Abstract
The response of two root associated bacteria Pseudomonas pseudoalcaligenes and Bacillus pumilus were studied in the (salt-sensitive) rice GJ17 cultivar to salinity under controlled environmental growth conditions for protection of plant from adverse effect of salinity. Salinity affects the growth of salt-sensitive cultivar, but inoculation of plant growth promoting rhizobacteria (PGPR) reduces the harmful effect of salinity. The present study states that PGPR helps to reduce lipid peroxidation and superoxide dismutase activity in salt-sensitive GJ17 cultivar under salinity and play an important role in the growth regulation for positive adaptation of plants to salt stress. This study shows that inoculation of paddy (Oryza sativa) with such bacteria could provide salt-tolerant ability by reducing the toxicity of reactive oxygen species by reducing plant cell membrane index, cell caspase-like protease activity, and programmed cell death and hence resulted in increase cell viability. As these isolates remain associated with the roots, the effects of tolerance against salinity are observed here. Results also indicate that isolated PGPR strain help in alleviating up to 1.5 % salinity stress as well as improve tolerance.
Keywords: PGPR, Salinity, Cell caspase-like protease activity, Programmed cell death, Lipid peroxidation
Introduction
Lack of water in soil is a major factor limiting global crop yield, and thus, understanding of the molecular mechanisms controlling osmotic response has become useful to enhance osmotic tolerance in crop plants (Thapa et al. 2011). Osmotic stress inhibits plant growth and increases reactive oxygen species (ROS). ROS reacts with proteins, lipids, and DNA and initiates membrane lipid peroxidation (Basu et al. 2010) under osmotic stress. Plants respond to osmotic stress through physiological and biochemical adjustments, such as enhancement of osmolytes and antioxidant systems. The agricultural productivity of rice is decreased by both abiotic and biotic stresses.
In order to allow adjustment of the cellular redox state and to reduce toxic effect of salinity, plants should possess an efficient antioxidant system. Plants are protected against overproduced ROS and oxidative damage by an antioxidative defense system comprising of enzymatic and nonenzymatic antioxidants (Oztürk and Demir 2002). Such molecule acts as osmoprotectant of the cell membrane and could provide energy for growth and survival under stress conditions (Kavi Kishor et al. 2005).
Antioxidant enzymes control the biosynthesis and utilization of antioxidant metabolites such as glutathione and superoxide dismutase to detoxify ROS (Miller et al. 2010) and intricately regulate antioxidant capacity. ROS are thought to be key inducers of programmed cell death in plants (De Pinto et al. 2012), and antioxidants have an important role in this process (Li et al. 2007). Furthermore, programmed cell death that may be triggered by salt stress-induced oxidative stress in paddy may in part be controlled by caspase-like cysteine endopeptidase activity (Wang et al. 2010). Caspases belong to proteases of the cysteine endopeptidase (EC 3.4.22) family and are vital for the execution of programmed cell death in plant tissue (Groten et al. 2006). Cysteine endopeptidase activity is instrumental in the execution of programmed cell death in plants in response to salt stress (Wang et al. 2010), and in the mesophyll cells exposed to NaCl (Andronis and Roubelakis-Angelakis 2010). It thus appears that induction of caspase activity by abiotic stresses, including salt stress, may be transduced via ROS production in response to abiotic stresses. Despite this extensive number of reports on the role of antioxidant enzymatic activities in regulating plant responses to abiotic stresses, reports on caspase-like activity as a key regulator of salt stress responses are limited. Induction of antioxidant enzymes by PGPR strain increases the tolerance of lettuce grown under severe salt stress, and could serve as useful tool for alleviating salinity stress in salt-sensitive plants (Kohler et al. 2009). In the last decade, there were a number of reports on the beneficial effects of microorganisms such as Pseudomonas, Bacillus, Pantoea, Burkholderia, Rhizobium, etc. which help in enhancing the tolerance of crops such as sunflower, maize, wheat, chickpea, groundnut, spices, and grapes to drought, salinity, heat stress, and chilling injury under controlled conditions (Arshad et al. 2008; Vessey 2003). Association of plant growth promoting bacteria (PGPR) has been demonstrated for the successful restoration of the mangrove forest, indicating a beneficial close-microbial relationship mutually beneficially to both the host plant as well as the symbiotic organism (Bashan and Holguin 2002). Several earlier and recent reports demonstrates that the association of PGPR plays an important role towards the adaptation to stress in multiple crops such as Brassica napus (Bertrand et al. 2001), pepper (Del Amor and Cuadra-Crespo 2012), and Zea mays (Rojas-Tapias et al. 2012). Furthermore, short-term effects of salt stress on paddy biochemical and physiological responses are well documented but the long-term effects of salt stress (which are more reflective of field conditions) on such processes in paddy are rare. It was on this basis that we investigated lipid peroxidation, ROS accumulation, antioxidant enzyme activities, caspase-like enzymatic activities, cell death, and growth responses in rice cultivar to salt stress in the presence of PGPR to establish if any relationship exists between the level of salt stress tolerance and the physiological/biochemical processes due to PGPR studied here.
Materials and methods
Isolation and identification of PGPR
Bacterial strains were isolated from rhizosphere soil and roots of paddy as per our published method (Jha et al. 2011). The isolates were identified as Pseudomonas pseudoalcaligenes (GenBank Accession Number; EU921258) and Bacillus pumilus (GenBank Accession Number; EU921259).
Plant inoculation and treatment
Certified seeds of rice variety GJ17 (salinity sensitive) were obtained from Main Rice Research Center, Navagam, Anand, Gujarat and were inoculated with PGPR according to our published method (Jha and Subramanian 2012). The saline condition was maintained at five different salinity levels by adding (5, 10, 15, 20, and 25 g NaCl L−1) saline solution to the pots. The pot having 10 kg soil was planted with five transplants and each having three plants. To avoid osmotic shocks, NaCl concentration was gradually increased for four consecutive days. A plastic bag was put underneath each pot to collect excess water due to drainage. This water was reapplied to the respective pot. All seedlings were grown for 5 weeks without any fertilizer treatment. The experiment was conducted in a greenhouse at 20 to 25 °C and relative humidity at 70 to 80 %. Further experiment were conducted only with plants at 1.5 % salinity because plant inoculated with PGPR easily survive up to 1 % salinity as observed in our previous studies (Jha et al. 2011, Jha and Subramnain 2013).
Measurement of plant length and dry weight
Plants from each treatment after 35 days of sowing the seeds were collected carefully with plant roots. The shoot and root lengths were measured and plant dry weight was determined after oven drying at 70 °C until they reached constant weight.
Measurement of membrane stability index
Membrane stability index (MSI) was estimated by the method of Sairam et al. (1997). Fresh leaves (0.1 g) were taken in 10 cm3 of double-distilled water in two sets. One set was subjected to 40 °C for 30 min and its electrical conductivity was recorded using an electric conductivity meter (C1). Second set was kept in a boiling water bath (100 °C) for 10 min and its conductivity was also recorded (C2).
 |
Measurement of cell viability
Leaves from each treatment were assayed for cell viability as described by Sanevas et al. (2007). Leaves were stained with 0.25 % (w/v) Evans Blue for 15 min at room temperature and then washed for 30 min in distilled water, followed by extraction of the Evans Blue stain from leaves tissue using 1 % (w/v) SDS after incubation for 1 h at 55 °C. Absorbance of the extract was measured at 600 nm to determine the level of Evans Blue taken up by the leaves’ tissues. The cell viability assay is based on the uptake of Evans blue by nonviable cells. Ten milligrams per milliliter of Evans Blue Dye is used as standard used to quantify the amount of Evan’s blue taken up.
Determination of caspase-like activity
Caspase-like activity was assayed on the upcoming third youngest leaves from each treatment at the end of 35 days of salt treatment using the method of María et al. (2011_. Two hundred milligrams of leaf tissue was ground in liquid nitrogen into a fine powder and homogenized in 2 ml of assay buffer containing 100 mM Tris–HCl (pH 7.2), 5 mM MgCI2, 2 mM EDTA, 10 % (v/v) glycerol, 10 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride (PMSF). This mixture was centrifuged at 13,000 g for 30 min at 4 °C to obtain tissue extract, and 25 μl of the tissue extract was incubated in 70 μl of assay buffer at 37 °C for 5 min, followed by addition of 10 μl of 5 mM N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA) as substrate (dissolved in dimethyl sulfoxide) for caspase-like activity to a final concentration of 0.5 mM. A blank reaction was set up in which Ac-DEVD-pNA was substituted with 10 μl of DMSO. These reaction mixtures were incubated at 37 °C for 60 min, within which caspase-like activity was followed by measuring absorbance at 405 nm every 20 min during the 60-min incubation period. Caspase-like activity was calculated using the extinction coefficient of 9.6 mM−1 cm−1 for the p-nitroaniline.
Agarose gel analysis of DNA for programmed cell death
Hundred milligram of leaf tissue form each treatment was frozen in liquid nitrogen immediately after sampling and ground with a mortar and pestle to a fine powder. Isolation of DNA was performed using a DNeasy plant mini kit (QIAGEN, Mississauga, Ontario, Canada) according to the manufacturer’s instructions. To observe DNA fragmentation, samples (0.5 μg mL−1 lane−1, final concentration) were run with a 100-bp ladder on a 1 % ethidium bromide agarose gel at a constant 50 V.
Measurement of lipid peroxidation
Lipid peroxidation (reflected by MDA content) was measured by grinding leaf tissue (200 mg) into a fine powder in liquid nitrogen. The tissue was homogenized in 800 μl of cold 5 % (w/v) TCA. The homogenate was centrifuged at 12,000 g for 30 min and further process based on the method of Madhava Rao and Sresty (2000). The concentration of MDA was calculated using an extinction coefficient of 155 mM cm−1. All tests were carried out in triplicate.
Enzyme extractions
Two grams of leaves were homogenized with a mortar and pestle in 4 ml of ice-cold 50 mM Tris-acetate buffer pH 6.0, containing 0.1 mM ethylene diamine tetraacetic acid (EDTA), 5 mM cysteine, 2 % (w/v) polyvinylpyrrolidone (PVP), 0.1 mM phenyl methyl sulphonyl fluoride (PMSF), and 0.2 % (v/v) Triton X 100. The homogenate was centrifuged at 12,000 g for 20 min and the supernatant was filtered through Sephadex G-25 column equilibrated with the same buffer used for homogenization. The column elutes were used as an enzyme source for determination of enzyme activity. All operations were performed at 4 °C. Protein concentration was determined by taking OD at 595 nm by using bovine serum albumin as a standard.
Estimation of superoxide dismutase activity
Superoxide dismutase (SOD) activity was estimated spectrophotometrically as the inhibition of photochemical reduction of NBT at 560 nm. Three milliliters of reaction mixture contained 33 mM NBT, 10 mM l-methionine, 0.66 mM EDTANa2, and 0.0033 mM riboflavin in 0.05 M Na-phosphate buffer (pH 7.8) and 0.1 ml enzyme from plant. One unit of SOD is defined as the amount of enzyme that inhibits 50 % NBT photoreduction. Reactions were carried out at 25 °C, under light intensity of about 300 μmol−1 m−2 s−1 for 10 min. Modifications of the original methods are detailed by Costa et al. (2010).
Estimation of ascorbate peroxidase activity
Ascorbate peroxidase activity was estimated according to the method of Nakano and Asada, (1981). The reaction mixture consist of 0.05 mol L−1 Na-phosphate buffer (pH 7), 0.5 mmol L−1 ascorbate, 0.1 mmol L−1 EDTA.Na2, 1.2 mmol L−1 H2O2, and 0.1 ml enzyme extract in a final assay volume of 1 ml. The increase in absorption at A290 was recorded for 3 min. The concentration of oxidized ascorbate was calculated using extinction coefficient (=2.8 mM cm−1). One unit of APX was defined as micromoles per minute per milligram protein ascorbate oxidized per minute.
Statistical analysis
All experiments described were performed three times independently, with measurements taken from three (for all other measurements) or ten (for dry weight measurements) different plants for each treatment in each of the three independent experiments. One-way analysis of variance (ANOVA) test was used to analyze all data, and mean (of three independent experiments) was compared at 5 % level of significance.
Results
We compared plant’s root length, shoot length, and dry weights between the PGPR inoculated and noninoculated paddy GJ17 cultivar under normal and saline conditions. Plant shoot length, root length, dry weight, and membrane stability index were negatively affected by salinity but inoculation with PGPR definitely reduced the adverse affects of salinity in the cultivar as shown in Table 1. Root length in PGPR-inoculated plants increased by 10–37 % in nonsaline condition and 4.5–18 % under salinity; shoot length increased by 4–13 % in nonsaline condition and 3.4–8.3 % under salinity. PGPR-inoculated plants showed 18–57 % increased dry weight in nonsaline and 17–39 % under salinity. Membrane stability index also increased by 2.4–4.8 % in nonsaline and 2.6–7.8 % under salinity compared to respective control.
Table 1.
Effect of PGPR on the root length, shoot length, dry weight, and membrane stability index under normal and 1.5 % saline condition in paddy variety GJ17
| % Salinity | Treatment | Root | Shoot | Dry weight | Membrane |
|---|---|---|---|---|---|
| Length (cm) | Length (cm) | (gm plant-1) | Stability index | ||
| Normal | Control | 0.192cd | 43.3d | 0.71d | 82.4cd |
| Control + B. pumulis |
0.211c | 45.1c | 0.84bc | 84.1bc | |
| Control + P. pseudoalcaligenes |
0.254ab | 47.9ab | 0.92b | 84.7b | |
| Control + B. pumulis + P. pseudoalcaligenes |
0.263a | 49.2a | 1.12a | 86.3a | |
| Stressed | Control | 0.154cd | 32.2cd | 0.23cd | 38.4cd |
| Control + B. pumulis |
0.161c | 33.3c | 0.27bc | 39.1bc | |
| Control + P. pseudoalcaligenes |
0.179ab | 34.9ab | 0.32b | 40.7ab | |
| Control + B. pumulis + P. pseudoalcaligenes | 0.183a | 35.2a | 0.34a | 41.6a |
For each parameter, values in columns followed by the same letter are not significantly different at (P ≤ 0.05)
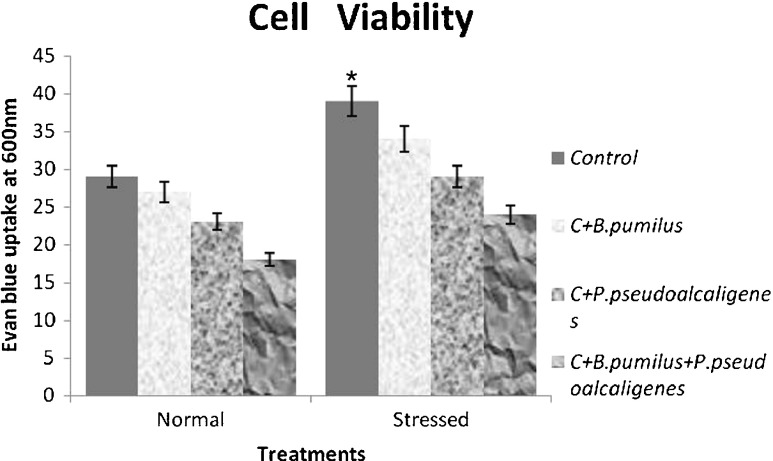
Plants treated with salt suffered a loss in cell viability, as indicated by an increase in the uptake of Evans Blue (which is indicative of cell death) (Fig. 1). The loss of cell viability was higher in GJ17 under salinity compared to the corresponding controls. Inoculation with PGPR reduced the loss of cell viability by 1–1.6 times in normal condition and 1.2–1.6 times under stressed condition in the cultivar compared to noninoculated plants. Inoculation with both the PGPR showed highest cell viability compared to individual ones.
Fig. 1.
Effect of inoculation with B. pumilus, P. pseudoalcaligenes alone, and in combination on cell viability in paddy GJ17 under normal and saline condition (n = 5)
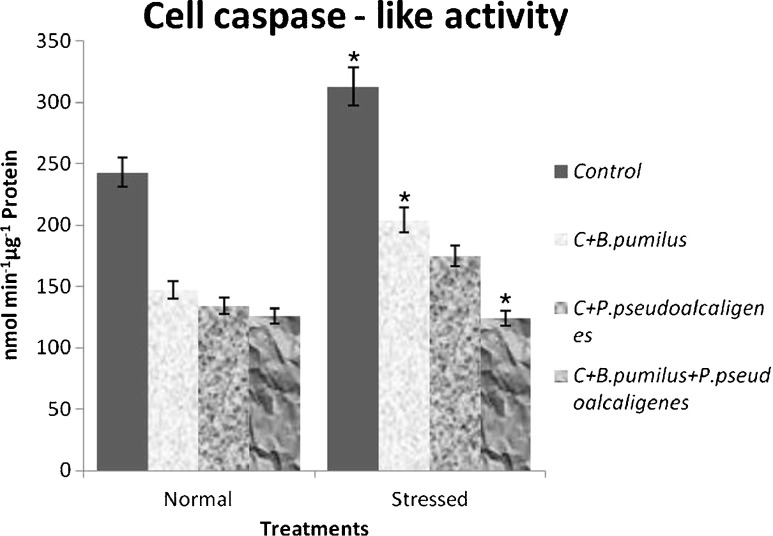
Salinity causes generation of ROS that can trigger the activation of caspase-like activity. On this basis, we investigated the level of caspase-like protease activity in paddy cultivar inoculated with PGPR under salinity. Cell caspase-like enzymatic activity increased in response to salt treatment and more in noninoculated plants than in inoculated ones (Fig. 2). Cell caspase-like protease activity reduced 1.3–1.6 times in normal condition, and under salinity, it reduced from 1.5 to 2.5 times in inoculated plants and similar to cell viability assay inoculation with both the PGPR showing least cell caspase-like protease activity compared to individual ones.
Fig. 2.
Effect of inoculation with B. pumilus, P. pseudoalcaligenes alone, and in combination on cell caspase-like activity in paddy GJ17 under normal and saline condition (n = 5)
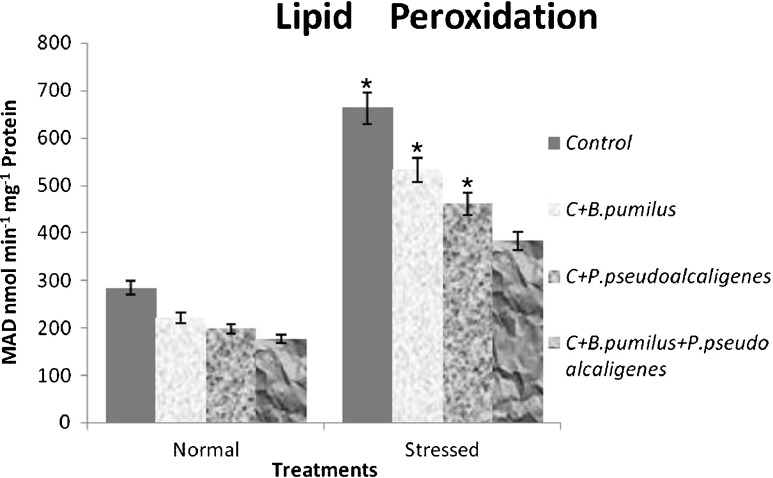
Excessive ROS levels result in oxidative stress, for which lipid peroxidation is one of the biochemical markers. So lipid peroxidation (estimated from MDA content) in the cultivar increased in response to salt treatment. MDA content increased in salt-treated GJ17, whereas decreased in PGPR-inoculated plants compared to the corresponding controls (Fig. 3). Inoculation with individual PGPR reduced lipid peroxidation by one time while mixture of both the PGPR reduced it by 1.6 times in stressed condition compared to control.
Fig. 3.
Effect of inoculation with B. pumilus, P. pseudoalcaligenes alone, and in combination on lipid peroxidation in paddy GJ17 under normal and saline condition (n = 5)
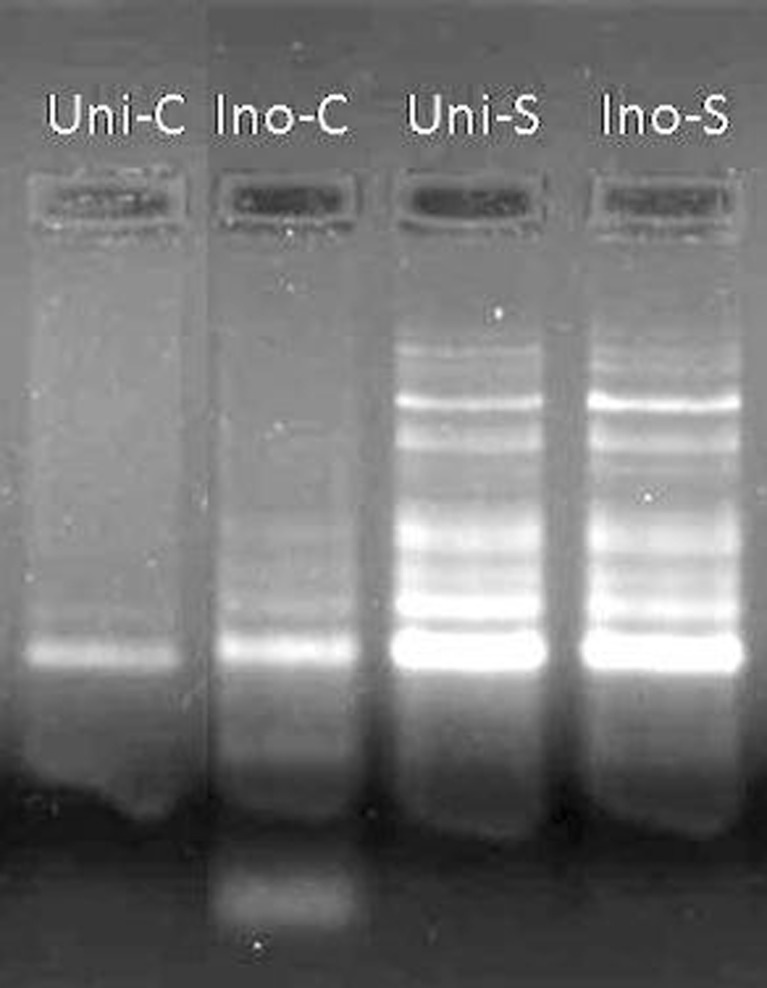
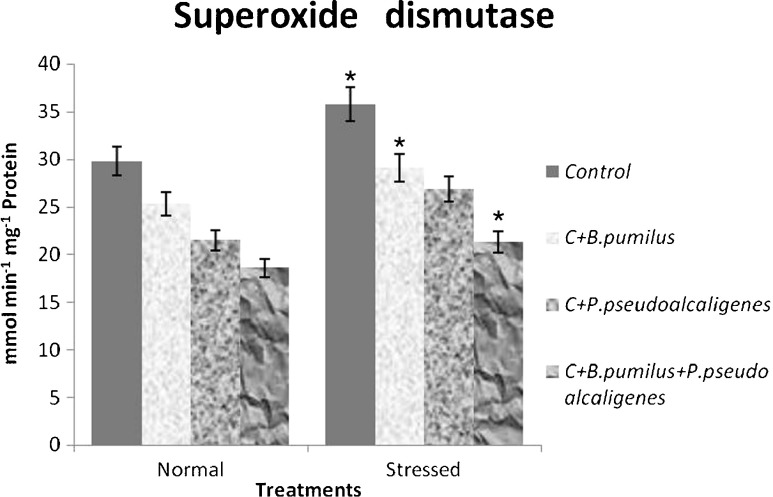
Genomic DNA was isolated from leaves of all treatments and separated in agarose gel electrophoresis to analyze programmed cell death in the form of DNA fragmentation (Fig. 4). Under salinity, extensive DNA fragmentation is detected in both the situations, i.e., under inoculated as well as uninoculated plants, indicating degradation of DNA. Few amounts of fragmentation were observed in the gel due to PGPR inoculation under nonsaline condition. SOD activity increased in GJ17 in response to salt treatment. PGPR-inoculated plants showed decrease of SOD activity in the paddy under both conditions compared to the corresponding untreated controls (Fig. 5).
Fig. 4.
Agarose gel electrophoresis showing the effect of B. pumilus, P. pseudoalcaligenes alone, and in combination on programmed cell death in paddy GJ17 under normal and saline condition (n = 5) visualized via DNA fragmentation. Uni-C pure control, Ino-C inoculated control, Uni-S uninoculated stressed, Ino-S inoculated stressed
Fig. 5.
Effect of inoculation with B. pumilus, P. pseudoalcaligenes alone, and in combination on superoxide dismutase in paddy GJ17 under normal and saline condition (n = 5)
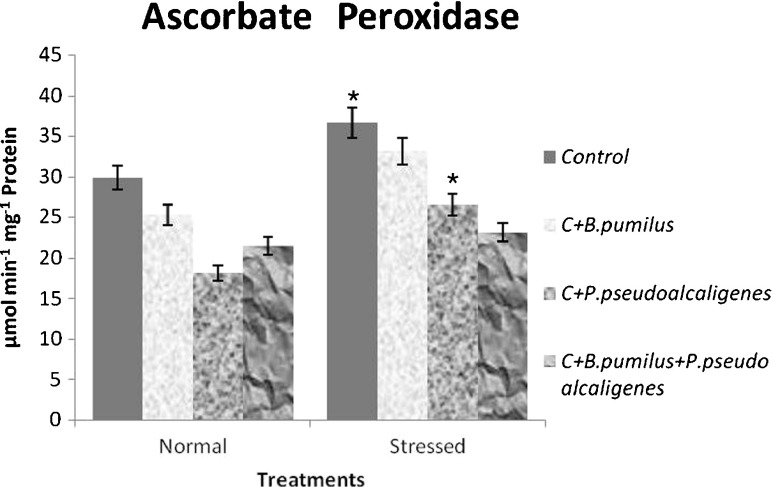
Accumulation of H2O2 can trigger ascorbate peroxidase (APX) activity in an attempt by the cells to detoxify the H2O2. We, thus, measured APX activity to establish if the trend of this enzymatic activity observed for SOD would be maintained for APX under the same conditions. The degree of increase in APX activity in plants was more pronounced in GJ17 in response to salt treatment (Fig. 6) and showed the same trend as SOD. It reflects proper coordination among the SOD and APX.
Fig. 6.
Effect of inoculation with B. pumilus, P. pseudoalcaligenes alone, and in combination on ascorbate peroxidase in paddy GJ17 under normal and saline condition (n = 5)
Discussion
Cultivation practices, in particular an inadequate irrigation management, have led to growing salinization of soil and groundwater resources (Mahajan and Tuteja 2005). Salinity affects the crop productivity and yield as salt stress negatively affects plant growth (Parida and Das 2005). In the present study, physiological and biochemical changes in paddy cultivars were studied in the presence of PGPR. However, when the plants were inoculated with PGPR, the extent of growth suppression was ameliorated and the treated plants showed greater growth response and dry weight than noninoculated control plants; this is supported by Han and Lee (2005) and Kohler et al. (2009).
Salinity produces oxidative stress by means of enhanced occurrence of reactive oxygen species (ROS) and also results in high H2O2 concentration (Zhu et al. 2007), acting both as the damaging toxic molecule and beneficial signal transduction molecule (Miller et al. 2010). In this study, salinity causes excessive levels of ROS which cause membrane damage, a major parameter to estimate the level of destruction under saline stress. A decrease in MSI reflects the extent of lipid peroxidation caused by ROS (Sairam and Srivastava 2001). In this study, salinity causes decrease in MSI, whereas inoculation of plants with PGPR results in increase in MSI in both conditions, i.e., normal and saline. Membrane stability is a widely used criterion to assess crop drought tolerance, since water stress caused water loss from plant tissues which seriously impairs both membrane structure and function (Buchanan et al. 2000). ROS cause oxidation of cellular macromolecules like lipids, nucleic acids, and proteins and can trigger activation of caspase-like activity (Wang et al. 2010). In this study, cell caspase-like activity has been observed under salinity conditions. Whether inoculation with PGPR decreased such activity in paddy under normal as well as stressed condition has not been reported till date.
ROS also act as signaling molecules that cause programmed cell death (PCD) in stress response (Mittler 2002). There are numerous examples of ROS being involved in the modulation of biological responses, including hormone signaling involving growth and development, the cell cycle, stress, and defense responses as well as PCD (Fortes et al. 2011). Such a dual function of ROS requires precise and efficient control over ROS production and scavenging (Mittler et al. 2011). Since the trend seen for cell death corresponded well with the trend observed for caspase-like activity across the treatments described here, it is likely that excessive H2O2 accumulation and ensuing macromolecular peroxidation resulting from salt stress triggers caspase-like activity that elicits a PCD pathway, leading to the observed loss in biomass. Determination of PCD-specific events like DNA fragmentation was also reported by Keyster et al. (2012). The observed increase in cell death in response to salt stress in the paddy cultivar due to caspase-like endopeptidase enzymatic activity results in programmed cell death. Wang et al. (2010) also reported the involvement of programmed cell death in plant responses to salt stress. This finding is very much interesting and study is in progress to find the possible mechanism for PGPR action under salinity.
MDA is a common and important index for evaluating the redox status of paddy. The lower the MDA content, the higher the antioxidative ability, as shown by Shao et al. (2005). Peroxidation can also greatly alter the physicochemical properties of membrane lipid bilayers, resulting in severe cellular dysfunction and biosynthesis of MDA. A previous study have reported higher activities of antioxidant enzymes and lower levels of lipid peroxidation in tolerant rice cultivar under salt stress (Dionisiosese and Tobita 1998). In this study, enhanced peroxidation was observed under salinity, but inoculation of PGPR decreased the peroxidation, thus protecting the cell from membrane destruction. The results suggested that inoculation with isolated PGPR strain to salt-stressed paddy could help to alleviate salinity stress and improve tolerance by reducing caspase-like endopeptidase enzymatic activity, decreasing lipid peroxidation, programmed cell death, and also regulating antioxidant enzymatic activity.
In order to allow adjustment of the cellular redox state and to reduce toxic effects of salinity, plant antioxidant system has to be activated as a possible mechanism in paddy. Accumulation of O2– can be countered by triggering SOD and APX activity to bring it to basal levels; the result of which is the production of H2O2 (Foyer and Noctor 2005). APX has higher affinity for H2O2 than CAT and POD, thus it may have more essential role in management of ROS during stress condition (Gill and Tuteja 2010). In this study, the PGPR-inoculated paddy showed decline in SOD and APX activities suggesting a lesser O2– scavenging and dismutating capacity in the GJ17 cultivar and signifies a possible involvement of the two enzymes in salt tolerance, which are in accordance with Dureja (2003).
Our results establish a direct relationship that exists between salt stress-induced oxidative damage and cell death-inducing caspase-like activity in salt-sensitive GJ17 cultivar, and the efficiency of the PGPR to regulate the plant antioxidant enzymes and thus limiting cell death-inducing caspase-like activity.
Contributor Information
Yachana Jha, Phone: +91-9426282152, Email: yachanajha@gmail.com.
R. B. Subramanian, Phone: +91-2692-234402, FAX: +91-2692-236475, Email: subramanianrb@gmail.com
References
- Andronis EA, Roubelakis-Angelakis KA. Short-term salinity stress in tobacco plants leads to the onset of animal-like PCD hallmarks in planta in contrast to long-term stress. Planta. 2010;231:437–448. doi: 10.1007/s00425-009-1060-x. [DOI] [PubMed] [Google Scholar]
- Arshad M, Sharoona B, Mahmood T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth yield and ripening of pea (Pisum sativum L) Pedosphere. 2008;18(5):611–620. doi: 10.1016/S1002-0160(08)60055-7. [DOI] [Google Scholar]
- Bashan Y, Holguin G. Plant growth-promoting bacteria: a potential tool for arid mangrove reforestation. Trees. 2002;16:159–166. doi: 10.1007/s00468-001-0152-4. [DOI] [Google Scholar]
- Basu S, Roychoudhury A, Saha PP, Sengupta DN. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol Plant. 2010;32:551–563. doi: 10.1007/s11738-009-0432-y. [DOI] [Google Scholar]
- Bertrand H, Nalin R, Bally R, Cleyet-Marcel JC. Isolation and identification of the most efficient growth-promoting bacteria associated with canola (Brassica napus) Biol Fertil Soils. 2001;33:152–156. doi: 10.1007/s003740000305. [DOI] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and Molecular Biology of Plants. Rockville, Maryland: American Society of Plant Physiologists; 2000. [Google Scholar]
- Costa MA, Pinheiro HA, Shimizu ESC, Fonseca FT, Santos Filho BGS, Moraes FKC, Figueiredo DM. Lipid peroxidation chloroplastic pigments and antioxidant strategies in Carapa guianensis (Aubl) subjected to water-deficit and short-term rewetting. Trees. 2010;24:275–283. doi: 10.1007/s00468-009-0397-x. [DOI] [Google Scholar]
- De Pinto MC, Locato V, De Gara L. Redox regulation in plant programmed cell death. Plant Cell Environ. 2012;35:234–244. doi: 10.1111/j.1365-3040.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- Del Amor FM, Cuadra-Crespo P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct Plant Biol. 2012;39:82–90. doi: 10.1071/FP11173. [DOI] [PubMed] [Google Scholar]
- Dionisiosese ML, Tobita S. Antioxidant response of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Dureja V (2003) Effect of salinity stress on antioxidant enzymes in salt-tolerant and salt-sensitive cultivar of rice (Oryza sativa L.) MSc Thesis. CCS Haryana Agricultural University, Hisar, India
- Fortes AM, Costa J, Santos F, Seguí-simarro JM, Palme K, Altabell T, Tiburcio AF, Pais S. Arginine decarboxylase expression polyamines biosynthesis and reactive oxygen species during organogenic nodule formation in hop. Plant Signal Behav. 2011;6:258–269. doi: 10.4161/psb.6.2.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in a biotic stress tolerance in crop plants. Annu Rev Plant Physiol Biochem. 2010;3:1–22. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Groten K, Dutilleul C, Van Heerden PDR, Vanackera H, Bernard S, Finkemeier I, Dietz KJ, Foyer CH. Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Lett. 2006;580:1269–1276. doi: 10.1016/j.febslet.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Han HS, Lee KD. Physiological responses of soybean—inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res J Agric Biol Sci. 2005;1(3):216–221. [Google Scholar]
- Jha Y, Subramanian RB. Paddy physiology and enzymes level is regulated by rhizobacteria under saline stress. J Appl Bot Food Qual. 2012;85:168–173. [Google Scholar]
- Jha Y, Subramanian RB (2013) Paddy innoculated with PGPR show better growth physiology and nutrient content under salinity. Chil J Agri Res 73(1):213–219
- Jha Y, Subramanian RB, Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant. 2011;33:797–802. doi: 10.1007/s11738-010-0604-9. [DOI] [Google Scholar]
- Kavi Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, Reddy KJ, Theriappan P, Sreenivaslu N. Regulation of proline biosynthesis degradation uptake and transport in higher plants its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88:424–438. [Google Scholar]
- Keyster M, Klein A, Ludidi N. Caspase-like enzymatic activity and the ascorbate-glutathione cycle participate in salt stress tolerance of maize conferred by exogenously applied nitric oxide. Plant Signal Behav. 2012;7(3):1–12. doi: 10.4161/psb.18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler J, Caravaca F, Alguacil MM, Roldán A. Elevated CO2 increases the effect of an arbuscular mycorrhizal fungus and a plant-growth-promoting rhizobacterium on structural stability of a semiarid agricultural soil under drought conditions. Soil Biol Biochem. 2009;41:1710–1716. doi: 10.1016/j.soilbio.2009.05.014. [DOI] [Google Scholar]
- Li JY, Jiang AL, Zhang W. Salt stress-induced programmed cell death in rice root tip cells. J Integr Plant Biol. 2007;49:481–486. doi: 10.1111/j.1744-7909.2007.00445.x. [DOI] [Google Scholar]
- Madhava Rao KV, Sresty TVS. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/S0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold salinity and drought stresses an overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- María Rodríguez-Serrano, Ivett Bárány, Deepak Prem, María-José, Coronado María C, Risueño, Pilar S, Testillano (2011) NO ROS and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot. doi: 10.1093/jxb/err400 [DOI] [PMC free article] [PubMed]
- Miller G, Suzuki N, Ciftci-Yilmazi N, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Breusegem FV. ROS signalling the new wave. Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Oztürk L, Demir Y. In vivo and in vitro protective role of proline. Plant Growth Regul. 2002;38:259–264. doi: 10.1023/A:1021579713832. [DOI] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants—a review. Ecotoxicol Environ Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Rojas-Tapias D, Moreno-Galván A, Pardo-Díaz S, Obando M, Rivera D, Bonilla R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl Soil Ecol. 2012;61:264–272. doi: 10.1016/j.apsoil.2012.01.006. [DOI] [Google Scholar]
- Sairam RK, Srivastava GC. Water stress tolerance of wheat (Triticum aestivum L) variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci. 2001;186:63–70. doi: 10.1046/j.1439-037x.2001.00461.x. [DOI] [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS (1997) Increased antioxidant enzyme activity in response to drought and temperature stress related with stress tolerance in wheat genotypes. Abstract National Seminar (ISSP) IARI New Delhi pp 69
- Sanevas N, Sunohara Y, Matsumoto H. Characterization of reactive oxygen species-involved oxidative damage in Hapalosiphon species crude extract-treated wheat and onion roots. Weed Biol Mana. 2007;7:172–177. doi: 10.1111/j.1445-6664.2007.00253.x. [DOI] [Google Scholar]
- Shao HB, Liang ZS, Shao MA, Wang BC. Impacts of PEG-6000 pretreatment for barley (Hordeum vulgare L) seeds on the effect of their mature embryo in vitro culture and primary investigation on its physiological mechanism. Colloids Surf B Biointerfaces. 2005;41(2–3):73–77. doi: 10.1016/j.colsurfb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Thapa G, Dey M, Sahoo L, Panda SK. An insight into the osmotic stress induced alterations in plants. Biol Plant. 2011;55:603–613. doi: 10.1007/s10535-011-0158-8. [DOI] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Wang J, Li X, Liu Y, Zhao X. Salt stress induces programmed cell death in Thellungiella halophila suspension-cultured cells. J Plant Physiol. 2010;167:1145–1151. doi: 10.1016/j.jplph.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Zhu J, Fu X, Koo YD, Zhu JK. An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol Cell Biol. 2007;27:5214–5224. doi: 10.1128/MCB.01989-06. [DOI] [PMC free article] [PubMed] [Google Scholar]