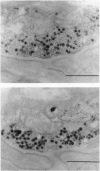
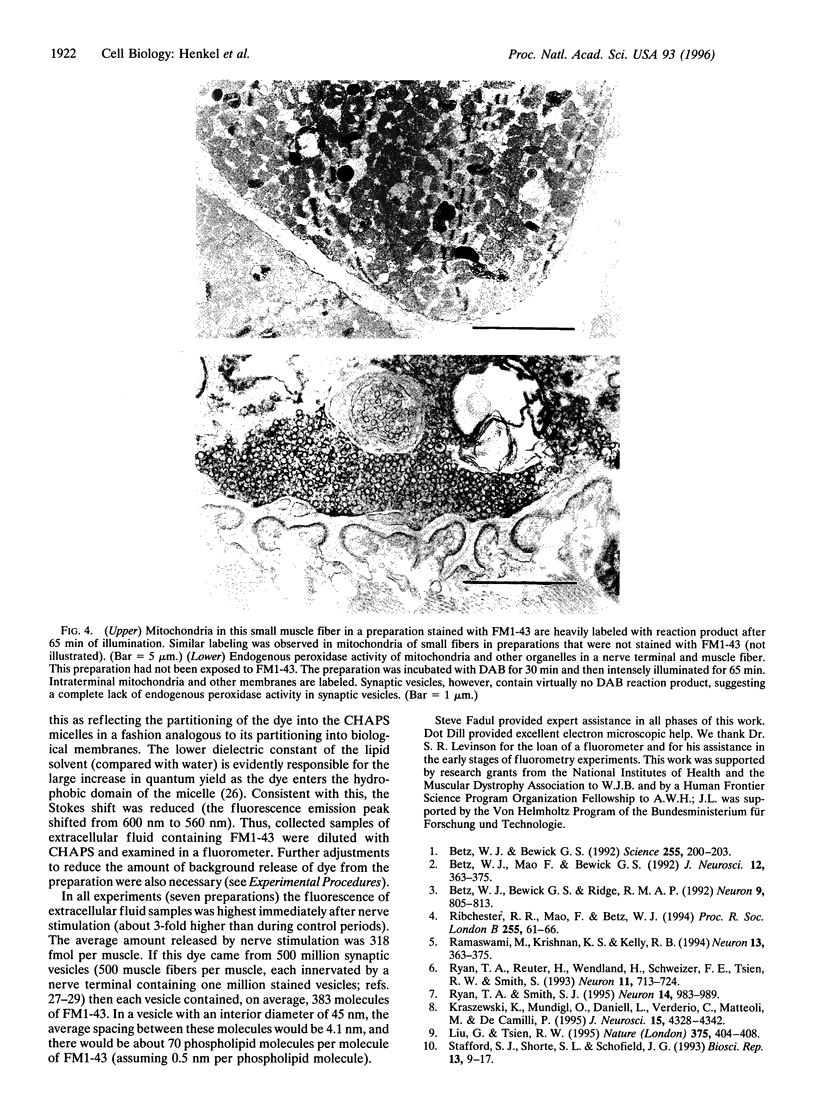
Abstract
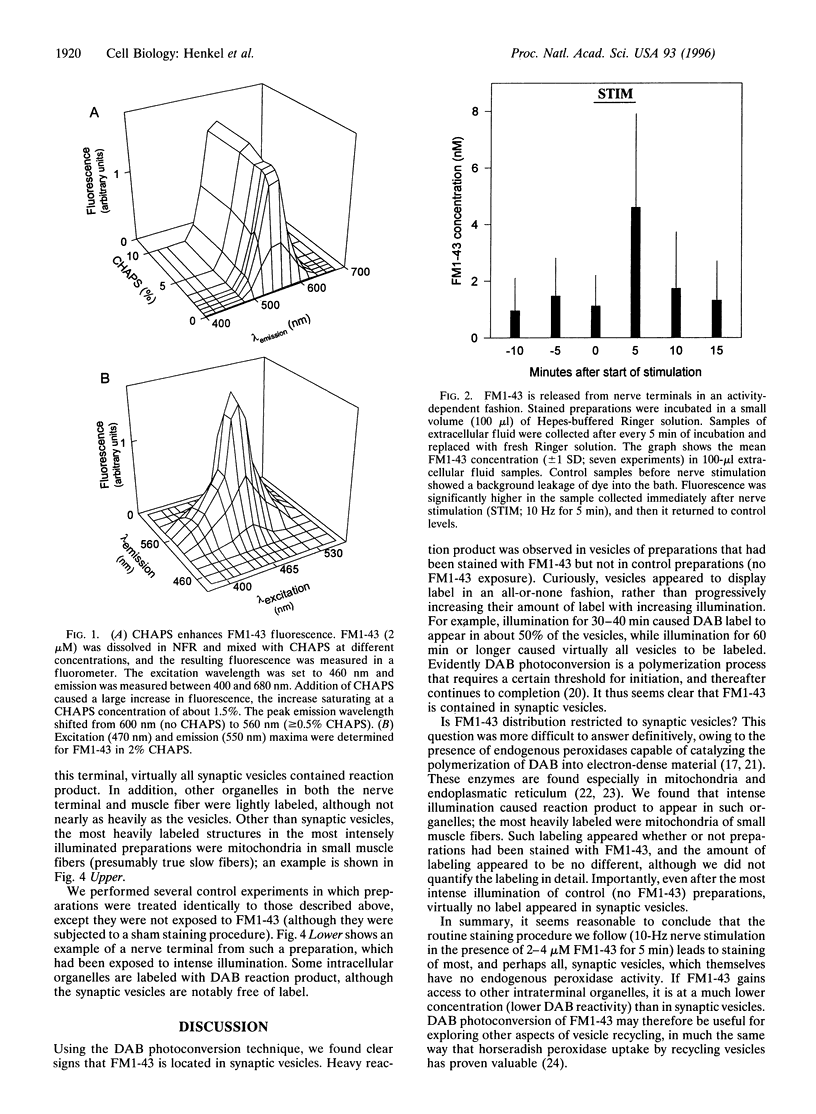
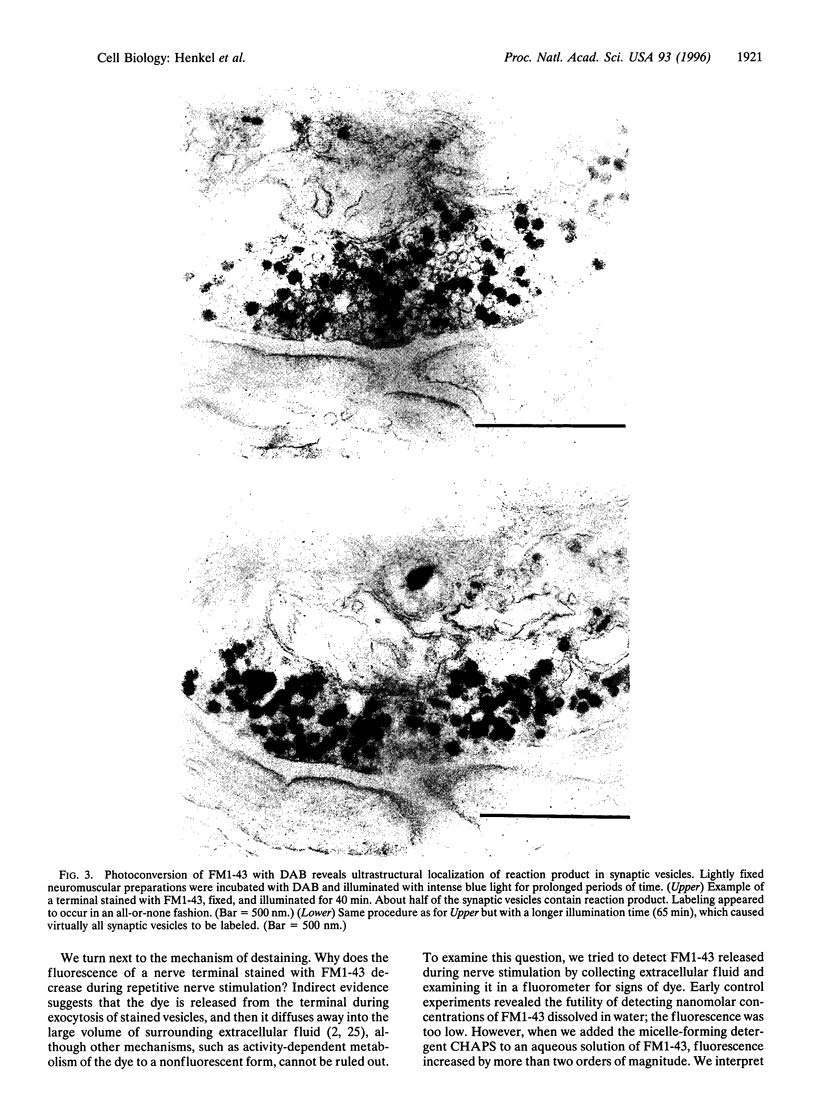
Previous work has shown that the fluorescent styryl dye FM1-43 stains nerve terminals in an activity-dependent fashion. This dye appears to label the membranes of recycled synaptic vesicles by being trapped during endocytosis. Stained terminals can subsequently be destained by repeating nerve stimulation in the absence of dye; the destaining evidently reflects escape of dye into the bathing medium from membranes of exocytosing synaptic vesicles. In the present study we tested two key aspects of this interpretation of FM1-43 behavior, namely: (i) that the dye is localized in synaptic vesicles, and (ii) that it is actually released into the bathing medium during destaining. To accomplish this, we first photolyzed the internalized dye in the presence of diaminobenzidine. This created an electron-dense reaction product that could be visualized in the electron microscope. Reaction product was confined to synaptic vesicles, as predicted. Second, using spectrofluorometry, we quantified the release of dye liberated into the medium from tubocurarine-treated nerve-muscle preparations. Nerve stimulation increased the amount of FM1-43 released, and we estimate that normally a stained synaptic vesicle contains a few hundred molecules of the dye. The key to the successful detection of released FM1-43 was to add the micelle-forming detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), which increased FM1-43 quantum yield by more than two orders of magnitude.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermüller S., Fahimi H. D. Selective cytochemical localization of peroxidase, cytochrome oxidase and catalase in rat liver with 3,3'-diaminobenzidine. Histochemistry. 1981;71(1):33–44. doi: 10.1007/BF00592568. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992 Jan 10;255(5041):200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol. 1993 Jan;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S., Ridge R. M. Intracellular movements of fluorescently labeled synaptic vesicles in frog motor nerve terminals during nerve stimulation. Neuron. 1992 Nov;9(5):805–813. doi: 10.1016/0896-6273(92)90235-6. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Mao F., Bewick G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992 Feb;12(2):363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Deerinck T. J., Martone M. E., Lev-Ram V., Green D. P., Tsien R. Y., Spector D. L., Huang S., Ellisman M. H. Fluorescence photooxidation with eosin: a method for high resolution immunolocalization and in situ hybridization detection for light and electron microscopy. J Cell Biol. 1994 Aug;126(4):901–910. doi: 10.1083/jcb.126.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Rubin L. L., Mauro A. Double mode of action of black widow spider venom on frog neuromuscular junction. J Neurocytol. 1978 Apr;7(2):193–202. doi: 10.1007/BF01217918. [DOI] [PubMed] [Google Scholar]
- Henkel A. W., Betz W. J. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995 Nov;34(11):1397–1406. doi: 10.1016/0028-3908(95)00126-q. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J Cell Biol. 1993 Jun;121(6):1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K., Mundigl O., Daniell L., Verderio C., Matteoli M., De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995 Jun;15(6):4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Tsien R. W. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995 Jun 1;375(6530):404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Edgington D. R., Kuffler D. P. Factors that influence regeneration of the neuromuscular junction. J Exp Biol. 1980 Dec;89:31–42. doi: 10.1242/jeb.89.1.31. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Premack B. A., Schulman H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron. 1994 Jun;12(6):1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Oehring H., Halbhuber K. J. Employment of merocyanine 540 fluorescence to form diaminobenzidine (DAB) oxidation product: a photoconversion method for the visualization of erythrocyte membrane fluidity for light and electron microscopy. Acta Histochem. 1991;90(2):127–134. doi: 10.1016/S0065-1281(11)80048-6. [DOI] [PubMed] [Google Scholar]
- Ramaswami M., Krishnan K. S., Kelly R. B. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994 Aug;13(2):363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Ribchester R. R., Mao F., Betz W. J. Optical measurements of activity-dependent membrane recycling in motor nerve terminals of mammalian skeletal muscle. Proc Biol Sci. 1994 Jan 22;255(1342):61–66. doi: 10.1098/rspb.1994.0009. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Reuter H., Wendland B., Schweizer F. E., Tsien R. W., Smith S. J. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993 Oct;11(4):713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Smith S. J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995 May;14(5):983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Sandell J. H., Masland R. H. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem. 1988 May;36(5):555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- Stafford S. J., Shorte S. L., Schofield J. G. Use of a fluorescent dye to measure secretion from intact bovine anterior pituitary cells. Biosci Rep. 1993 Feb;13(1):9–17. doi: 10.1007/BF01138174. [DOI] [PubMed] [Google Scholar]
- Walzer C., Frenk E. Ultrastructural demonstration of endogeneous peroxidase activity in mammalian epidermis. Histochemistry. 1983;78(4):491–501. doi: 10.1007/BF00496201. [DOI] [PubMed] [Google Scholar]
- van Bogaert L. J., Quinones J. A., van Craynest M. P. Difficulties involved in diaminobenzidine histochemistry of endogenous peroxidase. Acta Histochem. 1980;67(2):180–194. doi: 10.1016/s0065-1281(80)80022-5. [DOI] [PubMed] [Google Scholar]