Abstract
Background
Despite the increasing prevalence of the early discovery of small-sized non-small cell lung cancers (NSCLCs), particularly adenocarcinoma, sublobar resection has not yet gained acceptance for patients who can tolerate lobectomy.
Methods
We compared the outcomes of segmentectomy (n=155) and lobectomy (n=479) in 634 consecutive patients with clinical stage IA lung adenocarcinoma and in propensity score-matched pairs. Those who had undergone wedge resection were excluded.
Results
The 30-day postoperative mortality rate in this population was zero. Patients with large or right-sided tumors, high maximum standardized uptake value (SUVmax), pathologically invasive tumors (with lymphatic, vascular, or pleural invasion), and lymph node metastasis underwent lobectomy significantly more often. Three-year recurrence-free survival (RFS) was significantly higher after segmentectomy compared to lobectomy (92.7% vs. 86.9%, P=0.0394), whereas three-year overall survival (OS) did not significantly differ (95.7% vs. 94.1%, P=0.162). Multivariate analyses of RFS and OS revealed age and SUVmax as significant independent prognostic factors, whereas gender, tumor size and procedure (segmentectomy vs. lobectomy) were not. In 100 propensity score-matched pairs with variables adjusted for age, gender, tumor size, SUVmax, tumor location, the three-year RFS (90.2% vs. 91.5%) and OS (94.8% vs. 93.3%) after segmentectomy and lobectomy respectively were comparable.
Conclusions
Segmentectomy with reference to SUVmax should be considered as an alternative for clinical stage IA adenocarcinoma, even for low-risk patients.
Keywords: Adenocarcinoma, segmentectomy, sublobar resection, lung cancer, lobectomy
Introduction
Sublobar resection for intentionally treating patients with small non-small cell lung cancer (NSCLC) who are able to withstand lobectomy has remained highly controversial, although lobectomy is considered a standard procedure even for sub-centimeter lung cancers. The Lung Cancer Study Group (LCSG) revealed a three-fold increase in local recurrence rates and poorer survival in patients who had undergone sublobar resection rather than lobectomy in a singular randomized phase III study published in 1995 (1). The dogma that lobectomy is the standard of care for stage I NSCLC has been upheld until recently. However, several current investigations have found equivalent outcomes of sublobar resection and lobectomy when NSCLC are ≤2 cm (2-7).
Sublobar resection consists of segmentectomy and wedge resection, which are quite different from each other as curative surgery for lung cancer, since segmentectomy is more likely to provide sufficient margins and allows access to subsegmental and hilar lymph nodes. The present study retrospectively compared the outcomes of segmentectomy, not wedge resection and lobectomy among patients with clinical stage IA lung adenocarcinoma, and adjusted for clinical factors to minimize selection bias of patients. This analysis is an extended and updated version of our previous investigation (8).
Patients and methods
We analyzed data from 634 patients who had undergone lobectomy and segmentectomy for clinical T1N0M0 stage IA lung adenocarcinoma since October 2005. All patients were assessed using high-resolution computed tomography (HRCT) and F-18-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). Patients with incompletely resected (R1 or R2) or multiple tumors were excluded from the prospectively maintained database that was analyzed herein. All patients were staged according to the TNM Classification of Malignant Tumors, 7th edition (9). Platinum-based chemotherapy was administered to patients with pathological lymph node metastasis after surgery. The institutional review boards of the participating institutions approved the study and the requirement for informed consent from individual patients was waived because the study was a retrospective review of a database. Chest images were acquired by multi-detector HRCT independently of subsequent FDG-PET/CT examinations. Tumor sizes and maximum standardized uptake values (SUVmax) were determined by radiologists at each institution. Because of the heterogeneity of PET techniques and performance, we corrected inter-institutional errors in SUVmax resulting from PET/CT scanners of variable quality based on outcomes of a study using an anthropomorphic body phantom (NEMA NU2-2001, Data Spectrum Corp, Hillsborough, NC, USA) that conformed to National Electrical Manufacturers Association standards (10). A calibration factor was analyzed by dividing the actual SUV by the gauged mean SUV in the phantom background to decrease inter-institutional SUV inconsistencies. Postoperative follow-up of all patients from the day of surgery included physical examinations and chest X-rays every three months, as well as chest and abdominal CT and brain MRI assessments every six months for the first two years. Thereafter, the patients were assessed by physical examinations and chest X-rays every six months, and annual CT and MRI imaging.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software version 10.5 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using t-tests and Mann-Whitney U tests in all cohorts and Wilcoxon tests for propensity-matched pairs. Frequencies of categorical variables were compared using the χ2 test and propensity-matched pairs were analyzed using McNemar tests. Propensity score matching was applied to balance the assignments of the included patients and to correct for the operative procedures (lobectomy or segmentectomy) that confounded survival calculations. The variables of age, sex, tumor size, SUVmax, side and lobe were multiplied by a coefficient that was calculated from logistic regression analysis, and the sum of these values was taken as the propensity score for each patient. Lobectomy and segmentectomy pairs with equivalent propensity scores were selected by a 1-to-1 match.
We defined recurrence-free survival (RFS) as the time from the day of surgery until the first event (relapse or death from any cause) or last follow-up, and overall survival (OS) as the time from the day of surgery until death from any cause or the last follow-up. The durations of RFS and OS were analyzed using the Kaplan-Meier method, and differences in RFS and OS were assessed using the log-rank test. Both RFS and OS were assessed by multivariate analysis using the Cox proportional hazards model.
Results
Of the 634 patients analyzed in this study, 479 and 155 underwent lobectomy and segmentectomy, respectively (Table 1). Patients with large tumors, right-sided tumors, pathologically invasive tumors, (presence of lymphatic, vascular, or pleural invasion), high SUVmax, and lymph node involvement were significantly more often treated by lobectomy. However, age and gender did not differ significantly between the two procedures. Table 2 shows the segments that were removed during segmentectomy.
Table 1. Patient characteristics.
| Variables | Lobectomy (n=479) | Segmentectomy (n=155) | P value |
|---|---|---|---|
| Age | 66 [30-89] | 66 [31-89] | 0.37 |
| Gender | |||
| Male | 223 (46.6%) | 74 (48.1%) | 0.78 |
| Tumor size (cm) | 2.2 (0.7-3.0) | 1.5 (0.6-3.0) | <0.001 |
| SUVmax† | 2.1 (0-16.9) | 1.1 (0-9.8) | <0.001 |
| Side | |||
| Right | 325 (67.8%) | 81 (52.3%) | <0.001 |
| Lobe | <0.001 | ||
| Upper | 254 (53.0%) | 82 (52.9%) | |
| Middle | 48 (10.0%) | 0 (0%) | |
| Lower | 177 (37.0%) | 73 (47.1%) | |
| Lymphatic invasion | 97 (20.3%) | 10 (6.5%) | <0.001 |
| Vascular invasion | 111 (23.3%) | 10 (6.5%) | <0.001 |
| Pleural invasion | 66 (13.9%) | 8 (5.2%) | 0.0024 |
| Lymph node metastasis | 50 (10.6%) | 3 (1.9%) | <0.001 |
†, maximum standardized uptake value.
Table 2. Details of segmentectomy (n=155).
| Site | Number |
|---|---|
| Right (n=81) | |
| S1 | 11 |
| S1+2 | 1 |
| S2 | 13 |
| S3 | 7 |
| S6 | 31 |
| S7 | 3 |
| S8 | 8 |
| S9 | 1 |
| S10 | 1 |
| S7+8 | 1 |
| S8+9 | 2 |
| S9+10 | 1 |
| S7+8+9+10 | 1 |
| Left (n=74) | |
| S1+2 | 17 |
| S3 | 9 |
| S1+2+3 | 10 |
| S1+2+3c | 1 |
| S4 | 5 |
| S5 | 1 |
| S4+5 | 7 |
| S6 | 15 |
| S8 | 2 |
| S9 | 5 |
| S10 | 1 |
| S8+9+10 | 1 |
None of the patients died within 30 days of surgery, and tumors recurred in 54 patients at a median postoperative follow-up period of 34.2 months. Twenty recurrences were local only and 34 were distant (with or without local recurrence). Local recurrence occurred in 17 patients after lobectomy (hilar lymph node, n=1; mediastinal lymph node, n=11; pleura, n=2; hilar and mediastinal lymph nodes, n=1; bronchial stump and mediastinal lymph node, n=1; mediastinal lymph node and pleura, n=1) and in three patients after segmentectomy (bronchial stump, n=1; pleura, n=1; residual lung and mediastinal lymph node, n=1).
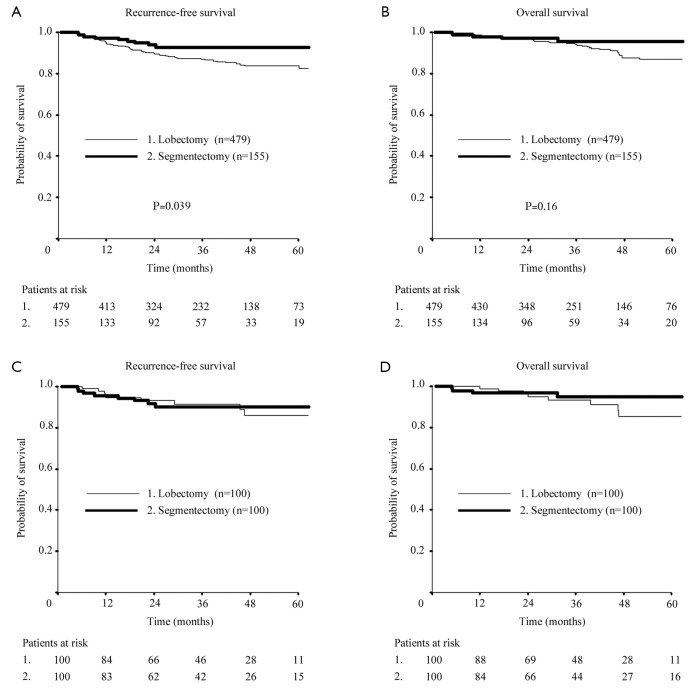
The 3-year OS rates between patients who underwent lobectomy and segmentectomy were similar (94.1% vs. 95.7%, P=0.162), whereas three-year RFS rates significantly differed (86.9% vs. 92.7%, P=0.0394; Figure 1). Table 3 shows that the multivariate analyses of RFS and OS selected age and SUVmax as significant independent prognostic factors, but not sex, tumor size, or procedure (lobectomy vs. segmentectomy).
Figure 1.
Recurrence-free (RFS) and overall survival (OS) curves of patients after lobectomy and segmentectomy. Three-year RFS (A) and OS (B) after lobectomy and segmentectomy were 86.9% vs. 92.7% (P=0.0394) and 94.1% vs. 95.7% (P=0.162), respectively, in all cohorts. Three-year RFS (C) and OS (D) in propensity score-matched patients after lobectomy and segmentectomy were 91.5% vs. 90.2% and 93.3% vs. 94.8%, respectively.
Table 3. Multivariate analyses for RFS and OS.
| Variables | HR (95% CI) | P value |
|---|---|---|
| Multivariate analysis for RFS† | ||
| Age | 1.04 (1.01-1.07) | 0.011 |
| Gender | ||
| Male vs. female | 1.20 (0.74-1.93) | 0.46 |
| Tumor size (cm) | 1.36 (0.86-2.14) | 0.19 |
| SUVmax‡ | 1.17 (1.09-1.25) | <0.001 |
| Procedure | ||
| Lobectomy vs. segmentectomy | 0.72 (0.34-1.52) | 0.39 |
| Multivariate analysis for OS# | ||
| Age | 1.05 (1.01-1.09) | 0.0082 |
| Gender | ||
| Male vs. female | 1.10 (0.49-1.70) | 0.78 |
| Tumor size (cm) | 1.23 (0.67-2.26) | 0.50 |
| SUVmax‡ | 1.13 (1.04-1.24) | 0.0068 |
| Procedure | ||
| Lobectomy vs. segmentectomy | 0.68 (0.25-1.82) | 0.44 |
RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval. †, recurrence-free survival; ‡, maximum standardized uptake value; #, overall survival.
Propensity score-matching based on clinical variables of age, gender, tumor size, SUVmax, side and lobe, allowed good matches of 100 lobectomy and segmentectomy pairs in terms of clinical and consequently pathological factors, except for more advanced age and higher SUVmax in the segmentectomy group (Table 4). Patients who underwent middle lobectomy were excluded from matching for a fair comparison, since tumors located in a middle lobe were never treated by segmentectomy. Figure 1 shows that the three-year RFS and OS did not significantly differ between propensity score-matched patients after lobectomy or segmentectomy (91.5% vs. 90.2% and 93.3% vs. 94.8%, respectively).
Table 4. Propensity score-matched comparison of clinical and pathologic factors between patients who underwent lobectomy and segmentectomy.
| Variables | Lobectomy (n=100) | Segmentectomy (n=100) | P value |
|---|---|---|---|
| Clinical factors | |||
| Age | 63 [33-82] | 66 [32-89] | 0.030 |
| Gender | |||
| Male | 46 (46%) | 50 (50%) | 0.67 |
| Tumor size (cm) | 1.6 (0.7-3.0) | 1.6 (0.6-3.0) | 0.28 |
| SUVmax† | 1.2 (0-8.7) | 1.2 (0-9.8) | 0.047 |
| Side | 0.27 | ||
| Right | 62 (62%) | 53 (53%) | |
| Lobe | 0.10 | ||
| Upper | 62 (62%) | 50 (50%) | |
| Lower | 38 (38%) | 50 (50%) | |
| Pathologic factors | |||
| Lymphatic invasion | 11 (11%) | 7 (7%) | 0.45 |
| Vascular invasion | 9 (9%) | 9 (9%) | 1.0 |
| Pleural invasion | 10 (10%) | 7 (7%) | 0.61 |
| Lymph node metastasis | 7 (7%) | 3 (3%) | 0.34 |
†, maximum standardized uptake value.
Discussion
The RFS and OS curves of patients with clinical stage IA lung adenocarcinoma seemed better after segmentectomy than lobectomy, although the clinical and pathological backgrounds significantly differed and would obviously affect their survival (11-16). Multivariate analyses of the clinical background for RFS and OS demonstrated that procedure (lobectomy vs. segmentectomy) was not a significant prognostic factor. The clinical features or pathological factors of lymphatic, vascular or pleural invasion, or lymph node metastasis were similar in propensity score-matching analyses that matched for potentially confounding variables of age, sex, tumor size, SUVmax, tumor location to minimize selection bias. Only age and SUVmax significantly differed. The three-year RFS and OS rates after segmentectomy and lobectomy group were similar in the matched model, although the former were significantly older and had a higher SUVmax. These data suggest that segmentectomy could be an alternative strategy for treating clinical stage IA lung adenocarcinoma when HRCT and FDG-PET/CT findings are taken into consideration.
This investigation has several limitations and the results should be interpreted with care. Information in the database analyzed herein included surgical procedures; however, further details such as indications for segmentectomy—that is, whether or not patients who were treated with segmentectomy could have tolerated lobectomy—are difficult to obtain. In addition, patients who underwent segmentectomy tended to have less invasive, smaller tumors, with small tumor size or low SUVmax, and thus a lower frequency of pathologically invasive factors such as lymphatic, vascular, pleural or nodal involvement. Therefore, we used propensity score-matched analysis to adjust the patients’ backgrounds as much as possible. However, we could not compare the surgical outcomes of patients with a relatively low SUVmax, implying that patients with a high SUVmax require close scrutiny. The database also did not include information about lung function. The key advantage of segmentectomy is the preservation of lung function, and several studies have shown that segmentectomy has functional advantages over lobectomy (5,17,18).
The target tumors of most previous studies that compared the outcomes of segmentectomy and lobectomy were T1 N0 M0 NSCLC of ≤2 cm (4-6). However, the present study included patients with clinical T1b tumors of 2 to 3 cm. Patients with T1b lung adenocarcinomas with a sufficient surgical margin could be candidates for sublobar resection if selected based on HRCT and FDG-PET/CT findings (12).
The ongoing, multicenter phase III clinical trials of propriety of radical segmentectomy in the United States (CALGB-140503) and Japan (JCOG0802/WJOG4607L) should be carefully monitored. The primary end-point of the Japanese study is OS (disease-free survival in the US study), and wedge resection is not permitted as a sublobar resection, as it differs from radical segmentectomy. The Japanese study (19) aims to compare the surgical outcomes of lobectomy and segmentectomy for T1 N0 M0 NSCLC measuring ≤2 cm, excluding radiologically less-invasive tumors such as ground-glass opacity (GGO)-dominant tumors on HRCT (20), and thus can show the true colors of segmentectomy compared with lobectomy. Segmentectomy is more procedurally demanding than either lobectomy or wedge resection, and thus incorrect outcomes of these clinical trials due to technical errors, such as recurrence at resection lines or excessive loss of lung function, might be a concern. Surgeons must carefully avoid local failure at the margin and fully expand adjacent segments to maximize postoperative lung function.
Current understanding of radical segmentectomy can be summarized as follows. Firstly, the indication for segmentectomy should be limited to T1 tumors ≤3 cm in diameter, and HRCT and PET-CT findings must be taken into consideration, particularly for T1b tumors (21-23). Whenever nodal involvement or an insufficient margin is confirmed intraoperatively, segmentectomy should be converted to lobectomy with complete nodal dissection. Secondly, radical (intentional) and compromising indications for segmentectomy must be independently discussed. The former is for low-risk patients who can tolerate lobectomy. Thirdly, segmentectomy is more valuable than wedge resection from an oncological perspective because it allows nodal dissection at the hilum. Thus, the decision of the most suitable procedure, such as whether or not to intraoperatively convert to lobectomy, should consider precise staging and the lower rate of local recurrence resulting from sufficient surgical margins. Therefore, segmentectomy must be clearly separated from wedge resection amongst the categories of sublobar resection for lung cancer. Surgeons must become adept and master segmentectomy as a keynote procedure because small lung cancers are being detected with increasing frequency.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3 [DOI] [PubMed] [Google Scholar]
- 2.Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50 [DOI] [PubMed] [Google Scholar]
- 3.Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72 [PubMed] [Google Scholar]
- 4.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961 [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg 2002;73:1055-8; discussion 1058-9 [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75 [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64 [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14 [DOI] [PubMed] [Google Scholar]
- 10.Mawlawi O, Podoloff DA, Kohlmyer S, et al. Performance characteristics of a newly developed PET/CT scanner using NEMA standards in 2D and 3D modes. J Nucl Med 2004;45:1734-42 [PubMed] [Google Scholar]
- 11.Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12 [DOI] [PubMed] [Google Scholar]
- 12.Tsutani Y, Miyata Y, Nakayama H, et al. Prediction of pathologic node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J Thorac Cardiovasc Surg 2012;144:1365-71 [DOI] [PubMed] [Google Scholar]
- 13.Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer 2010;116:3170-7 [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Nakayama H, Okumura S, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:1384-91 [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Tauchi S, Iwanaga K, et al. Associations among bronchioloalveolar carcinoma components, positron emission tomographic and computed tomographic findings, and malignant behavior in small lung adenocarcinomas. J Thorac Cardiovasc Surg 2007;133:1448-54 [DOI] [PubMed] [Google Scholar]
- 16.Tsutani Y, Miyata Y, Misumi K, et al. Difference in prognostic significance of maximum standardized uptake value on [18F]-fluoro-2-deoxyglucose positron emission tomography between adenocarcinoma and squamous cell carcinoma of the lung. Jpn J Clin Oncol 2011;41:890-6 [DOI] [PubMed] [Google Scholar]
- 17.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33 [DOI] [PubMed] [Google Scholar]
- 18.Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4 [DOI] [PubMed] [Google Scholar]
- 20.Tsutani Y, Miyata Y, Yamanaka T, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovasc Surg 2013;146:17-23 [DOI] [PubMed] [Google Scholar]
- 21.Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2013;146:580-5 [DOI] [PubMed] [Google Scholar]
- 22.Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71 [DOI] [PubMed] [Google Scholar]
- 23.Tsutani Y, Miyata Y, Nakayama H, et al. Solid tumor size on high-resolution computed tomography and maximum standardized uptake on positron emission tomography for new clinical T descriptors with T1 lung adenocarcinoma. Ann Oncol 2013;24:2376-81 [DOI] [PubMed] [Google Scholar]