Abstract
Purpose:
To evaluate the feasibility of 3D single breath-hold late gadolinium enhancement (LGE) of the left ventricle (LV) using supplemental oxygen and hyperventilation and compressed-sensing acceleration.
Methods:
Breath-hold metrics (breath-hold duration, diaphragmatic/LV position drift, and maximum variation of RR interval) without and with supplemental oxygen and hyperventilation were assessed in healthy adult subjects using a real time single shot acquisition. Ten healthy subjects and 13 patients then underwent assessment of the proposed 3D breath-hold LGE acquisition (FOV=320×320×100 mm3, resolution=1.6×1.6×5.0 mm3, acceleration rate of 4) and a free breathing acquisition with right hemidiaphragm navigator (NAV) respiratory gating. Semi-quantitative grading of overall image quality, motion artifact, myocardial nulling, and diagnostic value was performed by consensus of two blinded observers.
Results:
Supplemental oxygenation and hyperventilation increased the breath-hold duration (35±11 s to 58±21 s, p<0.0125) without significant impact on diaphragmatic/LV position drift or maximum variation of RR interval (both p>0.01). LGE images were of similar quality when compared to free breathing acquisitions but with reduced total scan time (85±22 s to 35±6 s, p<0.001).
Conclusions:
Supplemental oxygenation and hyperventilation allow for prolonged breath-holding and enable single breath-hold 3D accelerated LGE with similar image quality as free breathing with NAV.
Keywords: late gadolinium enhancement, myocardial viability, 3D acquisition, acceleration techniques, breath-hold, left ventricle, hyperventilation, compressed sensing
INTRODUCTION
Late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) allows for non-invasive assessment of fibrosis and scar (1,2). Two dimensional (2D) multi-slice LGE is commonly used for the clinical assessment of left ventricular (LV) scar/fibrosis (2). A series of 10-14 short-axis slices is necessary to cover the entire LV and is acquired in 5-7 consecutive breath-holds (2 slices per breath-hold). However, 2D multi-slice acquisitions are limited by a large (8-10 mm) slice thickness that leads to partial volume effects and slice registration errors due to variability of breath-holds. Three-dimensional (3D) LGE sequences provide a higher signal-to-noise ratio (SNR) and can thus be used to increase LGE spatial resolution, especially in the through-plane direction, leading to improved scar quantification (3,4) when compared to 2D acquisitions.
Due to their long acquisitions, 3D LGE acquisitions are most commonly performed using free breathing (5-9) with right hemidiaphragm (RHD) respiratory navigators (NAV) (5-8). However, free breathing navigator gated 3D LGE results in prolonged scan times due to navigator inefficiency. Drift of the respiratory pattern can also be observed leading to poor gating efficiency and potentially incomplete scans. Furthermore, application of the navigator restore pulse in LGE causes additional inflow artifacts (10). Finally, due to contrast wash out kinetics over the whole acquisition, the optimal inversion time to null healthy myocardial signal may shift over the duration of the prolonged acquisition and result in additional image artifacts. 3D phase-sensitive inversion recovery (PSIR) sequences (11) eliminates the sensitivity to an imperfect inversion time (11,12). However, this approach increases the scan time by a factor of 2, which is not desired in 3D imaging. As an alternative, self-gated acquisitions have been proposed where the acquired image data are directly used for respiratory motion estimation and gating. Although self-gating techniques have been successfully applied in several applications such as CINE (13) and coronary MR angiography (14,15), it has not yet been applied to 3D LGE imaging and remains challenging due to the temporal variation of the LGE signal.
3D breath-hold (BH) acquisitions covering the entire LV have been proposed for LGE, but are associated with relatively low spatial resolution due to the limitation of breath-hold duration (3,16-18). Segmented 3D breath-hold acquisitions where the LV dataset is acquired in 3 separate breath-holds has been proposed, but may lead to misalignment of the 3D volumes due to breath-hold variations (19). As a result, single breath-holds using a highly accelerated 3D acquisition would be desirable. Parallel imaging techniques such as SENSE (20) or GRAPPA (21) are widely available and have been used in 3D and 2D LGE with acceleration rates of up to 2 (22-25). Compressed-sensing (CS) (26,27) is an alternative acceleration technique that enables higher acceleration rates. The utility of CS to accelerate LGE acquisitions by a factor of 4 has been recently presented (28). Prolonged breath-hold duration may also enable longer acquisition time and increased spatial resolution. Supplemental oxygen combined with hyperventilation has been demonstrated to provide substantially longer breath-hold durations (29-32).
In this study, we sought to investigate the feasibility of a highly accelerated LGE acquisition in combination with a prolonged breath-hold facilitated by supplemental oxygenation and hyperventilation. The contribution of this study is twofold. Initially, we assessed the impact of supplemental oxygenation and hyperventilation on breath-hold duration, diaphragmatic/LV position drift, and maximum variation of RR interval during breath-hold in healthy subjects. Subsequently, the feasibility of a single breath-hold accelerated 3D LGE acquisition was demonstrated using supplemental oxygenation, hyperventilation, and CS-based acceleration technique.
METHODS
All imaging was performed on a 1.5T Philips Achieva (Philips Healthcare, Best, The Netherlands) scanner using a 32-channel cardiac phased array receiver coil. Written informed consent was obtained from all participants and the imaging protocol was approved by our institutional review board.
Breath-hold metrics (breath-hold duration, diaphragmatic position drift, LV position drift, and maximum variation of RR interval) were analyzed using a combination of real-time imaging and monitoring of motion using respiratory navigators,. Subsequently, the feasibility of single breath-hold 3D LGE imaging with supplemental oxygenation and hyperventilation was studied.
Breath-hold characterization without and with supplemental oxygenation and hyperventilation
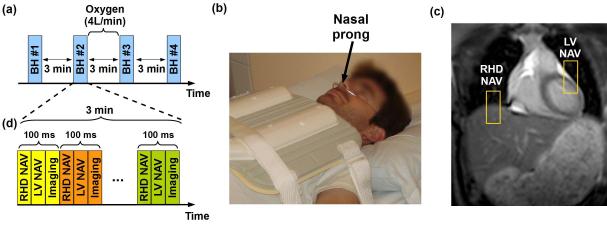
Ten healthy adult subjects (29±15 years, 3 male, “Healthy Group #1”) without any history of cardiovascular disease underwent MRI without and with supplemental oxygenation and hyperventilation. Figure 1 shows the imaging protocol used to evaluate breath-hold characteristics. In each subject, four different breath-hold scans were performed at end expiration (Figure 1a). End-inspiration breath-holds were not assessed since there have been shown to be associated with more complex motion drift/variation (33) undesirable for 3D LGE imaging. For the purpose of training, all subjects were asked to perform an initial breath-hold (BH#1) without supplemental oxygenation or hyperventilation. Subsequently, two breath-hold scans were performed without (BH#2) and with (BH#3) supplemental oxygenation and hyperventilation. Supplemental oxygen (4L/min, nasal prongs) was administered for 3 minutes (Figure 1b). Hyperventilation was performed immediately before the breath-hold by instructing the subject to take three rapid maximum capacity respirations. A fourth breath-hold scan was then performed without supplemental oxygenation or hyperventilation (BH#4). The time interval between each different breath-holds was 3 minutes to allow for subject recovery. In each breath-hold, subjects were asked to sustain their end-expiratory breath-hold as long as possible. To demonstrate if the increased breath-hold duration is associated with supplemental oxygen or a placebo effect, we repeated a similar study in a separate group of 10 healthy subjects (32±17 years, 1 male, “Healthy Group #2”). In this study, subjects were not instructed for hyperventilation and were told they will receive supplemental oxygen; however, room air (4L/min during 3 minutes) was administrated.
Figure 1.
Imaging protocol for breath-hold (BH) assessment. (a) Study protocol designed for BH assessment without (BH#1, BH#2, and BH#4) and with (BH#3) supplemental oxygen and hyperventilation. Oxygen (4 l/min) was administrated using nasal prolong as illustrated in (b), (c) and (d) show the sequence diagram used for BH monitoring. A real time steady-state free precession (SSFP) sequence was used to acquire a complete image every 100ms using a right hemidiaphragm (RHD) navigator (NAV), a NAV located at the left ventricle LV (LV NAV) and a 2D coronal imaging slice (Imaging) (d).
A 2D dynamic real time sequence was acquired during each breath-hold for both subject groups. Two pencil-beam navigators positioned on the dome of the RHD and the LV (Figure 1c) were acquired for each dynamic with a temporal resolution of 17 ms each. Navigators were positioned in SI direction and employed a 2D spiral pulse with the following parameter: spatial resolution =1 mm, flip angle=25°, TE=1.2 ms, and bandwidth=54 kHz. These navigator acquisitions were followed by a 2D dynamic real time steady-state free precession (SSFP) acquisition with the following parameters: TR/TE/α=2 ms/1 ms/50°, FOV=320×320 mm2, and spatial resolution=3.9×3.9 mm2, slice thickness=10 mm, and SENSE factor=2.35 (Figure 1d). Total scan time for the two navigators and real-time SSFP was 100 ms. This acquisition scheme was continuously repeated for 3 minutes (1800 dynamics) to monitor the patient breathing pattern during and immediately after their breath-hold. The 2D images were not used for breath-hold characteristic assessment, which was only based on the analysis of the two respiratory navigators and the recorded ECG signal.
NAVs and ECG signals of each acquisition were exported from the scanner and analyzed in Matlab (Mathworks, Natick MA). Breath-hold duration was measured by visual identification of the stable period of the RHD NAV signal. The diaphragmatic position drift was measured as the difference between the maximum and minimum RHD position over the breath-hold period. Since the LV navigator contains cardiac motion preventing an accurate detection of the respiratory induced drift, a 3rd order polynomial fit of the signal was first performed to remove the influence of the cardiac motion. The LV position drift was then measured as the difference between the maximum and minimum of the LV NAV fitted signal over the breath-hold period. The ECG R-wave was used to calculate the maximum variation of RR interval of each subject through each scan.
3D LGE imaging
The ten healthy subjects from “Healthy Group #1” and 13 patients (53±12 years, 9 male) referred to our center for evaluation of cardiovascular disease were recruited for this imaging study. Each subject was imaged for evaluation of scar using a) free-breathing LGE using RHD NAV and b) breath-hold LGE with supplemental oxygen and hyperventilation. Two scans were acquired in a random order to account for differences in contrast washout. LGE acquisitions were performed 16±4 min (in healthy subjects) and 21±8 min (in patients) after administration of 0.1 or 0.2 mmol/kg of gadobenate dimeglumine (MultiHance; Bracco Diagnostic Inc., Princeton, NJ). To comply with our IRB requirements, subjects with an estimated glomerular filtration rate (eGFR) of 45-60 mL/min/1.73m2 only received 0.1 mmol/kg of gadobenate dimeglumine. All healthy subjects and 5 out of 13 patients received an injection of 0.2 mmol/kg of gadobenate dimeglumine. Eight patients received a single dose of 0.1 mmol/kg of gadobenate dimeglumine. Before each 3D LGE acquisition, a Look-Locker sequence (34) was performed to obtain the optimal inversion time to provide the best nulling of the LV myocardium signal. Two 3D ECG-triggered inversion recovery prepared spoiled gradient echo sequences were used for LGE imaging with the following parameters: TR/TE/α=5.2ms/2.6ms/25°, FOV=320×320×100 mm3, acquisition matrix=200×200×25, resulting in a spatial resolution of 1.6×1.6×5.0 mm3, and acquisition in every heart beat (1 RR). All images were acquired during diastolic rest period with average duration of 126 ms (25 k-space lines) for healthy subjects and 146 ms (29 k-space lines) for patients. Both sequences used a 4-fold CS acceleration rate. The free breathing 3D LGE sequence employed a RHD NAV for gating and tracking the respiratory motion (gating window=7 mm, tracking factor=0.6 (35)). The breath-hold LGE sequence was performed with supplemental oxygenation (4 L/min for 3 minutes by nasal prong) and hyperventilation (three rapid maximum capacity breathes immediately before the end-expiratory breath-hold.
To accelerate imaging, a randomly undersampled pattern was developed for 3D LGE. The undersampling pattern was designed to fully acquire the k-space center lines and to randomly discard lines from the outer k-space so as to reach the desired acceleration factor. The number of k-space center-lines fully acquired was set to 21×9 lines (ky-kz). An identical randomly undersampled k-space profile was used for all the studies to minimize the effect of k-space weighting due to signal recovery after the inversion pulse in LGE. The raw k-space data were extracted and all image reconstructions were performed off-line using an improved CS reconstruction technique (36).
In a prior contrast-enhanced coronary (36) and LGE study (28), a radial k-space profile reordering (Supplementary material, Figure S1.a) was utilized to minimize the gradient jumps induced by the random pattern of the outer k-space (36). The acquisition is started with the blue segments and finished with the outer red segments. In this study, we propose an alternative profile reordering technique in which the center of k-space is acquired in a linear fashion immediately after the initiation of data acquisition (Supplementary material, Figure S1.b). The remaining outer k-space segments are then acquired in a radial fashion. This profile ordering provides improved robustness against motion, respiratory motion drift and incomplete breath-holds and is referred to as “inner linear-outer radial profile reordering”.
Data analysis
The statistical significance between breath-holds was evaluated for each metric using a one way analysis of variances (ANOVA) with significance threshold at p < 0.05. Additional paired t-tests with Bonferroni correction were performed for all pair of breath-holds when the ANOVA test was found significant. Statistical significance was considered at p < 0.05/6 = 0.008.
Reconstructed images obtained from the healthy adult subjects and the patients were exported in DICOM format and loaded into the Synedra View Personal (Synedra Information Technologies GmbH, Aachen, Germany) for image visualization and analysis. A subjective qualitative assessment of image quality was performed by consensus of two experienced cardiologists blinded to the acquisition scheme and all patient data. Each 3D dataset was scored for diagnostic value (yes/no) and image quality based on a 4-point scale: 1, poor/uninterpretable (severe motion/blurring artifact or poor nulling of the myocardium); 2-fair (moderate motion/blurring artifact or imperfect nulling of the myocardium); 3-good (mild motion/blurring artifact and good nulling of the myocardium); 4-excellent (sharply defined myocardium without motion/blurring artifact and excellent myocardial nulling). Motion artifact and myocardial nulling were also individually scored based on the same 4-point scale corresponding to each of these criteria. A Wilcoxon signed rank test was used to test the null hypothesis that the difference of image quality scores between both acquisitions was zero. Diagnostic values were compared between both sequences by means of McNemar’s test. Statistical significance was considered for p < 0.05.
RESULTS
Breath-hold characterization
Example of RHD NAV data, LV NAV data and RR interval variation are shown in Figure S2 (Supplementary material) during breath-holds without (S2a,S2c,S2e) and with (S2b,S2d,S2f) supplemental oxygenation and hyperventilation. Table 1 summarizes the breath-hold characteristics for all subjects of “Healthy Group 1”. One-way ANOVA indicated statistical difference in term of BH duration among the breath-holds (p=0.02). Additional paired t-tests revealed statistical differences between BH#1 vs. BH#3 (p=0.001), BH#2 vs. BH#3 (p=0.005), and BH#4 vs. BH#3 (p=0.007) demonstrating that supplemental oxygenation and hyperventilation significantly prolong breath-hold duration by ~20s. There was a trend for the second to be longer than the first breath-hold (~5 s, p=0.015) suggesting the utility of a training breath-hold. One breath-hold has been found sufficient for the training phase since no further increase was observed between the second and the fourth breath-hold (p=0.79). Finally, there were no statistically significant differences between breath-holds for all remaining metrics as determined by the one-way ANOVA (p>0.05 for all metrics). Finally, in the “Healthy Group #2”, breath-hold duration was 34±12s, 35±13s, 38±13s, and 36±14s for BH#1, BH#2, BH#3, and BH#4, respectively. No significant difference was observed between breath-holds in terms of breath-hold duration as indicated by the one way ANOVA (p=0.94).
Table 1.
Breath-hold (BH) duration, right hemidiaphragm (RHD) position drift, left ventricular (LV) position drift and maximal variation of RR interval variation measured from the BHs performed in a sequential order without (BH#1, BH#2, BH#4) and with (BH#3) supplemental oxygen (4 l/min nasal prong) and hyperventilation in 10 healthy adult subjects (“Healthy Group #1”). Minimum and maximum values are reported in bracket. The second breath-hold (BH#2; no supplemental oxygen or hyperventilation) duration was longer than the first breath-hold confirming the benefit of a training breath-hold with no further benefit of training (BH#4). Still longer breath-holds were achieved with supplemental oxygen and hyperventilation (BH#3). NS indicates no statistical difference.
| Breath-hold duration (s) |
RHD position drift (mm) |
LV position drift (mm) |
Maximum variation of RR interval (beats per minute) |
|
|---|---|---|---|---|
|
| ||||
| BH #1 | 35 ± 11(18-53) | 4.6 ± 3.3(1-10) | 2.7 ± 2.1(0-8) | 12 ± 6(5-25) |
| BH #2 | 40 ± 14(18-65) | 5.9 ± 4.0(2-15) | 3.8 ± 2.3(1-9) | 14 ± 8(5-30) |
| BH #3 | 58 ± 21(27-96) | 7.0 ± 4.6(1-18) | 4.9 ± 3.9(2-12) | 13 ± 5(8-25) |
| BH #4 | 41 ± 16(14-60) | 6.9 ± 4.2(3-16) | 3.4 ± 2.7(1-9) | 14 ± 5(8-20) |
|
| ||||
| P BH # vs. #2 | 0.015 | NS | NS | NS |
| P BH#2 vs. #3 | 0.005 | NS | NS | NS |
| P BH #3 vs. #4 | 0.007 | NS | NS | NS |
| P BH #2 vs. #4 | NS | NS | NS | NS |
|
| ||||
3D LGE imaging: free breathing vs. breath-hold acquisitions
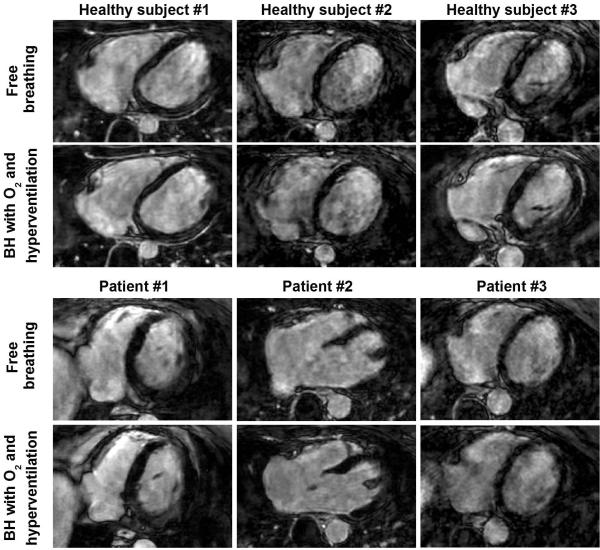
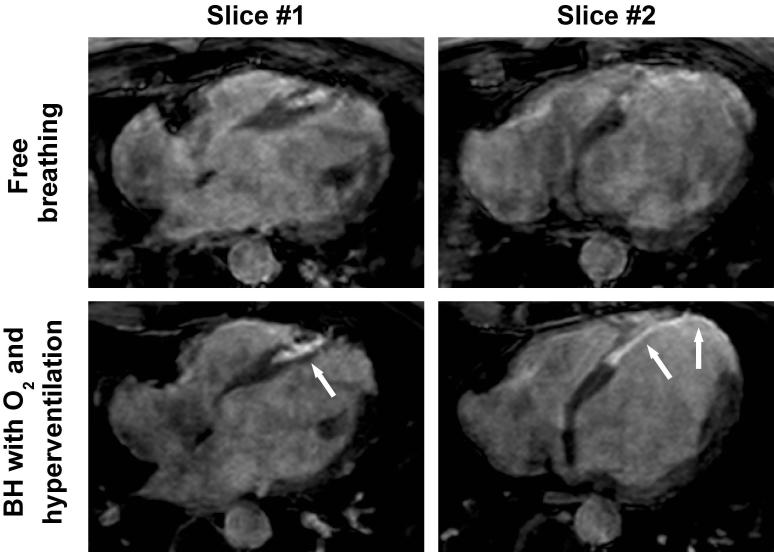
Figure 2 shows examples of 3D LGE images, acquired in three healthy subjects and three patients with both breath-hold and free-breathing acquisitions. Image quality obtained for both acquisitions appears similar in term of sharpness and motion/blurring artifact. Figure 3 shows two slices from the 3D LGE dataset acquired in a 44-year-old patient with a prior myocardial infarction using both free-breathing and breath-hold acquisitions. Both acquisitions were performed 45 minutes after injection of only 0.1 mmol/kg of contrast agent which resulted in reduced overall contrast when compared to other patient data in Figure 2. The free-breathing acquisition was completed in 2 min 26 s (25% gating efficiency). Severe motion artifacts are visible in all slices limiting accurate delineation of the scar area. The proposed 3D BH LGE provided improved image quality where the scar area could be defined in all slices.
Figure 2.
3D axial late gadolinium enhancement (LGE) images (undersampling rate=4) obtained in three healthy subjects and three patients using the breath-hold (BH) acquisition with supplemental oxygenation (O2) and hyperventilation and the free breathing acquisition. Similar image quality was obtained using both sequences.
Figure 3.
3D axial late gadolinium enhancement (LGE) images (undersampling rate=4) obtained in a 44-year-old gentleman with a history of myocardial infarction using free breathing NAV acquisition and BH acquisition with supplemental oxygen and hyperventilation. A 25% NAV gating efficiency resulted in total scan time of 2 min 26 s with images showing motion artifacts and incomplete myocardial nulling. The proposed 3D breath-hold (BH) LGE acquisition with supplemental oxygenation and hyperventilation provided better image quality with substantially reduced motion artifacts and myocardial nulling, allowing for better LGE assessment.
Table 2 summarizes the data from the healthy subjects, patients and the combined group. LGE signal enhancement was observed in only two patients. 3D LGE images were identified as diagnostic in all healthy subjects using both 3D LGE sequences. Three of 13 (23%) patients had non-diagnostic images using both acquisitions. Note that myocardial nulling was scored either 1 or 2 for both techniques in these 3 patients. Two additional patients had non-diagnostic images using the free-breathing acquisition and diagnostic images using the proposed 3D BH LGE sequence. No significant difference was found between the two sequences in terms of diagnostic value, motion artifact and myocardial nulling. Overall image quality in all subjects (healthy and patients) was similar for BH LGE and free breathing LGE (p=NS). Breath-hold LGE significantly reduced the overall scan time from 85±22 s (free breathing LGE) to 35±6 s (p<0.001).
Table 2.
Diagnostic value, image quality (overall, motion artifact, myocardial nulling), and acquisition time using free breathing and 3D breath-hold (BH) late gadolinium enhancement (LGE). Similar image quality is achieved using both sequences while 3D BH LGE provides a significant reduction of the acquisition time.
| Diagnostic (yes) |
Overall quality (1-4) |
Motion artifact (1-4) |
Myocardial nulling (1-4) |
Acquisition time (s) |
||
|---|---|---|---|---|---|---|
|
| ||||||
| Healthy | Free breathing LGE | 10/10 | 3.7±0.5 | 3.7±0.5 | 4.0±0.0 | 81±12 |
| BH LGE | 10/10 | 3.8±0.4 | 3.8±0.4 | 4.0±0.0 | 37±6 | |
| P value | NS | NS | NS | NS | <0.001 | |
|
| ||||||
| Patients | Free breathing LGE | 8/13 | 2.7±0.8 | 3.1±0.8 | 3.1±1.3 | 88±28 |
| BH LGE | 10/13 | 3.1±0.7 | 3.4±0.6 | 3.2±1.2 | 34±6 | |
| P value | NS | NS | NS | NS | <0.001 | |
|
| ||||||
| Overall | Free breathing LGE | 18/23 | 3.1±0.9 | 3.3±0.8 | 3.5±1.1 | 85±22 |
| BH LGE | 20/23 | 3.4±0.7 | 3.6±0.6 | 3.5±1.0 | 35±6 | |
| P value | NS | NS | NS | NS | <0.001 | |
|
| ||||||
DISCUSSION
In this study, we demonstrated the feasibility of a single breath-hold 3D LGE acquisition using a highly accelerated acquisition combined with supplemental oxygen and hyperventilation. The latter led to prolonged breath-holding performance in both healthy adults and patients with cardiovascular disease. For both healthy subjects and patients, the single breath-hold 3D LGE images were of similar quality when compared to a more conventional free breathing 3D respiratory NAV acquisition.
We observed a >50% increase in breath-hold duration when the breath-hold is performed with hyperventilation and supplemental oxygen. A finding in good agreement with prior data from Danias et al. (29) who noted an increase from 35 to 55 seconds in similar conditions. In the current study, we also observed that breath-hold training could provide an increase in BH duration; however these results would need to be confirmed in a larger subject group. We also showed that increase in breath-hold duration is attributed to supplemental oxygenation and hyper-ventilation of the subject and not to a placebo effect. These data suggest that supplemental oxygen and hyperventilation should be considered routine for optimum prolonged breath-hold performance and that a training breath-hold is beneficial.
Diaphragmatic position drift data were also in good agreement with previously published data of 6.6±3.5 mm (29). Despite our instructions for an end-expiratory breath-hold, NAV data demonstrated that our subject with the largest (18 mm) RHD drift held their breath at end-inspiration.
Inversion recovery LGE sequences are sensitive to RR interval variation. We found no significant difference in maximum variation of RR-interval with supplemental oxygen and hyperventilation. This data will need to be confirmed in a larger study.
Compared to previous 3D BH LGE approaches where the resolution in the third dimension was >8mm (3,17,18) using a relatively long acquisition window (180 ms to 326 ms) which are sensitive to motion artifacts, the proposed approach allows for increasing the resolution in the third dimension by a factor of ~2 while maintaining a shorter acquisition window of ~120 ms. Our MRI parameters also include a slightly longer TR (5.2 ms) when compared to prior 3D BH LGE studies (3.8-4.2 ms; (3,17,18)) which is expected to result in better SNR. SNR was not examined in our study due to CS reconstruction.
The employed inner linear-outer radial profile ordering technique allows for a virtual acquisition of the k-space center lines within a narrow gating window. Although, this profile reordering technique is expected to improve the robustness of the acquisition against RR interval variation, this was not evaluated in the study.
The scan time of the single breath-hold 3D LGE is approximately ~35 s with 1.6×1.6×5 mm3 spatial resolution and acquisition window of 120 ms. Although all healthy subjects and patients could sustain such long breath-hold, the proposed technique may not be applicable in all patients. For example, patients with chronic obstructive pulmonary disease or congestive heart failure may show reduced breath-holding abilities (37). Further acceleration is required to enable breath-hold 3D LGE in these patients. For patients who are unable to hold their breath, the free-breathing 3D LGE with NAV gating can still be used.
Quantitative or semi-quantitative assessment of LGE scar, including transmurality, scar volume, and the size of the peri-infarct zone have been widely used as metrics, with potential prognostic value (38). Higher spatial resolution LGE (28) could potentially improve our ability to better quantify these metrics, however higher resolution imaging is long and may not be needed if the patient does not have any scar. Thus, the proposed approach may have a role as an initial survey scan to determine the presence/absence of scar before initiating a longer dedicated 3D high-resolution scan. This will save 5-12 min of scan time in our current imaging protocol for scar assessment.
The physiology of breath-holding involves a complex mechanism driven by O2 and CO2 partial pressures in blood, which are detected by chemoreceptors in arteries and central nervous system (39-41). During breath-hold, the arterial pressure of oxygen falls while the carbon dioxide pressure increases. One hypothesis explaining breath-hold breakpoint is the detection of too low oxygen arterial partial pressure or too high carbon dioxide arterial pressure which would generate an involuntary breath. This hypothesis thus suggests the existence of a minimum O2 arterial pressure threshold and a maximum CO2 arterial pressure threshold. Supplemental oxygen may increase its partial pressure in the blood. Moreover, the hyperventilation induces a reduction of the carbon dioxide level in the blood (hypocapnia), which has an important role, as hypoxemia is thought to be less dyspnea-inducing than hypercapnia (39,40).
There are several limitations in this study. Variations in sex, age, contrast agent dose, and imaging time among healthy subjects and patients could have limited the assessment of differences in breath-hold metrics and LGE sequences. Our patient population was small and the impact of supplemental oxygen and hyperventilation needs to be further examined within the context of a much broader range of patients with cardiovascular disease. The number of subjects with LGE fibrosis/ scar was small and also needs to be confirmed in a larger series, as well as reproducibility of the prolonged single breath-hold as compared with RHD navigator gating. No quantitative LGE analysis was performed. Finally, we did not compare the breath-hold 3D LGE approach to conventional breath-hold 2D acquisitions.
CONCLUSION
Supplemental oxygenation and hyperventilation increase breath-hold duration by a factor of >1.5 without significant changes of diaphragm/LV position drift and maximum variation of RR interval. The proposed single breath-hold 3D accelerated LGE imaging using supplemental oxygenation and hyperventilation provides similar image quality as free breathing 3D accelerated LGE imaging with respiratory gating/tracking.
Supplementary Material
Acknowledgements
Supported by NIH R01EB008743-01A2.
REFERENCES
- 1.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 2.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 3.Goetti R, Kozerke S, Donati OF, Surder D, Stolzmann P, Kaufmann PA, Luscher TF, Corti R, Manka R. Acute, subacute, and chronic myocardial infarction: quantitative comparison of 2D and 3D late gadolinium enhancement MR imaging. Radiology. 2011;259(3):704–711. doi: 10.1148/radiol.11102216. [DOI] [PubMed] [Google Scholar]
- 4.Peters DC, Appelbaum EA, Nezafat R, Dokhan B, Han Y, Kissinger KV, Goddu B, Manning WJ. Left ventricular infarct size, peri-infarct zone, and papillary scar measurements: A comparison of high-resolution 3D and conventional 2D late gadolinium enhancement cardiac MR. J Magn Reson Imaging. 2009;30(4):794–800. doi: 10.1002/jmri.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TD, Spincemaille P, Weinsaft JW, Ho BY, Cham MD, Prince MR, Wang Y. A fast navigator-gated 3D sequence for delayed enhancement MRI of the myocardium: comparison with breathhold 2D imaging. J Magn Reson Imaging. 2008;27(4):802–808. doi: 10.1002/jmri.21296. [DOI] [PubMed] [Google Scholar]
- 6.Saranathan M, Rochitte CE, Foo TK. Fast, three-dimensional free-breathing MR imaging of myocardial infarction: a feasibility study. Magn Reson Med. 2004;51(5):1055–1060. doi: 10.1002/mrm.20061. [DOI] [PubMed] [Google Scholar]
- 7.Goldfarb JW, Shinnar M. Free-breathing delayed hyperenhanced imaging of the myocardium: a clinical application of real-time navigator echo imaging. J Magn Reson Imaging. 2006;24(1):66–71. doi: 10.1002/jmri.20609. [DOI] [PubMed] [Google Scholar]
- 8.Spuentrup E, Buecker A, Karassimos E, Gunther RW, Stuber M. Navigator-gated and real-time motion corrected free-breathing MR Imaging of myocardial late enhancement. Rofo. 2002;174(5):562–567. doi: 10.1055/s-2002-28271. [DOI] [PubMed] [Google Scholar]
- 9.Peters DC, Shaw JL, Knowles BR, Moghari MH, Manning WJ. Respiratory bellows-gated late gadolinium enhancement of the left atrium. J Magn Reson Imaging. 2012 doi: 10.1002/jmri.23954. In press. doi: 10.1002/jmri.23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghari MH, Peters DC, Smink J, Goepfert L, Kissinger KV, Goddu B, Hauser TH, Josephson ME, Manning WJ, Nezafat R. Pulmonary vein inflow artifact reduction for free-breathing left atrium late gadolinium enhancement. Magn Reson Med. 2011;66(1):180–186. doi: 10.1002/mrm.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47(2):372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber AM, Schoenberg SO, Hayes C, Spannagl B, Engelmann MG, Franz WM, Reiser MF. Phase-sensitive inversion-recovery MR imaging in the detection of myocardial infarction. Radiology. 2005;237(3):854–860. doi: 10.1148/radiol.2373041483. [DOI] [PubMed] [Google Scholar]
- 13.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51(1):93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai P, Larson AC, Park J, Carr JC, Li D. Respiratory self-gated four-dimensional coronary MR angiography: a feasibility study. Magn Reson Med. 2008;59(6):1378–1385. doi: 10.1002/mrm.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stehning C, Bornert P, Nehrke K, Eggers H, Stuber M. Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn Reson Med. 2005;54(2):476–480. doi: 10.1002/mrm.20557. [DOI] [PubMed] [Google Scholar]
- 16.Dewey M, Laule M, Taupitz M, Kaufels N, Hamm B, Kivelitz D. Myocardial viability: assessment with three-dimensional MR imaging in pigs and patients. Radiology. 2006;239(3):703–709. doi: 10.1148/radiol.2393050586. [DOI] [PubMed] [Google Scholar]
- 17.Foo TK, Stanley DW, Castillo E, Rochitte CE, Wang Y, Lima JA, Bluemke DA, Wu KC. Myocardial viability: breath-hold 3D MR imaging of delayed hyperenhancement with variable sampling in time. Radiology. 2004;230(3):845–851. doi: 10.1148/radiol.2303021411. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl HP, Papavasiliu TS, Beek AM, Hofman MB, Heusen NS, van Rossum AC. Myocardial viability: rapid assessment with delayed contrast-enhanced MR imaging with three-dimensional inversion-recovery prepared pulse sequence. Radiology. 2004;230(2):576–582. doi: 10.1148/radiol.2302021120. [DOI] [PubMed] [Google Scholar]
- 19.Bauner KU, Muehling O, Theisen D, Hayes C, Wintersperger BJ, Reiser MF, Huber AM. Assessment of Myocardial Viability with 3D MRI at 3 T. AJR Am J Roentgenol. 2009;192(6):1645–1650. doi: 10.2214/AJR.08.1394. [DOI] [PubMed] [Google Scholar]
- 20.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 21.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 22.van den Bosch HC, Westenberg JJ, Post JC, Yo G, Verwoerd J, Kroft LJ, de Roos A. Free-breathing MRI for the assessment of myocardial infarction: clinical validation. AJR Am J Roentgenol. 2009;192(6):W277–281. doi: 10.2214/AJR.08.1580. [DOI] [PubMed] [Google Scholar]
- 23.Kellman P, Larson AC, Hsu LY, Chung YC, Simonetti OP, McVeigh ER, Arai AE. Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn Reson Med. 2005;53(1):194–200. doi: 10.1002/mrm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52(15):1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 25.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 27.Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn Reson Med. 2007;57(6):1086–1098. doi: 10.1002/mrm.21236. [DOI] [PubMed] [Google Scholar]
- 28.Akcakaya M, Rayatzadeh H, Basha TA, Hong SN, Chan RH, Kissinger KV, Hauser TH, Josephson ME, Manning WJ, Nezafat R. Accelerated late gadolinium enhancement cardiac MR imaging with isotropic spatial resolution using compressed sensing: initial experience. Radiology. 2012;264(3):691–699. doi: 10.1148/radiol.12112489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danias PG, Stuber M, Botnar RM, Kissinger KV, Chuang ML, Manning WJ. Navigator assessment of breath-hold duration: impact of supplemental oxygen and hyperventilation. AJR Am J Roentgenol. 1998;171(2):395–397. doi: 10.2214/ajr.171.2.9694460. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy RM, Shea SM, Deshpande VS, Green JD, Pereles FS, Carr JC, Finn JP, Li D. Coronary MR angiography: true FISP imaging improved by prolonging breath holds with preoxygenation in healthy volunteers. Radiology. 2003;227(1):283–288. doi: 10.1148/radiol.2271011415. [DOI] [PubMed] [Google Scholar]
- 31.Klocke FJ, Rahn H. Breath holding after breathing of oxygen. J Appl Physiol. 1959;14:689–693. doi: 10.1152/jappl.1959.14.5.689. [DOI] [PubMed] [Google Scholar]
- 32.Marks B, Mitchell DG, Simelaro JP. Breath-holding in healthy and pulmonary-compromised populations: effects of hyperventilation and oxygen inspiration. J Magn Reson Imaging. 1997;7(3):595–597. doi: 10.1002/jmri.1880070323. [DOI] [PubMed] [Google Scholar]
- 33.Holland AE, Goldfarb JW, Edelman RR. Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology. 1998;209(2):483–489. doi: 10.1148/radiology.209.2.9807578. [DOI] [PubMed] [Google Scholar]
- 34.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Review of Scientific Instruments. 1970:41. [Google Scholar]
- 35.Wang Y, Riederer SJ, Ehman RL. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med. 1995;33(5):713–719. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- 36.Akcakaya M, Basha TA, Goddu B, Goepfert LA, Kissinger KV, Tarokh V, Manning WJ, Nezafat R. Low-dimensional-structure self-learning and thresholding: regularization beyond compressed sensing for MRI reconstruction. Magn Reson Med. 2011;66(3):756–767. doi: 10.1002/mrm.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gay SB, Sistrom CL, Holder CA, Suratt PM. Breath-holding capability of adults. Implications for spiral computed tomography, fast-acquisition magnetic resonance imaging, and angiography. Invest Radiol. 1994;29(9):848–851. [PubMed] [Google Scholar]
- 38.Ordovas KG, Higgins CB. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology. 2011;261(2):358–374. doi: 10.1148/radiol.11091882. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan GF, Richerson GB. Role of chemoreceptors in mediating dyspnea. Respir Physiol Neurobiol. 2009;167(1):9–19. doi: 10.1016/j.resp.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 41.Parkes MJ. Breath-holding and its breakpoint. Exp Physiol. 2006;91(1):1–15. doi: 10.1113/expphysiol.2005.031625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.