Abstract
Background
Surgical resection is the preferred treatment modality for eligible candidates with non-small cell lung cancer (NSCLC). However, the selection of sublobar resection versus lobectomy for early-stage NSCLC remains controversial. Previous meta-analyses comparing these two procedures presented data without considering the significant differences in the patient selection processes in individual studies. The present study aimed to compare the overall survival (OS) and disease-free survival (DFS) outcomes of patients who underwent sublobar resections who were also eligible for lobectomy procedures with those who underwent lobectomy.
Methods
An electronic search was conducted using five online databases from their dates of inception to December 2013. Studies were selected according to predefined inclusion criteria and meta-analyzed using hazard ratio (HR) calculations.
Results
Twelve studies met the selection criteria, including 1,078 patients who underwent sublobar resections and 1,667 patients who underwent lobectomies. From the available data, there was no significant differences in OS [HR 0.91; 95% confidence interval (CI) 0.64-1.29] or DFS (HR 0.82; 95% CI 0.60-1.12) between the two treatment arms. In addition, no significant OS difference was detected for patients who underwent segmentectomies compared to lobectomies (HR 1.04; 95% CI 0.66-1.63, P=0.86).
Conclusions
Using the available data in the current literature, patients who underwent sublobar resection for small, peripheral NSCLC after intentional selection rather than ineligibility for greater resections achieved similar long-term survival outcomes as those who underwent lobectomies. However, patients included for the present meta-analysis were a highly selected cohort and these results should be interpreted with caution. The importance of the patient selection process in individual studies must be acknowledged to avoid conflicting outcomes in future meta-analyses.
Keywords: Sublobar resection, segmentectomy, non-small cell lung cancer (NSCLC), meta-analysis
Introduction
The primary and preferred treatment of early stage non-small cell lung cancer (NSCLC) remains to be surgical resection for eligible candidates. Traditionally, this was performed by lobectomy or greater resection procedures (1). However, sublobar resections in the form of wedge resections or segmentectomies have been reported as an alternative surgical technique, especially in patients with significant comorbidities or limited pulmonary function.
Conflicting outcomes for sublobar resections versus lobectomies have been reported previously, and the issue remains controversial, despite a randomized-controlled trial published by the Lung Cancer Study Group (LCSG) in 1995 (2). Importantly, differences in patient selection and baseline characteristics in the two treatment groups have obscured the evidence for these surgical approaches. It is important to recognize that survival outcomes of patients who were allocated to sublobar resections due to significant comorbidities rather than intentional selection must be vastly different, and any analysis must take into account of the patient selection process to either the lobectomy or sublobar resection groups.
The aim of the present meta-analysis was to compare the overall survival (OS) and disease-free survival (DFS) outcomes of patients who underwent either a lobectomy or a sublobar resection in a population that could have tolerated either procedure. That is, assessing patients who were intentionally allocated to the sublobar resection group rather than deemed inoperable by the lobectomy approach. A subgroup analysis was performed to compare the OS of segmentectomy versus lobectomy in this study cohort.
Methods
Literature search strategy
A systematic electronic search was performed using Ovid Medline, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Database of Abstracts of Review of Effectiveness from their dates of inception to December 2013. To achieve the maximum sensitivity of the search strategy and identify all potentially relevant studies, we combined “segmentectomy” or “sublobar” or “limited” or “sublobectomy” or “wedge resection” as Medical Subject Headings (MeSH) terms or keywords with “lobectomy” and “survival” or “mortality” and “NSCLC” or “lung cancer”. All relevant articles identified were assessed with application of predefined selection criteria.
Selection criteria
Eligible studies included those in which comparative outcomes were presented for patients with early-stage NSCLC who underwent sublobar resections or lobectomies. Sublobar resections included anatomical segmentectomies or wedge resections, and subgroup analysis was performed for segmentectomies when data was available. To minimize differences between baseline patient characteristics, studies in which patients were allocated to the sublobar resection group due to increased comorbidities were excluded from analysis. When centers published duplicate trials with accumulating numbers of patients or increased lengths of follow-up, only the most updated reports were included for qualitative appraisal. When data were presented separately for different stages of disease, early-stage NSCLC were selected where possible. All publications were limited to human subjects and in English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded.
Data extraction and critical appraisal
The primary outcomes included OS and DFS. All data were extracted from article texts, tables, and figures. Two investigators (D.C. and S.G.) independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators (C.C. and T.D.Y.).
Statistical analysis
Meta-analysis was performed by combining the results of reported OS and DFS. Hazard ratio (HR) and associated variance were obtained or calculated from each selected study using techniques described by Tierney and Parmar (3,4). When direct calculations were not possible due to a lack of presented data, HRs were estimated using Kaplan-Meier graphs. Calculations were performed independently by two researchers (C.C. and D.H.T.) and discrepancies were discussed to reach consensus. The summary statistical analysis was conducted with Review Manager Version 5.1.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom). I2 statistic was used to estimate the percentage of total variation across studies, due to heterogeneity rather than chance.
Results
Quantity and quality of trials
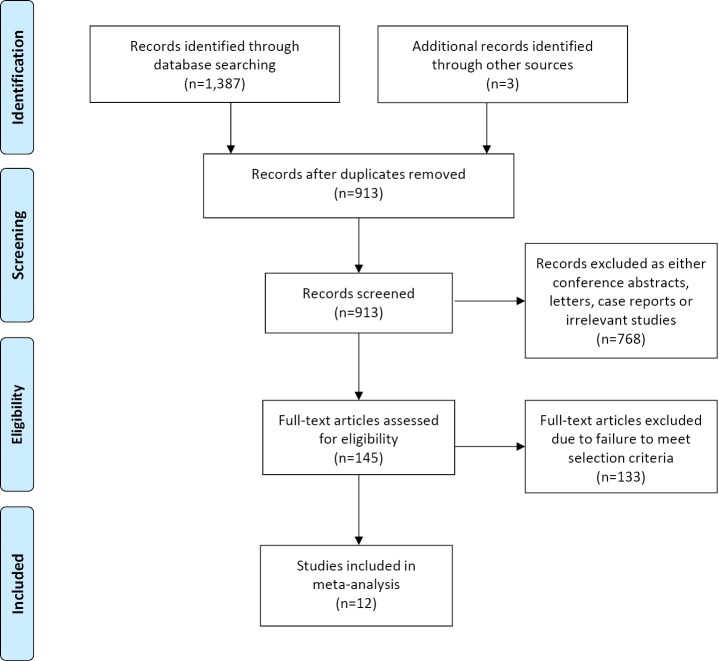
A total of 1,387 records were identified through the five electronic database searches, with three additional studies identified through other sources. After removal of duplicates and limiting the search to humans and English language, 913 articles remained to be screened. Exclusion of irrelevant studies resulted in 145 articles, which were retrieved for more detailed evaluation. After applying the selection criteria, 12 articles remained for assessment, including 1,078 patients who underwent sublobar resections and 1,667 patients who underwent lobectomies (2,5-15). A summary of the search strategy is presented in Figure 1 and a review of study characteristics is presented in Table 1. Baseline patient characteristics included in the present meta-analysis appeared to show similar age and gender distribution between the two surgical treatment groups. However, tumor size was found to be generally smaller in the patients who underwent sublobar resection. A summary of these findings are presented in Table 2. Adenocarcinomas accounted for the majority of pathological findings in all of the included studies, and nearly all studies were limited to stage I disease. A summary of histopathological and staging data for the selected studies is presented in Tables 3 and 4, respectively.
Figure 1.
Summary of search strategy performed to identify relevant comparative studies on sublobar resections vs. lobectomies for early-stage NSCLC. NSCLC, non-small cell lung cancer.
Table 1. Study characteristics of relevant articles identified for meta-analysis comparing sublobar resection versus lobectomy for patients with NSCLC.
| Author | Year | Institution | Study period | Sublobar (n) | Lobectomy (n) | Selection for sublobar approach | Follow-up (median months) |
|---|---|---|---|---|---|---|---|
| Read (5) | 1990 | McClellan Memorial Veterans Hospital, USA | 1966-1988 | 113 (S:107, W:6) | 131 | NR | Sub: 42, Lob: 54M |
| Warren (6) | 1994 | Rush-Presbyterian-St. Luke’s Medical Centre | 1980-1988 | 38 (S:38, W:0)* | 34* | Small and peripherally located lesions | NR |
| Ginsberg (2) | 1995 | North American Lung Cancer Study Group Institutions, USA | 1982-1988 | 122 (S:82, W:40) | 125 | Randomized study | >54 |
| Kodama (7) | 1997 | Osaka Medical Center, Japan | 1985-1996 | 46 (S:46, W:0) | 77 | Well-defined peripheral tumor <2 cm | Sub: 30, Lob: 83 |
| Koike (8) | 2003 | Niigata Cancer Centre Hospital, Japan | 1992-2000 | 74 (S:60, W:14) | 159 | Clinical T1 peripheral NSCLC <2 cm | Sub: 53±22M, Lob: 50±32M |
| Okada (9) | 2006 | Hyogo Medical Center for Adults, Niigata Cancer Centre Hospital & Osaka Medical Centre, Japan | 1992-2001 | 305 (S:NR, W:NR) | 262 | Clinical T1 peripheral NSCLC <2 cm | Sub: 72, Lob: 71 |
| Kodama (10) | 2008 | Osaka Medical Center, Japan | 1997-2002 | 58 (S:25, W:33) | 80 | <2 cm and GGO ratios | Sub: 91, Lob: 98 |
| Sugi (11) | 2010 | Yamaguchi-Ube Medical Centre, Japan | 2001-2004 | 33 (S:33, W:0) | 111 | Tumors <2 cm with high GGO and >2 cm from periphery | 60 |
| Ichiki (12) | 2011 | University of Occupational and Environmental Health, Japan | 2001-2008 | 35 (S:18, W:17) | 104* | Adenocarcinoma <10 or 11-20 mm in which >50% GGO | >60 |
| Yamashita (13) | 2012 | Oita University Hospital, Japan | 2003-2011 | 90 (S:90, W:0) | 124 | NR | 30 |
| Hamatake (14) | 2012 | Fukuoka University School of Medicine, Japan | 1995-2011 | 66 (S:32, W:34) | 77 | Pure GGO on CT and <1 cm in size. Wedge if close to pleura, segmentectomy if non-peripheral lesion | NR |
| Tsutani (15) | 2013 | Hiroshima University, Kanagawa Cancer Center, Cancer Institute Hospital & Hyogo Cancer Center, Japan | 2005-2010 | 98 (S:98, W:0) | 383 | Peripheral lesion that could be completely resected by segmentectomy | 43.2 |
NSCLC, non-small cell lung cancer; *, stage T1a disease analyzed; M, mean; S, segmentectomy; W, wedge resection; Sub, sublobar; Lob, lobectomy; GGO, ground glass opacity; NR, not reported.
Table 2. A summary of patient baseline characteristics in comparative studies on sublobar resection versus lobectomy for patients with NSCLC.
| Author | Age (mean) |
Male gender, n [%] |
Mean tumor size (cm) |
|||||
|---|---|---|---|---|---|---|---|---|
| Sublobar | Lobectomy | Sublobar | Lobectomy | Sublobar | Lobectomy | |||
| Read (5) | 62.4±7.5 | 242 [99] | 2.03±0.6 | |||||
| Warren (6) | 63.9±9.8 | 63.8±9.9 | 44 [67] | 67 [65] | 2.23±0.97 | 3.28±1.71 | ||
| Ginsberg (2) | >60M | >60M | 149 [61] | ≤3 | ||||
| Kodama (7) | 61M | 61M | 31 [67] | 46 [60] | 1.67±0.50 | 2.29±0.52 | ||
| Koike (8) | 64.2±7.2 | 65.3±9.5 | 38 [51] | 80 [50] | 1.5±0.4 | 1.7±0.4 | ||
| Okada (9) | 63.2 | 64 | 167 [55] | 146 [56] | 1.57 | 1.62 | ||
| Kodama* (10) | 60M | 90 [50] | NR | NR | ||||
| Sugi (11) | 61.6±9.4 | 64.8±9.4 | 19 [44] | 31 [33] | 1.42±0.44 | 2.33±0.69 | ||
| Ichiki (12) | 67.9 | 67.1 | 15 [43] | 64 [56] | <2 | <2 | ||
| Yamashita (13) | 69M | 68M | 41 [46] | 73 [59] | 1.5M | 2.0M | ||
| Hamatake (14) | 64 | 62 [43] | 0.8 | |||||
| Tsutani (15) | 67M | 66M | 45 [46] | 169 [44] | 1.7M | 2.2M | ||
Data is presented as numbers with percentage of study population in brackets. NSCLC, non-small cell lung cancer; M, median; NR, not reported; *, baseline characteristics in this study included patients operated on for reasons other than NSCLC.
Table 3. The histopathological subtype of tumors in comparative studies of sublobar resection versus lobectomy for patients with NSCLC.
| Author | Adenocarcinoma |
Squamous cell |
Bronchoalveolar |
Large cell |
Adenosquamous |
Neuroendocrine |
Other |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub | Lob | Sub | Lob | Sub | Lob | Sub | Lob | Sub | Lob | Sub | Lob | Sub | Lob | |||||||
| Read (5) | NR | NR | 137 [56] | NR | NR | NR | NR | NR | NR | NR | NR | 107 [44]§ | ||||||||
| Warren (6) | 44 [67] | 53 [51] | 15 [23] | 35 [34] | 0 [0] | 0 [0] | 2 [3] | 9 [9] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 5 [8] | 6 [6] | ||||||
| Ginsberg (2) | NR | NR | 30 [25] | 33 [26] | NR | NR | NR | NR | NR | NR | NR | NR | 92 [75]§ | 92 [74]§ | ||||||
| Kodama (7) | 36 [78] | 61 [79] | 8 [17] | 13 [17] | 0 [0] | 0 [0] | 2 [4] | 3 [4] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||||
| Koike (8) | 68 [92] | 141 [89] | 5 [7] | 17 [11] | NR | NR | NR | NR | NR | NR | NR | NR | 1 [1] | 1 [1] | ||||||
| Okada (9) | 276 [90] | 229 [87] | 27 [9] | 30 [12] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 2 [1] | 3 [1] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||||
| Kodama (10) | 58 [100] | 70 [88] | 0 [0] | 4 [5] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 6 [8] | ||||||
| Sugi (11) | 2 [5] | 21 [22] | 2 [5] | 8 [8] | NR | NR | 0 [0] | 4 [4] | 0 [0] | 2 [2] | NR | NR | 39 [91]* | 60 [63]* | ||||||
| Ichiki (12) | 35 [100] | 114 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||||
| Yamashita (13) | 26 [29] | 51 [41] | 11 [12] | 20 [16] | 48 [53] | 52 [42] | NR | NR | NR | NR | NR | NR | 5 [6] | 1 [1] | ||||||
| Hamatake (14) | 127 [89] | 7 [5] | 0 [0] | 0 [0] | 1 [1] | 0 [0] | 9 [6] | |||||||||||||
| Tsutani (15) | 98 [100] | 383 [100] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | ||||||
Data is presented as numbers with percentage of study population in brackets. NSCLC, non-small cell lung cancer; §, non-squamous origin; *, tumors classified according to the Noguchi classification system; NR, not reported.
Table 4. Staging of lung cancer in comparative studies for sublobar resection versus lobectomy.
| Author | Sublobar resection |
Lobectomy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage IA | pT1a [<2 cm] | pT1b [2-3 cm] | Stage IB | >Stage I | Stage I | Stage IA | pT1a [< 2 cm] | pT1b [2-3 cm] | Stage IB | >Stage I | ||
| Read (5) | 113 [100] | NR | NR | NR | NR | 0 [0] | 131 [100] | NR | NR | NR | NR | 0 [0] | |
| Warren (6) | 66 [100] | 51 [77] | 38 [58] | 13 [20] | 15 [23] | 0 [0] | 103 [100] | 44 [42] | 34 [33] | 10 [10] | 59 [56] | 0 [0] | |
| Ginsberg (2) | 122 [100] | 122 [100] | NR | NR | NR | 0 [0] | 125 [100] | 125 [100] | NR | NR | NR | 0 [0] | |
| Kodama (7) | 46 [100] | 46 [100] | 46 [100] | 0 [0] | 0 [0] | 0 [0] | 77 [100] | 77 [100] | NR | NR | 0 [0] | 0 [0] | |
| Koike (8) | 74 [100] | 74 [100] | 74 [100] | 0 [0] | 0 [0] | 0 [0] | 159 [100] | 159 [100] | 159 [100] | 0 [0] | 0 [0] | 0 [0] | |
| Okada (9) | 273 [90] | 266 [87] | 305 [100] | 0 [0] | 7 [2] | 32 [10] | 227 [87] | 217 [83] | 262 [100] | 0 [0] | 10 [4] | 35 [13] | |
| Kodama (10) | 58 [100] | 58 [100] | 58 [100] | 0 [0] | 0 [0] | 0 [0] | 62 [78] | 62 [78] | 80 [100] | 0 [0] | NR | 18 [22] | |
| Sugi (11) | 40 [93] | 40 [93] | 40 [93] | 0 [0] | 0 [0] | 0 [0] | 80 [84] | 80 [84] | NR | NR | NR | 9 [9] | |
| Ichiki (12) | 35 [100] | 35 [100] | 35 [100] | 0 [0] | 0 [0] | 0 [0] | 99 [87] | 96 [84] | 114 [100] | 0 [0] | 3 [3] | 15 [13] | |
| Yamashita (13) | 90 [100] | 90 [100] | 76 [84] | 14 [16] | 0 [0] | 0 [0] | 124 [100] | 124 [100] | 72 [58] | 52 [42] | 0 [0] | 0 [0] | |
| Hamatake (14) | NS* | NS* | 66 [100] | 0 [0] | NR | NR | NS* | NS* | 77 [100] | 0 [0] | NR | NR | |
| Tsutani (15) | 97 [99] | 97 [99] | NR | NR | 0 [0] | 1 [1] | 339 [89] | 339 [89] | NR | NR | 0 [0] | 44 [11] | |
Data is presented as numbers with percentage of study population in brackets. *, 136 (95%) of the entire cohort (sublobar and lobectomy patients) were stage I & stage IA; NR, not reported; NS, not specified.
Of the twelve studies identified for inclusion in the present meta-analysis, one study was a randomized controlled trial that compared 122 patients who underwent sublobar resections with 125 patients who underwent lobectomy (2). The remaining 11 studies were observational comparative studies, including three studies that reported prospectively collected data (10,11,15). One recent report by Tsutani et al. utilized propensity score analysis to adjust for potential differences in patient characteristics between the segmentectomy and lobectomy treatment groups (15). Reported median follow-up periods ranged from 30 to 98 months, but there was variation according to the treatment group and a lack of routine imaging to detect disease recurrence. Individual studies were also limited by the population size, which was generally less than 150 patients in each treatment arm, as summarized in Table 1.
Sublobar resections vs. lobectomies
Using the available data in the existing literature, 12 studies involving 1,078 patients who underwent sublobar resections were compared to 1,667 patients who underwent lobectomies to assess the OS from the date of surgery. The combined HR for OS was 0.91 [95% confidence interval (CI) 0.64-1.29; P=0.61], as shown in Figure 2. DFS was reported in five studies involving 600 patients who underwent sublobar resections and 1,039 patients who underwent lobectomies. Comparative data demonstrated no significant differences as the HR for DFS was 0.82 (95% CI 0.60-1.12; P=0.21), as shown in Figure 3.
Figure 2.
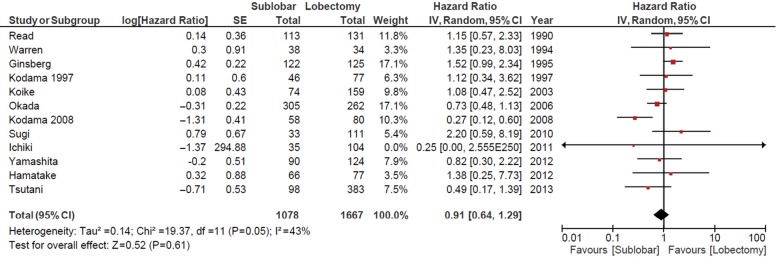
Overall survival: sublobar vs. lobectomy. CI, confidence interval.
Figure 3.
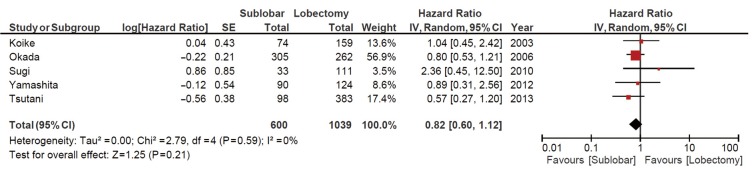
Disease-free survival: sublobar vs. lobectomy. CI, confidence interval.
Segmentectomies vs. lobectomies
A subgroup analysis was performed for segmentectomies versus lobectomies, which included seven studies involving 551 patients in the segmentectomy group and 999 patients who underwent lobectomies. There was no statistically significant difference between the two surgical intervention groups, and the combined HR for OS was 1.04 (95% CI 0.66-1.63, P=0.86), as shown in Figure 4.
Figure 4.
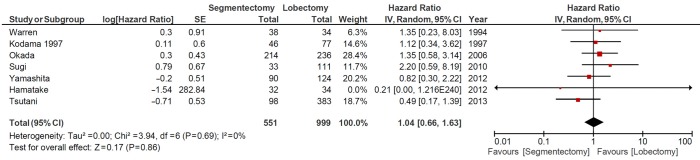
Overall survival: segmentectomy vs. lobectomy. CI, confidence interval.
Discussion
The selection of the appropriate surgical resection procedure for patients with small, peripheral NSCLC remains controversial. On one hand, lobectomy is commonly considered to be the standardized approach to achieve long-term oncological efficacy and minimize the risks of local recurrence (16). Conversely, sublobar resections have been demonstrated to preserve lung function without compromising DFS (9). Unfortunately, the presentation of the clinical evidence on long-term outcomes has been unclear, partly due to the collation of clinical data without considering the variable patient selection processes of comparative studies. The primary focus of the present meta-analysis was to compare patients who underwent sublobar resections who were also eligible for lobectomy procedures. Patients who underwent segmentectomy or wedge resection because they were considered too frail or had insufficient lung capacity for lobectomy resection were excluded from analysis. This analytical approach for NSCLC has not been performed previously in the medical literature.
According to our findings, patients who intentionally underwent sublobar resections did not demonstrate any significant OS or DFS differences compared to patients who underwent lobectomy. Furthermore, patients who underwent segmentectomy also had similar survival outcomes compared to the lobectomy approach. It is important to emphasize that patients included in the individual comparative studies selected for the present analysis generally had early-stage NSCLC and often with ground glass opacities. This cohort of patients is increasingly being diagnosed after the initiation of more aggressive and accurate imaging screening programs in selected countries (17,18). In addition, the level of evidence was relatively low, with only one RCT and the rest of the studies consisting of level IV evidence. Our findings contradict previous meta-analyses that combined patients who underwent sublobar resections due to significant comorbidity or limited pulmonary functions with those who underwent intentional resection for comparison with lobectomy procedures (19,20).
The only completed randomized controlled trial was conducted by the LCSG from 1982 to 1988 (2). Computed tomography was not routinely performed and positron emission tomography was not available. In addition, T1N0 criteria at the time included tumors less than 3 cm, and patients who underwent sublobar resections were not differentiated between segmentectomies and wedge resections. Furthermore, data was unavailable for almost a third of the patients, and the initial presented data were inaccurate, as highlighted by a recent letter by Detterbeck (21). The updated results of this study found lobectomy to confer a significant survival benefit as well as a decrease in the recurrence rate compared to the sublobar resection group. Despite its many limitations, results of the LCSG study formed the basis of many current guidelines.
More recently, a number of case series reports have demonstrated encouraging outcomes for patients undergoing sublobar resections following strict patient selection protocols. A number of Japanese studies have shown that patients with small, peripheral lesions with various degrees of GGO can achieve similar or superior survival outcomes (10-12,14). These results have revived interest in the debate of lobectomy versus sublobar resections in T1N0M0 NSCLC. Currently, RCTs are underway to compare patients who undergo segmentectomy (22) or sublobar resection (CALGB 140503) versus lobectomy. Outcomes of these trials will no doubt have a strong impact on the surgical management of patients with small, peripheral NSCLC. Furthermore, in an era of growing enthusiasm for minimally invasive surgery, the comparison of clinical outcomes after video assisted thoracoscopic (VATS) sublobar resections versus VATS lobectomies may be of immense value.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3 [DOI] [PubMed] [Google Scholar]
- 3.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34 [DOI] [PubMed] [Google Scholar]
- 5.Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 1990;49:391-8; discussion 399-400 [DOI] [PubMed] [Google Scholar]
- 6.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4 [PubMed] [Google Scholar]
- 7.Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53 [DOI] [PubMed] [Google Scholar]
- 8.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8 [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75 [DOI] [PubMed] [Google Scholar]
- 10.Kodama K, Higashiyama M, Takami K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg 2008;34:1068-74 [DOI] [PubMed] [Google Scholar]
- 11.Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60 [DOI] [PubMed] [Google Scholar]
- 12.Ichiki Y, Hanagiri T, Baba T, et al. Limited pulmonary resection for peripheral small-sized adenocarcinoma of the lung. Int J Surg 2011;9:155-9 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8 [DOI] [PubMed] [Google Scholar]
- 14.Hamatake D, Yoshida Y, Miyahara S, et al. Surgical outcomes of lung cancer measuring less than 1 cm in diameter. Interact Cardiovasc Thorac Surg 2012;15:854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64 [DOI] [PubMed] [Google Scholar]
- 16.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24Suppl 6:vi89-98 [DOI] [PubMed] [Google Scholar]
- 17.Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20 [DOI] [PubMed] [Google Scholar]
- 18.Wisnivesky JP, Mushlin AI, Sicherman N, et al. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003;124:614-21 [DOI] [PubMed] [Google Scholar]
- 19.Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg. 2013 doi: 10.1093/ejcts/ezt554. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8 [DOI] [PubMed] [Google Scholar]
- 21.Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg 2013;96:742-4 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4 [DOI] [PubMed] [Google Scholar]