Abstract
Reproductive sinks regulate monocarpic senescence in crop plants. Monocarpic senescence was studied in wheat fertile (cv. HW 2041) and its isonuclear cytoplasmic male sterile (CMS) line. CMS plants exhibited slower rate of senescence accompanied by longer green leaf area duration and slower deceleration in chlorophyll, protein content, PN and rubisco content coupled with lower protease activities than fertile (F) plants. CMS plants also exhibited lower ROS levels and less membrane damage than F plants. CMS plants maintained better antioxidant defense, less oxidative damage in chloroplast and higher transcript levels of both rbcL and rbcS genes during senescence than F plants. F plants exhibited early induction and higher expression of SAGs like serine and cysteine proteases, glutamine synthetases GS1 and GS2, WRKY53 transcription factor and decline in transcript levels of CAT1 and CAT2 genes than CMS plants. Hence, using genetically fertile and its CMS line of wheat it is confirmed that delayed senescence in the absence of reproductive sinks is linked with slower protein oxidation, rubisco degradation and delayed activation of SAGs. Better antioxidant defense in chloroplasts at later stages of senescence was able to mitigate the deleterious effects of ROS in CMS plants. We propose that delayed increase in ROS in cytoplasmic male sterile wheat plants resulted in delayed activation of WRKY53, SAGs and the associated biochemical changes than fertile plants.
Keywords: Senescence, Oxidative stress, Protein carbonylation, CMS wheat
Introduction
Senescence is an orderly genetically controlled process of nutrient relocation and cell death. It is regulated by both endogenous and environmental factors. Endogenous factors include reproductive development, hormones, reactive oxygen species (ROS) and environmental factors include biotic and abiotic stresses. Senescence involves the active turnover and recapture of cellular materials for use in the growing parts such as developing seeds (Lim et al. 2007). Leaf senescence is an important determinant of crop productivity. Delaying leaf senescence resulted in prolongation of greenness and photosynthesis that increased carbon filling into seeds thus improving grain yield and quality (Derkx et al. 2012; Uauy et al. 2006).
Reactive oxygen species levels increase during leaf senescence resulting in higher lipid peroxidation and protein oxidation (Zimmermann and Zentgraf 2005). Antioxidants and ROS scavenging enzymes play an important role in ROS detoxification in the cell (Foyer and Noctor 2009). Balance between production and scavenging of ROS, which normally is tightly regulated, appears to be disrupted during the progression of senescence in different cellular compartments due to depletion of antioxidants and/or decline in activities of ROS scavenging enzymes (Zimmermann and Zentgraf 2005; Srivalli and Khanna-Chopra 2009).
Chloroplasts are the main site of ROS production due to the photooxidative nature of their components (Asada 2006). ROS produced in the chloroplasts are scavenged by an efficient antioxidant defense system including both enzymes and metabolites. In chloroplasts both stromal and thylakoid bound isozymes of superoxide dismutase (SOD) and ascorbate peroxidase (APX) are present to detoxify ROS. However, the equilibrium between production and scavenging of ROS may be perturbed during senescence as the activity of stromal and thylakoidal SOD and APX declined during senescence in wheat (Srivalli and Khanna-Chopra 2009). High ROS accumulation in chloroplasts resulted in protein oxidation and degradation especially of large subunit of rubisco (Feller et al. 2008).
Senescence process requires up-regulation and/or activation of many different transcription factors (TFs) including NAM, NAC, and WRKY (Guo et al. 2004). WRKY53 was identified as a key player in the regulation of leaf senescence in Arabidopsis and is proposed to regulate expression of downstream senescence associated genes SAGs (Miao et al. 2004). Expression of many SAGs is induced when accumulation of ROS increases (Bieker et al. 2012). All catalase (CAT) genes are known to be down regulated during senescence however CAT2 is the major CAT isozyme downregulated (Zimmermann et al. 2006).
In wheat, salvaged nitrogen (N) from the leaves accounts for up to 90 % of the total grain N content (Kichey et al. 2007). rubisco is the major source of N in leaves and both rubisco subunit genes rbcL and rbcS are known to show altered expression during senescence (Suzuki et al. 2010). Nitrogen is mainly mobilized to grains in the form of glutamine. Hence the extent of glutamine synthetase (GS) activity which is enhanced during senescence is considered an important determinant of total grain N in crops (Kawakami and Watanabe 1988; Goodall et al. 2013). Both GS1 and GS2 are upregulated during senescence.
We have shown earlier that flag leaf senescence is delayed on removal of spikelets in wheat (Srivalli and Khanna-Chopra 2004, 2009). Physical removal of spikelets may cause ROS accumulation as wounding in plants transiently produces ROS in the damaged tissue (Leon et al. 2000). Hence, in the present study we used wheat fertile and its isonuclear cytoplasmic male sterile line to examine the regulation of senescence through reproductive sink and its influence on oxidative stress, damage and expression of SAGs in the flag leaf. Here we show that longevity in cytoplasmic male sterile wheat plants is associated with delayed increase in ROS, WRKY53, senescence associated genes and better antioxidant defense in chloroplasts than fertile plants.
Materials and methods
Wheat cv. HW2041 (fertile, F) and its isonuclear CMS line obtained from the Division of Genetics, IARI, New Delhi, were sown in the fields of Water Technology Center, IARI, New Delhi, India in the last week of November, 2011. The CMS lines of HW2041 has been developed by crossing between wheat Triticum aestivum cv. HW2041 and Triticum timopheevi. T. timopheevi was used as female parent and HW2041 was used as male parent. This cross results in cytoplasmic male sterile F1 hybrids as described in Tomar and Anbalagan (2004). The CMS line was developed through repeated backcross breeding with T. timopheevi (Sinha et al. 2013).
The plant-to-plant and row-to-row distances were 10 cm and 25 cm, respectively in a plot size of 3 × 2 m. Fertilizers were applied at the rate of 100 N:40P:40 K kg/ha as urea: single super phosphate: muriate of potash respectively. Plants were well-watered throughout the experiment. The ears of CMS plants were covered with paper bags in both the main shoot and the tillers to avoid any external pollination. Flag leaf was sampled from anthesis to grain maturity at 7 days interval. For the biochemical assays, the leaves were sampled and processed as described in Srivalli and Khanna-Chopra (2009).
Green flag-leaf area was determined by using nondestructive method based on the formula: leaf area = length × maximum breadth × 0.7 (Aggarwal and Sinha 1987). Chlorophyll (Chl) content was measured according to Lichtenthaler (1987). Total soluble proteins were measured according to Lowry et al. (1951) using BSA as a standard.
Rubisco protein content was measured by western blot analysis as described in Chauhan et al. (2009). Leaf samples frozen in liquid nitrogen were ground in a mortar and a pestle with liquid nitrogen and then extracted [3 ml per 0.25 g(FW)] in 30 mM Tris buffer, pH 7.8, containing 1 mM ascorbic acid, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, and 0.5 mM PMSF (Zivy et al. 1983). PVPP [4 % (w/w)] was added at the time of grinding. The extracted samples were passed through four layers of cheesecloth and centrifuged at 10,000×g for 20 min. To the supernatant, eight volumes of acetone were added and the protein precipitated overnight. The samples were again centrifuged at 10,000×g for 20 min. To the pellet, electrophoresis sample solution [125 mM Tris–HCl buffer, pH 6.8, 10 % glycerol, 5 % 2-mercaptoethanol (β-ME), and 2 % SDS] was added and boiled for 4 min. Aliquots of the protein samples were subjected to electrophoresis on a 10 % SDS PAGE (Laemmli 1970). In all cases, 10 μg of total soluble protein was loaded. Western blot analysis of rubisco large subunit (LSU) was done according to Towbin et al. (1979). After electrophoresis, the separated polypeptides were electrotransferred at 4 °C onto a nitrocellulose membrane (0.45 μm, Bio-Rad, Richmond, CA, USA) at 50 V for 1 h in transfer buffer using a transblot unit (Bio-Rad, Richmond, CA, USA). The membrane was probed with 1:2,000 dilution of polyclonal antibodies against rubisco LSU. The antibodies were prepared as reported earlier (Srivalli et al. 2001). The immunoreactive protein bands were visualized using anti-rabbit IgG (whole molecule) alkaline phosphatase conjugate antiserum in 1:20,000 dilution with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) as substrate. Rubisco LSU was quantified by scanning the blots using a gel documentation system (GelDocMega system, Biosystematica, Wales, UK).
Endopeptidase activity was measured following the modified version of Peoples et al. (1983). Samples (0.5 g) frozen in liquid nitrogen were ground using a mortar and pestle in liquid nitrogen and after that suspended in 2 ml of 250 mM Tris–HCl buffer, pH 7.0, containing 10 mM β-ME. PVP [2 % (w/v)] was added during homogenization and the extract was centrifuged at 10,000×g, 4 °C for 20 min. The supernatant was collected and passed through three layers of cheesecloth. The supernatant was dialysed overnight against 25 mM Tris–HCl buffer, pH 7.0, containing 10 mM β-ME. The reaction mixture contained 50 μl of crude extract, 125 μl of 250 mM sodium acetate buffer, pH 4.8, containing 10 mM β-ME and 75 μl of rubisco [0.45 % (w/v)] (Sigma-Aldrich) as a substrate. After incubation at 50 °C for 1 h, the reaction was stopped by adding 1 ml of 10 % TCA solution and incubated at 4 °C for 1 h. After centrifugation at 25,000×g for 10 min, the absorbance by TCA-soluble peptides generated during the reaction were estimated of the supernatants at 340 nm using a spectrophotometer (Lambda 2S UV/VIS spectrophotometer, Perkin Elmer, Massachusetts, USA). Controls were kept for zero time, i.e. the reaction was stopped immediately without further incubation and without substrate. One unit of proteolytic activity in 1 cm cuvette was defined as an increment of 0.01 in A340 in 1 h. For inhibitor studies, the reaction mixture containing 50 μl of the crude extract was preincubated for 10 min with one of the following inhibitors −3.8 μl of 2 mM PMSF, 18.8 μl of 10 mM EDTA, 9.4 μl of 25 μM DL-norleucine and 3.8 μl of 10 mM iodoacetamide for serine-, metallo-, aspartate-, and cysteine proteases, respectively (Ye and Varner 1996). The remaining procedure was the same as for the endopeptidase activity. All the inhibitors were dissolved in water except PMSF which was dissolved in isopropanol.
Net photosynthetic rate (PN) was measured in flag leaves from anthesis to maturity in the morning between 10:00 and 11:00 using LI-COR-6400 portable photosynthesis instrument (LI-COR Inc., Lincoln, NE, USA). The measurements were done on the adaxial surface of the flag leaf in the field under saturating photosynthetic photon flux density (PPFD) 1,200–1,500 μmol m–2 s–1, CO2 concentration ~380 ppm, air temperature 24–29 °C and air humidity 50 ± 5 %.
Chloroplasts were isolated from flag leaf using a 40–80 % discontinuous Percoll density gradient method as previously described in Khanna-Chopra and Sabarinath (2004). Two green bands were generated. The upper band contained broken chloroplasts and the lower intact. The intact chloroplasts were transferred to a clean Corex tube and diluted with a cold buffer containing 50 mM Hepes–KOH pH 7.5, 330 mM sorbitol, 4 mM ascorbate, 2 mM EDTA, 1 mM MgCl2 and 1 mM MnCl2 and then centrifuged at 4,300 g for 3 min. Resuspended the chloroplast pellets in a smaller volume of the above buffer. Intactness of purified chloroplasts was measured according to ferricyanide reduction test (Lilley et al. 1975) using Clark-type O2 electrode (Hansatech Instruments Ltd., England). The percentage intactness of chloroplasts was 80–85 % up to 21 DAA and 68–74 % at 28 DAA. The remaining chloroplasts were again centrifuged at 4,300 g for 3 min and the chloroplast pellets were resuspended in a hypotonic medium containing 50 mM Hepes–KOH, pH 7.5 and 4 mM ascorbate to lyse them. The lysed chloroplasts were used for carbonyl assay, SOD ativity and APX activity assay.
H2O2 was extracted according to the procedure of Veljovic-Jovanovic et al. (2002). 0.2 g fresh leaf material was ground in liquid nitrogen and extracted with 2 ml of 5 % trichloroacetic acid. Four percentage of insoluble PVPP was added at the time of grinding. The homogenate was centrifuged at 18,000 g for 5 min, and the supernatant was passed through a Dowex anion exchange resin (1 × 8–400, Sigma-Aldrich Co., St. Louis, MO, USA) equilibrated with 2 ml of 5 % TCA to remove the coloured compounds. After centrifugation for 5 min at 1,000 g, the extracts were neutralized to pH 5.6 with K2CO3. One unit of ascorbate oxidase was added to the samples, which were then incubated for 10 min at room temperature. The reaction mixture contained 50 μL test solution, 50 μL of 0.5 mM luminol in 0.2 M NH4OH (pH 9.5), and 800 μL of 0.2 M NH4OH in 1 mL test tubes which were placed in Luminoskan TL Plus luminometer (Labsystems). Chemiluminescence was initiated by the addition of 100 μL of 0.5 mM K3Fe(CN)6 in 0.2 M NH4OH and the photons emitted were counted over 5 s. H2O2 content was determined using a calibration curve.
Lipid peroxidation was estimated by measuring the content of 2-thiobarbituric acid-reactive substances in leaf homogenates, prepared in 20 % TCA containing 0.5 % of 2-thiobarbituric acid, and heated at 95 °C for 25 min (Heath and Packer 1968). Malondialdehyde (MDA) (e = 155 mM−1 cm−1) content was determined spectrophotometrically at 532 nm and corrected for non-specific turbidity at 600 nm. Carbonyl groups in the proteins of chloroplasts fractions were determined by spectrophotometeric assay according to the method of (Levine et al. 1994). Samples with a concentration of 10 μg of total soluble proteins were mixed with an equal volume of 12 % SDS and then with two volumes of 20 mM dinitrophenylhydrazine dissolved in 10 % trifluoroacetic acid (TFA). As controls, samples were treated only with 10 % TFA. The mixture was incubated for 25 min at room temperature and the reaction was stopped by adding 1.5 sample volumes of 2 M Tris/30 % (v/v) glycerol. The volumes mentioned above always refer to the volume of the sample, before the addition of the derivatizing agent. The absorbance at 370 nm was measured. The carbonyl content was calculated using molar absorption coefficient of 22,000 M−1 cm−1.
For gene expression study leaf samples were collected at three stages i.e. 7, 21 and 28 DAA for RNA. Total RNA was isolated using TRIZOL reagent in an RNAse-free centrifuge tubes as per manufacturers protocol (Invitrogen). To eliminate DNA from aqueous RNA extractions, samples were treated with 10 units of RNase-free DNaseI (Qiagen, USA). Total RNA was quantified spectrophotometrically by measuring absorbance at 260 nm. RNA was fractionated on 1 % agarose gel to check the quantity and integrity.
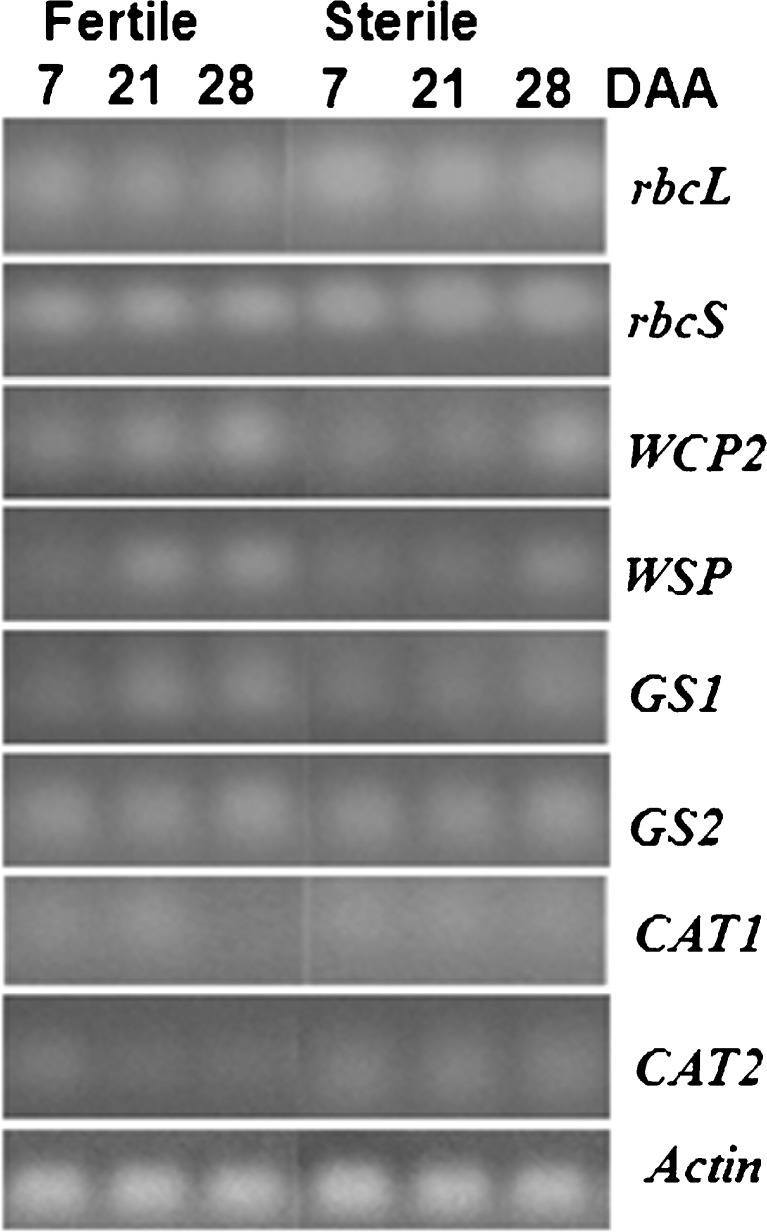
Reverse transcription polymerase chain reaction (RT-PCR) was used to study the expression of genes related to senescence namely rbcL, rbcS, WCP2, WSP, GS1, GS2, CAT1, CAT2 and WRKY53 (Table 1). To ensure that equal amounts of RNA template were added to each RT-PCR reaction, amplification of Actin gene was performed (Fig. 2). Primers used for different genes were taken from previously reported papers (Table 1). For RT-PCR expression analysis, the first-strand cDNAs were synthesized using Superscript III reverse transcriptase following the manufacturer’s protocol (Invitrogen). Second strand PCRs were performed in 50 μL reaction mixture with 1:5 times diluted first strand reaction (cDNA) as template. The reaction mix contained cDNA template, 200 μM dNTPs, 2.5 mM MgCl2, 0.5 μM gene-specific primers, and 2 unit Taq DNA polymerase in 1× PCR buffer. The temperature profile for PCR was as follows: 94 °C for 3 min followed by 30 cycles of 94 °C for 30 s, 44–61 °C (specific for different primer sets, Table 1) for 30 s and 72 °C for 1 min. The final extension was for 10 min at 72 °C. For all the RT-PCR reactions, actin was used as an internal control. PCR products were fractionated in a 3 % agarose gel containing 0.5 μg/mL ethidium bromide. The gels were scanned using gel documentation system (Biosystematica, Wales, UK) and the bands obtained on the gels were quantified by TotalLab software.
Table 1.
Primers sequences used and PCR conditions for quantitative reverse transcriptase analysis of different SAGs
| Gene | Primer sequence | Tm | Reference |
|---|---|---|---|
| WRKY53 | F-5′-GACTCTCGAAAAATCTCGCTGCTC-3′ R-5′-ACATGTAAACGCCACAGGGGAAC-3′ |
54 °C | Wu et al. 2008 |
| rbcL | F-5′-CGCCTCATGGTATCCAAGTTG-3′ R-5′- CGATTAGCTGCTGCACCAGGTG-3′ |
60 °C | Almeselmani et al. 2012 |
| rbcS | F-5′-CTGTGATGGCTTCCTCGG-3′ R-5′-TTAGGCCTTGCCGGACTC-3′ |
61 °C | Rampino et al. 2006 |
| WCP2 | F-5′-TTCCGCTCGTTGGCTCTCCTC-3′ R-5′-CCGCCCCCTCGACAACATCTC-3′ |
61 °C | Simova et al. 2010 |
| WSP | F-5′-CAGCGGAAGCAACATATCATT-3′ R-5′-GGGTACTTCCGTCTGACCAT-3′ |
50 °C | Simova et al. 2010 |
| GS 1 | F-5′-TCCCCTGTCCCGCATTTCCCAGAGA-3′ R-5′-GGAGCTTGCTGGGGTCATCAACGGG-3′ |
51 °C | Grabowska et al. 2012 |
| GS 2 | F-5′-AGGTCGCCCCGCCCCCTTCCCTCCTC-3′ R-5′-GGCCAGCACCTTGAAGCCGGAGGT-3′ |
60 °C | Grabowska et al. 2012 |
| CAT 1 | F-5′-ACTACGACGGGCTCATG-3′ R-5′-GGAGCTGAGACGGCTTC-3′ |
46 °C | Luna et al. 2005 |
| CAT 2 | F-5′-CCTTAATCAGCAGGGATG-3′ R-5′-AGATAGAACACGCGGAG-3′ |
46 °C | Luna et al. 2005 |
| Actin | F-5′-GAGAAGATGACTCAGATC-3′ R-5′-ATCCTTCCTGATATCGAC-3′ |
44 °C | Luna et al. 2005 |
Fig. 2.
Expression pattern of rbcL, rbcS, WCP2, WSP, GS1, GS2, CAT1 and CAT2 genes in flag leaf of wheat during monocarpic senescence
ANOVA and mean comparisons (CRD, two-factor analysis) were conducted using the MSTAT-C (CIMMYT, Mexico, Version 1.00/EM 1988). Critical difference (CD) was calculated using students ‘t’ test (P 0.05).
Results
In the present experiment, senescence was monitored in the flag leaf of wheat cv. HW2041 and its isonuclear CMS line. CMS plants maintained greener canopy for longer period of time than F plants which was also apparent visually (Table 2). Chlorophyll content and PN was higher in CMS plants upto 28 DAA than F plants. PN declined after 14 DAA and 21 DAA in F and CMS plants respectively. Hence, CMS plants exhibited delayed senescence than F plants.
Table 2.
Senescence related parameters in flag leaf of wheat fertile and sterile isolines during monocarpic senescence
| Days after anthesis → | 14 | 21 | 28 | CD | ||||
|---|---|---|---|---|---|---|---|---|
| Parameters↓ | Fertile | Sterile | Fertile | Sterile | Fertile | Sterile | ||
| Green fag leaf area (% of anthesis) | 94 | 92 | 79 | 76 | 5 | 40 | 9.8 | |
| Chlorophyll content (mgg−1 FW) | 4.28 | 4.91 | 3.76 | 4.54* | 0.58 | 2.95* | 0.88 | |
| Photosynthesis rate (μmolCO2m−1 s−1) | 19.6 | 20.4 | 12.3 | 19.1* | 1.7 | 11.8* | 3.12 | |
| Protein content (mgg−1FW) | 21.7 | 20.42 | 8.84 | 15.68* | 2.3 | 8* | 2.11 | |
| Rubisco LSU content (relative units) | 19.3 | 20.3 | 12.5 | 19.1* | 2.2 | 11.1* | 2.12 | |
| Endopeptidase activity (units mg−1protein) | 1164 | 902* | 1485 | 944* | 2359 | 1968* | 95 | |
| Endopeptidase characterization (% of the activity) | Cysteine | – | – | 67 | 84 | 57 | 68 | – |
| Serine | – | – | 25 | 10 | 29 | 29 | – | |
Values represent means of three independent replicates. Asterisks indicate significant differences between fertile and sterile plants (P < 0.05). Critical difference (CD) value for all enzymes/metabolites (treatment × stages) was calculated at 5 % level (P < 0.05)
Protein and rubisco content declined sharply after 14 DAA in F plants (Table 2). CMS plants maintained higher levels of protein and rubisco content at 21 and 28 DAA than F plants. The decline in leaf protein and rubisco content was parallel with increase in endopeptidase activity in both the lines. F plants exhibited significantly higher endopeptidase activity than CMS plants at all stages studied. Cysteine proteases (CP) followed by serine proteases (SP) contributed to the increase in protease activity at 21 DAA and 28 DAA in both isolines (Table 2).
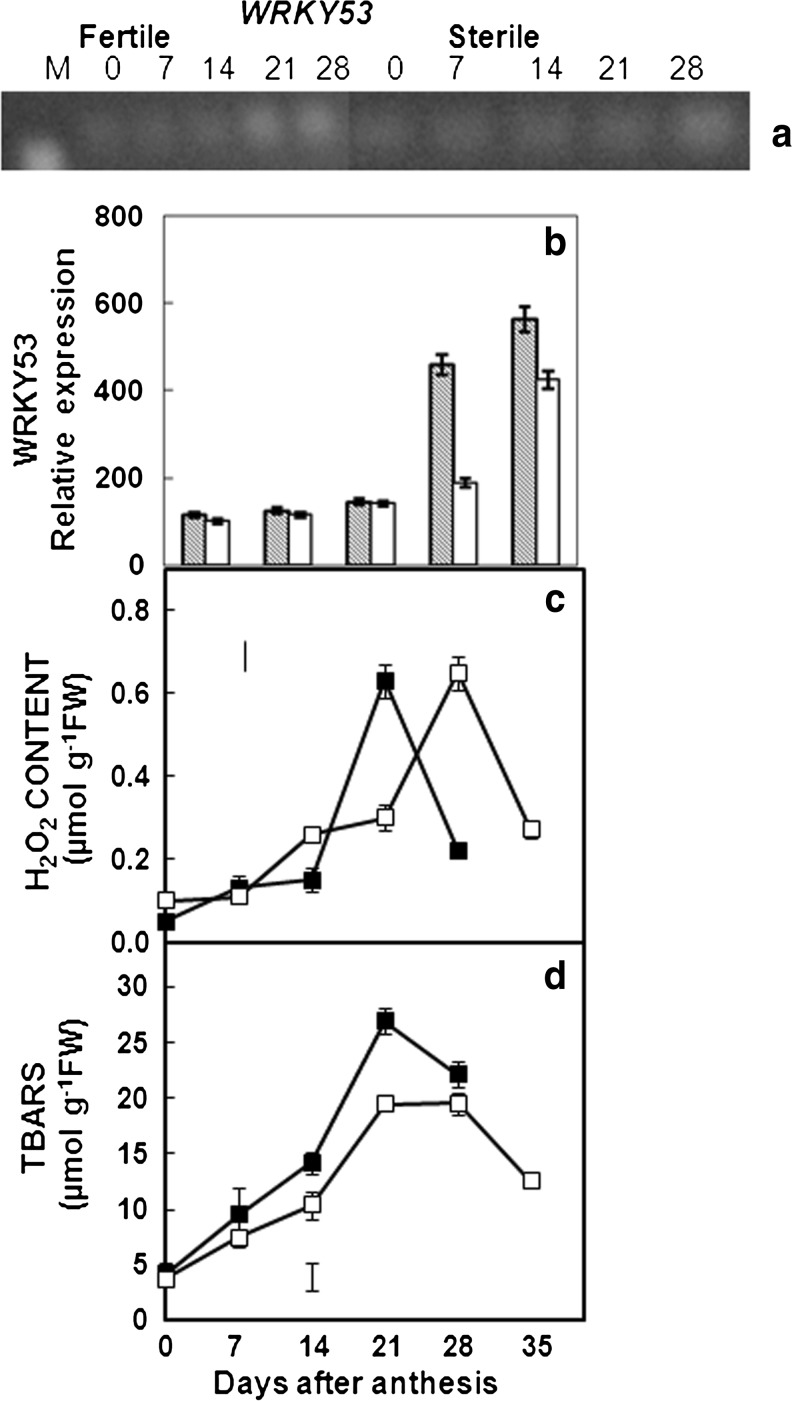
Reactive oxygen species (ROS) content increased significantly during senescence in both wheat isolines (Fig. 1). H2O2 content increased earlier in F plants reaching a peak at 21 DAA while CMS plants exhibited maximum H2O2 content at 28 DAA. F plants exhibited higher membrane damage than CMS plants at all stages studied. At 21 DAA lipid peroxidation increased by 7 fold and 5 fold in F and its CMS line respectively. F plants showed early and higher induction of the transcription factor WRKY53 and maximum WRKY53 expression was observed at the same time when H2O2 content was at the peak in both F and CMS plants i.e. 21 and 28 DAA respectively (Fig. 1).
Fig. 1.
Effect of reproductive sinks on expression of WRKY53 transcript gel analysis (a) and relative transcript level as calculated using gel documentation (b), H2O2 content (c) and lipid peroxidation (d). Fertile (crossed bar) and CMS (open bar) for (b) and fertile (filled box), CMS (open box) for (c) and (d). Error bars indicate mean ± SE (n = 3). In some cases error bars are smaller than the symbol. Vertical bars indicate critical difference (CD) treatments × stages (P < 0.05)
The expression of SAGS was also different in the F and its CMS line. CMS plants had higher mRNA levels of both rbcL and rbcS throughout the study (Figs. 2 and 3). No significant change in rbcS and rbcL mRNA levels was observed during senescence in both F and CMS plants. Expression of both WCP2 and WSP increased earlier in F than CMS plants. F plants exhibited about 4 fold and 6 fold increase in mRNA levels of WCP2 and WSP at 21 and 28 DAA respectively while CMS plants showed significant increase in mRNA levels of WCP2 and WSP only at 28 DAA. GS1 and GS2 mRNA levels increased in both F and CMS plants. However, increase was prominent in F plants. Both CAT1 and CAT2 mRNA levels were higher during senescence in CMS plants compared to F plants (Figs. 2 and 3).
Fig. 3.
Expression pattern of rbcL, rbcS, WCP2, WSP, GS1, GS2, CAT1 and CAT2 genes in flag leaf of wheat during monocarpic senescence. The gels were scanned using gel documentation system to calculate relative transcript level. Fertile (filled bar) and CMS (open bar). Three separate gels were scanned. Error bars indicate mean ± SE (n = 3)
Chloroplasts in both F and CMS plants showed increase in SOD and APX activity with increase in age up to 21 DAA. However, at 28 DAA CMS plants exhibited significantly higher SOD and APX activities than F plants (Fig. 4). At 28 DAA protein carbonyls in chloroplasts declined sharply in F plants but remained higher in CMS plants.
Fig. 4.
Oxidative damage to proteins as protein carbonyls (a), SOD activity (b) and APX activity (c) in chloroplast from the flag leaf of fertile and its CMS isoline of wheat during monocarpic senescence. Fertile (crossed bar) and CMS (open bar). Error bars indicate mean ± SE (n = 3). In some cases error bars are smaller than the symbol. Vertical bars indicate critical difference (CD) treatments × stages (P < 0.05)
Discussion
Reproductive sinks regulate leaf senescence in monocarpic plants and manipulation of sink strength can accelerate/delay the rate of senescence (Nooden 1988). CMS plants exhibited delayed senescence (Table 2). It has been reported earlier that de-sinking results in delayed leaf senescence in wheat and soybean (Srivalli and Khanna-Chopra 2004, 2009; Crafts-Brandner and Egli 1987).
In monocarpic plants such as wheat senescence is associated with nutrient mobilization (including N) from leaves to developing grains (Khanna-Chopra 2012). CMS plants maintained higher levels of protein and rubisco content and less protease activity than F plants during senescence. In soyabean also leaf N and rubisco protein content declined faster in plants with sinks compared to de-sinked plants (Crafts-Brandner and Egli 1987). A net degradation of rubisco and other chloroplast proteins can be observed during endogenously initiated leaf senescence as well as during or after abiotic stresses which allows the reutilization of N in other organs (Feller et al. 2008). During early stages of senescence, rubisco accounts for about 90 % of the degraded proteins (Miller and Huffaker 1985).
Despite the sharp decline in rubisco LSU protein content after 14 DAA in F and 21 DAA in CMS plants both isolines maintained mRNA levels of both rbcL and rbcS at all stages (Figs. 2 and 3). CMS plants had higher expression of rbcL and rbcS than F plants at all stages (Table 2, Figs. 2 and 3). This suggests that post-transcriptional regulation of rubisco content may occur in senescing wheat leaves. It was reported recently that rubisco content in rice was regulated at transcription level in young and post-transcriptional level in old senescent leaves (Suzuki and Makino 2013). Expression of rbcL and rbcS generally declines during senescence (Breeze et al. 2011) and decline in rbcL mRNA levels was lesser and slower than rbcS (Suzuki et al. 2010).
It is known that both cysteine and serine proteases help in protein mobilization in wheat leaves during monocarpic senescence (Table 2, Martínez et al. 2007; Chauhan et al. 2009). F plants showed higher expression of WCP2 and WSP than CMS plants during senescence (Figs. 2 and 3). Transcriptome study of Arabidopsis senescent leaves also revealed that cysteine and serine proteases are predominantly induced during natural and/or abiotic stress induced senescence (Buchanan-Wollaston et al. 2005). Roberts et al. (2003) reported a serine protease activity in senescing wheat leaves for which rubisco was a target protein.
GS is involved in the synthesis of glutamine which is the major form of mobilized N from senescing tissues to grains/pods (Finnemann and Schjoerring 2000). GS1 and GS2 in wheat and its homologue in maize, barley and Arabidopsis showed upregulation during senescence which was associated with high grain protein (Buchanan-Wollaston et al. 2005; Martin et al. 2006; Goodall et al. 2013). Increased mRNA levels of GS1 and GS2 may lead to higher activity of respective proteins in F plants than CMS plants which is needed for N mobilization to developing grains. In CMS plants stem and other vegetative parts acted as alternate sinks, which was evident from their better vegetative growth than F plants. In fact, in the absence of reproductive sinks vegetative parts act as alternate sinks in plants (del Molino et al. 1995; Srivalli and Khanna-Chopra 2004).
WRKY53 expression peaked with accumulation of H2O2 in both F and CMS plants which was at 21 and 28 DAA in F plants and CMS plants respectively (Figs. 2 and 3). WRKY53 transcription factor is known to regulate leaf senescence in plants (Miao et al. 2004). Induction of WRKY53 expression was observed at early stages of senescence before expression of many SAGs (Hinderhofer and Zentgraf 2001; Miao et al. 2004). At later stages of senescence WRKY53 expression was required for the continued expression of SAGs and was controlled by ethylene responsive factors (Koyama et al. 2013). Regulator of WRKY53 expression and DNA binding of corresponding protein itself is involved in signal transduction from H2O2 to WRKY53 promoter (Miao et al. 2007). Expression of WRKY53 induced rapidly in Arabidopsis on treatment with H2O2. WRKY53 is known to regulate expression of other members of WRKY family genes, ROS defense related genes i.e. CAT1 and CAT2, SAGs including SAG12, and receptor kinases involved in senescence induced downstream signaling in Arabidopsis (Miao et al. 2004). It is likely that the early oxidative burst in F plants compared to CMS plants may serve as a signal triggering early senescence as observed in other PCD processes (Fig. 1, Zentgraf et al. 2012).
Senescence like other PCD processes is associated with enhanced ROS accumulation culminating in death in plants (Foyer and Noctor 2009). Early ROS accumulation in F plants than CMS plants may be due to reproductive sinks, which act as a stress in F plants and serve as a signal for start of the senescence program (Srivalli and Khanna-Chopra 2004; Zentgraf et al. 2012). ROS accumulation increases during and is associated with increased expression of senescence associated genes (Bieker et al. 2012; Navabpour et al. 2003). In Arabidopsis, 15 senescence-associated NAC domain TFs are upregulated by H2O2 and an oxidative burst played a role in ANAC092-mediated senescence (Balazadeh et al. 2010; Petrov and Van Breusegem 2012).
Membrane damage is the key signal of cellular deterioration and death as maintaining cell membrane integrity is essential for survival in plants (Khanna-Chopra 2012). It was clear that CMS plants could maintain membrane integrity for a longer period during monocarpic senescence than F plants. The increase in the leaf H2O2 level occurs in parallel with increase in lipid peroxidation and protein oxidation in senescent leaves (Vanacker et al. 2006). CMS plants also showed higher activities of antioxidant enzymes, better redox state than F plants (data not shown). This also contributed to the lesser membrane damage in CMS plants. Similar results have been observed in our studies in wheat wherein spikelets were removed and plants without spikelets showed better antioxidant defense and less oxidative damage than the control (Srivalli and Khanna-Chopra 2004, 2009).
CMS chloroplasts exhibited better antioxidant defense than F plants while protein carbonyls in chloroplasts declined sharply in F plants but remained higher in CMS plants during senescence (Fig. 4). Chloroplasts are also the major source of ROS in plants because of photosynthesis in an aerobic environment, and are the first organelles to show visible symptoms of degradation processes during senescence (Munné and Alegre 2002; Foyer and Noctor 2009). It is likely that damaged proteins in chloroplasts are mobilized quickly in F plants while CMS plants accumulate oxidized proteins in absence of reproductive sinks. Chloroplastic proteins are major source of nitrogen in leaves for mobilization towards the developing seeds/plant parts (Wada et al. 2009) and reproductive sinks drive N mobilization from leaves to grains (Srivalli and Khanna-Chopra 2004, 2009; del Molino et al. 1995; Mǿller et al. 2007). It is tempting to speculate that the better antioxidant defense in chloroplasts of the CMS plants may help in maintaining chloroplasts integrity for a longer period of time than F plants during senescence.
This study using wheat genetically fertile and its cytoplasmic male sterile line shows that reproductive sinks regulate monocarpic senescence through enhanced ROS, rubisco degradation and proteolysis in wheat. Delayed increase in ROS in cytoplasmic male sterile plants resulted in delayed activation of senescence events such as cellular damage, WRKY53 and other senescence associated genes than F plants. Better antioxidant defense in chloroplasts at later stages of senescence was able to mitigate the deleterious effects of ROS in cytoplasmic male sterile plants, which may contribute towards longevity. Hence, we propose that longevity in cytoplasmic male sterile wheat plants is associated with delayed increase in ROS, WRKY53, senescence associated genes and better antioxidant defense in chloroplasts than fertile plants.
Acknowledgments
This research was supported by the financial grants of CSIR Emeritus Scientist Scheme awarded to Dr. (Mrs.) R. K. Chopra.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- AsA
Ascorbate
- APX
Ascorbate peroxidase
- CP
Cysteine protease
- CAT
Catalase
- DAA
Days after anthesis
- GS
Glutamine synthetase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- SAGs
Senescence associated genes
- SP
Serine protease
References
- Aggarwal PK, Sinha SK. Performance of wheat and tritcale varieties in a variable soil water environment. IV. Yield components and their association with grain yield. Field Crop Res. 1987;17:45–53. doi: 10.1016/0378-4290(87)90081-5. [DOI] [Google Scholar]
- Almeselmani M, Deshmukh PS, Chinnusamy V. Effects of prolonged high temperature stress on respiration, photosynthesis and gene expression in wheat (Triticum aestivum L.) varieties differing in their thermotolerance. Plant Stress. 2012;6:25–32. [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Wu A, Mueller-Roeber B. Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal Behav. 2010;5:733–735. doi: 10.4161/psb.5.6.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker S, Riester L, Stahl M, Franzaring J, Zentgraf U. Senescence-specific alteration of hydrogen peroxide levels in Arabidopsis thaliana and oilseed rape spring variety Brassica napus L cv. Mozart. J Integr Plant Biol. 2012;54:540–554. doi: 10.1111/j.1744-7909.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23:873–894. doi: 10.1105/tpc.111.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signaling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Srivalli S, Nautiyal AR, Khanna-Chopra R. Wheat cultivars differing in heat tolerance show a differential response to monocarpic senescence under high-temperature stress and the involvement of serine proteases. Photosynthetica. 2009;47:536–547. doi: 10.1007/s11099-009-0079-3. [DOI] [Google Scholar]
- Crafts-Brandner SJ, Egli DB. Sink removal and leaf senescence in soybean: cultivar effect. Plant Physiol. 1987;85:662–666. doi: 10.1104/pp.85.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Molino IMM, Martinez-Carrasco R, Perez P, Hernandez L, Morcuende R. Influence of nitrogen supply and sink strength on changes in leaf nitrogen compounds during senescence in two wheat cultivars. Physiol Plant. 1995;95:51–58. doi: 10.1111/j.1399-3054.1995.tb00807.x. [DOI] [Google Scholar]
- Derkx AP, Orford S, Griffiths S, Foulkes MJ, Hawkesford MJ. Identification of differentially senescing mutants of wheat and impacts on yield, biomass and nitrogen partitioning. J Integr Plant Biol. 2012;54:555–566. doi: 10.1111/j.1744-7909.2012.01144.x. [DOI] [PubMed] [Google Scholar]
- Feller U, Anders I, Mae T. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot. 2008;59:1615–1624. doi: 10.1093/jxb/erm242. [DOI] [PubMed] [Google Scholar]
- Finnemann J, Schjoerring JK. Post-translational regulation of cytosolic glutamine synthetase by reversible phosphorylation and 14-3-3 protein interaction. Plant J. 2000;24:171–181. doi: 10.1046/j.1365-313x.2000.00863.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Goodall AJ, Kumar P, Alyson KT. Identification and expression analysis of cytosolic glutamine synthase genes in barley (Hordeum vulgare L.) Plant Cell Physiol. 2013;54:492–505. doi: 10.1093/pcp/pct006. [DOI] [PubMed] [Google Scholar]
- Grabowska A, Kwinta J, Bielawski W. Glutamine synthetase and glutamate dehydrogenase in triticale seeds: molecular cloning and genes expression. Acta Physiol Plant. 2012;34:2393–2406. doi: 10.1007/s11738-012-1085-9. [DOI] [Google Scholar]
- Guo Y, Cai Z, Gan S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ. 2004;27:521–549. doi: 10.1111/j.1365-3040.2003.01158.x. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: 1 Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hinderhofer K, Zentgraf U. Identification of a tran-scription factor specifically expressed at the onset of leaf senescence. Planta. 2001;213:469–473. doi: 10.1007/s004250000512. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Watanabe A. Senescence-specific increase in cytosolic glutamine synthetase and its mRNA in radish cotyledons. Plant Physiol. 1988;88:1430–1434. doi: 10.1104/pp.88.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Chopra R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma. 2012;249:469–481. doi: 10.1007/s00709-011-0308-z. [DOI] [PubMed] [Google Scholar]
- Khanna-Chopra R, Sabarinath S. Heat stable chloroplastic Cu/Zn SOD in Chenopodium murale. Biochem Biophys Res Commun. 2004;320:1187–1192. doi: 10.1016/j.bbrc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Kichey T, Hirel B, Heumez E, Dubois F, Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crop Res. 2007;102:22–32. doi: 10.1016/j.fcr.2007.01.002. [DOI] [Google Scholar]
- Koyama T, Nii H, Mitsuda N, Masaru O, Sakihito K, Ohme-Takagi M, Sato F. A regulatory cascade involving class II ethylene response factor transcriptional repressors operates in the progression of leaf senescence. Plant Physiol. 2013;162:991–1005. doi: 10.1104/pp.113.218115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Sanchez-Serrano JJ. Wound signaling in plants. J Exp Bot. 2000;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Lilley RM, Fitzgerald MP, Rienits KG, Walker DA. Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 1975;75:1–10. doi: 10.1111/j.1469-8137.1975.tb01365.x. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot. 2005;56:417–423. doi: 10.1093/jxb/eri039. [DOI] [PubMed] [Google Scholar]
- Martin A, Lee J, Kichey T, Gerentes D, Zivy M, Tatout C, et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell. 2006;18:3252–3274. doi: 10.1105/tpc.106.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez DE, Bartoli CG, Grbic V, Guiamet JJ. Vacuolar cysteine proteases of wheat (Triticum aestivum L.) are common to leaf senescence induced by different factors. J Exp Bot. 2007;58:1099–1107. doi: 10.1093/jxb/erl270. [DOI] [PubMed] [Google Scholar]
- Miao Y, Laun TM, Smykowski A, Zentgraf U. Arabidopsis MEKK1 can take a 4 short cut: it can directly interact with senescence-related WRKY53 transcription factor 5 on the protein level and can bind to its promoter. Plant Mol Biol. 2007;65:63–76. doi: 10.1007/s11103-007-9198-z. [DOI] [PubMed] [Google Scholar]
- Miao Y, Laun TM, Zimmermann P, Zentgraf U. Targets of WRKY53 transcription factor and its role durin leaf senescence in Arabidopsis. Plant Mol Biol. 2004;55:853–867. doi: 10.1007/s11103-005-2142-1. [DOI] [PubMed] [Google Scholar]
- Miller BL, Huffaker RC. Differential induction of endoproteinases during senescence of attached and detached barley leaves. Plant Physiol. 1985;78:442–446. doi: 10.1104/pp.78.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mǿller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Munné B, Alegre L. Plant aging increases oxidative stress in chloroplasts. Planta. 2002;214:608–615. doi: 10.1007/s004250100646. [DOI] [PubMed] [Google Scholar]
- Navabpour S, Morris K, Allen R, Harrison E, Mackerness SAH, Buchanan-Wollaston V. Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot. 2003;54:2285–2292. doi: 10.1093/jxb/erg267. [DOI] [PubMed] [Google Scholar]
- Nooden LD. Whole plant senescence. In: Nooden LD, Leopold AC, editors. Senescence and aging in plants. San Diego: Academic; 1988. pp. 391–439. [Google Scholar]
- Peoples MB, Pate JS, Atkins CA. Mobilization of nitrogen in fruiting plants of a cultivar of cowpea. J Exp Bot. 1983;34:563–578. doi: 10.1093/jxb/34.5.563. [DOI] [Google Scholar]
- Petrov VD, Van Breusegem F (2012) Hydrogen peroxide- a central hub for information flow in plants. AoB Plants pls014. doi:10.1093/aobpla/pls014 [DOI] [PMC free article] [PubMed]
- Rampino P, Spano G, Pataleo S, Mita G, Napier JA, Di Fonzo N, Shewry PR, Perrotta C. Molecular analysis of a durum wheat ‘stay green’mutant: expression pattern of photosynthesis-related genes. J Cereal Sci. 2006;43:160–168. doi: 10.1016/j.jcs.2005.07.004. [DOI] [Google Scholar]
- Roberts IN, Murray PF, Caputo CP, Passeron S, Barneix AJ. Purification and characterization of a subtilisin-like serine protease induced during the senescence of wheat leaves. Physiol Plant. 2003;118:483–490. doi: 10.1034/j.1399-3054.2003.00142.x. [DOI] [Google Scholar]
- Simova SL, Vaseva I, Grigorova B, Demirevska K, Feller U. Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol Biochem. 2010;48:200–206. doi: 10.1016/j.plaphy.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Sinha P, Tomar SMS, Vinod, Singh VK, Balyan HS. Genetic analysis and molecular mapping of a new fertility restorer gene Rf8 for Triticum timopheevi cytoplasm in wheat (Triticum aestivum L.) using SSR markers. Genetica. 2013 doi: 10.1007/s10709-013-9742-5. [DOI] [PubMed] [Google Scholar]
- Srivalli B, Bharti S, Khanna-Chopra R. Vacuolar cysteine proteases and ribulose-1,5-bisphosphate carboxylase/ oxygenase degradation during monocarpic senescence in cowpea leaves. Photosynthetica. 2001;39:87–93. doi: 10.1023/A:1012400104001. [DOI] [Google Scholar]
- Srivalli B, Khanna-Chopra R. The developing reproductive sink induces oxidative stress to mediate nitrogen mobilization during monocarpicsenescence in wheat. Biochem Biophys Res Commun. 2004;325:198–202. doi: 10.1016/j.bbrc.2004.09.221. [DOI] [PubMed] [Google Scholar]
- Srivalli S, Khanna-Chopra R. Delayed wheat flag leaf senescence due to removal of spikelets is associated with increased activities of leaf antioxidant enzymes, reduced glutathione/oxidized glutathione ratio and oxidative damage to mitochondrial proteins. Plant Physiol Biochem. 2009;47:663–670. doi: 10.1016/j.plaphy.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kihara-Doi T, Kawazu T, Miyake C, Makino A. Differences in Rubisco content and its synthesis in leaves at different positions in Eucalyptus globulus seedlings. Plant Cell Environ. 2010;33:1314–1323. doi: 10.1111/j.1365-3040.2010.02149.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Makino A. Translational downregulation of RBCL is operative in the coordinated expression of Rubisco genes in senescent leaves in rice. J Exp Bot. 2013 doi: 10.1093/jxb/ers398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SMS, Anbalagan S. Characterization of cytoplasmic male sterile lines in wheat (Triticum aestivum L.) Indian J Genet. 2004;64:189–195. [Google Scholar]
- Towbin H, Staehelin T, Gorgon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Sandalio LM, Jimenez A, Palma JM, Corpas FJ, Meseguer V, Gomez M, Sevilla F, Leterrir M, Foyer CH, del Rio LA. Role of redox regulation in leaf senescence of pea plants grown in different sources of nitrogen nutrition. J Exp Bot. 2006;57:1735–1745. doi: 10.1093/jxb/erl012. [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Noctor G, Foyer CH. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem. 2002;40:501–507. doi: 10.1016/S0981-9428(02)01417-1. [DOI] [Google Scholar]
- Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino A. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 2009;149:885–893. doi: 10.1104/pp.108.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ni Z, Yao Y, Guo G, Sun Q. Cloning and expression profiles of 15 genes encoding WRKY transcription factor in wheat (Triticum aestivem L.) Prog Nat Sci. 2008;18:697–705. doi: 10.1016/j.pnsc.2007.12.006. [DOI] [Google Scholar]
- Ye ZH, Varner JE. Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol Biol. 1996;30:1233–1246. doi: 10.1007/BF00019555. [DOI] [PubMed] [Google Scholar]
- Zentgraf U, Zimmermann P Smykowski (2012) A role of intracellular hydrogen peroxide as signalling molecule for plant senescence. In: Nagata T (eds) Senescence, ISBN: 978-953-51-0144-4. In Tech publishing, Rijeka, Croatia, pp 31–50
- Zimmermann P, Orendi G, Heinlein C, Zentgraf U. Senescence specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006;29:1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zentgraf U. The correlation between oxidative stress and leaf senescence during plant development. Cell Mol Biol Lett. 2005;10:515–534. [PubMed] [Google Scholar]
- Zivy M, Thiellement H, Devienne D, Hofmann JP. Study on molecular and cytoplasmic genome expression in wheat by two-dimensional gel electrophoresis. 1. 1st results on 18 alloplasmic lines. Theor Appl Genet. 1983;66:1–7. doi: 10.1007/BF00281838. [DOI] [PubMed] [Google Scholar]