Abstract
Purpose
The effect of cyclin D1 overexpression on breast cancer outcomes and prognosis is controversial, even though amplification of the cyclin D1 gene, CCND1, has been shown to be associated with early relapse and poor prognosis. In this study, we examined the relationship between cyclin D1 overexpression and disease-specific survival (DSS). We also analyzed survival in patients who experienced recurrence.
Methods
We retrospectively analyzed data from patients diagnosed with ductal carcinoma between April 2005 and December 2010. We examined clinicopathologic factors associated with cyclin D1 overexpression and analyzed the influence of cyclin D1 on recurrence-free survival and DSS.
Results
We identified 236 patients diagnosed with primary breast cancer who completed all phases of their primary treatment. Cyclin D1 overexpression was significantly associated with longer DSS (5-year DSS, 89.9% in patients without cyclin D1 overexpression vs. 98.9% in patients with cyclin D1 overexpression; p=0.008). Multivariate analysis also found that patients with cyclin D1 overexpressing tumors had significantly longer disease-specific survival than patients whose tumors did not overexpress cyclin D1, with a hazard ratio for disease-specific mortality of 7.97 (1.17-54.22, p=0.034). However, in the group of patients who experienced recurrence, cyclin D1 overexpression was not significantly associated with recurrence-free survival. Cyclin D1 overexpression was significantly associated with increased survival after disease recurrence, indicating that cyclin D1 overexpression might be indicative of more indolent disease progression after metastasis.
Conclusion
Cyclin D1 overexpression is associated with longer DSS, but not recurrence-free survival, in patients with breast cancer. Longer postrecurrence survival could explain the apparent inconsistency between DSS and recurrence-free survival. Patients with cyclin D1-overexpressing tumors survive longer, but with metastatic disease after recurrence. This information should spark the urgent development of tailored therapies to cure these patients.
Keywords: Breast neoplasms, Cyclin D1, Disease-specific survival, Recurrence
INTRODUCTION
Breast cancer is a clinically diverse disease, with differences among tumors that are driven by multiple genetic alterations and molecular events [1]. One such genetic alteration in breast cancer is amplification of the chromosome locus 11q13, which harbors the cyclin D1 gene, CCND1 [2].
Cyclin D1 is a crucial cell cycle regulator that promotes progression through G1 and into S phase. Previous studies have established that cyclin D1 has oncogenic capacity [3]. Over 50% of all primary breast cancers overexpress cyclin D1. The CCND1 gene, which produces cyclin D1, is only amplified in approximately 15% of breast tumors that overexpress cyclin D1 [3,4,5]. The prognostic and predictive value of cyclin D1 overexpression is still controversial because past studies have found that cyclin D1 overexpression can be related to either good [6,7,8] or poor [9,10] prognosis, while CCND1 gene amplification has been consistently shown to correlate with early relapse and poor prognosis.
To resolve the inconsistencies of previous studies, we examined the relationship between cyclin D1 overexpression and disease specific survival (DSS), recurrence-free survival, and postrecurrence survival.
METHODS
Patients
We retrospectively identified patients diagnosed with primary breast cancer who completed all phases of their primary treatment at Wonju Severance Christian Hospital between April 2005 and December 2010. The study was approved by the respective Institutional Review Board (0000-12-5-035).
Primary treatment for patients with breast cancer was determined according to the National Comprehensive Cancer Network guidelines and the Health Insurance Review & Assessment Service of Korea (HIRA, http://www.hira.or.kr). In brief, the guidelines for breast cancer patients recommend the use of anthracycline-based regimens for patients without nodal metastasis, anthracycline plus taxane-based regimens for patients with lymph node metastasis, antihormonal therapy for patients with estrogen receptor (ER)-positive cancer, and trastuzumab for patients with human epidermal growth factor receptor 2 (HER2)-positive cancer.
Medical records were used to ascertain patients' medical histories, including age, sex, and pathology results such as tumor size, lymph node status (number of positive lymph nodes, number of nodes examined), hormonal receptor status, and HER2 status. We obtained survival data from the breast cancer database at Wonju Severance Christian Hospital and the Korean National Cancer Center database. Patients with bilateral disease, stage IV disease, or inflammatory breast cancer, and patients lacking pathology results, were excluded from this study. For disease specific survival mortality, only patients who died specifically from breast cancer, and not as the result of a different disease, were included. Recurrence-free survival was defined as the time from the start of primary treatment to the time of first locoregional recurrence, distant recurrence, or contralateral disease.
Pathological characteristics
Immunohistochemical staining for ER, progesterone receptor, and HER2
All immunohistochemical staining was observed by light microscopy. A cutoff value of 1% or more positively stained nuclei was used to determine ER and progesterone receptor (PR) positivity. HER2 staining was analyzed according to the American Society of Clinical Oncology and College of American Pathologists guidelines using the following categories: 0, no staining; 1+, weak, incomplete membranous staining in less than 10% of tumor cells; 2+, complete membranous staining, either uniform or weak, in at least 10% of tumor cells; and 3+, uniform, intense membranous staining in at least 30% of tumor cells. HER2 immunostaining was considered to be "positive" in specimens that received a score of 3+, whereas scores of 0 to 1+ were regarded as negative. Cases given a score of 2+ were evaluated for HER2 amplification by fluorescence in situ hybridization.
Immunohistochemical detection of cyclin D1
Sections 4 µm thick were serially cut from formalin-fixed, paraffin-embedded tissue samples and mounted on precoated slides. The anticyclin D1 rabbit monoclonal antibody SP4 (100 µL, dilution 1:50; LabVision, Fremont, USA) was used to detect cyclin D1. Immunohistochemical staining was performed using the Ventana HX BenchMark platform (Ventana Medical Systems, Tucson, USA) according to the manufacturer's protocol for automated staining.
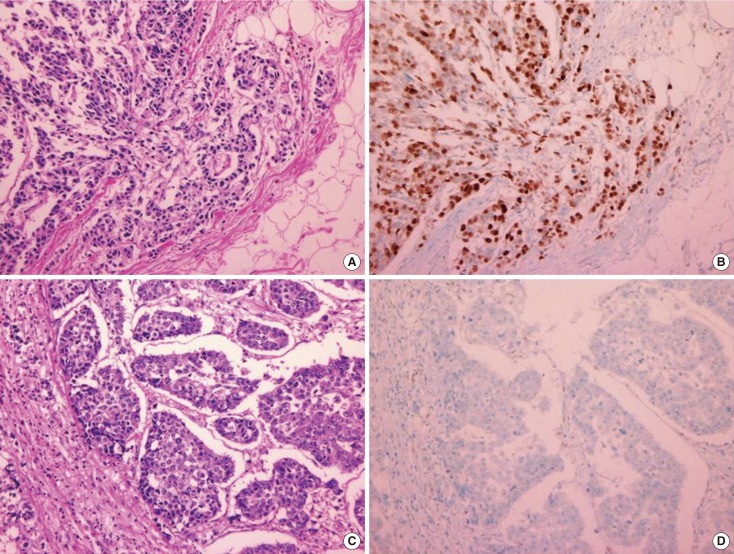
Cyclin D1 expression levels were determined semiquantitatively based on the nuclear staining intensity and positive nuclear staining fraction of tumor cells. The staining intensity was given a score from 0 to 3: 0, negative (no staining of any nuclei, even at high magnification); 1, weak (only visible at high magnification); 2, moderate (readily visible at low magnification); or 3, strong (striking positive staining, even at low magnification). The tumor cells were also graded on a scale of 0 to 5+ based on the percentage of cells with nuclear staining: 0, no cells with nuclear staining; 1+, <1% of cells; 2+, 1% to 9% of cells; 3+, 10% to 32% of cells; 4+, 33% to 67% of cells; or 5+, >67% of cells. We used the staining intensity scores and the scores from the positive nuclear fraction analysis to calculate Allred scores (Figure 1) [2,11].
Figure 1.
Microphotographs showing cyclin D1 positive and negative tumors. (A) Cyclin D1 positive breast cancer with histologic grade 1 (H&E stain, ×40) and (B) same tumor with immunohistochemical staining of cyclin D1 and it showed more than 60% tumor cells were cyclin D1 positive (immunohistochemical stain for cyclin D1, ×400). (C) Cyclin D1 negative breast cancer with histologic grade 3 (H&E stain, ×40) and (D) same tumor with immunohistochemical staining showed no cyclin D1 positive cells (immunohistochemical stain for cyclin D1, ×400).
Statistical analysis
Frequency distributions of categorical variables among various groups were compared using chi-square tests. Fisher exact tests were used if the expected frequencies were <5. Disease-specific survival curves were calculated using the Kaplan-Meier method with log-rank tests. The Cox proportional hazards model was used for multivariate analyses. Statistical analyses were performed using SPSS software version 20.0 (IBM, Armonk, USA). p-values <0.05 were considered statistically significant.
RESULTS
Patient demographics
We identified 253 patients diagnosed with breast cancer who completed treatment, and 236 of these patients were eligible for analysis. Patients with bilateral disease (n=1), stage IV disease (n=8), or inflammatory breast cancer (n=4), and those lacking pathology results (n=4), were excluded from our analysis. Baseline patient characteristics are summarized in Table 1. The mean age at diagnosis was 51.6 years. The average tumor size was 2.55 cm, and 63.6% of patients were node-negative. In our population, 66.1% of patients were ER-positive, 69.5% were PR-positive, and 22.9% were HER2-positive. Cyclin D1 was overexpressed in 81.4% of the patients.
Table 1.
Patients' characteristics (n=236)
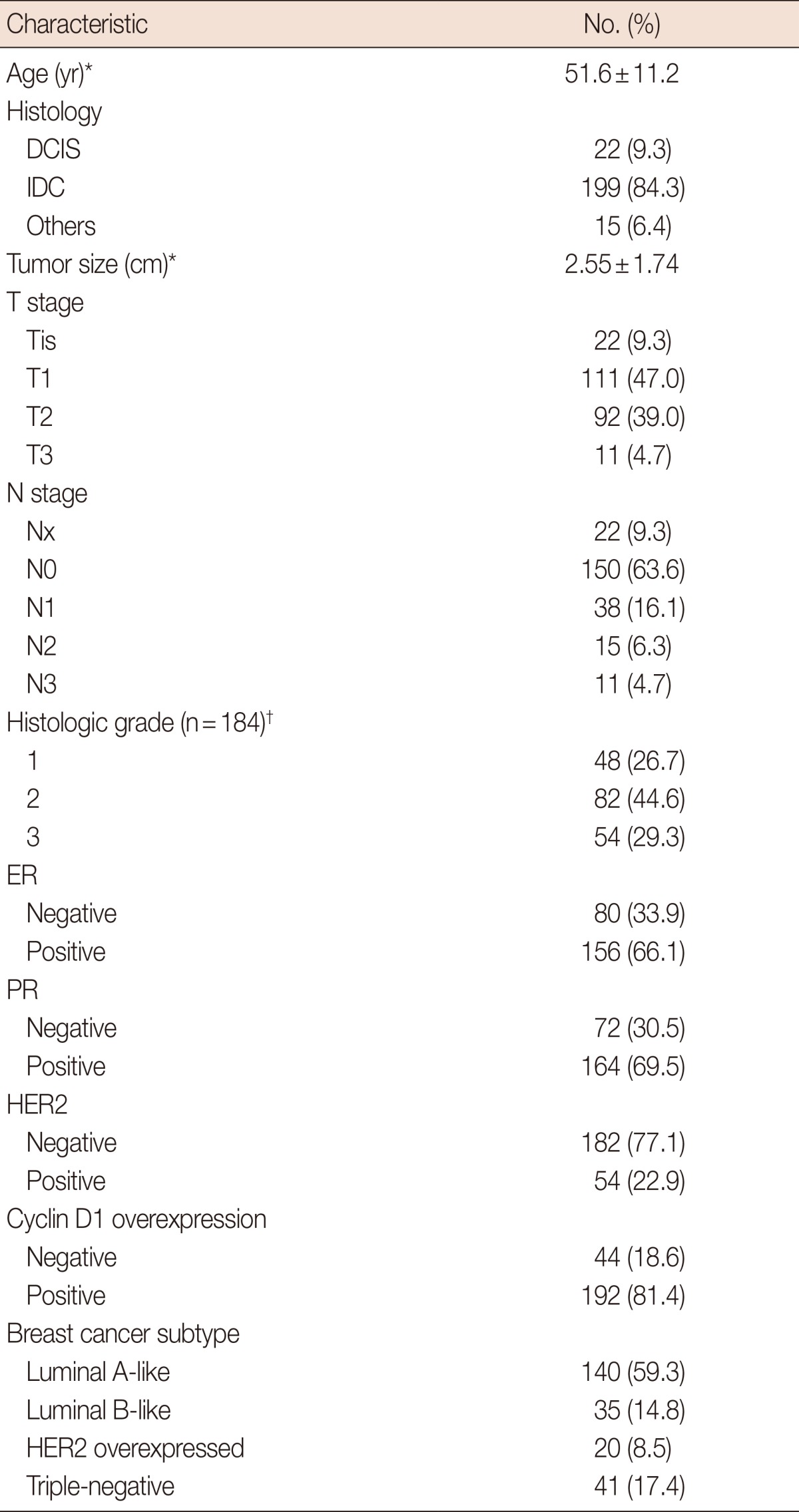
DCIS=ductal carcinoma in situ; IDC=invasive ductal carcinoma; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD; †Histologic grade was available from 184 patients.
Cyclin D1 and clinicopathologic factors
Cyclin D1 overexpression was related to lower histologic grade (p=0.001), ER-positivity (p<0.001), PR-positivity (p<0.001), and non-triple negative breast cancer (TNBC) (p<0.001) (Table 2).
Table 2.
Baseline characteristics by cyclin D1 overexpression
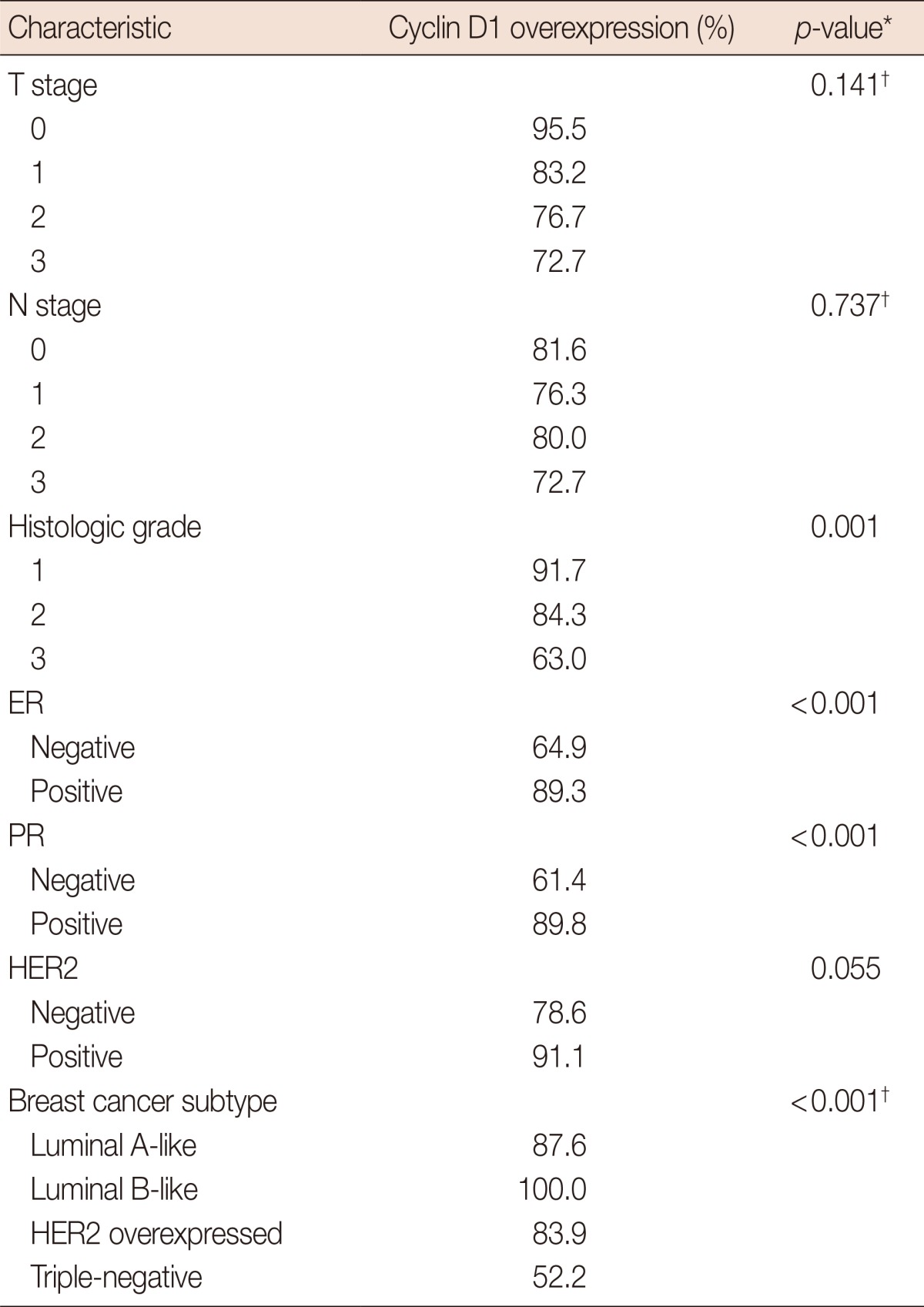
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*chi-square test; †Fisher exact test.
Disease-specific survival
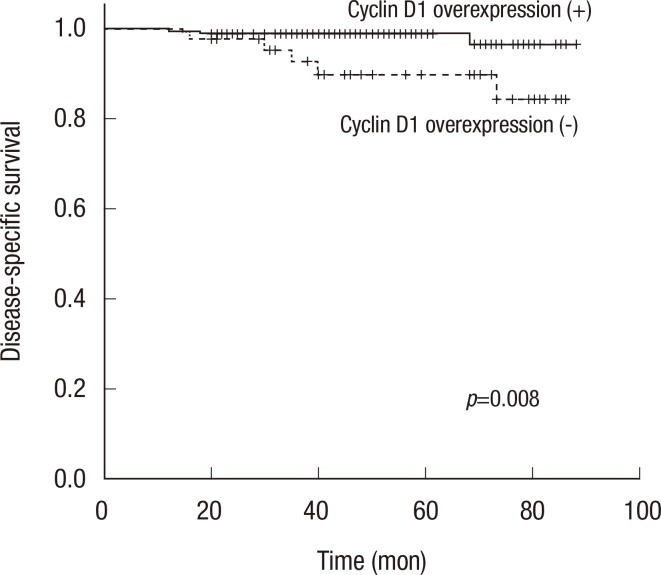
DSS was significantly lower in patients with a higher T stage (p=0.002) or higher N stage (p<0.001). Cyclin D1 overexpression was significantly associated with longer DSS (5-year DSS, 89.9% of patients without overexpressing tumors vs. 98.9% of patients with overexpressing tumors, p=0.008) (Table 3, Figure 2). In a multivariate analysis of T stage and N stage tumors, cyclin D1 overexpression remained significantly associated with better DSS. The hazard ratio for disease-specific mortality of the group without cyclin D1 overexpression was 7.97 (CI, 1.17-54.22; p=0.034) (Table 4).
Table 3.
Univariate Cox regression analysis for disease-specific mortality
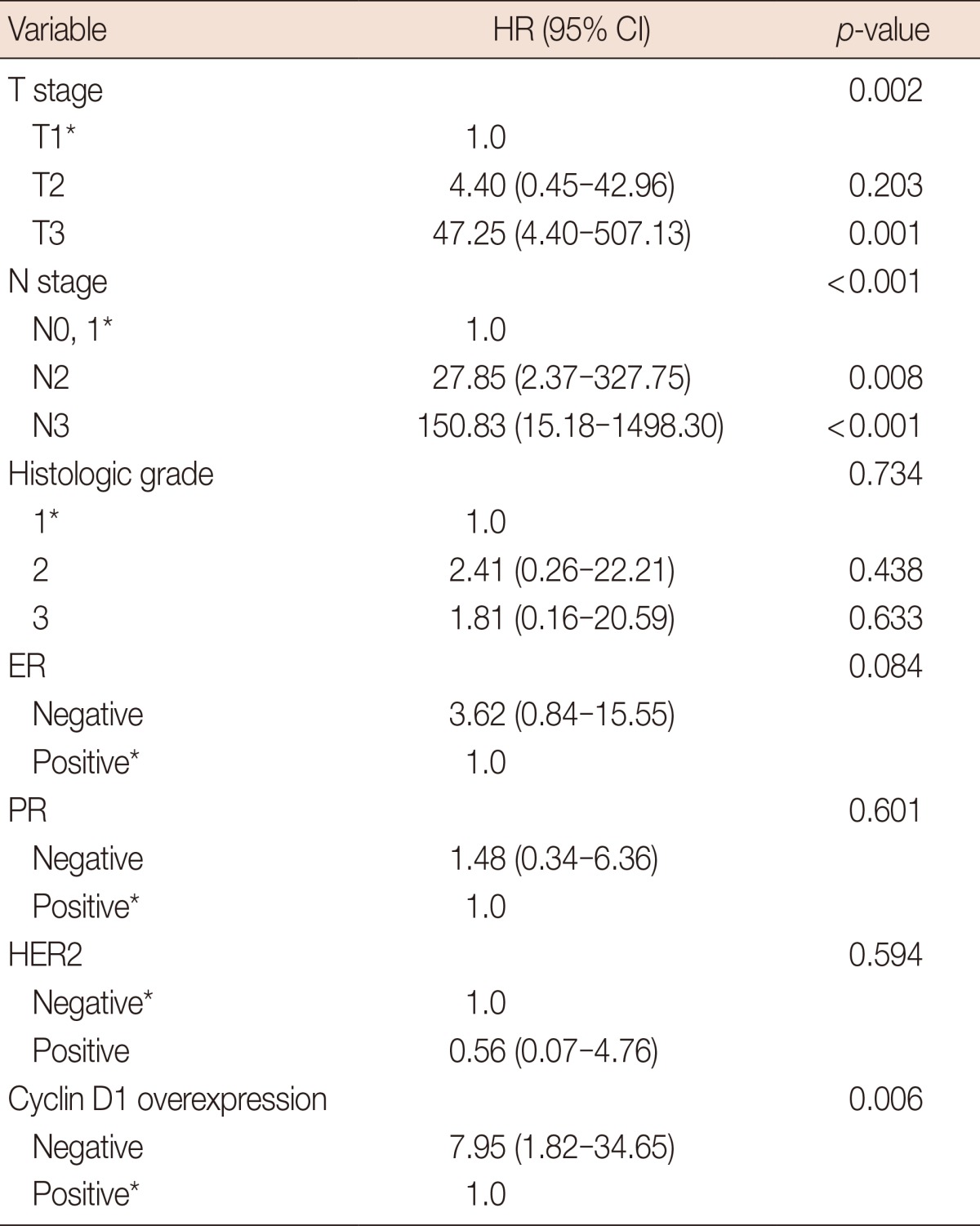
HR=hazard ratio; CI=confidence interval; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Reference.
Figure 2.
Kaplan-Meier curves for disease specific survival stratified by cyclin D1 overexpresson. Kaplan-Meier survival curve for patients with (solid line) or without (dotted line) cyclin D1 overexpression showed statistically significant different overall survival (p=0.008).
Table 4.
Cox proportional multivariate hazard model for disease-specific mortality
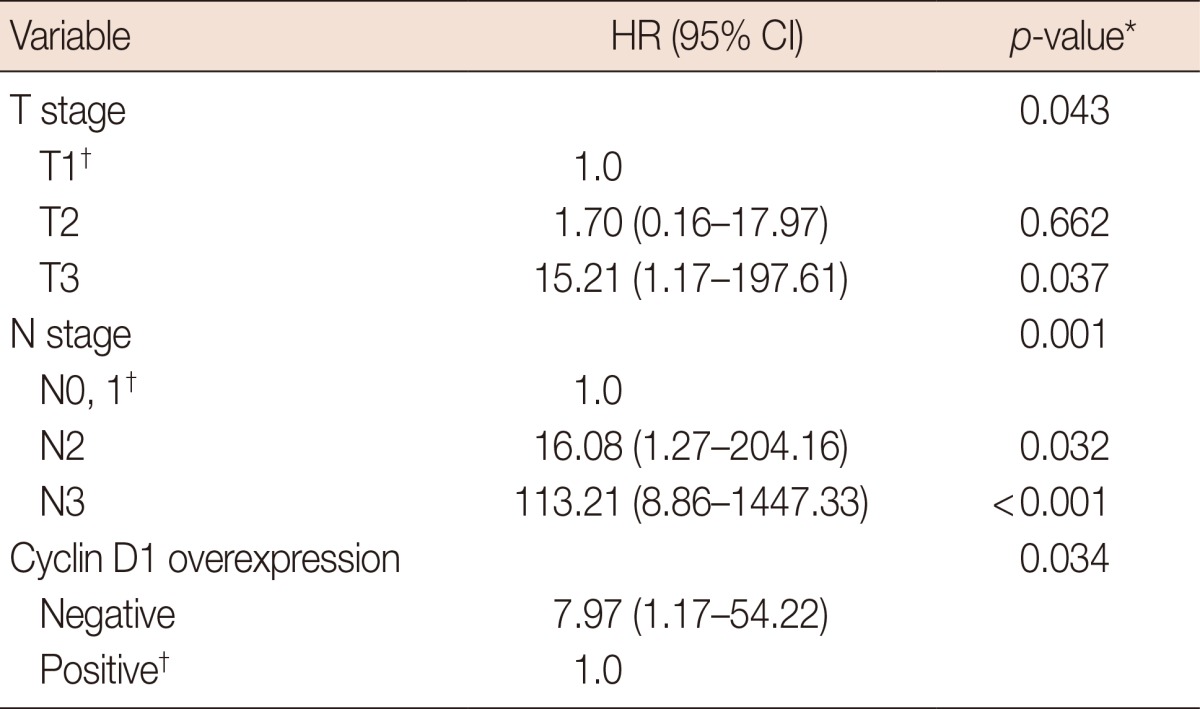
HR=hazard ratio; CI=confidence interval.
*Cox multivariate regression analysis; †Reference.
Cyclin D1 and recurrence-free survival, indolence, and postrecurrence survival
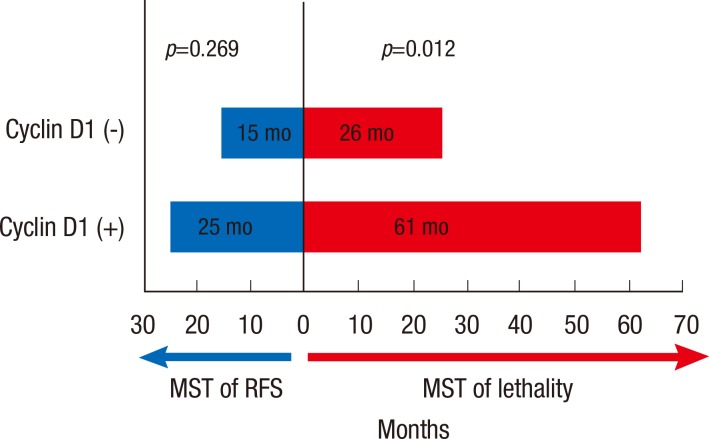
Cyclin D1 overexpression did not significantly correlate with a difference in recurrence-free survival (median of recurrence-free survival time [MST], 15 months for patients with tumors expressing cyclin D1 vs. 25 months for patients with tumors that did not overexpress cyclin D1, p=0.269) (Figure 3). Also, cyclin D1 overexpression was not associated with any site-specific predisposition for metastasis. For example, cyclin D1 expression was not correlated with more cases of bone metastasis, or more cases of visceral metastasis (p=0.580).
Figure 3.
Median survival time (MST) of recurrence free survival and survival after recurrence by cyclin D1 overexpression in patients with experienced recurrence. Cyclin D1 overexpression was not correlated with a difference in recurrence free survival (p=0.269) but significantly associated with increased survival after disease recurrence (p=0.012).
RFS=recurrence free survival.
Finally, we found that patients with cyclin D1 overexpression survived significantly longer after disease recurrence than patients without cyclin D1 overexpression (MST, 61 months vs. 26 months, p=0.012), implying that cyclin D1 overexpression may indicate more indolent postrecurrence disease progression (Figure 3).
DISCUSSION
Previous studies have found associations between overexpression of cyclin D1 and breast cancer subtypes that are more indolent, are ER-positive, and have a better prognosis [3,12,13]. In this study, we found that cyclin D1 overexpression was significantly correlated with ER- and PR-positivity, lower histologic grade, and the non-TNBC subtype. These relationships indicate that cyclin D1 overexpression is associated with types of breast cancer that have good prognostic features. Cyclin D1 is known to play a pivotal role in estrogen-induced breast cancer, since estrogen action is mediated through transcriptional activation of cyclin D1 and c-myc [3,12,14,15,16]. This evidence suggests that cyclin D1 plays a critical role in human breast cancer cell cycle control. Cyclin D1 is also known to regulate the growth of estrogen responsive tissues through ligand-independent ER activation [3,17,18]. Cyclin D1 can bind to the hormone-binding domain of ER, promoting ER association with one of its coactivators and resulting in upregulation of ER-mediated transcription through a cyclin dependent kinase (CDK)-independent mechanism. This estrogen-independent ER-agonistic role of cyclin D1 could underlie the oncogenic pathway in ER-positive breast cancer [3,17,18,19]. However, the prognostic significance of cyclin D1 overexpression for breast cancer seems to be inconsistent [2,12,20]. Amplification of the CCND1 gene at the chromosome locus 11q13 has even been reported to be a potentially negative prognostic factor. Our findings indicate that cyclin D1 overexpression is significantly correlated with increased disease specific survival.
The conflicting views of the prognostic significance of cyclin D1 stem from the fact that cyclin D1 is thought to play a pivotal role in breast cancer progression [3,5,21]. Patients with cyclin D1 overexpressing breast cancer have a better prognosis in terms of longer disease-free survival and overall survival, but cyclin D1 is thought to play a pivotal role in breast cancer progression, making it indicative of poor prognosis when progression is the factor being considered [2,12,20,22]. We believe a reason for the conflicting information found in the literature has to do with the fact that breast cancer that overexpresses cyclin D1 may progress through more indolent evolutional pathway among several various evolutionary pathways [11], resulting in less aggressive or more dormant breast cancer. It was initially believed that a CDK-dependent function of cyclin D1 resulted in progression through the G1 phase of the cell cycle, and that the associated enhanced cellular proliferation was the mechanism of its oncogenic potential [3,5,21,23]; however, evidence from various clinical studies has failed to support this hypothesis. Therefore, it has been suggested that there may be an alternative, CDK-independent function that results in tumorigenesis [3]. Recent studies have shown that cyclin D1 can interact with a variety of transcription factors such as androgen receptor, dentin matrix acidic phosphoprotein 1, CCAAT enhancer binding protein b, and histone acetylases and deacetylases that are independent of CDKS, suggesting that cyclin D1 may function as a transcriptional regulator in addition to its well-established CDK-dependent role in cell cycle progression [3,8,13,24,25,26,27,28]. From these past studies, we reasoned that breast cancer that overexpresses cyclin D1 may progress through one of these evolutionary pathways [13,29,30], resulting in less aggressive or more dormant breast cancer.
We analyzed recurrence-free survival because most breast cancer deaths are due to metastatic disease, not from the primary tumor, and we suspected that cyclin D1 overexpression was associated with a difference in recurrence-free survival. However, we found that there was no difference in recurrence-free survival between patients that had tumors that overexpressed cyclin D1 and those that did not.
Next, we analyzed survival after disease recurrence to try to understand why there was a statistical difference in DSS, but not in recurrence-free survival. We found that patients with cyclin D1-overexpressing breast cancer lived longer, though this did not mean they lived a disease-free life. Other previous studies that reported that cyclin D1 overexpression was significantly associated with longer overall survival also failed to show a significant difference in disease-free survival.
Though metastatic breast cancer is currently an incurable disease, it is treatable with serial administration of endocrine, cytotoxic, and biologic therapies. It is critical that we gain a better understanding of the features that underlie the clinical course after disease recurrence in order to identify potential candidate biomarkers or targets for the subgroup of patients with more indolent disease progression, even after disease recurrence. We suggest that our results give a reasonable basis for future studies of cyclin D1 as a candidate target for patients who have longer survival even though they are not disease-free.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Chang JC, Hilsenbeck SG. Prognostic and predictive markers. In: Harris JR, Lippman ME, Osborne CK, Morrow M, editors. Diseases of the Breast. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 443–457. [Google Scholar]
- 2.Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res. 2012;14:R57. doi: 10.1186/bcr3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–727. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Velasco-Velázquez MA, Li Z, Casimiro M, Loro E, Homsi N, Pestell RG. Examining the role of cyclin D1 in breast cancer. Future Oncol. 2011;7:753–765. doi: 10.2217/fon.11.56. [DOI] [PubMed] [Google Scholar]
- 5.Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005;23:4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, et al. A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer. 1996;73:728–734. doi: 10.1038/bjc.1996.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stendahl M, Kronblad A, Rydén L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004;90:1942–1948. doi: 10.1038/sj.bjc.6601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002;9:409–416. [PubMed] [Google Scholar]
- 9.McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, et al. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995;11:885–891. [PubMed] [Google Scholar]
- 10.Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5:2069–2076. [PubMed] [Google Scholar]
- 11.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 12.Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013;15:R5. doi: 10.1186/bcr3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS. Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int. 2003;53:74–80. doi: 10.1046/j.1440-1827.2003.01441.x. [DOI] [PubMed] [Google Scholar]
- 14.Prall OW, Rogan EM, Musgrove EA, Watts CK, Sutherland RL. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster JS, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996;10:488–498. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 16.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 17.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 18.Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musgrove EA, Wakeling AE, Sutherland RL. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989;49:2398–2404. [PubMed] [Google Scholar]
- 20.Quintayo MA, Munro AF, Thomas J, Kunkler IH, Jack W, Kerr GR, et al. GSK3β and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Res Treat. 2012;136:161–168. doi: 10.1007/s10549-012-2229-8. [DOI] [PubMed] [Google Scholar]
- 21.Caldon CE, Sutherland RL, Musgrove E. Cell cycle proteins in epithelial cell differentiation: implications for breast cancer. Cell Cycle. 2010;9:1918–1928. doi: 10.4161/cc.9.10.11474. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Park WC, Yim HW, Lee MA, Park G, Lee KY. Expression of c-erbB2, cyclin D1 and estrogen receptor and their clinical implications in the invasive ductal carcinoma of the breast. Jpn J Clin Oncol. 2007;37:708–714. doi: 10.1093/jjco/hym082. [DOI] [PubMed] [Google Scholar]
- 23.Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr Relat Cancer. 2011;18:C19–C24. doi: 10.1530/ERC-11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995;55:4257–4263. [PubMed] [Google Scholar]
- 25.Zukerberg LR, Yang WI, Gadd M, Thor AD, Koerner FC, Schmidt EV, et al. Cyclin D1 (PRAD1) protein expression in breast cancer: approximately one-third of infiltrating mammary carcinomas show overexpression of the cyclin D1 oncogene. Mod Pathol. 1995;8:560–567. [PubMed] [Google Scholar]
- 26.Nielsen NH, Lodén M, Cajander J, Emdin SO, Landberg G. G1-S transition defects occur in most breast cancers and predict outcome. Breast Cancer Res Treat. 1999;56:105–112. doi: 10.1023/a:1006208419350. [DOI] [PubMed] [Google Scholar]
- 27.Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer. 2001;84:1064–1069. doi: 10.1054/bjoc.2001.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong JS, van Diest PJ, Michalides RJ, Baak JP. Concerted overexpression of the genes encoding p21 and cyclin D1 is associated with growth inhibition and differentiation in various carcinomas. Mol Pathol. 1999;52:78–83. doi: 10.1136/mp.52.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins FC, De S, Almendro V, Gönen M, Park SY, Blum JL, et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2012;2:503–511. doi: 10.1158/2159-8290.CD-11-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]