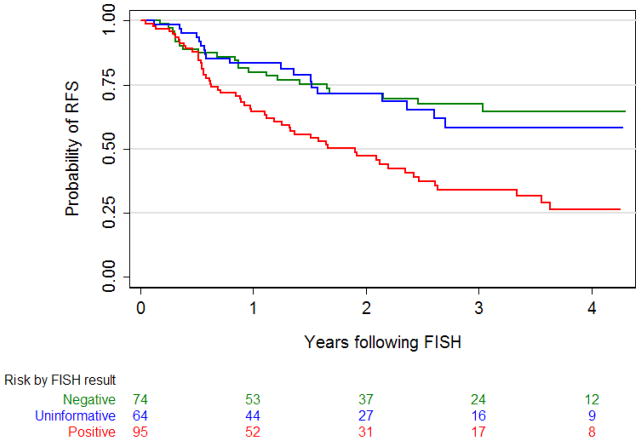
Recurrence occurred in 97 patients (40%). Median time to recurrence for patients with negative, uninformative, and positive FISH was 16 months (95% CI 6.5, 29.5), 13 months (95% CI 6.4, 25.8), and 12 months (95% CI 6.2, 25.5), respectively. In 10 patients (4%), first recurrence after reflex FISH was detected in the upper tract. Overall, FISH results were significantly associated with risk of recurrence (p=0.001). A positive FISH significantly increased the risk of recurrence (hazard ratio 2.35; 95% CI 1.42, 3.90; p=0.001) compared to negative FISH on multivariable analysis. Uninformative FISH was not associated significantly with recurrence (hazard ratio 1.18; 95% CI 0.64, 2.19; p=0.6) compared to negative FISH. None of the other predictors analyzed were significantly associated with recurrence (all p≥0.1) (Table 2). Differences in RFS by FISH result were statistically significant (p<0.001). Probability of three-year RFS was 67% (95% CI 54, 78), 58% (95% CI 41, 72), and 34% (95% CI 23, 45) for patients with negative, uninformative, and positive FISH, respectively (Figure 1).
Table 2.
Multivariable Cox regression model for recurrence after initial FISH
| Predictor | HR | 95% CI | p value |
|---|---|---|---|
|
| |||
| Carcinoma in situ | 1.12 | 0.65, 1.91 | 0.7 |
|
| |||
| High grade tumor | 1.20 | 0.60, 2.42 | 0.6 |
|
| |||
| IVT prior to FISH | 1.55 | 0.92, 2.59 | 0.1 |
|
| |||
| T Stage | |||
| Ta | Reference | 0.1 | |
| T1 | 1.13 | 0.70, 1.84 | |
| Tis | 0.59 | 0.31, 1.11 | |
|
| |||
| FISH result | |||
| Negative | Reference | 0.001 | |
| Uninformative | 1.18 | 0.64, 2.19 | |
| Positive | 2.35 | 1.42, 3.90 | |
HR = hazard ratio; CI = confidence interval; IVT = intravesical therapy; FISH = fluorescence in situ hybridization
Figure 1.
Kaplan-Meier probability curves for recurrence-free survival (RFS) after reflex FISH stratified by FISH result. Differences in RFS are statistically significant (p<0.001, log-rank test).
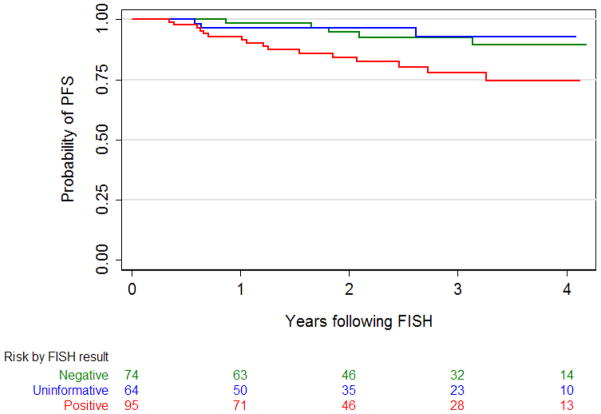
Tumor progression occurred in 24 patients. FISH results were significantly associated with risk of progression (p=0.02). Positive FISH was significantly associated with increased risk of progression (hazard ratio 3.01; 95% CI 1.10, 8.21; p=0.03) while uninformative FISH was not significant (hazard ratio 0.79; 95% CI 0.19, 3.33; p=0.8), compared to negative FISH. The presence of any carcinoma in situ was also a significant predictor of progression on univariate analysis (hazard ratio 11.37, 95% CI 2.67, 48.35, p=0.001). The models also suggest that prior intravesical therapy was a predictor for progression (HR 2.92; 95% CI 1.00, 8.55; p=0.05) (Table 3). Differences in PFS by FISH result were statistically significant (p=0.01). Three-year PFS was 93% (95% CI 81, 97), 93% (95% CI 78, 98), and 78% (95% CI 65, 86) for patients whose FISH results were negative, uninformative, and positive, respectively (Figure 2).
Table 3.
Univariate Cox regression model for progression after initial FISH
| Predictor | HR | 95% CI | p value |
|---|---|---|---|
|
| |||
| Carcinoma in situ | 11.37 | 2.67, 48.35 | 0.001 |
|
| |||
| IVT prior to FISH | 2.92 | 1.00, 8.55 | 0.05 |
|
| |||
| T Stage | |||
| Ta | Reference | 0.1 | |
| T1 | 1.55 | 0.58, 4.18 | |
| Tis | 2.85 | 1.03, 7.86 | |
|
| |||
| FISH result | |||
| Negative | Reference | 0.02 | |
| Uninformative | 0.79 | 0.19, 3.33 | |
| Positive | 3.01 | 1.10, 8.21 | |
HR = hazard ratio; CI = confidence interval; IVT = intravesical therapy; FISH = fluorescence in situ hybridization
Figure 2.
Kaplan-Meier probability curves for progression-free survival (PFS) after reflex FISH stratified by FISH result. Differences in PFS are statistically significant (p=0.01, log-rank test).
We identified a subgroup of 125 patients who had their subsequent surveillance cystoscopy performed at our institution between two and six months after the reflex FISH assay. Cystoscopy was negative in 108 patients and positive in 17. Clinical and tumor characteristics are shown in Table 4. FISH results were not significantly associated with the results of the next cystoscopy (p=1). While the association was not significant, the 95% CI indicates that a positive FISH can potentially increase the odds of having a positive subsequent cystoscopy by 2.74 times over a negative FISH (odds ratio 0.84, 95% CI 0.26, 2.74). None of the other characteristics analyzed (CIS, tumor grade, history of IVT, tumor stage) were significantly associated with result of subsequent cystoscopy (all p≥0.2) (Table 5).
Table 4.
Characteristics for patients included in outcome model for subsequent surveillance cystoscopy after reflex FISH, stratified by cystoscopy result (n = 125)
| Variable | Negative cystoscopy | Positive cystoscopy |
|---|---|---|
| No. patients | 108 | 17 |
| Age at FISH, yr, median (IQR) | 71 (65, 79) | 69 (64, 77) |
| Sex: males, no. (%) | 94 (87) | 12 (71) |
| T stage (%) | ||
| Ta | 46 (43) | 10 (59) |
| T1 | 39 (36) | 5 (29) |
| Tis | 23 (21) | 2 (12) |
| Carcinoma in situ (%) | 62 (57) | 7 (41) |
| Tumor grade (%) | ||
| Low | 20 (19) | 2 (12) |
| High | 88 (81) | 15 (88) |
| IVT prior to FISH (%) | 73 (68) | 12 (71) |
| FISH result (%) | ||
| Negative | 34 (31) | 6 (35) |
| Uninformative | 27 (25) | 4 (24) |
| Positive | 47 (44) | 7 (41) |
| Time from FISH to cystoscopy, mo, median (IQR) | 3.7 (3.2–4.6) | 3.7 (3.2–4.2) |
IQR = interquartile range; FISH = fluorescence in situ hybridization; IVT = intravesical therapy
Table 5.
Univariate logistic regression model predicting outcome of subsequent surveillance cystoscopy after reflex FISH
| Predictor | OR | 95% CI | p value |
|---|---|---|---|
|
| |||
| Carcinoma in situ | 0.52 | 0.18, 1.47 | 0.2 |
|
| |||
| High grade tumor | 1.70 | 0.36, 8.06 | 0.5 |
|
| |||
| IVT prior to FISH | 1.15 | 0.38, 3.52 | 0.8 |
|
| |||
| T Stage | |||
| Ta | Reference | 0.4 | |
| T1 | 0.59 | 0.19, 1.87 | |
| Tis | 0.40 | 0.08, 1.98 | |
|
| |||
| FISH result | |||
| Negative | Reference | 1 | |
| Uninformative | 0.84 | 0.21, 3.28 | |
| Positive | 0.84 | 0.26, 2.74 | |
OR = odds ratio; CI = confidence interval; IVT = intravesical therapy; FISH = fluorescence in situ hybridization
Discussion
In this study, a positive UroVysion FISH assay was found to be a predictor of recurrence and progression in patients with negative cystoscopy but suspicious urinary cytology during surveillance for NMIBC. However, we were unable to identify a significant association between FISH results and cystoscopic evidence of recurrence in the immediate follow-up period. Thus, while FISH appears to be reliable in demonstrating tumor biology and natural history of NMIBC, we cannot determine an obvious clinical role for reflex FISH testing in these patients at the current time.
Prior studies have demonstrated the predictive ability of FISH in patients with surveillance cytology demonstrating varying degrees of atypia, based on the premise that chromosomal changes may precede visual evidence of recurrent disease. (11–13) Sarosdy et al. introduced the term “anticipatory positive” to describe this scenario. In their multicenter study, 15 of 36 (41%) surveillance patients with negative cystoscopy but positive FISH eventually recurred, at a mean time to recurrence of 6 months. (11) In a larger study, Yoder et al. evaluated recurrence in 250 patients receiving reflex FISH for equivocal or negative urinary cytology. Disease recurred during surveillance in 63% of patients with negative cystoscopy within 29 months, with an estimated recurrence risk of 48% (95%CI 34, 62) and 54% (95% CI 40, 67) at six and 10 months, respectively.(13)
However, our focus was specifically on patients with suspicious cytology, a group that represents a diagnostic and therapeutic challenge. While not definitively positive for malignancy, a suspicious cytology still puts patients at high risk for tumor recurrence. (22) When cytology is suspicious but cystoscopy does not reveal any obvious tumor, clinicians could continue routine surveillance without additional testing—but at the risk of missing cancer diagnosis. However, a prior report from our institution revealed that the positive predictive value of a positive reflex FISH in any patient with suspicious cytology was only 56.8%, indicating that additional workup may be unnecessary for all patients. (16)
In our cohort of 243 cystoscopically negative patients with suspicious cytology, 103 (42%) had anticipatory positive FISH, and 55 patients (23%) experienced recurrence. A positive FISH result, a significant predictor of recurrence and progression in our study, could therefore be used to reinforce the importance of strict surveillance to detect these events and initiate appropriate therapy. Positive FISH was also associated with earlier time to recurrence and progression, compared to uninformative or negative FISH. However, it is important to note that all of these events in our study were diagnosed under standard cystoscopic surveillance protocols, regardless of reflex FISH result. Therefore, the predictive ability of a reflex FISH, by itself, appears to have limited clinical utility.
We hypothesized that FISH may have increased clinical impact if a positive assay result could reliably identify patients likely to recur in the immediate follow-up period. Similarly, if a negative FISH definitively predicted for negative immediate cystoscopy, a case could be made for extending the interval to subsequent follow-up despite the suspicious urinary cytology. This would decrease the number of surveillance cystoscopies, sparing some patients unnecessary procedures and reducing overall surveillance costs. However, we were unable to detect a significant association between FISH results – positive or negative – and immediate follow-up cystoscopy. In the absence of evidence to the contrary, long-term follow-up with cystoscopy at regular intervals remains the mainstay of NMIBC surveillance. The added cost of FISH in the surveillance of bladder cancer must also be taken into consideration. Bladder UC is already one of the costliest cancers to treat, largely due to the demands for lifelong surveillance, and in prospective trials FISH has not shown increased cost-effectiveness over cystoscopy alone for tumor detection. (23, 24)
Our results should be interpreted in the context of the study design and its limitations. First, while FISH was ordered for suspicious cytology in surveillance patients in a prospective, unbiased manner, our data review and analysis was retrospective. Second, FISH was performed reflexively and a positive result by itself did not trigger additional diagnostic testing, nor did a negative result delay future surveillance studies. However, because treating physicians were not blinded to FISH results, it is possible that bias and test results could have influenced treatment decisions. There was also variability in patient follow-up – some patients discontinued surveillance at our institution, and others did not receive follow-up at recommended intervals. Differences in prior treatment for NMIBC may have influenced FISH results as well (single-agent versus combination therapy, number of treatment cycles, and patterns of treatment response). Despite differences in choice and duration of agents used, intravesical therapy has generally been shown to decrease early recurrence in both primary and recurrent NMIBC. (25, 26) In contrast, positive FISH after bacillus Calmette-Guérin treatment has been shown to predict for recurrence and treatment failure. (27–29) However, we did not find a significant association between prior intravesical therapy and likelihood of positive cystoscopy in the early follow-up period after FISH (p=0.8). Finally, the diagnosis of atypical or suspicious cytology can vary from institution to institution. (4, 30) Our study population may therefore differ from other groups, limiting the generalizability of our results.
In conclusion, anticipatory positive urinary FISH predicts for recurrence and progression in NMIBC patients in the setting of negative cystoscopy and suspicious cytology. These results can be used to counsel patients and reinforce the need for strict surveillance. However, the lack of an association between FISH result and cystoscopic tumor recurrence in the immediate follow-up period appears to limit the utility of the assay in modifying NMIBC surveillance regimens.
Acknowledgments
Sources of funding: This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. Dr. Sfakianos is a research fellow in urologic oncology supported by an NIH/NCI training grant under award number T32-CA82088.
Contributor Information
Philip H. Kim, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY
Ranjit Sukhu, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY.
Billy H. Cordon, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY
John P. Sfakianos, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY
Daniel D. Sjoberg, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY
A. Ari Hakimi, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY.
Guido Dalbagni, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY.
Oscar Lin, Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, NY.
Harry W. Herr, Department of Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, New York, NY
References
- 1.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. European urology. 2011;59(6):997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Donat SM. Evaluation and follow-up strategies for superficial bladder cancer. The Urologic clinics of North America. 2003;30(4):765–76. doi: 10.1016/s0094-0143(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 3.Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP, 3rd, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66(6 Suppl 1):35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Brimo F, Vollmer RT, Case B, Aprikian A, Kassouf W, Auger M. Accuracy of urine cytology and the significance of an atypical category. American journal of clinical pathology. 2009;132(5):785–93. doi: 10.1309/AJCPPRZLG9KT9AXL. [DOI] [PubMed] [Google Scholar]
- 5.Yafi FA, Brimo F, Auger M, Aprikian A, Tanguay S, Kassouf W. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urologic oncology. 2013 doi: 10.1016/j.urolonc.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Karakiewicz PI, Benayoun S, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU international. 2006;97(5):997–1001. doi: 10.1111/j.1464-410X.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 7.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. The Journal of urology. 2007;178(6):2314–30. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Nabi G, Greene D, O’Donnell MO. Suspicious urinary cytology with negative evaluation for malignancy in the diagnostic investigation of haematuria: how to follow up? Journal of clinical pathology. 2004;57(4):365–8. doi: 10.1136/jcp.2003.009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halling KC, Kipp BR. Bladder cancer detection using FISH (UroVysion assay) Advances in anatomic pathology. 2008;15(5):279–86. doi: 10.1097/PAP.0b013e3181832320. [DOI] [PubMed] [Google Scholar]
- 10.Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urologic oncology. 2008;26(6):646–51. doi: 10.1016/j.urolonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, Yore L, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. The Journal of urology. 2002;168(5):1950–4. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]
- 12.Skacel M, Fahmy M, Brainard JA, Pettay JD, Biscotti CV, Liou LS, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. The Journal of urology. 2003;169(6):2101–5. doi: 10.1097/01.ju.0000066842.45464.cc. [DOI] [PubMed] [Google Scholar]
- 13.Yoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. American journal of clinical pathology. 2007;127(2):295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed] [Google Scholar]
- 14.Caraway NP, Khanna A, Fernandez RL, Payne L, Bassett RL, Jr, Zhang HZ, et al. Fluorescence in situ hybridization for detecting urothelial carcinoma: a clinicopathologic study. Cancer cytopathology. 2010;118(5):259–68. doi: 10.1002/cncy.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan AB, Salido M, Espinet B, Placer J, Pijuan L, Juanpere N, et al. A multicolor fluorescence in situ hybridization assay: A monitoring tool in the surveillance of patients with a history of non-muscle-invasive urothelial cell carcinoma: A prospective study. Cancer cytopathology. 2011;119(6):395–403. doi: 10.1002/cncy.20168. [DOI] [PubMed] [Google Scholar]
- 16.Ferra S, Denley R, Herr H, Dalbagni G, Jhanwar S, Lin O. Reflex UroVysion testing in suspicious urine cytology cases. Cancer. 2009;117(1):7–14. doi: 10.1002/cncy.20016. [DOI] [PubMed] [Google Scholar]
- 17.Lotan Y, Bensalah K, Ruddell T, Shariat SF, Sagalowsky AI, Ashfaq R. Prospective evaluation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. The Journal of urology. 2008;179(6):2164–9. doi: 10.1016/j.juro.2008.01.105. [DOI] [PubMed] [Google Scholar]
- 18.Schlomer BJ, Ho R, Sagalowsky A, Ashfaq R, Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. The Journal of urology. 2010;183(1):62–7. doi: 10.1016/j.juro.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 19.Bollmann M, Heller H, Bankfalvi A, Griefingholt H, Bollmann R. Quantitative molecular urinary cytology by fluorescence in situ hybridization: a tool for tailoring surveillance of patients with superficial bladder cancer? BJU international. 2005;95(9):1219–25. doi: 10.1111/j.1464-410X.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. American Joint Committee on Cancer; p. xiv.p. 648. [DOI] [PubMed] [Google Scholar]
- 21.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. International journal of surgical pathology. 2005;13(2):143–53. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 22.Raitanen MP, Aine RA, Kaasinen ES, Liukkonen TJ, Kylmala TM, Huhtala H, et al. Suspicious urine cytology (class III) in patients with bladder cancer: should it be considered as negative or positive? Scandinavian journal of urology and nephrology. 2002;36(3):213–7. doi: 10.1080/003655902320131901. [DOI] [PubMed] [Google Scholar]
- 23.Lotan Y, Shariat SF, Schmitz-Drager BJ, Sanchez-Carbayo M, Jankevicius F, Racioppi M, et al. Considerations on implementing diagnostic markers into clinical decision making in bladder cancer. Urologic oncology. 2010;28(4):441–8. doi: 10.1016/j.urolonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Kamat AM, Karam JA, Grossman HB, Kader AK, Munsell M, Dinney CP. Prospective trial to identify optimal bladder cancer surveillance protocol: reducing costs while maximizing sensitivity. BJU international. 2011;108(7):1119–23. doi: 10.1111/j.1464-410X.2010.10026.x. [DOI] [PubMed] [Google Scholar]
- 25.Huncharek M, Geschwind JF, Witherspoon B, McGarry R, Adcock D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. Journal of clinical epidemiology. 2000;53(7):676–80. doi: 10.1016/s0895-4356(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 26.Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer research. 2001;21(1B):765–9. [PubMed] [Google Scholar]
- 27.Mengual L, Marin-Aguilera M, Ribal MJ, Burset M, Villavicencio H, Oliver A, et al. Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guerin therapy. European urology. 2007;52(3):752–9. doi: 10.1016/j.eururo.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Savic S, Zlobec I, Thalmann GN, Engeler D, Schmauss M, Lehmann K, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin therapy. International journal of cancer Journal international du cancer. 2009;124(12):2899–904. doi: 10.1002/ijc.24258. [DOI] [PubMed] [Google Scholar]
- 29.Kamat AM, Dickstein RJ, Messetti F, Anderson R, Pretzsch SM, Gonzalez GN, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guerin therapy for bladder cancer: results of a prospective trial. The Journal of urology. 2012;187(3):862–7. doi: 10.1016/j.juro.2011.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raab SS, Grzybicki DM, Vrbin CM, Geisinger KR. Urine cytology discrepancies: frequency, causes, and outcomes. American journal of clinical pathology. 2007;127(6):946–53. doi: 10.1309/XUVXFXMFPL7TELCE. [DOI] [PubMed] [Google Scholar]