Abstract
Genetic resources of landraces (84 cultivars) were collected from various agro-ecological regions of West Bengal and adjoining areas and characterized based on qualitative and quantitative agro-morphological descriptors along with zinc (Zn) and iron (Fe) content. The DUS protocol was employed to study 16 agro-morphological passport data such as: vegetative data (anthocyanin pigmentation, plant habit), reproductive data (flag leaf attitude, stigma colour, panicle attitude), including eight grain quality traits: grain length, grain width, 1000 grains weight, kernel length, kernel breadth etc. Highest seed weight was found in cultivar Khechri (32.04 g/1000 seeds), collected from Sundarban and least seed weight was 9.6 g/1000seeds in Katharibhog. Maturity duration was found very short (<100 days) in Jumla Marshi (97 days) collected from world’s coldest rice growing area, Jumla, Nepal. Penultimate leaves breadth was observed broad (>2 cm) in one cultivar Jungli (2.3 cm). Seeds per panicle were 180 in Chinisakkar (medium range), 177 in Dudheswar, and 151 in Ladua. Flag leaf was found in erect condition in late observation in Dudheswar, Enda and Ghiosh. Seventeen cultivars were grouped in the aromatic rice category out of total 84 local landraces. Twenty-one cultivars were with awn, whose length ranges from 1.6 mm (Anandi) to 22.5 mm (Tulaipanji). Kernel colour varies from red, yellowish, brownish, creamy white to white. Kernel length varies from 4 mm to 8 mm and breadth 1.90 mm to 3 mm. Kernel length/breadth ration varied from 1.6 to 3.9. Highest ratio of L/B was found in Pusa Basmati 1(3.9) and lowest in Dudhey (1.6). Elongation ration was highest in Kalokure (2.07) and lowest in Phoolpakri (0.62). Nutritional values of mineral contents of iron (Fe) and zinc (Zn) were estimated in all cultivars by Atomic Absorption Spectrophotometic method. Iron concentration varies from 0.25 μg/g to 34.8 μg/g and zinc from 0.85 μg/g to 195.3 μg/g in the landraces. Highest iron containing rice was Swetonunia with 34.8 μg/g and highest amount of Zn was found in Nepali Kalam which was 195.3 μg/g. Anaerobic germination (AG) was observed in 18 cultivars among 84 land races (viz. Jungli, Kumrogore, Dudheshwar, Rambhog and Tulsi etc.), the trait is highly desired by the rice breeder for the introgression of this gene (QTL) to the HYV for direct seeding in the field for saving labour cost and reduced maturity time. Dendrogram showed genetic diversity among 84 landraces by grouping them into five major clusters. All the descriptors evaluated in this study have showed that there is enough genetic diversity among landraces and this information can be helpful to the breeders to choose the right parent for crop improvement.
Keywords: Rice landraces, Aromatic rice, Genetic diversity, Agro-morpho-quality traits, Anaerobic germination, Micronutrient-Zinc and Iron
Introduction
Rice (Oryza sativa L.) is the most important staple food crop in the world and provides food and livelihood security to over half (~3.5 billion) of the global population. Approximately 150 million tons of rice is needed by 2030 to feed 1.378 billion Indian people (Goyal and Singh 2002) and ~850 million tons to feed 5 billion rice consumers in the world (at least 1.1 % yield increase is required every year) (Subudhi et al. 2006). Different breeding strategies such as introduction, selection, recombination breeding, heterosis breeding etc. were practiced during and after green revolution to increase the yield levels of rice. Although, yield was improved using these breeding strategies, the yield levels have stagnated subsequently. Breaking the yield ceiling through genetic improvement becomes the priority in rice research (Khush 2005). Aggressive introduction of modern high yielding varieties (HYV) has resulted in the loss of a large number of landraces especially from irrigated lands, which leads to narrowing down the gene pool of the rice diversity (Rana et al. 2000). The diversity of rice genetic resources is depleting. The diversity of rice has been well used in efforts to solve today’s food problems (Khush and Virk 2000) since the green revolution. Rice landraces were collected over several decades to become ‘parents’ of the high-yielding, pest-resistant, and well-adapted varieties that resulted in unprecedented increase in rice yields. India is accomplished with a great diversity of rice germplasm in its vast territorial land areas (agro-ecological area). Any crop improvement program should aim at broadening the genetic base of the breeding stock (Collard et al. 2005). Wide genetic resources may be required to either increase the genepool for germplasm improvement or for the development of new cultivated varieties (Subudhi et al. 2006). Different agro-morphological traits (passport data) play very important roles for their characterization and varietal identification which ultimately helps rice breeder for its improvement (Laxuman et al. 2011). Agro-morphological properties of rice cultivars determine their yield potential, local agronomic suitability and ability to escape from or to tolerate biotic and abiotic stresses. As for example, one local rice cultivar, FR13A has revealed tolerance to submergence for 10 to 12 days and was used as donor to improve rice cultivars with tolerance to submergence (Xu and MacKill 1996). Rice is a poor source of essential micronutrients such as Fe and Zn (Bouis and Welch 2010). Diet deficient in minerals mainly Fe and Zn in staple food crops causes ‘hidden hunger’ or micronutrient malnutrition in developing countries (Welch and Graham 2004). Some landrace may have high micronutrient content and can be screened from the germplasm. Recent trend in rice production is marked with the shift from transplanting to direct seeding notably in areas where there is scarcity of irrigation water and high cost of labor. It is important to choose varieties that have anaerobic germination and exceptional seedling vigor for use in direct seeding. Improving the productivity of rice cultivation shall involve screening of available germplasm for tolerance to biotic and abiotic stresses, and intensifying of research on the genetics and physiological mechanisms of tolerance to these stresses would be important. Thus accurate assessment of the levels and patterns of genetic diversity can be invaluable in crop breeding for diverse applications including introgression desirable genes from diverse germplasm into the available genetic base and for widening the narrow genetic base of the developed varieties. It is therefore, pertinent to capture and conserve the genetic diversity existing in farmers land either in ex situ, in the form of seed or on farm and also for protecting the sovereign rights.
Thus, the present investigation was intended to characterize the genetic resources of local landraces of rice [Oryza sativa L.] on the basis of agro-morpho-quality traits (passport data) for genetic diversity analysis including the genetic evaluation of micronutrient content and anaerobic germination.
Materials and methods
Experimental site
The present investigation was carried out during kharif 2010–2012 at Experimental Rice Field, Department of Botany, University of North Bengal, WB, India, representing the low land with sandy-loam soil, acidic pH which is located at latitude of 26° 84′ North and longitude of 88° 44′ East.
Experimental material
The experimental material for the present investigation comprised of 84 local landraces of rice (Oryza sativa L.) cultivars of West Bengal and adjoining areas. Landraces were collected from the farmer’s field, threshing yard of West Bengal and adjoining areas. Relevant habitat information, unique features, common name and traditional knowledge about the landraces were gathered from local farmers during end of kharif season in 2010 and 2011.
Recording of observation
The data on morphological, physicochemical and other traits are recorded from three randomly selected representative plants in all the genotypes in each replication. The standard method of DUS test (Distinctiveness, Uniformity and Stability, Govt. of India) was used for recording observation for each of the character which includes the following- plant height, stem thickness, penultimate leaf length, penultimate leaf breadth, flag leaf length, flag leaf breadth, panicle length, panicle branching, seed/panicle, grain weight, grain length, awn length (Bioversity protocols: www.bioversity.org). The software STATISTICA 12 was run to construct the dendrogram based on morpho-quality traits of 84 landraces for their genetic diversity assessment.
Measurement of physico-chemical and cooking quality traits
The following physical and cooking quality characters viz., kernel length (KL), kernel breadth (KB), ratio of KL/KB, cooked kernel length (CKL), cooked kernel breadth (CKB), ratio of CKL/CKB, linear elongation ratio, cooking time was measured as per the descriptors suggested by IRRI.
Alkali spreading value/gelatinization temperature
Gelatinization temperature (GT) was estimated based on alkali spreading value (ASV). The alkali spreading value (ASV) was determined by the method of Little et al. (1958). Two sets of seven polished rice kernels of each cultivar was placed in Petri plates containing 10 ml of freshly prepared 1.7 % KOH solution. The kernels were arranged in such a way to provide space between kernels for spreading. The plates were covered and incubated at 30 °C for 23 h. The appearance and disintegration of kernels were rated visually as follows- Score 1: kernels unaffected, score 2: kernel swollen, score 3: kernel swollen with collar incomplete and narrow, score 4: kernel swollen with collar complete and wide, score 5: kernel split or segmented with collar complete and wide, score 6: kernel dispersed, merging with collar and score 7: kernels were dispersed, intermingled and disappeared completely. A low AVS corresponds to a high GT, conversely, a high ASV indicates a low GT. The aroma of polished rice grains was determined by a sensory evaluation protocol according to the method of Sood and Siddiq (1978). Ten milled rice grains were placed in a 50 mm Petri plate containing 10 ml of 1.7 % KOH and incubated at room temperature for 10 min with lids on. The lids were then opened one by one and samples were smelt and rated for aroma by sensory evaluation in a scale of 0 to 3, where 0 means non-aromatic and 3 means highly aromatic. Two blind checks, Pusa Basmati 1(moderately aromatic) and Ranjit (non aromatic) were included in this observation.
Gel consistency
The GC was measured according to the method of Cagampang et al. (1973). Briefly, 100 mg rice flour was weighted in duplicate into 13 mm × 100 mm culture tubes, to which 200 μl of ethyl alcohol (95 %) containing 0.025 % thymol blue was added to prevent clumping of the powder during gelatinization. Two milliliter of 1 N KOH was added. The contents of the tube were mixed thoroughly using a Vortex Genie mixer. The test tubes were placed in a vigorously boiling water bath for 8 min. Tubes were removed from the water bath and left at room temperature for 5 min. Tubes were cooled in an ice-water bath for 15 min then laid down horizontally on a table surface. The blue gel length was measured 1 h later as the distance from the bottom of the tube to the front of the gel migration. The gel length thus obtained provides a measurement of the gel consistency (GC): the longer the distance, softer the gel and classified into three categories: hard GC (30–40 mm length), medium GC (40–60 mm length), and soft GC (61–100 mm length).
Amylose estimation by spectrophotometer
The simplified method of Juliano (1971) was used for estimating the amylose content. One hundred (100) mg flour was transferred to volumetric flask and homogenized with 1 ml of 95 % ethanol and 9 ml of 1 N NaOH. The samples were heated for 10 min in the water bath to gelatinize the starch. After cooling, it was made up 100 ml with distilled water. Half ml aliquots of each test solution were separately placed in two test tubes. Five ml of water, 0.10 ml of acetic acid and 0.20 ml of iodine solution were added. An additional 4.20 ml of water was added into each tube to make the total volume of reaction mixture to 10 ml. Absorbance was measured using a spectrophotometer (Systronic, India) at a wavelength of 620 nm. The amylsoe content was determined using a standard curve developed from known quantities of purified potato amylose from Sigma, USA.
Fe and Zn content estimation
Iron (Fe) and Zinc (Zn) content of 84 local cultivars were estimated according to standard method (Lindsey and Norwell 1969) by Atomic Absorption Spectrophotometer (Varian Spectra AA50B). Seeds from 84 cultivars were dehusked gently using a palm dehusker. One gram oven dried ground dehusked seed samples were placed in a 150 ml conical flask. To this 25–30 ml diacidic mixture (HNO3:HClO4; 5:1 v/v) was added and kept overnight. Next day, it was digested by heating till clear white precipitates settled down at the bottom. The crystals were dissolved by diluting in double distilled water. The contents were filtered through Whatman No. 42 filter paper. The filtrates were made to 50 ml with double distilled water and used for the determination of iron and zinc contents. Concentration was expressed in μg/g of rice against standard curve.
Anaerobic germination method
Seed dormancy was broken by incubating the seeds at 50 °C for 5 days. The seeds were subjected to imbibitions overnight by soaking them in water and incubate for synchronous germination in an incubator at 28 ° C in the dark (Boamfa et al. 2003). Germinating seeds at the pigeon breast stage (pre-germinated seeds of about 3 days old) were seeded for anaerobic experiment (Manangkil et al. 2008).
-
i.
Test Tube Method
In this method pre- germinated seeds were kept in a glass test tube (25 mm in diameter and 150 mm in height) filled with 10 cm deep distilled water. At the 7th day of incubation, shoot (coleoptiles) lengths were measured.
-
ii.
Water logged soil Method
Pre- germinated seeds were sown in paddled soil filled in plastic cup of 4 × 5 cm at the depth of 3 cm from the soil surface. Each cup then immersed inside a plastic flat tray filled with 3 cm deep water above the soil surface and placed in a green house. To obtain comparable data with those from the test tube method, seedlings were removed from the soil at the 5th day and their shoot lengths were measured.
Results and discussion
Morpho-metric analysis
Rice germplasm were evaluated to access genetic variability on the basis of 16 important agro- morphological parameters, and eight quality characters. All the (84 cultivars) local landraces of rice under study showed wide range of genetic variation in respect to these traits. These traits belong to three categories viz., qualitative - measured through visual observation like anthocyanin coloration (present or absent), quantitative- measurable traits (leaf length, leaf width etc.), pseudo-qualitative- characters whose range of expression is at least partly continuous but may vary in one measurement (intensity of green coloration: light, medium and dark). The frequency distributions of 16 agro-morphological characters showed variable range of characters (Table 1). Out of 16 morphological characters, basal leaf sheath colour, leaf blade colour, ligule colour, and plant habit showed more frequent variation among the rice landraces (genotypes). Majority of landraces were found to possess colourless coleoptiles (98.81 %) on tenth days in coleoptile colour character category, basal leaf sheath colour on booting stage was green (96.6 %), intensity of leaf green colour on booting stage was dark (98.02 %), colour of ligule on booting stage was white (97.7 %), in leaf sheath character: intensity of anthocyanin colouration was very weak (96.5 %), in spikelet character: colour of stigma was white (95.4 %), colour of tip of lemma white (86.36 %), density of pubescence medium (52.27 %); auricle was without anthocyanin colouration (97.7 %); leaf: pubescence of blade surface on booting stage was weak (51.3 %), leaf collar was present on booting stage (100 %), stem length varies from very short (45.97 %), short (39.08 %), to medium (5.74 %). Heading time varies from (50 % of plants with panicles) very early (28.73 %), early (26.43 %), medium (48.27 %), late (17.24 %), and very late (2.29 %). On late observation it was noticed that flag leaf attitude was erect (54.4 %), semi-erect (31.81 %), horizontal (2.27 %), and deflexed (13.63 %) (Table 1).
Table 1.
Characterization of 84local rice landraces based on 16 agro-morphological traits
| Agro-morphological characters | Colour pattern/type | Frequency | Percent |
|---|---|---|---|
| 1. Coleoptile colour on tenth days | Colourless | 83 | 98.81 |
| Green | – | – | |
| Purple | 1 | 1.19 | |
| 2. Basal leaf sheath colour on booting stage | Uniform purple | 3 | 3.57 |
| Green | 81 | 96.43 | |
| Light purple | – | – | |
| Purple lines | – | ||
| 3. Leaf intensity of green colour on booting stage | Light | 14 | 16.6 |
| Medium | 2 | 2.38 | |
| Dark | 68 | 80.95 | |
| 4. Colour of ligule on booting stage | White | 87 | 97.71 |
| Light purple | 1 | 2.29 | |
| Purple | – | ||
| 5. Leaf sheath: Intensity of anthocyanin colouration | Very weak | 81 | 96.43 |
| Weak | 2 | 2.38 | |
| Medium | – | – | |
| Strong | 1 | 1.19 | |
| Very strong | – | ||
| 6. Spikelet colour of stigma | White | 80 | 95.24 |
| Light green | – | 4.76 | |
| Yellow | – | ||
| Light purple | – | ||
| Purple | 4 | 4.76 | |
| 7. Anthocyanin colouration of auricles | Colourless | 83 | 98.8 |
| Light purple | – | – | |
| Purple | 1 | 1.19 | |
| 8. Spikelet colour of tip of lemma | White | 72 | 85.71 |
| Yellowish | – | – | |
| Brown | 7 | 8.33 | |
| Red | 5 | 5.95 | |
| Purple | – | – | |
| Black | – | – | |
| 9. Time of heading (50 % of plants with panicles) | Very early (<71 days) | 5 | 5.95 |
| Early (71–90 days) | 22 | 26.19 | |
| Medium (91–110 days) | 42 | 50.00 | |
| Late (111–130 days) | 13 | 15.47 | |
| Very late (>131 days) | 2 | 2.38 | |
| 10. Spikelet density of pubescence | Absent | 18 | 21.42 |
| Weak | 42 | 50.00 | |
| Medium | 24 | 28.57 | |
| Strong | – | – | |
| Very strong | |||
| 11. Stem length | Very short (<91 cm) | 38 | 45.23 |
| Short (91–110 cm) | 32 | 38.09 | |
| Medium (111–130 cm) | 9 | 10.71 | |
| Long (131–150 cm) | 5 | 5.95 | |
| Very long (>150 cm) | – | ||
| 12. Leaf: Pubescence of blade surface on booting stage | Weak | 43 | 51.19 |
| Absent | 37 | 44.04 | |
| Medium | 4 | 4.7 | |
| 13. Panicle curvature of main axis | Straight | 25 | 29.76 |
| Semi-straight | 24 | 28.57 | |
| Deflexed | 28 | 33.33 | |
| Dropping | 7 | 8.33 | |
| 14. Leaf collar present/Absent on booting stage | Absent | 0 | 0 |
| Present | 85 | 100 | |
| 15. Culm: attitude | Erect | 15 | 17.85 |
| Semi-erect | 64 | 76.19 | |
| Opened | – | – | |
| Spreading | 5 | 5.95 | |
| 16. Flag leaf attitude in late observation | Erect | 44 | 52.38 |
| Semi-erect | 28 | 33.33 | |
| Horizontal | 2 | 2.38 | |
| Deflexed | 10 | 11.90 |
Parameters pertaining to grain characters were recorded (Table 2) using standard method (www.bioversity.org) for genetic variation analysis. Grain weight, grain length, grain breadth, awn length, bran colour, and grain shape were measured. Out of 84 local landraces highest grain weight was found in cultivar, Khechri (32.49 mg) collected from Sundarban, and lowest grain weight was found in Kataribhog (9.34 mg). According to length/breadth ratio, rice grains have been grouped into three categories (USDA protocol; Adair 1972), short grains L/B ratio is ≤2.2:1, medium grain L/B ratio is 2.3:1 to 3.3:1 and long grain L/B ratio is ≥3.4:1 (Table 2). Seventeen cultivars were grouped in the aromatic rice category out of total 84 local landraces. Among these categories, Tulaipamji cultivar of North Dinajpur district was considered as best quality and preferred by consumers for its fragrance and soft fluffy grain quality. One cultivar Chinisakkar showed highest spikelet branching (11), seeds per panicle (180), and panicle length (23.48 cm) among other aromatic rices. Twenty-one cultivars were with awn, whose lengths were ranges from 1.6 mm (Anandi) to 22.25 mm (Tulaipanji). Bran colour ranges from red, yellowish, brownish, creamy white to white (Table 2). Some landraces Newlodhan, Dudheswar, and Bhangeri showed stout stem and remain in green condition for long periods. Dendrogram was constructed based on agro-morpho-quality characters of 84 local landraces of rice which showed five clusters with genetic variation (Fig. 1). This genetic variation is the inherent characteristic of the individual landrace. The principal component analysis (PCA) showed the scattered plot of the dendrogram as graphical representation with genetic relationship among 84 local rice landraces (Fig. 2).
Table 2.
Characterization of 84 landraces of rice based on grain quality traits
| Sl No. | Name of cultivars | Grain length (GL) mm | Grain breadth (GB) mm | GL/GB ratio | 1000Grain wt.(gm) | Awn length (mm) | Bran color | Shape of grain |
|---|---|---|---|---|---|---|---|---|
| 1 | Anandi | 7.7 | 2.9 | 2.6:1 | 28.52 | 1.6 | White | Medium |
| 2 | Attey | 8.1 | 2.0 | 3.2:1 | 21.53 | – | White | Medium |
| 3 | Aichung | 7.6 | 2.4 | 3.1:1 | 16.24 | – | White | Medium |
| 4 | Swetonunia | 7.1 | 3.0 | 2.3:1 | 21.89 | – | White | Medium |
| 5 | Bharlang | 7.2 | 2.8 | 2.5:1 | 24.49 | – | White | Medium |
| 6 | Badshabhog | 6.0 | 3.0 | 3:1 | 25.09 | – | White | Medium |
| 7 | Bhangeri | 8.0 | 2.9 | 2.7:1 | 21.87 | – | White | Medium |
| 8 | Bagrakalam | 7.6 | 2.0 | 3.8:1 | 26.05 | – | White | Long |
| 9 | Bhadoi | 7.7 | 3.0 | 2.5:1 | 21.27 | – | Red | Medium |
| 10 | Chinisakkar | 5.7 | 2.0 | 2.8:1 | 24.00 | – | White | Medium |
| 11 | Champa | 9.0 | 3.0 | 3:1 | 24.10 | 2.2 | White | Medium |
| 12 | Champsali | 8.7 | 3.0 | 2.9:1 | 21.27 | 4 | Red | Medium |
| 13 | Charinagrey | 8.8 | 2.0 | 4.4:1 | 17.11 | 3.4 | White | Long |
| 14 | Chunakathi | 8.7 | 2.5 | 3.4:1 | 18.39 | – | CW | Long |
| 15 | Chirakhey | 6.2 | 3.0 | 2.06:1 | 22.08 | – | White | Short |
| 16 | Chiniatop | 6.1 | 2.0 | 2.4:1 | 10.43 | – | White | Medium |
| 17 | Chunia | 6.2 | 2.5 | 2.4:1 | 16.26 | – | YW | Medium |
| 18 | Dhanraj | 7.8 | 3.0 | 2.6:1 | 21.26 | – | White | Medium |
| 19 | Dudheswar | 7.8 | 2.0 | 3.9:1 | 18.12 | – | White | Long |
| 20 | Dangimarua | 6.8 | 3.0 | 2.2:1 | 19.28 | 3 | CW | Medium |
| 21 | Dharia | 7.8 | 3.0 | 2.6:1 | 21.19 | 15.3 | White/red | Medium |
| 22 | Dasnunia | 7.8 | 2.5 | 3.1:1 | 25.37 | 9 | White | Medium |
| 23 | Dudhey | 6.6 | 2.5 | 2.6:1 | 17.44 | – | White | Medium |
| 24 | Dudheykalam | 8.6 | 2.5 | 3.4:1 | 26.15 | 8 | CW | Long |
| 25 | Enda | 7.5 | 3.0 | 2.5:1 | 25.88 | 12.5 | Red | Medium |
| 26 | Ghiosh | 6.8 | 2.8 | 2.4:1 | 26.98 | – | CW | Medium |
| 27 | Gokhraj | 7.03 | 2.4 | 2.9:1 | 22.07 | – | White | Medium |
| 28 | Gobindobhog | 6.03 | 2.5 | 2.4:1 | 10.68 | – | CW | Medium |
| 29 | Begunbeej | 7 | 2.0 | 2.5:1 | 9.75 | – | CW | Medium |
| 30 | IR64 | 8.6 | 2.5 | 3.4:1 | 24.12 | – | White | Long |
| 31 | Jumla Marsi | 8.2 | 3.0 | 2.7:1 | 26.22 | – | CW | Medium |
| 32 | Phoolpakri | 7.4 | 3.2 | 4:1 | 22.94 | – | CW | Medium |
| 33 | Jungli | 7.6 | 2.5 | 3.04:1 | 20.24 | – | CW | Medium |
| 34 | Jhulur | 8.2 | 3.0 | 2.7:1 | 20.01 | – | CW | Medium |
| 35 | Jeerasari | 7.1 | 2.5 | 2.8:1 | 18.16 | – | White | Medium |
| 36 | Kalonunia | 7.5 | 2.0 | 3.7:1 | 21.21 | 12.5 | Red/white | Long |
| 37 | Kattaka | 6.9 | 2.33 | 3:1 | 21.14 | – | White | Medium |
| 38 | Katharibhog | 6.3 | 2.0 | 3.1:1 | 9.34 | – | White | Medium |
| 39 | Kaberi | 9.7 | 3.0 | 3.2:1 | 26.44 | – | White | Medium |
| 40 | Koshia binni | 8.5 | 2.5 | 3.4:1 | 21.05 | – | Red | Long |
| 41 | Kumrogore | 7.5 | 3.0 | 2.5:1 | 21.02 | – | Red | Medium |
| 42 | Kabiraj | 9.2 | 2.0 | 4.6:1 | 25.86 | 22.2 | CW | Long |
| 43 | Khasha | 6.3 | 2.0 | 3.1:1 | 12.35 | – | CW | Medium |
| 44 | Khamtilai | 7.9 | 3.4 | 2.8:1 | 21.73 | – | White | Medium |
| 45 | Kalojera | 6.1 | 2.5 | 2.4:1 | 24.65 | – | Red | Medium |
| 46 | Kalobhog | 6.5 | 2.0 | 3.2:1 | 13.17 | – | White | Medium |
| 47 | Khechri | 7.8 | 3.0 | 2.6:1 | 32.49 | 18 | CW | Medium |
| 48 | Kantarangi | 9.06 | 3.0 | 3.02:1 | 30.48 | – | Red | Medium |
| 49 | Khal khajara | 7.4 | 2.6 | 2.8:1 | 20.43 | – | White | Medium |
| 50 | Kantajinghasal | 9.2 | 2.5 | 3.6:1 | 26.08 | 4.5 | Red/CW | Long |
| 51 | Kholako dhan | 9.2 | 4.1 | 2.2:1 | 28.12 | 4.8 | White | Medium |
| 52 | Kalokure | 7.7 | 2.3 | 2.3:1 | 20.87 | – | White | Medium |
| 53 | Laxmansal | 8.5 | 3.4 | 2.5:1 | 24.08 | – | White | Medium |
| 54 | Laxmikajol | 9.3 | 3.0 | 3.1:1 | 29.86 | – | CW | Medium |
| 55 | Ladua | 7.6 | 3.0 | 2.5:1 | 22.53 | 2.4 | White | Medium |
| 56 | Malsira | 7.2 | 2.5 | 2.8:1 | 20.95 | 15.2 | CW | Medium |
| 57 | Metung | 8.2 | 2.8 | 2.9:1 | 17.37 | – | White | Medium |
| 58 | Marshi | 7.03 | 2.5 | 2.8:1 | 26.05 | – | CW | Medium |
| 59 | Maipali | 8.1 | 2.5 | 3.2:1 | 19.3 | – | White | Medium |
| 60 | Mahukal | 7.9 | 3.0 | 2.6:1 | 29.27 | – | Red | Medium |
| 61 | Mala dhan | 8.1 | 2.1 | 3.8:1 | 20.60 | 16.6 | CW | Long |
| 62 | Magursal | 7.4 | 2.5 | 2.9:1 | 23.95 | – | Brown | Medium |
| 63 | Nav dhan | 6.0 | 2.1 | 2.8:1 | 14.21 | – | White | Medium |
| 64 | Newlo dhan | 8.1 | 2.6 | 3.1:1 | 27.05 | – | CW | Medium |
| 65 | Nageswari | 7.7 | 3.0 | 2.5:1 | 25.95 | 12 | Red | Medium |
| 66 | Nepali Kalam | 5.5 | 2.1 | 2.6:1 | 25.49 | – | White | Medium |
| 67 | Nagra | 7.7 | 2.5 | 3.08:1 | 24.00 | – | White | Medium |
| 68 | Pusa Basmati 1 | 9.5 | 2.0 | 4.7:1 | 22.29 | 7.8 | CW | Long |
| 69 | Pijam | 8.3 | 2.5 | 3.2:1 | 16.82 | – | White | Medium |
| 70 | Pahalman | 9.9 | 2.5 | 3.9:1 | 26.95 | – | CW | Long |
| 71 | Radhunipagal | 5.7 | 2.0 | 2.8:1 | 11.72 | – | CW | Medium |
| 72 | Ranjit | 8.2 | 2.5 | 3.2:1 | 14.69 | – | White | Medium |
| 73 | Sanu Addey | 5.3 | 2.5 | 2.1:1 | 21.64 | – | CW | Short |
| 74 | Solapatnai | 10.4 | 2.5 | 4.1:1 | 28.12 | – | Red/white | Long |
| 75 | Sukha dhan | 7.7 | 2.5 | 3.08:1 | 12.25 | – | Red | Medium |
| 76 | Sita bhog | 5.4 | 2.0 | 2.7:1 | 22.45 | – | CW | Medium |
| 77 | Sarulalat | 9.3 | 2.5 | 3.7:1 | 21.24 | 4 | CW | Medium |
| 78 | Tulaipanji | 9.0 | 2.0 | 4.5:1 | 15.44 | 22.25 | White | Long |
| 79 | Tulsi | 5.7 | 2.5 | 2.2:1 | 16.24 | – | White | Medium |
| 80 | Thulo addey | 6.7 | 2.7 | 2.4:1 | 23.86 | – | CW | Medium |
| 81 | Tangrey | 8.0 | 2.5 | 3.2:1 | 20.83 | – | White | Medium |
| 82 | Thakur binni | 8.1 | 2.5 | 3.2:1 | 17.66 | – | CW | Medium |
| 83 | Birimphole | 7.8 | 2.5 | 3.1:1 | 18.77 | – | White | Medium |
| 84 | Rambhog | 5.3 | 2.5 | 2.1:1 | 21.64 | – | CW | Short |
| Mean | 7.69 | 2.59 | 0.13 | 21.96 | ||||
| SE | 0.12 | 0.04 | 0.01 | 0.529 | ||||
| SD | 1.120 | 0.41 | 0.02 | 4.848 | ||||
| Variance | 1.255 | 0.174 | 0.004 | 23.508 | ||||
| CD | 0.039 | 0.012 | – | 0.255 |
CW creamy white, YW yellowish white
Fig. 1.
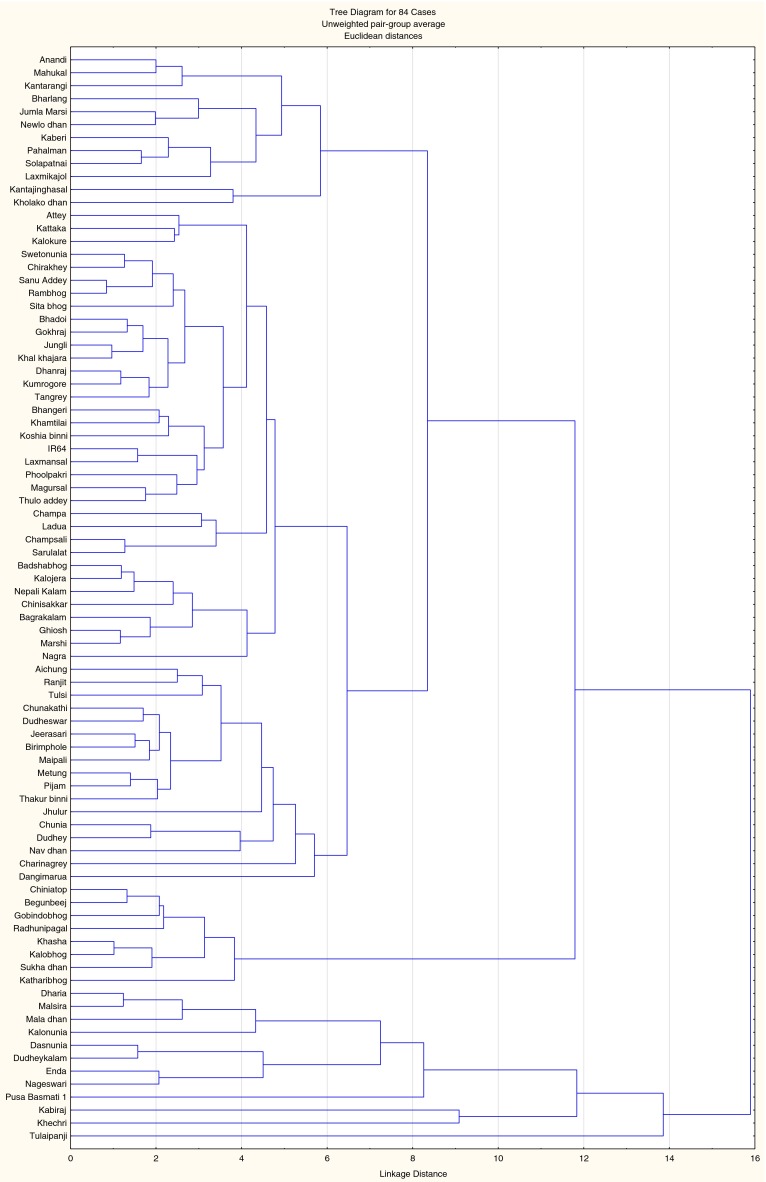
Dendrogram of 84 local rice landraces constructed based on agro-morphology traits
Fig. 2.
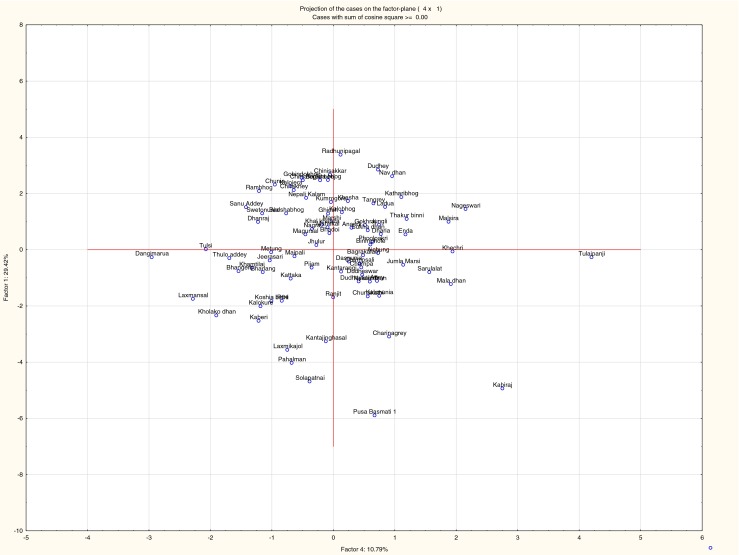
The principal component analysis (PCA) of the 84 local rice landraces showing genetic relationship in a graphical representation scatter plot
Physico-chemical analysis
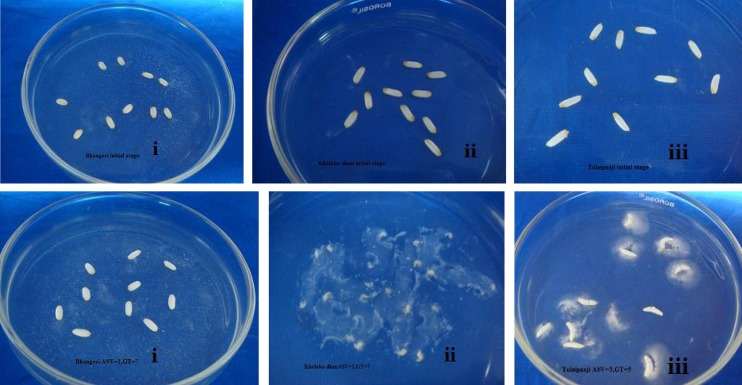
Kernel length (KL) was measured according to Govt. of India notification (No 67, 23 Jan, 2003, Ministry of Commerce). Grain was considered as medium slender A grade basmati rice with 7 mm minimum length, while its minimum LBR is 3.5, kernel breadth (KB) 1.95 mm and KL/KB ratio is 3.07. Other quality parameters were recorded for basmati rice as per DUS protocol. Knowledge accumulated in the past decades indicates that the poor cooking and eating quality is directly related to three attributes of the physico-chemical characteristics of the starch in the endosperm; namely, amylose content (AC) (Juliano 1971), gel consistency (GC) (Cagampang et al. 1973) and gelatinization temperature (GT). Alkali spreading value (ASV) was showed distinct genetic variability among the land races (Fig. 3). Cultivar Kholakodhan showed low ASV (1), and GT (7), this character is preferred for grain quality improvement (IRRI) by the breeders. The ASV depends on the nature of the amylopectin molecules and is reported to be dependent on soluble starch synthase gene on the chromosome 6. Thirty-six rice grains were found with higher length elongation ratio (LER) greater than 1.32 which is desirable as good quality grains (Table 3). Among collected cultivars highest linear elongation ratio (LER) was found in Kalokure (2.07), lowest in Phoolpakri (0.62). Breadth wise highest elongation ratio (BWER) was found in Birimphole (2.17) and lowest in Magursal (0.89). The cultivar Tulaipanji showed a significantly lower ELR of 1.40 with KL 6 mm and width 1.96 mm (Table 3). Kernel length was found to have positive correlation with KB, KL/KB, CKL, CKL/CKB and negatively correlated with CKB and LER (Table 4). Cooking time for rice is the time when 90 % of the starch in the grain no longer show opaque center when pressed between two glass plates. Rice differs in optimum cooking time in excess water between 15 and 25 min without pre-soaking. Landraces like Govindobhog, Tulaipanji, Chiniatap, Begunbeej, Chanachur, Bhadaore, Bhangeri takes 5 min to cook in excess water and Ghiosh takes 25 min to cook. The lower the cooking time better is the quality in terms of fuel and energy consumption during cooking. Amylose content of the rice grain determines whether it will be firm and fluffy on cooking, or it will turn sticky and glutinous. Apparent amylose content (ACC) falls into the following four categories: glutinous = 0 to 5 %, Low = 5 to 19 %, Intermediate = 19 to 23 %, and High >23 %. Glutinous category was absent in this study. Basmati type varieties have intermediate amylose content (AC) of 20–25 % and their grains remain firm and separated after cooking, at the same time they give a soft mouth feel while eating. Tulaipanji grain showed the same amylose content (20 %) and giving the same mouth feel as Basmati with aroma. Both the traits (GT and ASV) are known to be governed by the enzymes of granule bound starch synthase I (GBSSI) encoded by the Waxy gene locus on short arm of chromosome 6 (Umemoto et al. 2002 and 2004). The GBSSI, is responsible for amylose biosynthesis and also has a role in the elongation of long chains in amylopectin. Mutations in the Waxy locus leading to loss of GBSSI activity result in amylose free (waxy) starch.
Fig. 3.
Some example of ASV and GT experiment. First row showing (i–iii above) the initial stage of Alkali spreading value (ASV) and Gelatinization Temperature (GT) and second row showing (i–iii below) after 23 h stage of Alkali spreading value (ASV) and Gelatinization Temperature (GT). i. High gelatinization: No dispersion (Alkali spreading value-1). ii. Low gelatinization: almost complete dispersion (Alkali spreading value-7). iii. Intermediate gelatinization: moderate dispersion (alkali spreading value-4)
Table 3.
Characterization of 84 local rice landraces based on grain quality
| Sl no. | Cultivar name | Kernel length (KL) mm | Kernel breadth (KB) mm | KL/KB ratio | Cooked kernel length (CKL) mm | Cooked kernel breadth (CKB) mm | CKL/CKB ratio | Linear elongation ratio (LER) | Breadth wise elongation ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Anandi | 6.0 | 2.6 | 2.3:1 | 7.6 | 3.5 | 2.1:1 | 1.2 | 1.3 |
| 2 | Attey | 5.5 | 2.3 | 2.3:1 | 10.2 | 3.0 | 3.4:1 | 1.8 | 1.3 |
| 3 | Aichung | 5.4 | 2.5 | 2.1:1 | 8.9 | 3.0 | 2.9:1 | 1.6 | 1.2 |
| 4 | Swetonunia | 5.03 | 2.03 | 2.4:1 | 7.2 | 3.0 | 2.4:1 | 1.4 | 1.4 |
| 5 | Bharlang | 5.2 | 2.03 | 2.5:1 | 10.3 | 2.7 | 3.8:1 | 1.9 | 1.3 |
| 6 | Badshabhog | 4.5 | 2.0 | 2.2:1 | 6.8 | 2.5 | 2.7:1 | 1.5 | 1.2 |
| 7 | Bhangeri | 6.3 | 2.0 | 3.1:1 | 7.03 | 2.3 | 3.05:1 | 1.1 | 1.1 |
| 8 | Bhagrakalam | 5.4 | 2.0 | 2.7:1 | 7.8 | 3.0 | 2.6:1 | 1.4 | 1.5 |
| 9 | Bhadoi | 5.2 | 2.5 | 2.08:1 | 8.2 | 3.0 | 2.7:1 | 1.5 | 1.2 |
| 10 | Chinisakkar | 4.2 | 2.0 | 2.1:1 | 5.1 | 2.5 | 2.04:1 | 1.2 | 1.2 |
| 11 | Champa | 5.9 | 2.5 | 2.3:1 | 8.1 | 3.0 | 2.7:1 | 1.3 | 1.2 |
| 12 | Champsali | 6.1 | 2.5 | 2.4:1 | 7.8 | 3.0 | 2.6:1 | 1.2 | 1.2 |
| 13 | Charinagrey | 6.3 | 2.0 | 3.1:1 | 9.5 | 2.5 | 3.8:1 | 1.5 | 1.2 |
| 14 | Chunakathi | 6.07 | 2.5 | 2.4:1 | 8.8 | 2.5 | 3.5:1 | 1.4 | 1.0 |
| 15 | Chirakhey | 5.2 | 2.5 | 2.08:1 | 6.8 | 3.0 | 2.2:1 | 1.3 | 1.2 |
| 16 | Chiniatop | 4.6 | 2.0 | 2.3:1 | 5.2 | 2.5 | 2.08:1 | 1.1 | 1.2 |
| 17 | Chunia | 4.8 | 2.0 | 2.4:1 | 5.03 | 2.5 | 2.01:1 | 1.04 | 1.2 |
| 18 | Dhanraj | 5.7 | 2.0 | 2.8:1 | 6.1 | 3.0 | 2.03:1 | 1.07 | 1.5 |
| 19 | Dudheswar | 5.6 | 2.0 | 2.8:1 | 8.7 | 3.0 | 2.9:1 | 1.5 | 1.5 |
| 20 | Dangimarua | 6.03 | 2.0 | 5.7:1 | 6.8 | 3.0 | 2.2:1 | 1.1 | 1.5 |
| 21 | Dharia | 5.6 | 2.0 | 2.08 | 7.1 | 3.0 | 2.3:1 | 1.2 | 1.5 |
| 22 | Dasnunia | 6.03 | 2.0 | 3.01:1 | 7.6 | 3.0 | 2.5:1 | 1.2 | 1.5 |
| 23 | Dudhey | 4.2 | 2.5 | 1.6:1 | 5.6 | 3.0 | 1.8 | 1.3 | 1.2 |
| 24 | Dudheykalam | 6.2 | 2.0 | 3.1:1 | 7.9 | 3.0 | 2.6:1 | 1.2 | 1.5 |
| 25 | Enda | 5.2 | 2.5 | 2.08:1 | 7.9 | 3.0 | 2.6:1 | 1.2 | 1.2 |
| 26 | Ghiosh | 5.1 | 2.5 | 2.04:1 | 7.7 | 3.0 | 2.5:1 | 1.5 | 1.2 |
| 27 | Gokhraj | 5.0 | 2.4 | 2.08:1 | 7.9 | 3.0 | 2.6:1 | 1.5 | 1.2 |
| 28 | Gobindobhog | 4.03 | 2.0 | 2.01:1 | 6.7 | 3.0 | 2.2:1 | 1.6 | 1.5 |
| 29 | Begunbeej | 4.0 | 1.90 | 2.1 | 5.1 | 2.5 | 2.04:1 | 1.28 | 1.32 |
| 30 | IR64 | 7.1 | 2.0 | 3.5:1 | 8.6 | 3.0 | 2.8:1 | 1.2 | 1.5 |
| 31 | Jumla Marsi | 5.7 | 3.0 | 1.9:1 | 11.17 | 3.8 | 2.9:1 | 1.9 | 1.2 |
| 32 | Phoolpakri | 5.5 | 2.5 | 2.2:1 | 7.1 | 3.0 | 2.3:1 | 0.62 | 1.2 |
| 33 | Jungli | 5.1 | 2.5 | 2.04:1 | 7.2 | 3.0 | 2.4:1 | 1.4 | 1.2 |
| 34 | Jhulur | 6.9 | 2.5 | 2.7:1 | 9.0 | 3.0 | 3:1 | 1.3 | 1.2 |
| 35 | Jeerasari | 5.8 | 2.0 | 2.9:1 | 7.9 | 2.5 | 3.1:1 | 1.5 | 1.2 |
| 36 | Kalonunia | 7.5 | 2.0 | 3.7:1 | 7.6 | 3.0 | 2.5:1 | 1.01 | 1.5 |
| 37 | Kattaka | 5.1 | 2.0 | 2.5:1 | 8.7 | 2.0 | 4.3:1 | 1.7 | 1.0 |
| 38 | Kataribhog | 4.0 | 2.0 | 2:1 | 6.6 | 2.0 | 3.3:1 | 1.6 | 1.0 |
| 39 | Kaberi | 7.1 | 2.0 | 3.5:1 | 9.3 | 3.0 | 3.1:1 | 1.3 | 1.5 |
| 40 | Koshia binni | 7.4 | 2.0 | 3.7:1 | 8.2 | 3.0 | 2.7:1 | 1.1 | 1.5 |
| 41 | Kumrogore | 5.2 | 2.5 | 2.08:1 | 6.07 | 3.0 | 2.02:1 | 1.1 | 1.2 |
| 42 | Kabiraj | 7.1 | 2.03 | 3.4:1 | 10.8 | 2.5 | 4.3:1 | 1.5 | 1.2 |
| 43 | Khashadhan | 4.4 | 2.0 | 2.2:1 | 7.0 | 3.0 | 2.3 | 1.5 | 1.5 |
| 44 | Khamtilai | 6.0 | 2.1 | 2.8:1 | 8.8 | 3.0 | 2.9:1 | 1.4 | 1.4 |
| 45 | Kalojera | 4.4 | 2.0 | 2.2:1 | 6.8 | 3.0 | 2.2:1 | 1.5 | 1.5 |
| 46 | Kalobhog | 4.9 | 2.0 | 2.4:1 | 6.9 | 3.0 | 2.3:1 | 1.4 | 1.5 |
| 47 | Khechri | 5.1 | 2.5 | 2.04:1 | 8.5 | 3.0 | 2.8:1 | 1.6 | 1.2 |
| 48 | Kantarangi | 6.2 | 2.5 | 2.4:1 | 8.1 | 3.0 | 2.7:1 | 1.3 | 1.2 |
| 49 | Khal khajara | 4.7 | 2.1 | 2.2:1 | 7.8 | 3.0 | 2.6:1 | 1.5 | 1.4 |
| 50 | Katajinghasal | 7.6 | 2.0 | 3.8:1 | 9.4 | 3.0 | 3.1:1 | 1.2 | 1.0 |
| 51 | Kholako dhan | 6.3 | 2.2 | 2.8:1 | 8.7 | 2.0 | 4.3:1 | 1.3 | 0.9 |
| 52 | Kalokure | 5.1 | 2.0 | 2.5:1 | 10.6 | 2.0 | 5.3:1 | 2.07 | 1.0 |
| 53 | Laxmansal | 7.1 | 2.0 | 3.5:1 | 8.05 | 2.5 | 3.2:1 | 1.4 | 1.2 |
| 54 | Laxmikajol | 7.8 | 2.5 | 3.1:1 | 10.3 | 2.5 | 4.1:1 | 1.3 | 1.0 |
| 55 | Ladua | 4.9 | 2.8 | 1.7:1 | 6.4 | 2.8 | 2.2:1 | 1.3 | 1.0 |
| 56 | Malsira | 4.8 | 2.3 | 2.08:1 | 7.1 | 3.0 | 2.3:1 | 1.4 | 1.3 |
| 57 | Metung | 6.06 | 2.0 | 3.03:1 | 7.2 | 3.0 | 2.4:1 | 1.1 | 1.5 |
| 58 | Marshi | 5.0 | 2.2 | 2.2:1 | 7.5 | 3.0 | 2.5:1 | 1.5 | 1.3 |
| 59 | Maipali | 6.2 | 2.0 | 3.1:1 | 7.1 | 3.0 | 2.3:1 | 1.1 | 1.5 |
| 60 | Mahukal | 5.5 | 2.5 | 2.2:1 | 7.1 | 3.0 | 2.3:1 | 1.2 | 1.2 |
| 61 | Mala dhan | 6.1 | 2.0 | 3.0:1 | 7.8 | 3.0 | 2.6:1 | 1.2 | 1.5 |
| 62 | Magursal | 5.1 | 2.0 | 2.5:1 | 7.7 | 3.0 | 2.7:1 | 1.5 | 1.5 |
| 63 | Nav dhan | 4.0 | 2.0 | 2:1 | 6.9 | 2.5 | 2.7:1 | 1.7 | 1.2 |
| 64 | Newlo dhan | 6.0 | 2.6 | 2.2:1 | 9.9 | 3.0 | 3.3:1 | 1.5 | 1.1 |
| 65 | Nageswari | 5.2 | 3.0 | 1.7:1 | 6.1 | 3.0 | 2.03:1 | 1.1 | 1.0 |
| 66 | Nepali Kalam | 5.0 | 1.98 | 2.1:1 | 7.1 | 2.75 | 2.3:1 | 1.0 | 1.1 |
| 67 | Nagra | 5.7 | 2.0 | 2.8 | 9.0 | 3.0 | 3:1 | 1.5 | 1.5 |
| 68 | Pusa Basmati | 8.0 | 2.0 | 3.9 | 10.4 | 2.0 | 5.2:1 | 1.3 | 1.0 |
| 69 | Pijam | 5.4 | 2.0 | 2.7:1 | 7.6 | 2.5 | 3.04:1 | 1.4 | 1.2 |
| 70 | Pahalman | 7.8 | 2.0 | 3.8:1 | 10.6 | 3.0 | 3.5:1 | 1.3 | 1.5 |
| 71 | Radhunipagal | 4.1 | 2.0 | 2.05:1 | 4.9 | 3.0 | 1.6:1 | 1.1 | 1.5 |
| 72 | Ranjit | 6.9 | 2.5 | 2.7:1 | 8.5 | 2.5 | 3.4:1 | 1.2 | 1.0 |
| 73 | Sanu Addey | 4.8 | 2.0 | 2.4:1 | 7.6 | 2.5 | 3.04:1 | 1.5 | 1.2 |
| 74 | Solapatnai | 7.4 | 2.0 | 3.7:1 | 10.4 | 2.5 | 4.2:1 | 1.4 | 1.2 |
| 75 | Rambhog | 5.5 | 2.5 | 2.2:1 | 7.1 | 3.0 | 2.3:1 | 1.2 | 1.2 |
| 76 | Sukha dhan | 5.6 | 2.2 | 2.5:1 | 6.1 | 3.5 | 1.7:1 | 1.08 | 1.5 |
| 77 | Birimphole | 5.7 | 2.5 | 2.2:1 | 8.0 | 3.0 | 2.6:1 | 1.4 | 1.5 |
| 78 | Sita bhog | 4.1 | 2.0 | 2.05:1 | 5.6 | 2.5 | 2.2:1 | 1.3 | 1.2 |
| 79 | Sarulalat | 6.6 | 2.0 | 3.3:1 | 9.1 | 3.0 | 3.03:1 | 1.3 | 1.5 |
| 80 | Tulaipanji | 6.0 | 1.96 | 2.7:1 | 7.7 | 2.2 | 3.5:1 | 1.4 | 1.1 |
| 81 | Tulsi | 4.1 | 2.1 | 1.9:1 | 6.1 | 3.0 | 2.03:1 | 1.4 | 1.4 |
| 82 | Thulo addey | 4.7 | 2.5 | 1.8:1 | 6.7 | 3.0 | 2.2:1 | 1.4 | 1.2 |
| 83 | Tangrey | 5.6 | 2.5 | 2.2:1 | 7.8 | 3.0 | 2.6:1 | 1.3 | 1.2 |
| 84 | Thakur binni | 4.5 | 2.0 | 2.2:1 | 6.9 | 2.5 | 2.7:1 | 1.5 | 1.2 |
| Mean | 5.63 | 2.21 | 1.78 | 7.84 | 2.84 | 1.27 | 1.36 | 1.36 | |
| SE | 0.11 | 0.03 | 0.75 | 0.15 | 0.03 | 0.47 | 0.02 | 0.01 | |
| SD | 1.018 | 0.279 | 1.686 | 1.415 | 0.359 | 1.059 | 0.210 | 0.175 | |
| Variance | 1.038 | 0.078 | 2.844 | 2.003 | 0.129 | 1.123 | 0.044 | 0.030 | |
| CD | 0.038 | 0.012 | – | 0.041 | 0.006 | – | – | – |
Kernel shape (USDA): long means KL/KB ratio 3.0:1 and more; medium – 2.0:1 to 2.9:1; short- 1.9:1 and less
Table 4.
Correlation between eight physico-chemical parameters of rice grains
| Characters | KL | KB | KL/KB | CKL | CKB | CKL/CKB | LER | BWER |
|---|---|---|---|---|---|---|---|---|
| KL | 1 | 0.23 | 0.56 | 0.52 | −0.02 | 0.37 | −0.55 | −0.17 |
| KB | 1 | −0.64 | 0.06 | 0.53 | −0.33 | 0.05 | 0.53 | |
| KL/KB | 1 | 0.35 | −0.41 | 0.57 | −0.36 | 0.35 | ||
| CKL | 1 | 0.26 | 0.61 | 0.40 | 0.14 | |||
| CKB | 1 | −0.58 | 0.30 | 0.35 | ||||
| CKL/CKB | 1 | 0.03 | −0.29 | |||||
| LER | 1 | 0.29 | ||||||
| BWER | 1 |
Anaerobic germination (AG)
Some cultivars showed significant difference in anaerobic germination under submergence condition (Table 5). Correlation analysis was conducted among the cultivars using three parameters by two bioassay methods and R values ranging from 0.29 to 0.98 (Table 6). The test tube method showed significant correlations with all other parameters (i.e. water logged soil method-surface and subsurface; and vigor index) (Figs. 4, 5 and 6). Based on this correlation study, the Test tube method was considered to be the most simple, rapid and reliable bioassay for the anaerobic germination (AG). The seed vigor index (SVI) was calculated by multiplying seed germination percentage with seedling length (in mm). The seed lot showing the higher seed vigour index (SVI) is considered to be more vigorous. The SVI values vary among the cultivars which were as follows - Nageswari (80 × 98 = 7840), Ghiosh (98 × 25 = 2450), Dudheswar (60 × 25 = 1500), Sitabhog (90 × 23 = 2070), Tulsi (99 × 35 = 3465), Chunia (50 × 12 = 600), and Rambhog (90 × 74 = 6660). Seedling vigor plays a key role in the submergence avoidance mechanism (Manangkil et al. 2008). Because, low oxygen (O2) level enhances coleoptile elongation during the germination of rice seeds (Redona and MacKill 1996). Several other investigators have taken this as evidence and tried to relate coleoptile elongation or biochemical changes that are initiated by water uptake with tolerance to anaerobic conditions. Rice is well-known for its ability to germinate without oxygen. In the present study it was observed that the cultivar Ghiosh collected from Sundarban showed anaerobically better germinating performance without an anchoring rootlet followed by cultivars Tulsi and Nageswari (Tables 5 and 6). They showed some kind of submergence avoidance mechanism. Compared to transplanted culture, direct seeding method can reduce labor input by as much as 90 % and can shorten crop growth duration up to 14 days. Submergence is a major stress causing yield loss particularly in the direct seeded rice cultivation system. High germination rate and fast shoot and root elongation are the major traits that are closely related to seedling vigor that is a determinant of the optimum crop establishment in flood prone areas.
Table 5.
Seedling performance of some rice landraces in submergence anaerobic conditions
| Name of varieties | Test tube (on 7th day) (cm) | Waterlogged soil method | ||||
|---|---|---|---|---|---|---|
| Surface (on 14th day) | Sub-surface (on 14th day) | |||||
| Plumule | Radicle | Plumule | Radicle | Plumule | Radicle | |
| Nageswari | 2.1 | 7.7 | 5.26 | 4.14 | 6.78 | 3.98 |
| Ghiosh | 2.5 | Nil | 8.02 | 2.48 | 9.92 | 5.12 |
| Dudheswar | 1.3 | 1.3 | 6.88 | 2.34 | 4.78 | 4.28 |
| Sitabhog | 1.1 | Nil | 3.38 | 1.52 | Nil | Nil |
| Tulshi | 2.3 | 1.2 | 7.32 | 3.38 | 7.36 | 4.52 |
| Chunia | 1.2 | Nil | 4.38 | 3.28 | 5.28 | 1.78 |
| Rambhog | 2.9 | 1.6 | 7.4 | 3.32 | 4.04 | 2.26 |
| SD | 0.71 | 3.17 | 1.76 | 0.86 | 1.68 | 1.46 |
| SE | 0.27 | 1.20 | 0.66 | 0.33 | 0.63 | 0.55 |
Mean ± SE (Three replicates)
Table 6.
Correlation among three parameters obtained by two bioassay methods
| Characters | Test tube | Waterlogged soil method | Vigor index | |
|---|---|---|---|---|
| Surface | Sub-surface | |||
| Test tube | 1 | 0.54 | 0.29 | 0.98 |
| Surface | 1 | 0.43 | 0.57 | |
| Sub-surface | 1 | 0.37 | ||
| Vigor index | 1 | |||
R values ranging from 0.29 to 0.98
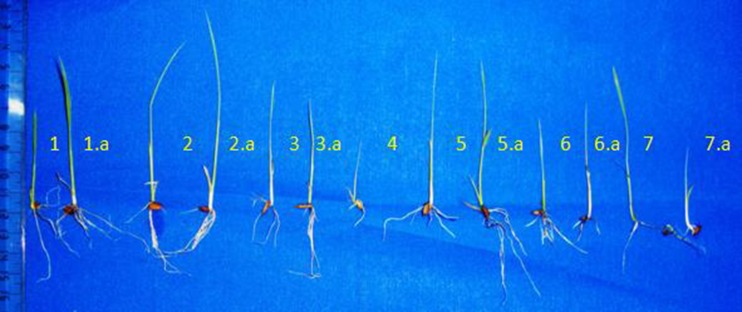
Fig. 4.
Measurement of seedling length under anaerobic condition. [1. Nageswari- surface, 1.a. Nageswari- subsurface, 2. Ghiosh- suface, 2.a. Ghiosh-subsuface, 3. Dudheswar-surface, 3.a. Dudheswar- subsurface, 4. Sitabhog-surface, 5. Tulshi- surface, 5.a. Tulshi-subsurface, 6. Chunia-surface, 6.a. Chunia- subsurface, 7. Rambhog-surface, 7.a. Rambhog- subsurface]
Fig. 5.
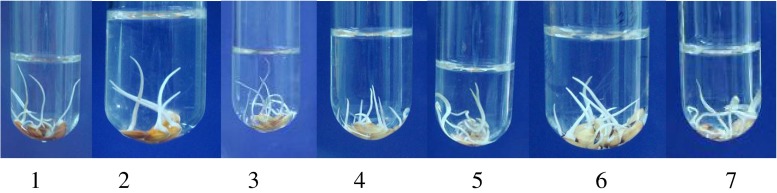
Test tube bioassay method for evaluating seedling vigor under anaerobic germination. [1-Nageswari, 2- Ghiosh, 3-Dudheswar, 4-Sitabhog, 5- Tulshi, 6-Chunia, 7-Rambhog]
Fig. 6.

An overview of Water-logged soil method under anaerobic germination
Iron and zinc content in 84 local rice landraces
Iron content varied between 0.25 μg/g to 34.8 μg/g and Zinc content varied from 0.85 μg/g to 195.3 μg/g (Table 7). Local cultivar Swetonunia had highest iron content of 34.8 μg/g followed by the other cultivars Gobindobhog 3.1 μg/g, and Attey 2.05 μg/g. Nepali Kalam had the highest Zinc content 195.3 μg/g followed by Govindobhog 138.6 μg/g, Begunbeej 20.4 μg/g and Ghiosh16.15 μg/g. Iron content in all the local landraces were very poor but Zinc content in some of the landraces was promising containing 195.3 μg/g in Nepali Kalam and 138.6 μg/g in Gobindobhog. Most of the commercially cultivated indica and japonica rice cultivars are deficient in iron and zinc compared to the other staple food crops such as wheat and maize (Gregorio et al. 2000). Zinc deficiency is probably the most widespread micronutrient deficiency in cereals. Since rice is the principal food of the Asian continent (Developing world), a lot of efforts are being made to develop nutritionally improved genotypes of rice. Breeding for enhancing bio-available minerals in edible portions through increasing the concentrations of metal-binding proteins have been studied by many researchers (Zhang et al. 2005). The first pre-requisite for initiating a breeding program to develop micronutrient-rich genotypes, is to screen the available germplasm and identify the source of genetic variation for the target trait, which can be used in crosses, genetic studies, molecular marker development and to understand the basis of enhanced micronutrient accumulation. Iron and Zinc contents in edible portions also depend on the efficiency of translocation of minerals from root tissues to edible plant organs and accumulation thereof. Mineral-rich and mineral poor rice genotypes identified in this study may be used in breeding program for introgression of high Fe and Zn content gene or QTLs in the improved varieties. Notably, there was about many fold difference in Fe and Zn content suggesting the existence of genetic potential to increase the concentration of these micronutrients in rice grain. Identified cultivar Nepali Kalam (195.3 μg/g Zinc) and Gobindobhog (138.6 μg/g Zinc) may be used as zinc donor in future.
Table 7.
Characterization of 84 landraces of rice based on Iron and Zinc content
| Rice landraces | Fe (μg/g) | Zn (μg/g) |
|---|---|---|
| Bhagrakalam | 2.05 ± 0.011 | 9.77 ± 0.011 |
| Aichung | 4.3 ± 0.003 | 3.8 ± 0.029 |
| Anandi | 0.75 ± 0.025 | 2.55 ± 0.003 |
| Dharia | 0.7 ± 0.029 | 3.4 ± 0.003 |
| Attey | 7.9 ± 0.006 | 4.1 ± 0.002 |
| Badsabhog | 6.2 ± 0.001 | 2.1 ± 0.008 |
| Solapatnai | 4.3 ± 0.003 | 3.8 ± 0.029 |
| Begunbeej | 7.0 ± 0.002 | 2.1 ± 0.008 |
| Jumla Marshi | 1.6 ± 0.021 | 20.4 ± 0.020 |
| Champa | 3.25 ± 0.068 | 0.85 ± 0.28 |
| Champasali | 0.6 ± 0.024 | 2.55 ± 0.21 |
| Charinagrey | 6.8 ± 0.004 | 1.5 ± 0.001 |
| Chiniatop | 0.33 ± 0.032 | 3.4 ± 0.24 |
| Chinisakkar | 13.4 ± 0.008 | 4.4 ± 0.003 |
| Chirakhey | 6.0 ± 0.000 | 2.3 ± 0.001 |
| Chunakathi | 7.0 ± 0.000 | 3.4 ± 0.004 |
| Chunia | 7.1 ± 0.003 | 2.1 ±0.003 |
| Dangimarua | 7.3 ± 0.001 | 2.7 ± 0.002 |
| Rambhog | 6.7 ± 0.002 | 2.5 ± 0.009 |
| Dhanraj | 4.7 ± 0.011 | 2.7 ± 0.000 |
| Sitabhog | 9.7 ± 0.002 | 6.3 ± 0.14 |
| Sarulalat | 11.9 ± 0.001 | 3.2 ± 0.006 |
| Tulaipanji | 0.45 ± 0.04 | 2.21 ± 0.14 |
| Khamtilai | 4.6 ± 0.002 | 1.7 ± 0.13 |
| Thulo addey | 5.1 ± 0.001 | 1.4 ± 0.006 |
| Kalobhog | 7.1 ± 0.001 | 2.4 ± 0.12 |
| Kalojera | 6.9 ± 0.001 | 1.6 ± 0.003 |
| Kalokure | 10.7 ± 0.002 | 1.9 ± 0.16 |
| Kalonunia | 6.0 ± 0.005 | 2.55 ± 0.266 |
| Kantarangi | 4.9 ± 0.002 | 2.6 ± 0.009 |
| Kantajinghasal | 3.8 ± 0.003 | 3.9 ± 0.003 |
| Katharibhog | 5.5 ± 0.001 | 2.6 ± 0.009 |
| Kattaka | 5.1 ± 0.003 | 2.5 ± 0.006 |
| Khalkhajara | 6.9 ± 0.001 | 2.5 ± 0.009 |
| Khasa dhan | 6.9 ± 0.004 | 2.2 ± 0.001 |
| Sanu addey | 6.9 ± 0.002 | 2.2 ± 0.004 |
| Kholako Dhan | 0.25 ± 0.07 | 2.12 ± 0.385 |
| Khechri | 1.73 ± 0.014 | 4.67 ± 0.137 |
| Koshia Binni | 4.3 ± 0.001 | 2.1 ± 0.017 |
| Kumrogore | 7.2 ± 0.001 | 1.6 ± 0.007 |
| Ladua | 8.0 ± 0.001 | 2.9 ± 0.006 |
| Sukhadhan | 12.1 ± 0.001 | 6.7 ± 0.001 |
| Bhadoi | 0.5 ± 0.038 | 1.7 ± 0.002 |
| Bhangeri | 6.7 ± 0.000 | 1.7 ± 0.001 |
| Bharlang | 6.7 ± 0.001 | 1.8 ± 0.003 |
| Birimphole | 0.4 ± 0.026 | 0.85 ± 0.70 |
| Tulshi | 7.0 ± 0.002 | 2.1 ± 0.008 |
| Dasnunia | 6.9 ± 0.004 | 3.8 ± 0.001 |
| Dudheykalam | 4.4 ± 0.001 | 2.0 ± 0.008 |
| Dudheswar | 5.8 ± 0.005 | 2.4 ± 0.005 |
| Dudhey | 65.4 ± 0.055 | 8.5 ± 0.008 |
| Enda | 0.4 ± 0.021 | 0.85 ± 0.35 |
| Ghiosh | 0.55 ± 0.13 | 8.5 ± 0.177 |
| Gokhraj | 8.6 ± 0.002 | 2.0 ± 0.006 |
| Gobindobhog | 6.2 ± 0.002 | 138.6 ± 0.071 |
| IR64 | 5.00 ± 0.003 | 2.0 ± 0.12 |
| Ranjit | 0.5 ± 0.029 | 1.27 ± 0.199 |
| Metung | 7.0 ± 0.002 | 3.2 ± 0.006 |
| Jeerasari | 4.4 ± 0.001 | 2.0 ± 0.006 |
| Maipali | 4.4 ± 0.004 | 1.9 ± 0.11 |
| Mahukal | 5.2 ± 0.003 | 2.1 ± 0.002 |
| Jhulur | 6.5 ± 0.002 | 4.6 ± 0.14 |
| Jungli | 1.8 ± 0.023 | 15.4 ± 0.077 |
| Kaberi | 7.1 ± 0.002 | 1.8 ± 0.11 |
| Kabiraj | 0.35 ± 0.049 | 0.85 ± 0.99 |
| Pijam | 8.3 ± 0.009 | 3.2 ± 0.008 |
| Laxmansal | 6.8 ± 0.004 | 1.3 ± 0.003 |
| Laxmikajal | 7.3 ± 0.001 | 2.5 ± 0.008 |
| Magursal | 7.0 ± 0.003 | 2.9 ± 0.007 |
| Maladhan | 6.6 ± 0.003 | 4.2 ± 0.007 |
| Malsira | 7.0 ± 0.003 | 1.7 ± 0.004 |
| Marshi | 9.8 ± 0.003 | 1.5 ± 0.006 |
| Nageswari | 6.6 ± 0.004 | 2.2 ± 0.11 |
| Nagra | 6.3 ± 0.003 | 2.4 ± 0.003 |
| Nav dhan | 6.6 ± 00.03 | 1.7 ± 0.006 |
| Nepali Kalam | 12.3 ± 0.001 | 195.3 ± 0.3 |
| Pahalman | 6.7 ± 0.001 | 2.0 ± 0.005 |
| Newlodhan | 4.8 ± 0.006 | 2.8 ± 0.009 |
| Phoolpakri | 7.4 ± 0.003 | 3.4 ± 0.001 |
| Pusa Basmati | 6.2 ± 0.004 | 1.9.009 |
| Radhunipagal | 6.2 ± 0.003 | 2.0 ± 0.005 |
| Swetonunia | 34.8 ± 0.003 | 35.2 ± o.oo2 |
| Tangrey | 0.43 ± 0.06 | 1.27 ± 0.34 |
| Thakurbinni | 6.4 ± 0.008 | 3.0 ± 0.001 |
Key observations of this study are summarized in Table 8. Agronomically important traits were identified which can be used by plant breeders for introgression into the elite cultivar for widening the gene pool as well as their improvement. Landraces such as, Newlodhan, Dudheswar and Bhangeri have stout stems and remain in green condition for long days, so breeders can consider them to be included in breeding program. Anaerobic germination traits can be transferred to elite HYV from Nageswary, Ghios, Dudheswar, and Tulshi for high seedling vigor. Root characters for long and highly branched traits can be accumulating into HYV from Jungli and Dudheswar. Disease tolerance traits (based on field observation data) mainly bacterial blight may be taken from Kholakodhan, Bharlang, Jungli, Newlodhan, and Dudheswar. Flag leaf erect trait was observed in Chiniatop, Ghios, Kantarangi, and Laxmansal which can be utilized by breeder for physiological efficiency. Most important traits identified in this investigation: very early maturity (<100 days) plant, Jumla Marshi (Black) collected from Jumla, Nepal which was also cold resistant cultivar. Broad penultimate leaf width was found in Jungli (2.3 cm). Time of heading very early (<71 days) was observed in Jumla marshi black, and Dudheykalam. High amount of micronutrients were found in some landraces as such Nepali Kalam contained highest amount of Zinc (195.3 μg/g) and highest amount of Iron was found in Swetonunia (34.8 μg/g) those can be considered by the breeder to alleviate hidden hunger. The cultivar Tulaipanji can be considered for grain quality trait with aroma. Thus, the present study gives detail characterization of 84 local landraces of rice with agronomic passport data so that breeders can choose donor parent according to their genetic improvement program as well as for conservation of the genetic resources.
Table 8.
Genetic diversity observed in the 84 local landraces of rice for specific traits preferred by breeders
| Characteristics/specific traits | Breeders choice | Name of local rice (Oryza sativa L.) cultivars |
|---|---|---|
| Culm attitude | Erect | Champa, Chunia, Chiniatop, Dharia, Kalobhog, Khechri, Gobindobhog, Kumrogore, |
| Time of heading (50 % of plants with panicles) | Very early (<71 days) | Jumla marshi, Dudhey kalam |
| Stem thickness | >0.55 cm | Anandi, Bharlang, Bhangeri, Chinisakkar, Champa, Champasali, Charinagrey, Chunakathi, Chirakhey, Dudheswar, Enda, Ghiosh, Jumla marshi, Jungli, Jhulur, Jeerasari, Kalonunia, Kattaka, Koshiabinni, Kumrogore, Kabiraj, Sarulalat, Tulaipanji, Tulshi, Newlodhan |
| Stem length | Very short <91 cm | Badshabhog, Bhadoi, Dhanraj, Gobindobhog, Jumla marshi, Jhulur, Jeerasari, Kattaka, Kaberi, Khechri, Laxmansal, Kalojera, Khashadhan |
| Panicle: Length of main axis | Medium (21–25 cm) | Chunia (22.98 cm), Bhangeri (23.88 cm), Chinisakkar (25.26 cm), Dudheswar (25.94), Newodhan (22.64 cm), Ladua (22.6 cm), Sukhadhan (22.4 cm), Sarulalat (25.28 cm). |
| Long (26–30 cm) | Not available in the collected germplasm | |
| Very long (>30 cm) | Not available in the collected germplasm | |
| Panicle curvature of main axis | Straight | Chiniatop, Dudheswar, Dharia, Dasnunia, Kalonunia, Kattaka, and Kataribhog |
| Panicle number per plant | Many >20 | Not available in the collected germplasm |
| Medium (11–20) | Dudheswar (14.8), Chinisakkar (11), Newlodhan (11.2), Ladua (11.4), | |
| No. of seeds per panicle | 240 | Chinisakkar (180), Dudheswar (177), Jungli (129), Chirakhey (101), Koshiabinni (101.8), Kabiraj (117), Ladua (151), Newlodhan (116), Maipali (131), Sukha dhan (110), Sarulalat (111) |
| Penultimate leaf width | Broad >2 cm | Jungli (2.3 cm) |
| Medium (1-2 cm) | Anandi (1.36 cm), Bharlang (1.02 cm), Bhangeri (1.12 cm), Dudheswar (1.14 cm), Kantajingasal (1.18 cm), Laxmikajol (1.04 cm), Maipali (1.12 cm), Newlodhan (1.34 cm), Sarulalat (1.02 cm), | |
| Penaltimate leaf blade length | Medium (30–45 cm) | Attey (35.74 cm), Jumla marshi (34 cm), Jhulur (42.46 cm), Jeerasari (47.94 cm), Katharibhog (18.54 cm), Khechri (44.2 cm), Kholako dhan (40.7 cm), Tulshi (43.6 cm) |
| Time of maturation | Very early (<100) | Jumla marshi black (97 days) |
| Flag leaf in early and late observation | Erect | Anandi, Aichung, Chiniatop, Dudheswar, Enda, Ghiosh, Jungli, Jhulur, Kantarangi, Laxmansal |
| Disease resistance | Bacterial blight (based on frequency observation in the field) | Dudheswar, Bharlang, Anandi, Aichung, Kholakodhan, Jungli, Newlodhan (These are least affected by diseases) |
| Abiotic stress tolerance | Cold tolerance-Jumla Marshi black | |
| i) cold tolerance | Jumla Marshi (White) | |
| ii) salinity tolerance | Salinity tolerance- Ghiosh | |
| iii) Anaerobic germination | Anaerobic germination- Tulshi, Nageswari, Ghiosh, Dudheswar | |
| Root character | Long and highly branched in Jungli, Dudheswar |
Acknowledgments
We are thankful to the authority of the University of North Bengal [Ref. No. 4031/R-2013 dt. 13.08.2013] for financial support and providing experimental rice field with irrigation system in the NBU Campus to carry out this research work. Thanks are due to Dr. B. Sinha, Department of Chemistry, NBU for helping in Atomic Absorption Spectrophotometry.
References
- Adair CR (1972) Rice in the United States: varieties and production. USDA. Handbook No. 289 (Rev.), 124
- Boamfa EI, Ram PC, Jackson MB, Reuss J, Harren FJM. Dynamic aspects of alcoholic fermentation of rice seedlings in response to anaerobiosis and to complete submergence: relationship to Submergence tolerance. Ann Bot. 2003;91:279–290. doi: 10.1093/aob/mcf205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis HE, Welch RM. Biofortification -a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:20–32. doi: 10.2135/cropsci2009.09.0531. [DOI] [Google Scholar]
- Cagampang GB, Perez CM, Juliano BO. A gel consistency test for eating quality of rice. J Sci Food Agric. 1973;24(12):1589–1594. doi: 10.1002/jsfa.2740241214. [DOI] [PubMed] [Google Scholar]
- Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica. 2005;142:169–196. doi: 10.1007/s10681-005-1681-5. [DOI] [Google Scholar]
- Goyal S K, Singh JP (2002) Demand versus supply of foodgrains in India: Implications to food security. Paper presentation at the 13th International Farm Management Congress, Wageningen, The Netherlands, July 7–12, 2002, pp. 20
- Gregorio GB, Senadhira D, Htut H, Graham RD. Breeding for trace mineral density in rice. Food Nutr Bull. 2000;21:382–386. [Google Scholar]
- Juliano BO. A simplified assay for milled rice amylose. Cereal Sci Today. 1971;16:334–338. [Google Scholar]
- Khush GS. What it will take to feed five billion rice consumers by 2030. Plant Mol Biol. 2005;59:1–6. doi: 10.1007/s11103-005-2159-5. [DOI] [PubMed] [Google Scholar]
- Khush GS, Virk PS. Rice breeding achievements and future strategies. Crop Improv. 2000;27:115–144. [Google Scholar]
- Laxuman C, Salimath P, Varma M. Molecular Mapping and Tagging of Quantitative Trait Loci in Rice- Molecular Breeding in Rice. Germany: Lambert Academic Publishing GmbH & Co; 2011. [Google Scholar]
- Lindsey WL, Norwell MA. A new DPTA-TEA soil test for zinc and ion. Agron Abstr. 1969;61:84–90. [Google Scholar]
- Little RR, Hilder GB, Dawson EH. Differential effect of dilute alkali on 25 varieties of milled white rice. Cereal Chem. 1958;35:111–126. [Google Scholar]
- Manangkil OE, Hien TTV, Yoshida S, Mori N, Nakamura C. A simple, rapid and reliable bioassay for evaluating seedling vigor under submergence in indica and japonica rice (Oryza sativa L.) Euphytica. 2008;163:267–274. doi: 10.1007/s10681-008-9645-1. [DOI] [Google Scholar]
- Rana JC, Sharma BD, Gautam PL. Agri-diversity erosion in the north-west Indian Himalayas-some case studies. Indain J Plant Genet Resour. 2000;13:252–258. [Google Scholar]
- Redona ED, Mackill DJ. Genetic variation for seeding vigor traits in rice. Crop Sci. 1996;36:285–290. doi: 10.2135/cropsci1996.0011183X003600020012x. [DOI] [Google Scholar]
- Sood BC, Siddiq EA. A rapid technique for scent determination in rice. Indian J Genet Plant Breed. 1978;38:268–271. [Google Scholar]
- Subudhi PK, Sasaki T, Khush GS. Rice. In: Kole C, editor. Genome mapping and molecular breeding in plants. Berlin: Springer Verlag; 2006. pp. 1–78. [Google Scholar]
- Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor Appl Genet. 2002;104:1–8. doi: 10.1007/s001220200000. [DOI] [PubMed] [Google Scholar]
- Umemoto T, Aoki N, Lin HX, Nakamura Y, Inouchi N, Sato Y, Yano M, Hirabayashi H, Maruyama S. Natural variation in rice starch synthase IIa affects enzyme and starch properties. Funct Plant Biol. 2004;31:671–684. doi: 10.1071/FP04009. [DOI] [PubMed] [Google Scholar]
- Xu KN, MacKill DJ. A major locus for submergence tolerance mapped on rice chromosome 9. Mol Breed. 1996;2:219–224. doi: 10.1007/BF00564199. [DOI] [Google Scholar]
- Zhang MW, Guo BJ, Peng ZM. Genetic effects on grain characteristics of indica black rice and their uses on indirect selections for some mineral element contents in grains. Genet Resour Crop Evol. 2005;52:1121–1128. doi: 10.1007/s10722-004-6114-0. [DOI] [Google Scholar]