Abstract
Severe childhood epilepsy is commonly associated with intellectual developmental disabilities. The reasons for these cognitive deficits are likely multifactorial and will vary between epilepsy syndromes and even among children with the same syndrome. However, one factor these children have in common is the recurring seizures they experience - sometimes on a daily basis. Supporting the idea that the seizures themselves can contribute to intellectual disabilities are laboratory result demonstrating spatial learning and memory deficits in normal mice and rats that have experienced recurrent seizures in infancy. Studies reviewed here have shown that seizures in vivo and electrographic seizure activity in vitro both suppress the growth of hippocampal pyramidal cell dendrites. A simplification of dendritic arborization and resulting decrease in the number of excitatory synapses could help explain the observed cognitive disabilities. There are a wide variety of candidate mechanisms that could be involved in seizure-induced growth suppression. The challenge is designing experiments that will help focus research on a limited number of potential molecular events. Thus far results suggest that growth suppression is NMDA receptor-dependent and associated with a decrease in activation of the transcription factor CREB. The latter result is intriguing since CREB is known to play an important role in dendrite growth. Seizure-induced dendrite growth suppression may not occur as a single process in which pyramidal cells dendrites simply stop growing or grow slower compared to normal neurons. Instead, recent results suggest that after only a few hours of synchronized epileptiform in vitro dendrites appear to partially retract. This acute response is also NMDA receptor dependent and appears to be mediated by the Ca+2/calmodulin-dependent phosphatase, calcineurin. An understanding of the staging of seizure-induced growth suppression and the underlying molecular mechanisms will likely prove crucial for developing therapeutic strategies aimed at ameliorating the intellectual developmental disabilities associated with intractable childhood epilepsy.
Keywords: Epilepsy, Epileptiform Activity, Hippocampus, Learning, Memory
1. Introduction
Integrating the myriad of synaptic inputs that dendrites receive is critical for not only proper neuronal function but also network operations and ultimately animal and human behavior. For instance, neurons in somatosensory cortex assimilate not only peripheral information relayed through ascending sensory pathways but also local circuit synaptic inputs to interpret environmental stimuli. Within the hippocampus, physiological signals arising from the occiptal, temporal and parietal lobes, posterior cingulate cortex and the contralateral hippocampus all converge on hippocampal neurons through the medial, lateral perforant pathways and the anterior commissure (Aggleton, 2012). Within the hippocampus, additional synaptic integration arising from local recurrent excitatory networks and feedforward and recurrent inhibitory connections are thought to contribute to the acquisition of new episodic memories (Scoville & Milner, 1957; Schmolck et al., 2002). It is thought that the dendrites of principle excitatory neurons of the hippocampus and their attendant synapses play a particularly important role in learning and the formation of memories. The soma of these neurons have a characteristic pyramidal morphology with dendritic processes extending from the pyramidal apex and base termed apical and basolateral dendrites, respectively. Often in human epilepsy, alterations in these dendritic trees are observed. For instance, histopathological observations of pyramidal cells from tissue, surgically resected to stop seizures, have described markedly reduced dendrite branching complexity (Multani et al., 1994; von Campe et al., 1997). In addition, the small actin rich dendritic protrusions that receive presynaptic signals, termed spines, are often reduced in number and density throughout the dendritic tree (Swann et al., 2000). Such anatomical alterations could help to explain the cognitive disabilities often associated with intractable seizure disorders.
With the development of imaging technologies such as magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI), our appreciation of the neuroanatomical abnormalities associated with epilepsy has increased. Despite the variety of etiologies that can lead to the synchronous neuronal hyperactivity that characterizes seizures, volumetric alterations of the white and grey matter of the neocortex have been commonly observed (Hermann et al., 2002). For instance, in pediatric patients diagnosed with temporal lobe epilepsy, reduced white matter tracks (Kimiwada et al., 2006) of the temporal lobe have been observed. Additionally, pediatric epilepsy is also associated with reduced hippocampal and temporal grey matter volumes (Cormack et al., 2005). Some of these effects appear to be progressive as recent longitudinal studies have illustrated significant reductions in cortical white matter volume after a two year follow-up (Hermann et al., 2010). These results suggest that seizures impair normal brain growth which conceivably could contribute to learning and memory deficits.
Often, seizures are co-morbid with other debilitating neurodevelopmental disorders. For instance, the coincidence of seizures in Fragile X syndrome is ~20% and ~81% in pediatric patients diagnosed with Rett syndrome (Musumeci SA et al.; Incorpora et al., 2002; Berry-Kravis, 2002; Jian et al., 2006). Interestingly, even within the prototypical classical phenotype of Rett syndrome, a recent study found 30% of seizures to be treatment resistant (Pintaudi et al., 2010). Dendritic alterations in spine and dendritic morphology are observed in both of these syndromes (Belichenko et al., 1994; Irwin et al., 2001; Chapleau et al., 2009). For example, pathological samples of fragile X patients have revealed an increase in spine density but the spines appear to be immature or underdeveloped (Irwin et al., 2001). Conversely, dendritic spines are eliminated as Rett syndrome progresses following 6-18 months of normal dendrite growth and development (Chapleau et al., 2009). Interestingly, when the gene responsible for Rett syndrome, methyl-CpG binding protein 2 (Mecp2) is removed from mice following normal development, dendritic branching and spines density are reduced (Van den Veyver & Zoghbi, 2001; Nguyen et al., 2012); suggesting that Mecp2 is also important for proper maintenance of dendrites. Surprisingly, little is known about the combined effects of seizures with these neurodevelopmental syndromes. Whether seizures exacerbate the clinical conditions produced by these and other gene mutations is unknown. Nonetheless, the aberrations of the dendritic structure apparent in epilepsy, fragile X, and Rett Syndrome may contribute to the learning and memory disabilities observed in each clinical situation.
2. Synaptic Plasticity and Activity-Dependent Dendrite Growth: Shared Molecular Mechanisms
The molecular correlates of learned experiences involve both presynaptic changes that modify transmitter release and postsynaptic modifications of spines that cover the dendritic trees. In vivo and in vitro animal models utilizing hippocampal slices have demonstrated rapid (<30mins) increases in post synaptic spine density and spine head volume follow repetitive stimulation (Matsuzaki et al., 2004). These increases correlate with increased AMPA receptor subunit insertion and increased AMPAr channel conductances (Malenka & Bear, 2004). Modifications of the pre-synaptic machinery following a learned experience include increases in synaptic quantal size, release probability and the readily releasable pool (Emptage et al., 2003; Lauri et al., 2007). Together the pre- and post-synaptic modifications increase the efficiency of synaptic transmission and have been termed long term potentiation, LTP (Malenka & Bear, 2004). Conversely, by reducing the strength and duration of the applied stimulation, modifications such as increased AMPAr endocytosis and reductions in spine head volume are thought to limit the strength of synaptic efficiency (Winder & Sweatt, 2001; Colledge et al., 2003; Zhou et al., 2004). This has been referred to as long term depression (LTD) and together with LTP provide support for a Hebbian model of synaptic plasticity.
Our understanding of how changes in the activity of numerous kinases, phosphatases, and proteases are orchestrated to produce the biphasic alterations in synaptic efficacy of LTP and LTD have been greatly expanded over the past two decades. Upon the release of glutamate from presynaptic nerve terminals and its binding to post-synaptic AMPA receptors, the influx of sodium depolarizes the postsynaptic plasma membrane and alleviates the noncompetitive extracellular magnesium ion block of NMDA receptors, which subsequently allows calcium entry into the postsynaptic neuron. The elevation of calcium through NMDA receptor activation and voltage-dependent calcium channels leads to the activation of numerous, highly regulated signaling pathways culminating in the structural and molecular changes associated with synaptic plasticity. One widely studied molecule, the serine/threonine protein kinase, Ca2+/calmodulin-dependent protein kinase (CAMKII) is activated when calcium bound calmodulin alters the CaMKII conformation allowing for its autophospholyation at T286 (Kolodziej et al., 2000; Rellos et al., 2010; Lisman et al., 2012). Activated CAMKII then phosphorylates the AMPA binding protein stargazin allowing for its translocation to the post-synaptic density (Diaz, 2010). To accommodate the newly inserted AMPA receptors at the active zone, f-actin is thought to be polymerized and results in an increase in spine head volume and a concomitant potentiation of the synapse (Shen et al., 1998; Lin et al., 2005; Diaz, 2010).
Much like the activity-dependent synaptic alterations underlying LTP and LTD, synaptic activity is also thought to regulate dendrite development. Dendrites have been proposed to proceed through 3 distinct stages of development (Wu et al., 1999). Stage 1 is the initial stage of neuronal differentiation when an axon is formed and begins growth. During this stage, dendrites are rudimentary and demonstrate modest growth. Stage 2 is the period of very rapid dendrite growth. During stage 3, dendrite growth slows dramatically and dendritic arbor can also be remodeled by the pruning of some branches. Synaptic inputs during the early phase of dendrite development are initially localized to dendritic shafts. As stage 2 progresses, dendritic spines are formed and become a predominant location for excitatory synapses (Fiala et al., 1998). Time-lapse imaging in Xenopus and murine developmental models has also revealed dendrite and spine development as a highly stochastic process. For instance, time-lapse multi-photon and confocal imaging has repeatedly shown that dendritic filopodia, nascent spines and branches rapidly extend and retract in a seeming trial and error fashion (Dailey & Smith, 1996; Hua & Smith, 2004; Cline & Haas, 2008). New branch nodes appear to be stabilized by contact with presynaptic nerve terminal and a resulting accumulation of the postsynaptic proteins. The scaffolding protein PSD-95 has been particularly well studied in this regard (Marrs et al., 2001; Niell et al., 2004). Results like these support the synaptotrophic hypothesis of dendrite development, which extends the Hebbian model of synaptic plasticity and suggests that the activity of glutamatergic synapses on growing dendrites contribute to dendrite branch formation, selection and extension (Vaughn J.E. et al., 1988). Indeed, a body of literature ranging from visual deprivation in kittens to genetic manipulations and live cell imaging in Xenopus support the notion that synaptic activity is necessary for proper axon and dendrite growth and maintenance (Wiesel & Hubel, 1965; Cline & Haas, 2008). Recent studies of cultured hippocampal neuronal and cerebellar granule neurons in vivo have provided further support for a role for both CaMKII isoforms, α and β, in dendrite arbor development (Puram et al., 2011; Ghiretti et al., 2013). This follows the pioneering work of Wu and Cline in Xenopus that suggests a critical role for CAMKII in dendrite growth. (Wu & Cline, 1998; Zou & Cline, 1999) For instance, these experiments showed that the over-expression of a constitutively active form of CaMKII led to a significant decrease in dendritic branching complexity while the RNAi mediated knock down increased dendritic complexity and length (Puram et al., 2011; Ghiretti et al., 2013). These results further emphasize the importance of CaMKII is regulating dendrite arbor development.
3. The Impact of Seizures on Developing Dendrites and Learning and Memory
As reviewed earlier, individuals with a history of intractable epilepsy earlier in life have been reported to have reduced gray and white matter volumes (Hermann et al., 2002; Cormack et al., 2005). One possible explanation for these neuroanatomical abnormalities is that recurring seizures alter neuronal development. Since the plasticity of glutamatergic synapses on dendrites is thought to contribute in important ways to processes of learning and memory, if seizures could be shown to suppress dendrite growth, it could be one possible mechanism contributing intellectual disabilities.
To explore this possibility, it was first necessary to establish that early-life seizures in animal models also produce cognitive deficits. This has turned out to be the case since multiple laboratories over the past 20 years have shown that seizures in infancy lead to learning and memory deficits later in life. For example, when a single (kainate-induced) prolonged (3 hours) episode of intermittent seizures was induced in a p7 rat pups, deficits in working memory were observed in adulthood (Cornejo et al., 2007). Moreover, when brief recurrent seizures have been induced by the volatile convulsant, flurothyl, several laboratories have demonstrated dramatic impairments in hippocampal-based spatial learning and memory (Holmes et al., 1998; Lee et al., 2001; Cornejo et al., 2007; Nishimura et al., 2011). Most often spatial learning has been tested in a Morris water maze where hippocampal function is evaluated by the ability of rodents to learn the location of a submerged platform within a pool of water by utilizing spatial cues located in the testing environment. In keeping with the role of CA1 pyramidal cells in spatial learning are recent studies of seizure-induced alteration of the properties of CA1 hippocampal place cells (Karnam et al., 2009). Place cells are thought to provide an animal with a spatial map of its environment and serve as surrogates makers for spatial memory. Among other observations, the investigators showed that place cells were unable to form stable spatial maps in animals that experienced early-life seizures (Karnam et al., 2009). One simple explanation for the learning deficits could be that the seizures kill some hippocampal neurons. However, numerous investigators have shown this is not the case (Nitecka et al., 1984; Liu et al., 1999; Lee et al., 2001; Riviello et al., 2002; Nishimura et al., 2008).
With advances in molecular genetics our laboratory has been able to use mice engineered to express a green or yellow fluorescent protein (GFP or YFP) in subsets of hippocampal pyramidal cells in order to begin to explore the effects early-life seizures might have not only on dendrite morphology but also growth. In keeping with results from previous studies, when flurothyl was administered three times a day in 7 to 11 day-old mice, spatial learning deficits were observed when these animals were tested as adults (Nishimura et al., 2011). These effects were found to be restricted to animals having experienced neonatal seizures -- since learning and memory was unaffected when the same seizures were induced starting at postnatal day 30 (Nishimura et al., 2011).
To determine if recurring seizures in infancy actually altered dendrite growth, we conducted a detailed analysis of the basilar dendrites of CA1 pyramidal cells at 6 ages from postnatal day 6 to 30. GFP positive pyramidal cells were confocally imaged and their dendrites reconstructed from image stacks. Results showed that the CA1 dendrites from control animals underwent rapid progressive growth between P6 and P30. Total dendritic length increased as did the number of branch points (Figure 1). In contrast, the growth of dendrites from mice having undergone neonatal seizures was significantly suppressed. While growth during the days of seizure induction from P7-11 was unaffected, in the weeks after seizures, the dendritic length and number of branch points did not increase as they did in dendrites from control mice. Results from these experiments clearly support the hypothesis that seizures in early-life can suppress the growth of developing dendrites (Nishimura et al., 2011).
Figure 1.
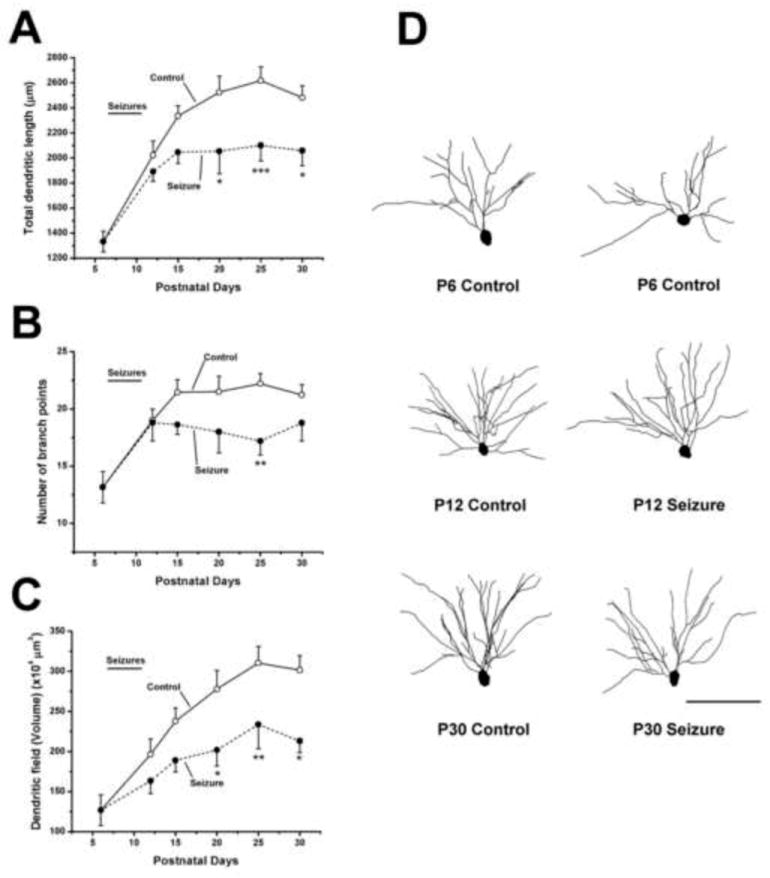
Growth of hippocampal dendrites is suppressed following the induction of seizures on postnatal days 7 through 11. Graphs summarize results from experiments in which the dendrites from control and flurothyl -treated CA1 hippocampal pyramidal cells were reconstructed and analyzed for differences in (A) total length, (B) numbers of branch points and (C) the volume of the dendritic field. Following 15 seizures between P7 to P11 (demarcated by a line labeled seizures above each graph) a dramatic decrease in the rate of dendrite growth were observed based on measures of the three parameters analyzed. (D) Representative Neurolucida reconstructions of dendrites of GFP positive CA1 pyramidal neurons at selected ages. The soma and basilar dendrites of these cells are shown and clearly illustrate that pyramidal cells from P30 control mice have more elaborate dendritic arbors than those from mice that experienced seizures in infancy. Differences in arbor complexity were not apparent at P12. Two control neurons from P6 mice are shown to illustrate baseline arbor branching and length. Results are presented as mean ± S.E.M. Control: n was between 12 and 29 neurons per age group, Seizure: n was 9–25 per age group.* p ≤ 0.05, ** p ≤ 0.01, *** p≤ 0.001 when comparing seizure groups to controls at the same age. Scale bar = 100 μm
To further explore the mechanisms of seizure-induced dendrite growth suppression, we utilized a hippocampal slice culture model. In these experiments, hippocampal cultures were prepared from P5 or P6 animals and cultured for up to one week. A subset of slices received bicuculline (100μM) in the culture media after three days in culture and were disinhibited throughout the duration of the experiment. Since recurrent excitatory synaptic transmission is sufficiently developed by this age, pharmacological blockade of GABAa receptors readily induces epileptiform ictal-like, activity. Given that slices were prepared from infant mice our hope was that dendrites would be growing at this time and thus we could examine the effects of seizure-like activity in vitro on dendrite growth. For these experiments we used slices from mice that expressed YFP in CA1 pyramidal cells. When dendrites from control (no bicuculline) slice cultures were reconstructed from confocal stacks, results demonstrated (as we had hoped) a rapid growth of CA1 pyramidal cell dendrites from DIV 3-7. Basilar dendrites grew both in length and branching complexity (Nishimura et al., 2008). In contrast, after as little as 24 hours of epileptiform activity, the length and branching complexity of slices treated with bicuculline was reduced and dendrites failed to grow over the next 4 days (Nishimura et al., 2008). Thus, much like our in vivo data, synchronized neuronal hyperactivity in slice cultures also suppresses the growth of developing dendrites.
Consistent with our anatomical results, whole cell electrophysiological recordings from pyramidal cells following chronic bicuculline treatment (7 days) showed reductions in the frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) mediated by both AMPA (2-amino-3-3-hydroxy-5-methyl-isoxazol-4-yl propanoic acid) and NMDA (N-methyl-D-aspartate) receptors (Swann et al., 2007a). In addition, both in vivo and in vitro models showed decreased expression of glutamatergic synaptic protein markers (Swann et al., 2007a; Swann et al., 2007b) as well as the synaptic scaffolding protein PSD-95 (post-synaptic density 95). Thus, results suggest that there is a reduction in the number of glutamatergic synapses on dendrites as a consequence of the seizure induced growth suppression of the dendritic arbors.
4. Molecular Mechanisms of Dendrite Growth
In recent years, a great deal has been learned about the basic mechanisms of dendrite growth (McAllister, 2000; de & Bonni, 2011; Bonini et al., 2011; Arikkath, 2012). Cell-intrinsic molecular programs have been shown to regulate dendrite development and are recognized to be highly complex, likely interactive and not fully understood. Classes of molecules shown to regulate dendrite development include: 1) secreted factors such as neurotrophins and cell adhesion molecules (Tan et al., 2010; Baj et al., 2011), 2) synaptic scaffolding proteins, e.g. PSD95 and Cypin (Charych et al., 2006; Kwon et al., 2011), 3) signaling molecules such as CAM Kinase 2b and CDK5 (Su & Tsai, 2011; Puram et al., 2011) 4) the cytoskeletal regulators Rac/Rho/Cdc42 (Chen & Firestein, 2007) and 5) transcription factors such as CREB and Nf-kB (Wayman et al., 2006; Bonini et al., 2011). With regards to CREB, several CREB binding partners have been identified and include CBP, CREST and CRTC1, which are co-activators of gene transcription and co-regulators of dendrite growth (Redmond et al., 2002; Aizawa et al., 2004; Li et al., 2009; Finsterwald et al., 2010). Moreover, molecules previously identified as critical players in other aspects of brain development are now though to contribute to dendrite growth. For instance, recently a number of extracellular secreted proteins, such as netrins, semaphorins and wnt, traditionally thought to influence axonal guidance, have also been implicated in dendrite development (Shelly et al., 2011; Smith et al., 2012; Niisato et al., 2013). In addition, notch signaling, which is known to be involved in non-autonomous cell proliferation, appears to contribute to dendritic development (Breunig et al., 2007; Terabayashi et al., 2007; Bonini et al., 2011).
5. Dendritic Growth Suppression: Roles for NMDA Receptors and the Transcription Factor CREB
Considering the growing list of potential candidate molecular mechanisms that could mediate seizure-induced dendrite growth suppression, it has become important to design experiments whose outcomes would assist in focusing future efforts on a limited number of potential molecular mechanisms. As previously reviewed, neuronal activity has been shown to play critical roles in dendrite development. Thus we suspected that seizure-induced growth suppression was also an activity-dependent process. When TTX was co-applied with bicuculline to slice cultures it is perhaps not very surprising that blockade of action potential sodium channels would inhibit seizure-induced growth suppression since TTX prevents seizure generation. However, when the NMDA receptor antagonist APV (2-amino-5-phosphonovaleric acid) was co-applied with bicuculline, seizure activity was unaltered yet the effects of seizure-like activity on dendrite growth suppression was abolished (Swann et al., 2007a; Nishimura et al., 2008). APV was also found to prevent seizure-induced alterations in the expression of a number of glutamatergic postsynaptic proteins including PSD95 (Swann et al., 2007a). Thus seizure induced growth suppression of hippocampal CA1 dendrites appears to be a NMDA receptor dependent process.
Calcium signaling though NMDA receptors triggers a variety of signaling mechanisms that impact a large number of biological processes from the cytoskeletal dynamics of dendrites to the transcription of genes important in dendrite growth. Brain derived neurotrophic factor (BDNF) is but one example of molecules whose expression is regulated by neuronal activity and that can induce the growth of pyramidal cells dendrites (McAllister et al., 1995; McAllister et al., 1996; Horch & Katz, 2002; Dijkhuizen & Ghosh, 2005). BDNF transcription has also been shown to be both calcium and NMDA receptor dependent (Hong et al., 2008). While BDNF transcription is regulated by numerous transcription factors including MeCP2 and MEF2, it is also regulated by CREB (Chen et al., 2003). To assess activation of CREB in our slice culture model we employed fluorescence immunohistochemistry to visualize pCREB (Ser-133) in hippocampal slice cultures after 4 days of bicuculline treatment (Figure 2). This is a time when dendrite growth is suppressed. Our results showed a dramatic reduction in the levels of pCREB in the nuclei of hippocampal pyramidal cells (Nishimura et al. 2008). Thus it is possible that suppression of signaling to CREB, which is well known to be important to dendrite growth, contributes to seizure-induced dendrite growth suppression. How seizure activity suppresses signaling to CREB is unknown at this time but suppression of upstream signaling cascades such as CaMKII, CaMKIV or MAPK pathways could play a role (Sheng et al., 1991; Wu & McMurray, 2001). Alternatively activation of a phosphatases such as serine/threonine phosphatase 1 (PP1) could dephosphorylate CREB and consequently suppress gene transcription (Bito et al., 1996; Redmond et al., 2002).
Figure 2.
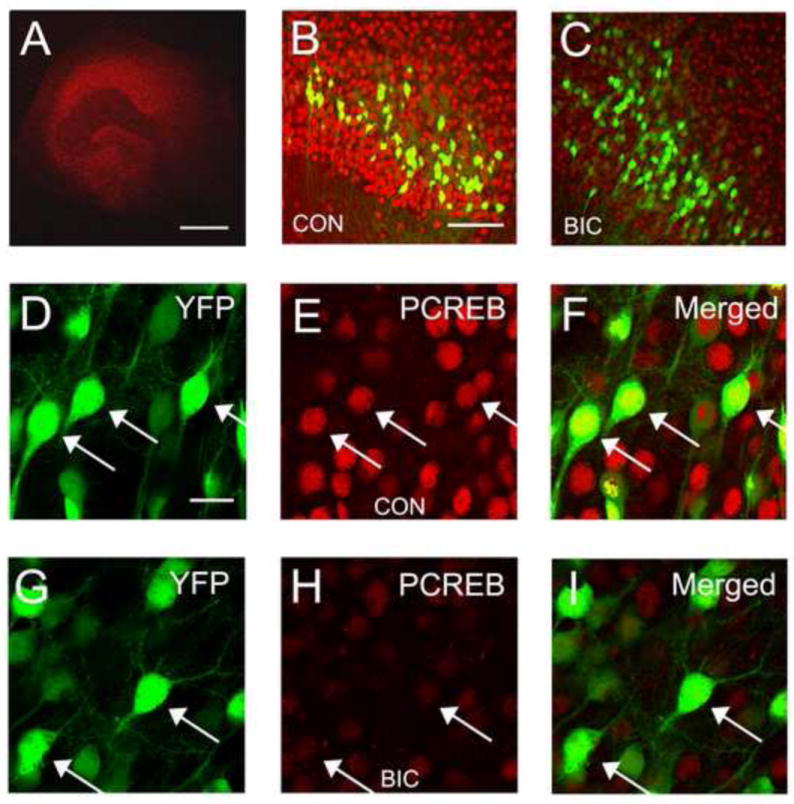
Chronic disinhibition reduces levels of phosphorylated CREB (pCREB) in CA1 hippocampal pyramidal cells. (A) Low magnification confocal image of a hippocampal slice culture showing typical pattern of immunohistochemical staining for pCREB. (B and C) Comparison of pCREB staining in hippocampal area CA1 in a control slice culture (B) and a slice treated for 4 days with bicuculline (C). Notice the dramatic reduction in expression levels of pCREB. Also shown are the subpopulation of CA1 pyramidal cells that express YFP in YFP-H mice. (D-F) The nuclear localization of pCREB is YFP-positive CA1 pyramidal cells is clearly illustrated in merged images from a control slice. (G-I) Following 4 days of bicuculline treatment pCREB immunoreactivity in CA1 pyramidal cells remains nuclear but is greatly reduced in intensity. The majority of pCREB positive –YFP negative cells are CA1 pyramidal cells that do not express YFP. Arrow heads denote sites of co-localization. Scale bars: A = 500 μm. B and C = 100 μm. D – I = 20 μm.
6. Early Events in Dendrite Growth Suppression
The activity-dependent alterations in dendrite anatomy and particularly spine morphology that accompanies LTP and LTD are well know to take place rapidly - over minutes and hours. Until recently, our studies of dendrite growth suppression have been on a time scale of many hours and days. Our thinking has been that seizure-induced dendrite growth suppression is likely a form of neuronal adaptation and that in response to intense and recurring seizures, pyramidal cells gradually compensate in an attempt to normalize neuronal and network excitability. Conceptually, this is reminiscent of studies of homeostatic plasticity in which synaptic strength and intrinsic neuronal excitability are thought to slowly adapt over periods of days to perturbations in network activity (Turrigiano & Nelson, 2004). For instance, when GABAa receptors are blocked in dissociated cultures, neuronal activity initially increases but over several days it returns to baseline levels (Turrigiano et al., 1998; Turrigiano & Nelson, 2004). This has been shown to be mediated at least in part by a reduction in the strength of excitatory synaptic transmission. However, despite the solid rationale for parallels between our studies of seizures and those of homeostatic plasticity, the marked difference in the intensity of network activity that occur during seizures lead us to examine the time course of the effects of seizure-like activity on hippocampal dendrites. Our initial experiments examined alterations in the expression of glutamatergic synaptic biomarkers NR2A and PSD95, which we had shown previously, were suppressed by prolonged treatment of slice cultures with bicuculline (Swann et al., 2007a). We were surprised to find a marked 30-40% decreases in synaptic protein expression after only four hours of network hyperactivity (Casanova et al., 2013). In parallel experiments, the reduction in synaptic markers corresponded with a decrease in the intensity of synchronized network activity. Network excitability as measured by the average duration of individual network bursts as well as the percent time networks were engaged in epileptiform activity were both reduced approximately 40% after four hours of bicuculline treatment. This rapid form of network adaptation was accompanied by a similar 50% reduction in the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) impinging on individual CA1 pyramidal neurons. One possibile explanation for these biochemical and electrophysiological observations was that the growth of the dendritic arbors were suppressed after only 4 hours of seizure-like activity.
However, our estimates of dendrite growth in slice cultures indicated that in 4 hours dendrite would only add on average ~50μm in length to the basilar dendrites which had an initial length of ~1000μm (Nishimura et al., 2008). If dendrite growth was completely suppressed we might anticipate only a 5% decrease in dendrite length – an order of magnitude less than the biochemical and electrophysiological differences observed. However, when the dendrites of CA1 neurons exposed to bicuculline for 4 hours were reconstructed, the number of branch points and total dendritic lengths were significantly decreased. Dendrite length was decreased ~230μm, nearly 5 fold more than would be expected due to growth suppression alone (Casanova et al., 2013). This leads us to suspect that seizure activity in early-life may initially induce dendrite branch retraction, which may be a forerunner of longer term growth suppression (Nishimura et al., 2008; Casanova et al., 2013).
The precise cellular and molecular mechanisms leading to this simplification of the hippocampal dendrite arbors are not known. However, experiments have shown that at least the reduction in the expression of synaptic markers is NMDA receptor dependent (Casanova et al. 2013). One calcium-dependent signaling molecule that is regulated by neuronal activity and capable of regulating dendrite cytoskeletal dynamics is the Ca+2/calmodulin-dependent serine/threonine phosphatase, calcineurin (Mulkey et al., 1994; Winder & Sweatt, 2001; Sarmiere & Bamburg, 2004). For example, studies have shown that LTD induction results in dendrite spines collapse and this is blocked by calcineurin antagonists such as FK506 and cyclosporine A (Zhou et al., 2004). In this regard, it is important to mention that under certain experimental preparations, LTD can be induced by epileptiform activity in hippocampal slices taken from immature animals (Smith & Swann, 1999) and that calcineurin activity is increased after seizures in severe adult animal models of epilepsy (Kurz et al., 2003; Sanchez et al., 2005). Thus we undertook experiments to examine a role for calcineurin in seizure-induced network adaptation and alterations in dendrite anatomy. Remarkably, when hippocampal slice cultures were co-treated with bicuculline and the calcineurin antagonist FK506 for four hours, all measures of neuronal and network adaptation induced by epileptiform activity were prevented. Antagonizing calcineurin prevented the decreases of the glutamatergic synaptic proteins PSD-95, NR2A, and NR1(Casanova et al., 2013). Functionally, the degree of network adaptation measured by percent time engaged in epileptiform activity was also prevented by FK506. Moreover, Figure 3 also suggests that calcineurin mediates the reduction in dendritic arborization induced by epileptiform activity since FK506 prevented both the reduction in branch points and total dendritic length of CA1 basilar dendrites (Casanova et al., 2013).
Figure 3.
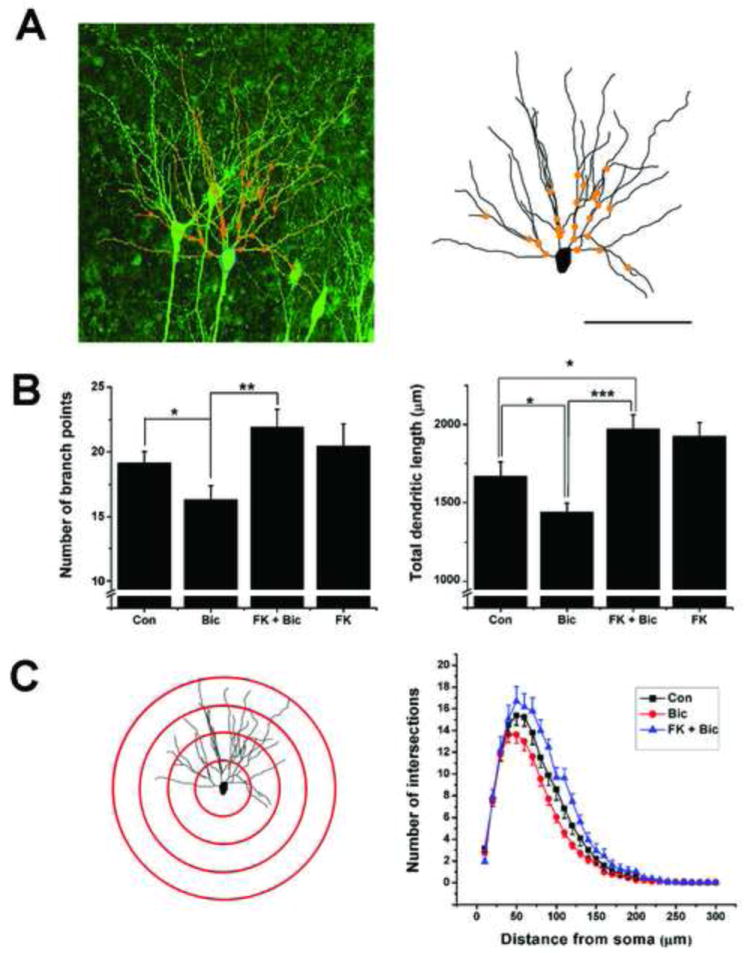
The calcineurin inhibitor, FK506, prevents bicuculline-induced reductions in dendritic arborization. (A) Image of CA1 hippocampal pyramidal cells in a slice culture taken from a GFP-M mouse. Superimposed on the image of one neuron is the computer reconstruction of its basilar dendrites (orange). Branch points are denoted by orange dots. For further clarity, this dendrite is shown to the right in A. (B). Bar graphs comparing basilar dendrite length and number of branch points in pyramidal cells. A 4 hour treatment with bicuculline produced a significant decrease in both branch number and dendrite length. This effect was abolished by co-treatment with FK506. FK506 alone increased dendrite length - likely reflecting calcineurin’s ability to partially suppress on-going dendrite growth. (C) Sholl analysis: left hand panel illustrates this type of analysis where the number of dendritic branches that intersect equally spaced concentric spheres were counted. In this drawing, cross sections of 4 spheres at 50μm intervals from the soma are shown. Right panel: bicuculline reduces the number of branch intersections at nearly all distances from the soma. FK506 blocks these effects of disinhibition on dendritic arbors. For clarity, data for FK506-only treatment are not plotted but did not differ from the FK+Bic plot. While bicuculline reduced both the length and number of dendrite branches, FK506 prevented these effects. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. n=13-25. For Sholl analysis a 2-way ANOVA with posthoc Scheffe test showed: Con vs Bic: F1, 1272 = 32.2, P ≤ 0.001: Bic vs Fk + Bic: F1, 971 = 66.9, P ≤ 0.001: Con vs FK + Bic: F1, 949 = 12.1, P ≤ 0.001) Scale bar in A = 100μm.
7. Conclusions
While the intellectual developmental disabilities associated with severe childhood epilepsy are a long-standing clinical problem, we are just beginning to understand the factors that contribute to these cognitive deficits. Research in animal models strongly supports the hypothesis that recurring seizures contribute to learning and memory deficits. Research from our laboratory has shown that seizures can suppress dendrite growth, which may occur in stages with acute dendrite retraction being a forerunner of longer-termed growth suppression. Since the activity-dependent plasticity of glutamatergic synapses (e.g. LTP and LTD) on hippocampal pyramidal cell dendrites are thought to be an underlying mechanisms of hippocampus-based learning and memory, a decrease in synapse number due to a reduction in dendritic arborization could be one mechanism contributing to learning disabilities. Dendrite growth suppression may be a way developing neurons attempt to compensate for the network hyperexcitability of epilepsy. By reducing arbor size and the number of glutamatergic synapses on neurons, network excitability could be reduced and seizures may become less frequent and severe. However, by eliminating excitatory synapses, which are thought to be anatomical substrates for learning and memory, these adaptive and presumably neuroprotective mechanisms could also result in deficiencies in learning and memory.
Acknowledgments
The work of our laboratory is supported by grants from NIH: NS018309, NS062992 and HD024064 and CURE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neuroscience & Biobehavioral Reviews. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Arikkath J. Molecular mechanisms of dendrite morphogenesis. Front Cell Neurosci. 2012;6:61. doi: 10.3389/fncel.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G, Leone E, Chao MV, Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci U S A. 2011;108:16813–16818. doi: 10.1073/pnas.1014168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Oldfors A, Hagberg B, Dahlstrom A. Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport. 1994;5:1509–1513. [PubMed] [Google Scholar]
- Berry-Kravis E. Epilepsy in fragile X syndrome. Developmental Medicine & Child Neurology. 2002;44:724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB Phosphorylation and Dephosphorylation: A Ca2+ and Stimulus Duration–Dependent Switch for Hippocampal Gene Expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bonini SA, Ferrari-Toninelli G, Uberti D, Montinaro M, Buizza L, Lanni C, Grilli M, Memo M. Nuclear factor kappaB-dependent neurite remodeling is mediated by Notch pathway. J Neurosci. 2011;31:11697–11705. doi: 10.1523/JNEUROSCI.1113-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JR, Nishimura M, Le JT, Tran TT, Swann JW. Rapid Hippocampal Network Adaptation to Recurring Synchronous Activity - a Role for Calcineurin. European Journal of Neuroscience. 2013 doi: 10.1111/ejn.12315. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiology of Disease. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci. 2006;26:10164–10176. doi: 10.1523/JNEUROSCI.2379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Firestein BL. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci. 2007;27:8378–8386. doi: 10.1523/JNEUROSCI.0872-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF Transcription Involves Calcium-Dependent Phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. The Journal of Physiology. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langerberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack F, Gadian DG, Vargha-Khadem F, Cross JH, Connelly A, Baldeweg T. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. NeuroImage. 2005;27:635–643. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de lT-U, Bonni A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron. 2011;72:22–40. doi: 10.1016/j.neuron.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. European Journal of Neuroscience. 2010;32:261–268. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen PA, Ghosh A. BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J Neurobiol. 2005;62:278–288. doi: 10.1002/neu.20100. [DOI] [PubMed] [Google Scholar]
- Dong-Jing Zou, Hollis TCline. Postsynaptic calcium/calmodulin-dependent protein kinase ll is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. The Journal of Neuroscience. 1999;19:8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A, Bliss TVP. Optical Quantal Analysis Reveals a Presynaptic Component of LTP at Hippocampal Schaffer-Associational Synapses. Neuron. 2003;38:797–804. doi: 10.1016/s0896-6273(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C, Fiumelli H, Cardinaux JR, Martin JL. Regulation of dendritic development by BDNF requires activation of CRTC1 by glutamate. J Biol Chem. 2010;285:28587–28595. doi: 10.1074/jbc.M110.125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiretti AE, Kenny K, Marr MT, Paradis S. CaMKII-Dependent Phosphorylation of the GTPase Rem2 Is Required to Restrict Dendritic Complexity. The Journal of Neuroscience. 2013;33:6504–6515. doi: 10.1523/JNEUROSCI.3861-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Dabbs K, Becker T, Jones JE, Gutierrez A, Wendt G, Koehn MA, Sheth R, Seidenberg M. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51:2038–2046. doi: 10.1111/j.1528-1167.2010.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- Holmes G, Gairsa J-L, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizure in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A Biological Function for the Neuronal Activity-Dependent Component of Bdnf Transcription in the Development of Cortical Inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Incorpora G, Sorge G, Sorge A, Pavone L. Epilepsy in fragile X syndrome. Brain Dev. 2002;24:766–769. doi: 10.1016/s0387-7604(02)00102-x. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Bower C, Anderson A, Williamson S, Christodoulou J, Leonard H. Predictors of seizure onset in Rett syndrome. The Journal of Pediatrics. 2006;149:542–547. doi: 10.1016/j.jpeds.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimiwada T, Juhász C, Makki M, Muzik O, Chugani DC, Asano E, Chugani HT. Hippocampal and Thalamic Diffusion Abnormalities in Children with Temporal Lobe Epilepsy. Epilepsia. 2006;47:167–175. doi: 10.1111/j.1528-1167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional Reconstructions of Calcium/Calmodulin-dependent (CaM) Kinase IIß and Truncated CaM Kinase IIß Reveal a Unique Organization for Its Structural Core and Functional Domains. Journal of Biological Chemistry. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- Kurz JE, Rana A, Parsons JT, Churn SB. Status epilepticus-induced changes in the subcellular distribution and activity of calcineurin in rat forebrain. Neurobiol Dis. 2003;14:483–493. doi: 10.1016/j.nbd.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Kwon M, Fernandez JR, Zegarek GF, Lo SB, Firestein BL. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J Neurosci. 2011;31:9735–9745. doi: 10.1523/JNEUROSCI.6785-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Palmer M, Segerstrale M, Vesikansa A, Taira T, Collingridge GL. Presynaptic mechanisms involved in the expression of STP and LTP at CA1 synapses in the hippocampus. Neurophar. 2007;52:1–11. doi: 10.1016/j.neuropharm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neurosci. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang C, Takemori H, Zhou Y, Xiong ZQ. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J Neurosci. 2009;29:2334–2343. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta Stimulation Polymerizes Actin in Dendritic Spines of Hippocampus. The Journal of Neuroscience. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature Reviews Neuroscience. 2012;13:168–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang L-T, Stafstrom CE, Holmes GL. Consequences of recurrent seizures during early brain development. Neurosci. 1999;92:1443–1454. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci. 2001;4:1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Multani P, Myers RH, Blume HW, Schomer DL, Sotrel A. Neocortical dendritic pathology in human partial epilepsy: a quantitative Golgi study. Epilepsia. 1994;35:728–736. doi: 10.1111/j.1528-1157.1994.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, FAU, Hagerman RJ, FAU, Ferri RF, Bosco PF, la Bernardina BF, Tassinari CA, FAU, De Sarro, De Sarro GB, FAU, Elia, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Nguyen MVC, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N. MeCP2 Is Critical for Maintaining Mature Neuronal Networks and Global Brain Anatomy during Late Stages of Postnatal Brain Development and in the Mature Adult Brain. The Journal of Neuroscience. 2012;32:10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Niisato E, Nagai J, Yamashita N, Nakamura F, Goshima Y, Ohshima T. Phosphorylation of CRMP2 is involved in proper bifurcation of the apical dendrite of hippocampal CA1 pyramidal neurons. Devel Neurobio. 2013;73:142–151. doi: 10.1002/dneu.22048. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Gu X, Swann JW. Seizures in early life suppress hippocampal dendrite growth while impairing spatial learning. Neurobiol Dis. 2011;44:205–214. doi: 10.1016/j.nbd.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Owens J, Swann JW. Effects of chronic network hyperexcitability on the growth of hippocampal dendrites. Neurobiol Dis. 2008;29:267–277. doi: 10.1016/j.nbd.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka LF, Tremblay E, FAU, Charton Charton GF, Bouillot JP, FAU, Berger ML, FAU, Ben-Ari, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II Histopathological sequelae. Neurosci. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Pintaudi M, Calevo MG, Vignoli A, Parodi E, Aiello F, Baglietto MG, Hayek Y, Buoni S, Renieri A, Russo S, Cogliati F, Giordano L, Canevini M, Veneselli E. Epilepsy in Rett syndrome: Clinical and genetic features. Epilepsy & Behavior. 2010;19:296–300. doi: 10.1016/j.yebeh.2010.06.051. [DOI] [PubMed] [Google Scholar]
- Puram SV, Kim AH, Ikeuchi Y, Wilson-Grady JT, Merdes A, Gygi SP, Bonni A. A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat Neurosci. 2011;14:973–983. doi: 10.1038/nn.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium Regulation of Dendritic Growth via CaM Kinase IV and CREB-Mediated Transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Rellos P, Pike ACW, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S. Structure of the CaMKIIδ/Calmodulin Complex Reveals the Molecular Mechanism of CaMKII Kinase Activation. PLoS Biol. 2010;8:e1000426. doi: 10.1371/journal.pbio.1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviello P, de Rogalski Landrot I, Holmes GL. Lack of cell loss following recurrent neonatal seizures. Developmental Brain Research. 2002;135:101–104. doi: 10.1016/s0165-3806(02)00302-4. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J Neurobiol. 2004;58:103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H.M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002;12:520–533. doi: 10.1002/hipo.10039. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hioopcampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Lim B, Popescu AT, Cheng Pl, Gao H, Poo Mm. Semaphorin3A Regulates Neuronal Polarization by Suppressing Axon Formation and Promoting Dendrite Growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIß Functions As an F-Actin Targeting Module that Localizes CaMKIIα/ß Heterooligomers to Dendritic Spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Watson JD, VanHoven MK, Colon-Ramos DA, Miller DM. Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci. 2012;15:731–737. doi: 10.1038/nn.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, Swann JW. Long-term depression of perforant path excitatory postsynaptic potentials following synchronous network bursting in area CA3 of immature hippocampus. Neurosci. 1999;89:625–630. doi: 10.1016/s0306-4522(98)00651-4. [DOI] [PubMed] [Google Scholar]
- Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Swann JW, Al Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Swann JW, Le JT, Lam TT, Owens J, Mayer AT. The impact of chronic network hyperexcitability on developing glutamatergic synapses. Eur J Neurosci. 2007a;26:975–991. doi: 10.1111/j.1460-9568.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- Swann JW, Le JT, Lee CL. Recurrent seizures and the molecular maturation of hippocampal and neocortical glutamatergic synapses. Dev Neurosci. 2007b;29:168–178. doi: 10.1159/000096221. [DOI] [PubMed] [Google Scholar]
- Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci U S A. 2010;107:9873–9878. doi: 10.1073/pnas.1003480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabayashi T, Itoh TJ, Yamaguchi H, Yoshimura Y, Funato Y, Ohno S, Miki H. Polarity-Regulating Kinase Partitioning-Defective 1/Microtubule Affinity-Regulating Kinase 2 Negatively Regulates Development of Dendrites on Hippocampal Neurons. The Journal of Neuroscience. 2007;27:13098–13107. doi: 10.1523/JNEUROSCI.3986-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Dasai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:845–846. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Van den Veyver IB, Zoghbi HY. Mutations in the gene encoding methyl-CpG-binding protein 2 cause Rett syndrome. Brain Dev. 2001;23(Supplement 1):S147–S151. doi: 10.1016/s0387-7604(01)00376-x. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Barber RP, Sims TJ. Dendritic development and preferential growth into synaptogenic fields: a quantitative study of Golgi-impregnated spinal motor neurons. Synapse. 1988;2:69–78. doi: 10.1002/syn.890020110. [DOI] [PubMed] [Google Scholar]
- von Campe G, Spencer DD, de Lanerolle NC. Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus. 1997;7:472–488. doi: 10.1002/(SICI)1098-1063(1997)7:5<472::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampel synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci. 1999;19:4472–4483. doi: 10.1523/JNEUROSCI.19-11-04472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, McMurray CT. Calmodulin Kinase II Attenuation of Gene Transcription by Preventing cAMP Response Element-binding Protein (CREB) Dimerization and Binding of the CREB-binding Protein. Journal of Biological Chemistry. 2001;276:1735–1741. doi: 10.1074/jbc.M006727200. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of Dendritic Spines Associated with Long-Term Depression of Hippocampal Synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]


