Abstract
Many experiments in plant molecular biology require processing of a large number of RNA samples and in some cases large quantities are required for a single application. In turmeric, a major spice and medicinal plant, a protocol for RNA isolation is not available. The major difficulty encountered while using other popular protocols is the low yield and quality of RNA which hampers the downstream applications like qRT-PCR, cDNA synthesis and micro RNA isolation. Commercial kits though available are costly and were found to be unsuccessful in case of rhizomes and root tissues that are rich in polyphenols, polysaccharides and alkaloids. It was thus felt that a quick, handy and cheap protocol of total RNA isolation from different tissues of turmeric was required for day to day working in our lab. The new protocol utilizes SDS based extraction buffer including β-mercaptoethanol and PVP with sequential acid phenol:chloroform extraction to remove polyphenols and proteins, followed by the purification with sodium acetate to eliminate polysaccharides. The protocol is simple and can be completed in less than 3 h. The RNA yield from rhizome was higher by more than fivefold with both A260/280 and A260/230 ratio in the range of 1.8–2.0. The protocol worked well with leaf, rhizome, pseudostem and root tissues with RIN >7.0 and the isolated RNA could be successfully used for cDNA synthesis, RT-PCR, qRT-PCR and small RNA isolation including microRNA.
Keywords: Curcumin, Polysaccharides, Nucleic acid isolation, RIN, sRNA, RT-PCR, qRT-PCR
Introduction
Turmeric (Curcuma longa L.) that belongs to the family Zingiberaceae is one of the most important crops with great medicinal and economic importance. The traditional uses in folk medicine are multiple and many of these have been confirmed by contemporary scientific research. These include antioxidant, antiarthritic, antimutagenic, antitumor, antithrombotic, antivenom, antibacterial, antifungal, antiviral, nematocidal, choleretic, antihepatotoxic and hemagglutinating activities (Kumar et al. 2006; Sangvanich et al. 2007). Turmeric also contains a wide variety of phytochemicals (Almedia et al. 2005; Abas et al. 2005). Due to its growing importance, turmeric is a target crop for gene expression and transcriptome analysis aimed at modifying curcuminoid biosynthetic pathway and identification of transcripts against various human diseases (Annadurai et al. 2012).
Deciphering these mechanisms demands substantially pure and un-degraded RNA for downstream applications like Northern blot hybridization, RT- PCR, cDNA library construction and RNA interference (Murillo et al. 1995; Hu et al. 2002; Wang et al. 2006). Isolation of RNA from certain plants is difficult due to the presence of high amounts of secondary metabolites, such as polysaccharides and polyphenolic compounds, which could co-precipitate or bind to RNA and result in poor yields (Gasic et al. 2004; Djami-Tchatchoua and Straker 2011). Thus, RNA recovery differs from tissue to tissue based on the levels of polyphenols and polysaccharides (Malnoy et al. 2001). This is particularly true in case of rhizome tissues of turmeric as it contains high levels of polyphenols, polysaccharides and alkaloids (Chempakam and Parthasarathy 2008). Most of the popular RNA isolation protocols including Trizol method and commercial kits (Plant RNeasy kit, Qiagen) did not give promising results in turmeric tissues. In our lab we are involved in cloning of genes of biosynthetic pathway for curcuminoid production, transcriptome analysis and association mapping for quality traits in turmeric. It was hence felt that a common protocol that yields RNA from different tissues will be a convenient and economical proposition. Thus the aim of the present study was to develop a simple, rapid and economical RNA isolation method that can give high yield and good quality total RNA from different tissues of turmeric suitable for subsequent applications.
Materials and methods
Plant material
A high curcumin containing released variety of the Indian Institute of Spices Research (IISR) viz., IISR Prathibha was used as the experimental material for the study. The plants were maintained in a greenhouse at IISR, Calicut. Before RNA isolation, the samples were thoroughly washed and frozen immediately using liquid nitrogen. A separate set of samples were stored in RNAlater (Ambion) to check the efficacy of the protocol in stored tissues.
RNA isolation methods
Five popular protocols for RNA extraction viz., Trizol method, Plant RNeasy kit (Qiagen), CTAB method (Chang et al. 1993), SDS- Tris saturated phenol method (Ghawana et al. 2011) and SDS-acid phenol based method (Hou et al. 2011) were tried for isolation of RNA. The optimised protocol is a modification of the SDS-acid phenol protocol (Hou et al. 2011). The reagents and the essential steps involved in the protocol is detailed below:
Solutions and reagents
Extraction buffer: 0–3 % SDS, 100 mM Tris-HCl, pH 8 and 25 mM EDTA - pH 8. PVP (0–3 %) in powder form and liquid form and β-mercaptoethanol (0–3 %)
0.1 % DEPC treated autoclaved water
Acid phenol: chloroform
Sodium acetate (0-5 M; adjusted to pH 5 with glacial acetic acid)
Cold isopropanol
Cold 70 % ethanol
Protocol
Optimized total RNA isolation
Grind 100 mg of tissue to a fine powder along with 2 % PVP (powder form) in a mortar and pestle using liquid nitrogen.
Add 2 ml of prewarmed (65 °C) extraction buffer (2 % SDS, 100 mM Tris-HCl - pH 8, 25 mM EDTA- pH 8 and 1 % β-mercaptoethanol), grind further and then transfer the contents equally to two, 2 ml centrifuge tubes.
Add equal volume of acid phenol: chloroform and vortex for 10 s. Leave it for 10 min at room temperature for the complete dissociation of nucleoprotein complexes.
Centrifuge at 15,000 g for 10 min at 4 °C
Transfer the supernatant to fresh tubes and add 0.3 volume of 5 M sodium acetate and 0.7 volume of acid phenol: chloroform. Mix by inverting the tubes and incubate in ice for 10 min.
Centrifuge at 15,000 g for 10 min at 4 °C and transfer the supernatant to fresh 1.5 ml centrifuge tubes.
Add 0.1 volume of 3 M sodium acetate and equal volume of isopropanol. Incubate the tubes at -20 °C for 1 h.
Centrifuge at 15,000 g for 10 min at 4 °C and discard the supernatant.
Wash the pellet with 70 % ethanol by centrifuging at 7,500 g for 5 min at 4 °C.
Air dry the pellet for 5–10 min and dissolve in 25 μl RNase free water.
Low molecular weight (LMW) RNA isolation
Small RNA including micro RNA fraction was precipitated from total RNA using polyethylene glycol and sodium chloride as described by Lu et al. 2007.
RNA quality
The quality of total RNA was analyzed using denaturing gel electrophoresis (Masek et al. 2005). Concentration and purity of total RNA was assessed by monitoring A260/280 ratio and A260/230 ratio using Biophotometer plus (Eppendorf, Germany). The integrity of total RNA was also determined using Agilent 2100 Bioanalyzer with Plant RNA Nano chip assay. The small RNA fractions were denatured by mixing it with equal volume of gel loading buffer II (Ambion), heated for 5 min at 95 °C and then chilled on ice. The integrity of small RNA was then analyzed using 15 % denaturing polyacrylamide gel containing 8 M urea (Yew and Kumar 2012; Lu et al. 2007) along with miRNA ladder (NEB). The gel was then stained using SYBR gold, Invitrogen.
RT-PCR/qRT- PCR
RT PCR and qRT-PCR were performed with the total RNA isolated from rhizome tissue following Chang et al. 1993; Ghawana et al. 2011 and the new protocol only, since the other three protocols either failed or yielded very low concentration of RNA. First strand cDNA was synthesized using total RNA (1 μg) in the presence of Oligo-(dT)18 primer and Revert Aid Reverse transcriptase (Thermo Scientific) after digesting with 1U of DNase I (Thermo Scientific) as described in supplier’s instruction. ~950 bp of actin was PCR amplified using forward (5′-GGT GTC ATG GTT GGT ATG GGT C-3′) and reverse (5′-TGC AAT CCA CAT CTGTTG GAA G-3′) primers. The PCR mixture was initially denatured at 94 °C for 2 min and then subjected to 30 cycles at the following conditions: 94 °C for 45 s, 58 °C for 1 min, 72 °C for 2 min with a final extension at 72 °C for 10 min. Inorder to test the efficacy of the new protocol, cDNA was synthesized and actin gene was amplified from leaf, pseudostem and root tissues also. The amplified products were separated on a 1 % agarose/ EtBr gel. 1 μl of cDNA, corresponding to 10 ng of total RNA from rhizomes of 4 month old turmeric plants was used for the qRT-PCR assay. qRT-PCR reaction was performed in triplicates with forward (5′-TAG GTG CCC AGA GGT TCT ATT-3′) and reverse (5′-ACC GCT AAG CAC CAC ATT AC-3′) primers targeting 125 bp of actin following QuantiFast SYBR Green PCR kit (Qiagen) protocol on Rotor-Gene Q (Qiagen).
Similarly, to analyze the microRNA expression profile, 1 μg of total RNA from different tissues of 4 month old turmeric plant was isolated using the present protocol and reverse transcribed with miScript Reverse Transcription kit (Qiagen). The amplification of turmeric specific miRNA was carried out using mature miRNA sequence as the forward primer (5′-TGC CTC AAG CTC GTT GCC TCC-3′) and miScript universal reverse primer as per the manufacturer’s instructions. A reverse transcription negative control (without reverse transcriptase) and a non-template negative control were included to confirm the absence of genomic DNA and to check for primer-dimer or any other in the reactions, respectively in both RT-PCR and qRT-PCR analysis.
Results and discussion
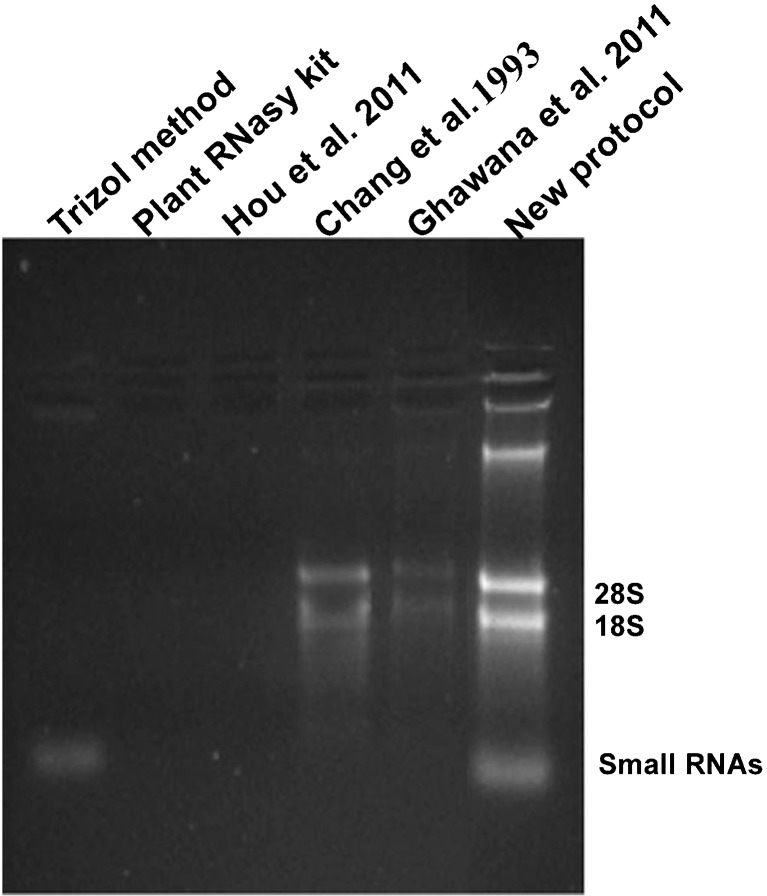
A major problem with RNA isolation from rhizome tissues of turmeric is the presence of polyphenols, polysaccharides and alkaloids which interfere with RNA extraction and complicate downstream applications. Polyphenolic compounds oxidize very quickly during RNA extraction and form quinones which bind with RNA (Loomis 1974) and polysaccharides co-precipitate with the RNA in low ionic concentration buffer (Birtic and Kranner 2006). The Trizol method, Plant RNeasy kit (Qiagen) and other three published protocols yielded RNA of low yield and purity from rhizomes (Fig. 1, Table 1). No RNA was obtained with Trizol method even when the recommended high salt solution procedure for tissues with high polysaccharide content was followed. RNA yield was lower when tried with Plant RNeasy kit (Qiagen) and Ghawana et al. 2011 method. The method of Chang et al. 1993 was time consuming (~16 h) even though it yielded RNA of comparatively better yield and quality. The quality and yield of RNA was very poor when tried with the method of Hou et al. 2011. Though Trizol yielded good quality of RNA from leaf tissues, it failed in case of rhizomes. DNA contamination was present in all the tested protocols with the least observed in the protocol of Chang et al. 1993, which involves addition of lithium chloride followed by overnight incubation. But this approach is time consuming. DNA contamination was removed by treating with RNase free DNase as reported in other protocols (Kiefer et al. 2000; Xu et al. 2010). Phenol has been used as an excellent alternative to Trizol, being cheap and reliable and has been demonstrated to be more suitable for high-throughput RNA extraction from a broad range of developmental stages (Box et al. 2011).
Fig. 1.
Agarose gel electrophoresis of RNA isolated from rhizome tissues of turmeric using different RNA isolation methods. For comparison, 5 μl of RNA was loaded. 100 mg of sample was used as the starting material and finally dissolved in 25 μl RNase free water in all the methods
Table 1.
Spectrophotometric analysis of yield and purity of RNA isolated using different protocols
| Method | Tissue used | A260/280 | A260/230 | RNA yield |
|---|---|---|---|---|
| (μg/g) | ||||
| Trizol method | Rhizome | _ | _ | _ |
| Plant RNeasy kit | Rhizome | 1.50 ± 0.07 | 0.22 ± 0.03 | 6.1 ± 0.06 |
| Ghawana et al. 2011 | Rhizome | 1.63 ± 0.07 | 1.64 ± 0.12 | 31 ± 6.2 |
| Chang et al. 1993 | Rhizome | 1.92 ± 0.05 | 1.87 ± 0.03 | 29 ± 2.7 |
| Hou et al. 2011 | Rhizome | _ | _ | _ |
| Present protocol | Rhizome | 1.80 ± 0.05 | 1.79 ± 0.02 | 180 ± 13 |
| Leaf | 1.82 ± 0.02 | 2.07 ± 0.05 | 163 ± 5 | |
| Shoot | 1.87 ± 0.02 | 2.08 ± 0.05 | 138 ± 6.5 | |
| Root | 1.75 ± 0.1 | 1.8 ± 0.15 | 102 ± 7.2 |
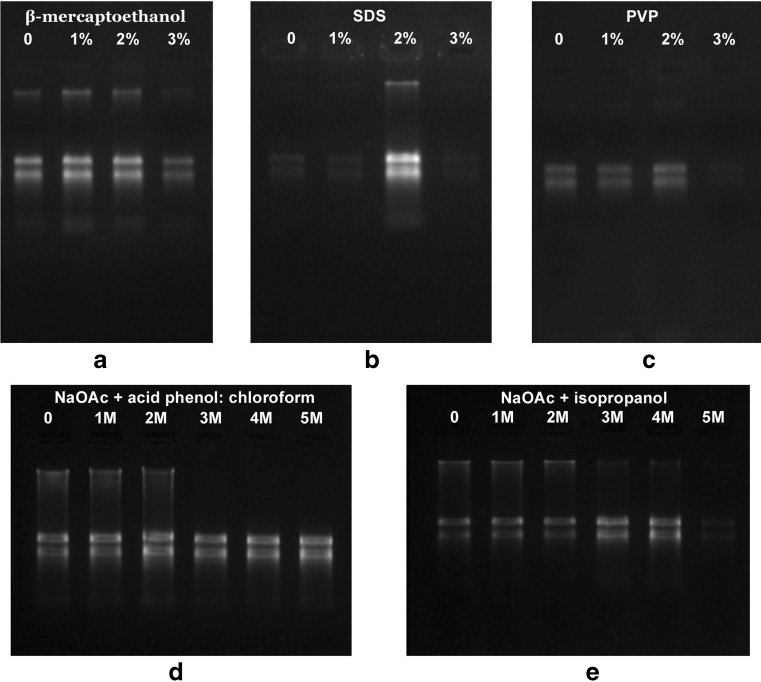
Hou et al. 2011 was selected for modification as the protocol is simple, rapid and also even a change in the sample: extraction buffer volume from 1.2 to 20 yielded RNA of comparable quantity and quality. The extraction buffer of Hou et al. 2011 contains Tris-HCl, sodium chloride, SDS and β-mercaptoethanol. We found that the presence of sodium chloride in the extraction buffer decreased the RNA yield but the addition of PVP and EDTA increased the yield. Hou et al. 2011 uses sodium chloride along with acid phenol:chloroform. Both sodium chloride and sodium acetate were reported to play an important role in nucleic acid extraction from polysaccharides (Rubio-Pina and Zapata-Perez 2011; Wang and Stegemann 2010). But in turmeric rhizome, the presence of sodium chloride even at 1 M decreased the yield. So we replaced the sodium chloride by sodium acetate. For the enhancement of RNA precipitation, we added sodium acetate along with isopropanol. We also optimized concentration of SDS (0–3 %), PVP (0–3 %), β-mercaptoethanol (0–3 %) and sodium acetate (0–5 %) individually in the protocol (steps 2–7). It was observed that 2 % SDS, 2 % PVP, 1 % β-mercaptoethanol were optimum in enhancing the yield (Fig. 2a, b and c). Addition of PVP in powder form was found better than the liquid form. A method employing PVP was successful in extracting RNA from Dendrobium perianth, flower buds and leaves (Champagne and Kuehnle 2000). SDS is widely used as a cell disrupting agent in RNA isolation (Ghawana et al. 2011; Hou et al. 2011; Gonzalez-Mendoza et al. 2008) and to inhibit RNase activity (Mendelsohn and Young 1978). β-mercaptoetanol was also used to inhibit RNase activity and to prevent sample oxidation (Gonzalez-Mendoza et al. 2008). Acidic pH was maintained by the addition of acid phenol as previously described to decrease the DNA contamination (Chomczynski and Sacchi 1987). Phenol: chloroform extraction aided in the protein precipitation (Perry and Kelley 1972). Re-extraction of the aqueous phase along with the sodium acetate and acid phenol:chloroform decreased the polysaccharide and protein contamination. The addition of sodium acetate increased the RNA yield (Fig. 2d). Further to enhance the RNA precipitation and for the removal of polysaccharides, 3 M sodium acetate was added along with the isopropanol in step 7. Here the addition of 3 M sodium acetate increases the RNA yield and any further increase decreased the yield (Fig. 2e). Sodium acetate (3 M) precipitation was reported to increase the RNA yield by eliminating polysaccharide contamination (Rubio-Pina and Zapata-Perez 2011; Singh et al. 2003; Suzuki et al. 2004; Wang et al. 2005).
Fig. 2.
Optimization of major components in RNA isolation
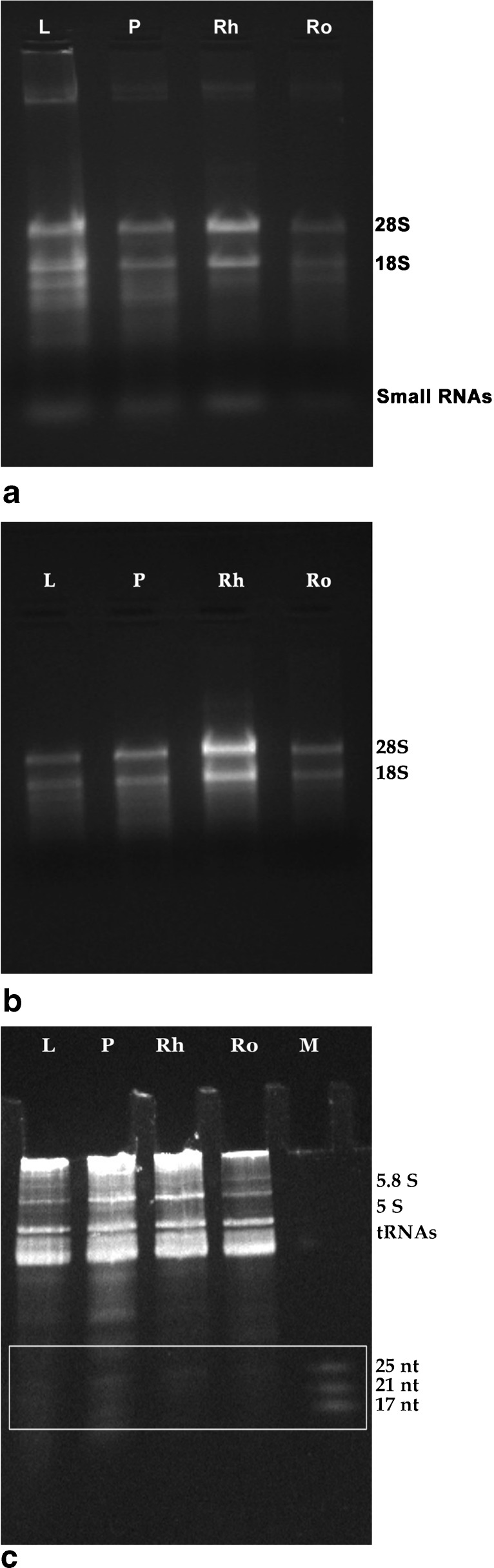
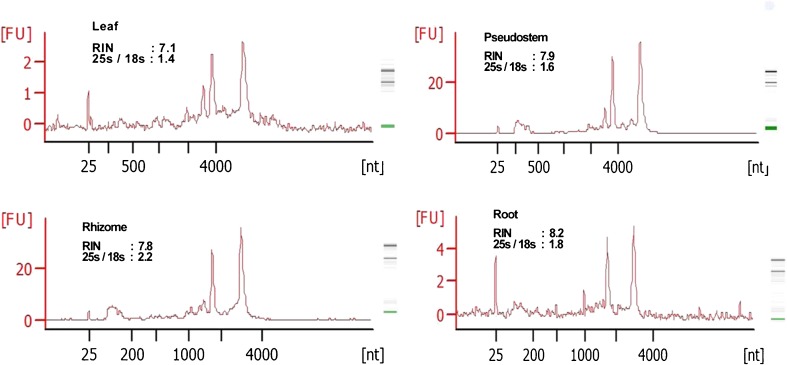
The same protocol was extended to isolate RNA from leaf, pseudostem and roots of turmeric. The integrity of RNA examined on 1.2 % denaturing TAE/agarose gel electrophoresis showed sharp, intact 28S and 18S rRNA bands, with the former having greater intensity than latter (Fig. 3a), suggesting little degradation of RNA (Salzman et al. 1999). The quality and yield of RNA was demonstrated by electrophoresis (Fig. 3) and UV-spectrometry. A260/A230 ratio ranged from 1.79 to 2.08, indicating minimal contamination by polyphenols and carbohydrates. Similarly, A260/A280 ratios exceeded 1.75, showing minimal protein contamination (Table 1). Analysis using Agilent Bioanalyzer 2100 further confirmed high quality RNA (RIN > 7) from different tissues of turmeric isolated by this protocol (Fig. 4). A RIN of greater than or equal to seven indicates the RNA is suitable for high stringency applications such as cDNA library construction and next generation sequencing (Schroeder et al. 2006). Also this protocol worked well with the tissues stored in RNAlater for 7 months (Fig. 3b). RNAlater was also found to be effective in preserving Arabidopsis thaliana seedlings for 10 months and the samples were suitable for qPCR analysis (Salvo-Chirnside et al. 2011). Additional rRNA bands were also obtained from the leaf and pseudostem tissues. These bands represent the abundant transcripts as these tissues are photosynthetically active (Masek et al. 2005). The intactness of RNA was established from high molecular weight double stranded cDNA (0.5–4 kb) synthesised using the new protocol.
Fig. 3.
a Denaturing TAE/agarose gel electrophoresis of RNA isolated from tissues of turmeric. b Denaturing TAE/agarose gel electrophoresis of RNA isolated from different tissues of turmeric stored in RNAlater c Analysis of small RNAs isolated from different tissues of turmeric: L Leaf, P Pseudostem, Rh Rhizome, Ro Root
Fig. 4.
Electropherogram of total RNA isolated from different tissues of turmeric generated by Agilent 2100 Bioanalyzer
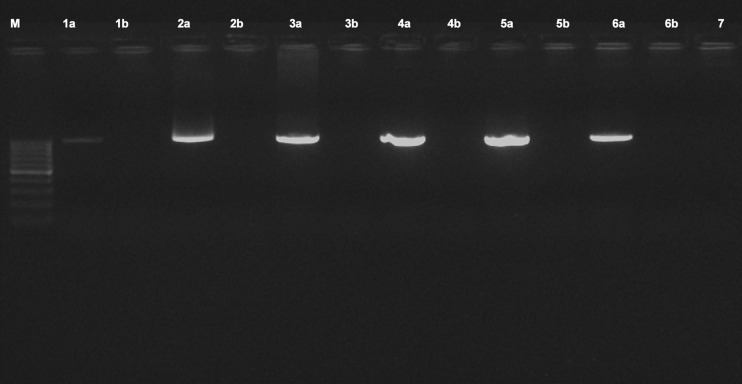
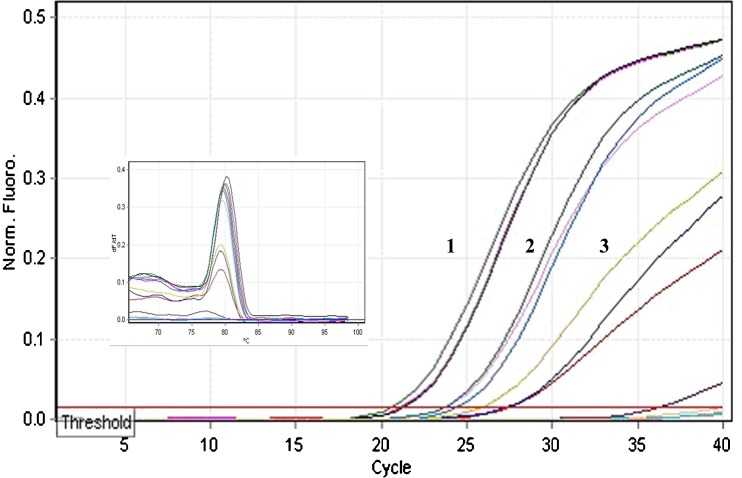
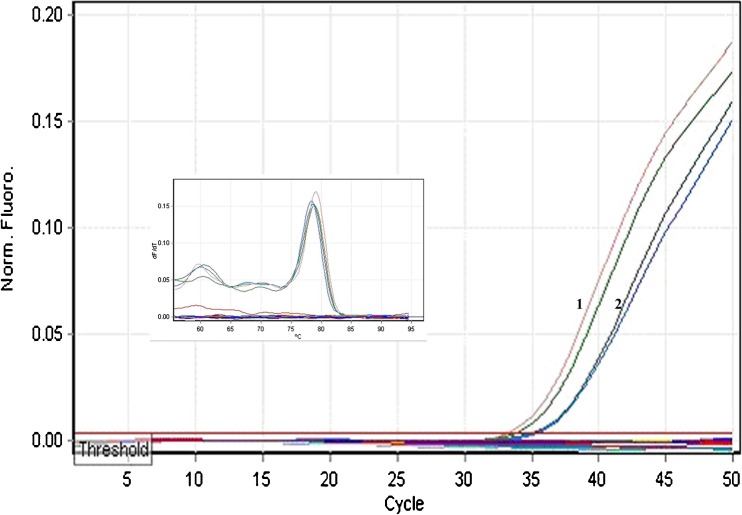
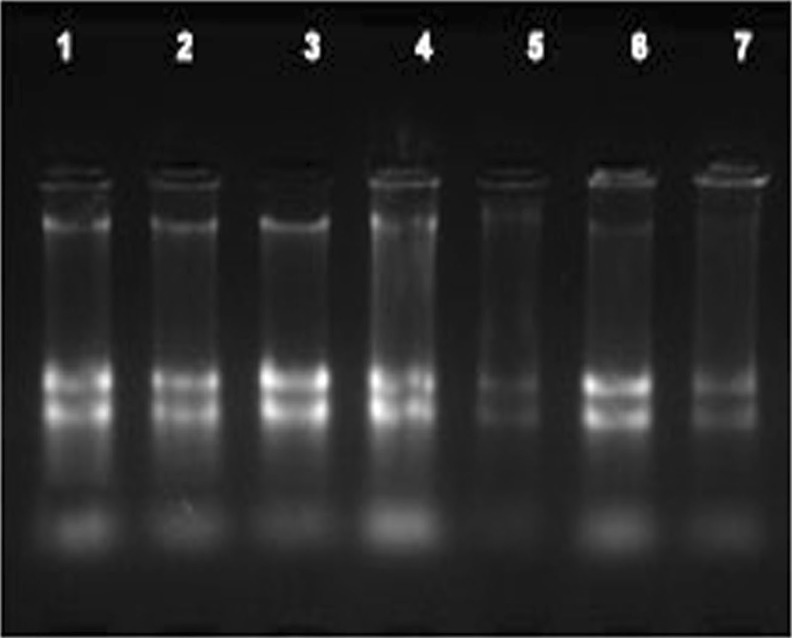
For the experimental analysis of microRNAs and other small RNAs, the first and most important step is the isolation of high-quality total RNA (Accerbi et al. 2010). Total RNA enriched with small RNA fraction was loaded on 15 % denaturing PAGE and intact bands corresponding to 5.8SrRNA, 5SrRNA, tRNA and microRNA marker (25, 21 and 17 nts) were observed, suggesting good recovery of small RNA including micro RNA fraction (Fig. 3c). The functional intactness of isolated mRNAs can be measured by RT-PCR analysis since reverse transcriptase is highly sensitive to impurities (Li et al. 2006; Ghangal et al. 2009). As shown in Fig. 5, ~950 bp of actin was successfully amplified from total RNA isolated from rhizome using three selected protocols. actin was amplified from all other tissues of turmeric by the new protocol and confirmed that the samples are PCR amplifiable and free of inhibitors. qRT-PCR was also performed with the cDNA synthesized from total RNA of rhizome following three selected protocols. All the samples showed amplification with normal Ct values with the standard deviations in the range that allowed for meaningful analysis of expression data. The difference in the Ct value between the samples could be related to the differences in the purity of total RNA (Deng et al. 2005). The expression was observed in reverse transcription negative control after 37 cycles in RNA isolated by Ghawana et al. 2011. However no expression was observed in our case in reverse transcription negative control and non-template negative control till 40 cycles indicating that the RNA isolated by our method was free of DNA contamination. A single peak in the melting curve indicated the purity and specificity of the amplified PCR fragment (Fig. 6). In order to authenticate the small RNAs, the expression profile of a novel turmeric specific microRNA identified from rhizomes (Santhi and Sheeja 2013) was analyzed by qRT-PCR. The rhizome and root tissues showed amplification but leaf and pseudostem tissues failed to show any expression. This indicates that the present protocol isolates good quality RNA suitable even for tissue specific expression analysis of miRNAs (Fig. 7). Further the new protocol was tested in three varieties of C. longa, three different species of Curcuma and ginger (Zingiber officinale) (Fig. 8). Intact 28S and 18S rRNA bands were observed in 1.2 % agarose gel showing the utility of this protocol in related species also.
Fig. 5.
PCR amplification of actin using the cDNA from rhizome following Ghawana et al. 2011 (1a), Chang et al. 1993 (2a) and the new protocol (5a). Lanes 3a, 4a and 6a correspond to actin amplification from cDNA of leaf, pseudostem and root respectively following the new protocol. 1b - 6b correspond to the reverse transcription negative control of the samples 1a - 6a respectively, 7- non template control. M-100 bp DNA ladder
Fig. 6.
Quality check of cDNA by qRT-PCR generated from rhizome using 1- New protocol (Mean Ct- 22), 2- Chang et al. 1993 (Mean Ct- 24.6), 3- Ghawana et al. 2011 (Mean Ct- 28.5)
Fig. 7.
Amplification plot and melt curve of amplicon generated using mature miRNA sequences from total RNA of rhizome (1) and root (2) during qRT-PCR
Fig. 8.
Agarose gel electrophoresis of RNA isolated from rhizomes of different plant species: 1- C. xanthorrhiza, 2- C. amada, 3- C. ceasia, 4- Zingiber officinale, 5- C. longa RH17, 6- C. longa Acc19, 7- C. longa Megaturmeric
In summary, a rapid method for isolation of good quality total RNA, which can facilitate studies on gene expression in turmeric is presented here. Using this protocol, there was a five fold increase in the yield of rhizome RNA with high purity when compared to other time consuming and expensive protocols. The remarkable feature of this protocol is the success in isolation of RNA from various tissues especially the rhizomes where most commonly used protocols failed. The new protocol was suited for tissue specific expression analysis of miRNAs and could be extended to other related species of Curcuma high in secondary metabolites.
Acknowledgments
We thank Director, IISR for providing necessary facilities to carry out this work.
Abbreviations
- DEPC
Diethylpyrocarbonate
- RT-PCR
Reverse transcription-polymerase chain reaction
- PVP
Polyvinylpyrrolidone
- SDS
Sodium dodecyl sulphate
- EDTA
Ethylene diamine tetraacetic acid
- NaOAc
Sodium acetate
- PEG
Polyethylene glycol
- RIN
RNA Integrity Number
- qRT-PCR
Quantitative real time polymerase chain reaction
References
- Abas F, Lajis NH, Shaari K, Israf DA, Stanslas J, Yusuf UK, Raof SM. A labdane diterpene glucoside from the rhizomes of Curcuma mangga. J Nat Prod. 2005;68:1090–1093. doi: 10.1021/np0500171. [DOI] [PubMed] [Google Scholar]
- Accerbi M, Schmidt SA, De PE, Park S, Jeong DH, Green PJ. Methods for isolation of total RNA to recover miRNAs and othersmall RNAs from diverse species. Methods Mol Biol. 2010;592:31–50. doi: 10.1007/978-1-60327-005-2_3. [DOI] [PubMed] [Google Scholar]
- Almedia LP, Cherubino APF, Alves RJ, Dufosse L, Gloria MBA. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res Int. 2005;38:1039–1044. doi: 10.1016/j.foodres.2005.02.021. [DOI] [Google Scholar]
- Annadurai RS, Neethiraj R, Jayakumar V, Damodaran AC, Rao SN, Katta MAVSK, Gopinathan S, Sarma SP, Senthilkumar V, Niranjan V, Gopinath A, Mugasimangalam RC. De Novo transcriptome assembly (NGS) of Curcuma longa L. rhizome reveals novel transcripts related to anticancer and antimalarial terpenoids. PLoS ONE. 2012;8(2):e56217. doi: 10.1371/journal.pone.0056217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtic S, Kranner I. Isolation of high -quality RNA from polyphenol, polysaccharide and lipid-rich seeds. Phytochem Anal. 2006;17:144–148. doi: 10.1002/pca.903. [DOI] [PubMed] [Google Scholar]
- Box MS, Coustham V, Dean C, Mylne JS. Protocol: A simple phenol-based method for 96-well extraction of high quality RNA from Arabidopsis. Plant Methods. 2011;7:7. doi: 10.1186/1746-4811-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne MM, Kuehnle AR. An effective method for isolating RNA from tissues of Dendrobium. Lindleyana. 2000;15(3):165–168. [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. doi: 10.1007/BF02670468. [DOI] [Google Scholar]
- Chempakam B, Parthasarathy VA (2008) Chemistry of Spices; Turmeric. Parthasarathy VA, Chempakam B, Zachariah TJ (Editors). Biddles Ltd., King’s Lynn, Oxfordshire, UK
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Deng MY, Wang H, Ward GB, Beckham TR, Mckenna TS. Comparison of six RNA extraction methods for the detection of classical swine fever virus by real-time and conventional reverse transcription–PCR. J VET Diagn Invest. 2005;17:574–578. doi: 10.1177/104063870501700609. [DOI] [PubMed] [Google Scholar]
- Djami-Tchatchoua AT, Straker CJ. The isolation of high quality RNA from the fruit of avocado (Persea americana Mill.) S Afr J Bot. 2011;78:44–46. doi: 10.1016/j.sajb.2011.04.009. [DOI] [Google Scholar]
- Gasic K, Hernandez A, Korban SS. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Rep. 2004;22(4):437–438. doi: 10.1007/BF02772687. [DOI] [Google Scholar]
- Ghangal R, Raghuvanshi S, Chand Sharma P. Isolation of good quality RNA from a medicinal plant sea buckthorn, rich in secondary metabolites. Plant Physiol Biochem. 2009;47(11):1113–1115. doi: 10.1016/j.plaphy.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Ghawana S, Paul A, Kumar H, Kumar A, Singh H, Bhardwaj PK, Rani A, Singh RS, Raizada J, Singh K, Kumar S. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Res Notes. 2011;4:85. doi: 10.1186/1756-0500-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mendoza D, Quiroz-Moreno A, Zapata-Perez O. An improved method for the isolation of total RNA from Avicennia germinans leaves. Verlag der Zeitschrift für Naturforschung. 2008;63(1):124–126. doi: 10.1515/znc-2008-1-222. [DOI] [PubMed] [Google Scholar]
- Hou P, Xie Z, Zhang L, Song Z, Mi J, He Y, Li Y. Comparison of three different methods for total RNA extraction from Fritillaria unibracteata: A rare Chinese medicinal plant. J Med Plants Res. 2011;5(13):2834–2838. [Google Scholar]
- Hu GC, Honda CH, Kita MY, Zhang Z, Tsuda T, Moriguchi T. A simple protocol for RNA isolation from fruit trees containing high levels of polysaccharides and polyphenol compounds. Plant Mol Biol Rep. 2002;20(1):69a–69g. doi: 10.1007/BF02801935. [DOI] [Google Scholar]
- Kiefer E, Heller W, Ernst D. A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep. 2000;18:33–39. doi: 10.1007/BF02825291. [DOI] [Google Scholar]
- Kumar GS, Nayaka H, Dharmesh SM, Salimath PV. Free and bound phenolic antioxidants in amla (Emblica officinalis) and turmeric (Curcuma longa) J Food Comp Anal. 2006;19:446–452. doi: 10.1016/j.jfca.2005.12.015. [DOI] [Google Scholar]
- Li JH, Tang CH, Song CY, Chen MJ, Feng ZY, Pan YJ. A simple, rapid and effective method for total RNA extraction from Lentinula edodes. Biotechnol Lett. 2006;28(15):1193–1197. doi: 10.1007/s10529-006-9074-y. [DOI] [PubMed] [Google Scholar]
- Loomis MD. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Lu C, Mayers BC, Green PJ. Construction of small RNA cDNA libraries for deep sequencing. Sci Direct Methods. 2007;43:110–117. doi: 10.1016/j.ymeth.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Malnoy M, Reynoird JP, Mourgues F, Chevreau E, Simoneau P. A method for isolating total RNA from pear leaves. Plant Mol Biol Rep. 2001;19:69a–69f. doi: 10.1007/BF02824081. [DOI] [Google Scholar]
- Masek T, Vopalensky V, Suchomelova P, Pospisek M. Denaturing RNA electrophoresis in TAE agarose gels. Anal Biochem. 2005;336:46–50. doi: 10.1016/j.ab.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mendelsohn SL, Young DA. Efficacy of sodium dodecyl sulfate, diethyl pyrocarbonate, proteinase K and heparin using a sensitive ribonuclease assay. Biochim Biophys Acta. 1978;519:461–473. doi: 10.1016/0005-2787(78)90099-0. [DOI] [PubMed] [Google Scholar]
- Murillo I, Raventos D, Jaeck E, San Segundo B. Isolation of total RNA and mRNA from plant tissues. Promega Notes Mag. 1995;54:2–7. [Google Scholar]
- Perry RP, Kelley DE. The production of ribosomal RNA from high molecular weight precursors. J Mol Biol. 1972;70:265–279. doi: 10.1016/0022-2836(72)90538-4. [DOI] [PubMed] [Google Scholar]
- Rubio-Pina JA, Zapata-Perez O. Isolation of total RNA from tissues rich in polyphenols and polysaccharides of mangrove plants. Electron J Biotechnol. 2011;14:5. [Google Scholar]
- Salvo-Chirnside E, Kane S, Kerr LE. Protocol: high throughput silica-based purification of RNA from Arabidopsis seedlings in a 96-well format. Plant Methods. 2011;7:40. doi: 10.1186/1746-4811-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman RA, Fujita T, Salzman Z, Hasegawa PM, Bressan RA. An improved RNA isolation method for plant tissues containing high levels of phenolic compounds or carbohydrates. Plant Mol Biol Rep. 1999;17:11–17. doi: 10.1023/A:1007520314478. [DOI] [Google Scholar]
- Sangvanich P, Kaeothip S, Srisomsap C, Thiptara P, Petsom A, Boonmee A, Svasti J. Hemagglutinating activity of Curcuma plants. Fitoterapia. 2007;78:29–31. doi: 10.1016/j.fitote.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Santhi R, Sheeja TE. Deep sequencing identifies candidate miRNAs from turmeric with possible regulatory roles on plant and human genes. In: Sasikumar B, Dinesh R, Prasath D, Biju CN, Srinivasan V, editors. National symposium on spices and aromatic crops (SYMSAC VII): Post-Harvest processing of spices and fruit crops. Kozhikode: Indian Society for spices; 2013. p. 210. [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3–16. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Kumar S, Singh P. A quick method to isolate RNA from wheat and other carbohydrate-rich seeds. Plant Mol Biol Rep. 2003;21:93a–93f. doi: 10.1007/BF02773401. [DOI] [Google Scholar]
- Suzuki Y, Kawazu T, Koyama H. RNA isolation from siliques, dry seeds and other tissues of Arabidopsis thaliana. Biotechniques. 2004;37:542–544. doi: 10.2144/04374BM03. [DOI] [PubMed] [Google Scholar]
- Wang L, Stegemann JP. Extraction of high quality RNA from polysaccharide matrices using cetlytrimethylammonium bromide. Biomaterials. 2010;31(7):1612–1618. doi: 10.1016/j.biomaterials.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang N, Du L. Isolation of RNA of high quality and yield from Ginkgo biloba leaves. Biotechnol Lett. 2005;27:9. doi: 10.1007/s10529-005-3629-1. [DOI] [PubMed] [Google Scholar]
- Wang D, Douglas D, Kreader C, Dinther JV, Valdes-Camin R. An integrated high-throughput system for mRNA purification and quantitation for use in identifying gene knockdown by RNA interference. J Assoc Lab Autom. 2006;11(5):314–318. doi: 10.1016/j.jala.2006.07.003. [DOI] [Google Scholar]
- Xu J, Aileni M, Abbagani S, Zhang P. A reliable and efficient method for total RNA isolation from various members of Spurge family (Euphorbiaceae) Phytochem Anal. 2010;21:395–398. doi: 10.1002/pca.1205. [DOI] [PubMed] [Google Scholar]
- Yew CW, Kumar VS. Isolation and cloning of microRNA from recalcitrant plant tissues with small amounts of total RNA: a step-by step approach. Mol Biol Rep. 2012;39:1783–1790. doi: 10.1007/s11033-011-0919-7. [DOI] [PubMed] [Google Scholar]