Abstract
Purpose
The aim of the study was to evaluate the efficacy and safety of combining sorafenib with chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer.
Methods
MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, American Society for Clinical Oncology abstracts, and European Society for Medical Oncology abstracts were searched. Randomized clinical trials that compared the efficacy and safety of sorafenib plus chemotherapy in patients with HER2-negative advanced breast cancer with placebo plus chemotherapy were eligible. The endpoints were progression-free survival (PFS), overall survival (OS), time to progression (TTP), duration of response (DOR), overall response rate (ORR), clinical benefits, and adverse effects. The meta-analysis was performed using Review Manager 5.2.6 (The Nordic Cochrane Centre), and the fixed-effect model weighted by the Mantel-Haenszel method was used. When considerable heterogeneity was found (p<0.1), further analysis (subgroup analysis, sensitivity analysis, or random-effect model) was performed to identify the potential cause. The results are expressed as hazard ratios or risk ratios, with their corresponding 95% confidence intervals.
Results
The final analysis included four trials comprising 844 patients. The results revealed longer PFS and TTP, and higher ORR and clinical benefit rates in patients receiving sorafenib combined with chemotherapy compared to those receiving chemotherapy and placebo. OS and DOR were similar in the two groups. Meanwhile, the incidence of some adverse effects, including hand-foot skin reaction/hand-foot syndrome, diarrhea, rash, and hypertension, were significantly higher in the sorafenib arm.
Conclusion
Sorafenib combined with chemotherapy may prolong PFS and TTP. This treatment was associated with manageable toxicities, but frequent dose interruptions and reductions were required.
Keywords: Breast neoplasms, Meta-analysis, Sorafenib, Treatment outcome
INTRODUCTION
Breast cancer is one of the leading causes of cancer deaths worldwide, with an estimated 460,000 deaths globally in 2008 [1]. Despite advances in treatment, many patients with breast cancer progress to locally advanced or metastatic disease. Individualized treatment strategies consider the patient's age, performance status, and disease stage, but rely primarily on human epidermal growth factor receptor 2 (HER2) and hormone receptor status [2,3]. Thus, treatment is especially challenging for patients with advanced disease that is HER2-negative or hormone receptor-negative, because there are fewer treatment options. Current pharmacological treatment options include hormone therapy, chemotherapy, and targeted therapy [4].
Angiogenesis plays a critical role in the development, transformation, and metastasis of breast cancer. Angiogenesis is associated with metastatic disease, disease recurrence, and reduced survival, and has hence become an attractive target in many treatment strategies [5,6].
Sorafenib is a multikinase inhibitor with both antiproliferative and antiangiogenic activities, and is indicated for use in unresectable hepatocellular and advanced renal cell carcinomas [7,8]. Breast cancer studies with sorafenib have focused on its use in combination with established chemotherapy or endocrine treatment regimens [9,10,11,12].
However, the efficacy and safety of combination treatments are still controversial, and to date, no meta-analysis has focused on this point. Hence, we conducted a meta-analysis to evaluate the efficacy and safety of sorafenib combined with chemotherapy in patients with HER2-negative advanced breast cancer.
METHODS
Inclusion criteria
Studies were considered eligible for review if they 1) were randomized controlled trials (RCTs); 2) included overall survival (OS), progression-free survival (PFS), time to progression (TTP), duration of response (DOR), overall response rate (ORR), clinical benefits, and adverse effects as outcomes; 3) compared sorafenib plus chemotherapy for patients with HER2-negative advanced breast cancer with placebo plus chemotherapy; 4) provided sufficient data for analysis; 5) could be accessed in full; and 6) were published in English.
If the above inclusion criteria were not met, the studies were excluded from the analysis.
Search strategy
We searched MEDLINE (1966 to October 2013), EMBASE (1974 to October 2013), the Cochrane Controlled Trials Register, American Society of Clinical Oncology abstracts, European Society for Medical Oncology abstracts, and the reference lists of the retrieved studies to identify RCTs comparing sorafenib plus chemotherapy for patients with HER2-negative advanced breast cancer with placebo plus chemotherapy. "Sorafenib AND breast neoplasms" was searched for the meta-analysis, and studies of randomized controlled design were included in the study after reading the titles, abstracts, and full texts.
Trial selection
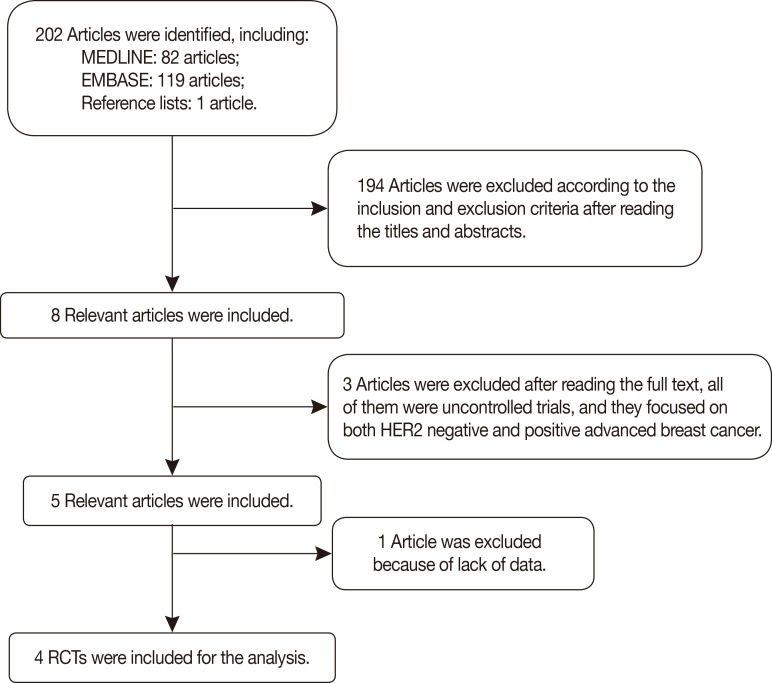
The authors independently reviewed the search results for relevant studies and retrieved the full articles containing the trials. Together, these authors then determined whether each of the selected RCTs fit the inclusion criteria, and studies were excluded accordingly with no discrepancies between the reviewers. The relevant trial selection process is presented in detail in Figure 1.
Figure 1.
Flow chart of trial selection for the meta-analysis.
HER2=human epidermal growth factor receptor 2; RCT=randomized controlled trial.
Quality assessment
The methodological quality of each trial was examined in terms of allocation generation, allocation concealment, blinding, and failure to perform follow-up examinations. Based on this assessment, studies were qualitatively classified according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions 5.2 [13]. Based on the quality assessment criteria, the quality of each study was broadly rated into the following three categories: (A) Adequate: all quality criteria were met, indicating a low risk of bias; (B) Unclear: one or more of the quality criteria were only partially met, indicating a moderate risk of bias; and (C) Inadequate: one or more criteria were not met, indicating a high risk of bias. Sensitivity analyses were subsequently performed on these quality factors, and differences were resolved by discussion among the reviewers.
Data extraction
Two reviewers (J.C. and C.X.T.) independently performed the data extraction. Types of outcome measure included OS, PFS, TTP, DOR, ORR, clinical benefits, and adverse effects. We used the methods of summarizing hazard ratio (HRs) of time-to-event data provided by Tierney et al. [14]. The HRs of time-to-event data (OS, PFS, TTP, and DOR) were extracted from the original studies, either directly from the reported number of events and the corresponding p-values of the log-rank statistics, or by reading of survival curves. We used the names of the first author and the year of publication of the article for identification.
Statistical analysis
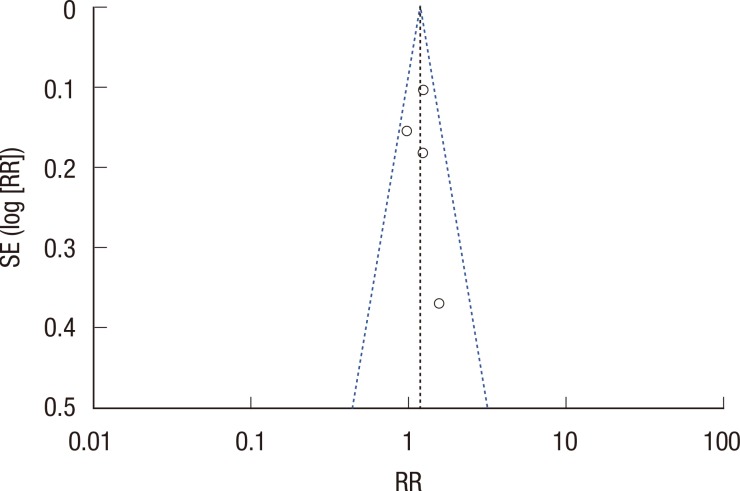
Meta-analysis was performed using Review Manager 5.2.6 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). If data were sufficiently similar, these data were presented as forest plots (Figure 2, 3, and 4). The funnel plot of the analysis did not provide evidence of publication bias (Figure 5).
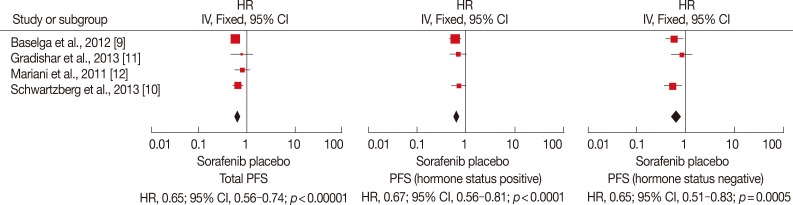
Figure 2.
Progression-free survival (PFS) analysis of sorafenib for human epidermal growth factor receptor 2-negative advanced breast cancer compared with placebo. Total PFS was significantly longer in sorafenib arm (hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.56-0.74; p<0.00001). No matter the hormone status is positive or negative, PFS is longer (when treatment) with sorafenib combined with chemotherapy (HR, 0.67, 95% CI, 0.56-0.81, p<0.0001; HR, 0.65, 95% CI, 0.51-0.83, p=0.0005).
IV=inverse variance.
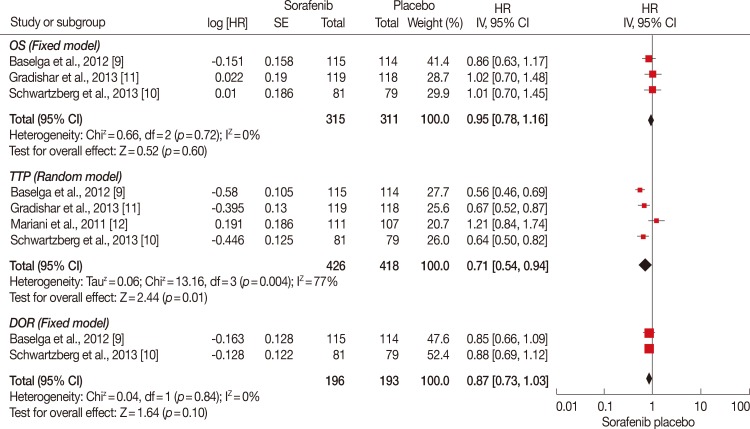
Figure 3.
Overall survival (OS), time to progression (TTP), and duration of response (DOR) analysis of sorafenib for human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer compared with placebo. TTP was significantly longer in sorafenib plus chemotherapy group (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.54-0.94; p=0.01). While OS and DOR were of no significance between the groups (HR, 0.95, 95% CI, 0.78-1.16, p=0.60; HR, 0.87, 95% CI, 0.73-1.03, p=0.10).
IV=inverse variance; SE=Standard Error.
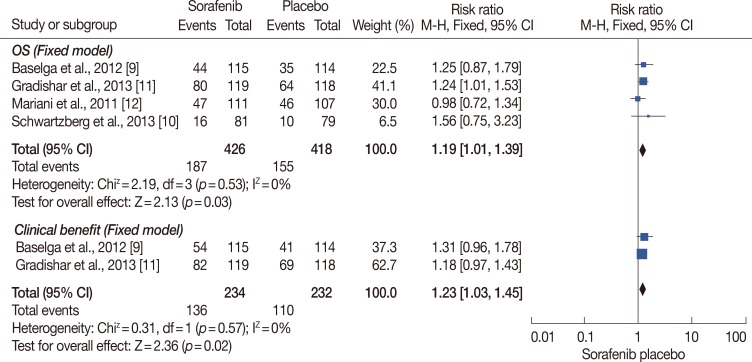
Figure 4.
Overall response rate (ORR) and clinical benefit analysis of sorafenib for human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer compared with placebo. ORR and clinical benefit rate were significantly higher in treatment group compared with placebo group (hazard ratio [HR], 1.19, 95% confidence interval [CI], 1.01-1.39, p=0.03; HR, 1.23, 95% CI, 1.03-1.45, p=0.02).
M-H=Mantel-Haenszel.
Figure 5.
Funnel plot of the included studies for the meta-analysis. The funnel plots did not provide evidence of publication bias.
SE=standard error; RR=risk ratio.
Time-to-event outcomes were compared using HRs. Results are expressed as risk ratios (RRs) for dichotomous outcomes, with 95% confidence intervals (CI). A "fixed-effect" approach was used if heterogeneity was not significant, or if significant, a "random-effects" statistical model was chosen. Tests for heterogeneity were carried out using the chi-square test with significance set at p<0.1 [15]. Sensitivity analysis was performed to explore whether the heterogeneity was caused by low quality; and if so, the lowest quality trials were excluded.
RESULTS
In total, four RCTs [9,10,11,12] involving 844 patients were included in the meta-analysis. The relevant trial selection process is presented in detail in Figure 1. The data were presented as forest plots, and the funnel plot of the analysis did not provide evidence of publication bias.
Characteristic of individual studies
The characteristics of each study are summarized in Table 1. The trials included in the analysis were conducted in nine different countries, located in Europe, North America, and Latin America. The majority (75%, 3/4) of the studies analyzed had a sample size larger than 200; the other 25% (1/4) had a sample size smaller than 200, but larger than 150.
Table 1.
Characteristics of randomized controlled trials included in the present meta-analysis
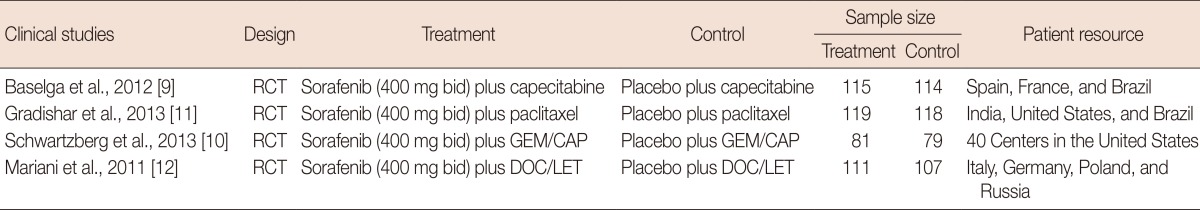
RCT=randomized controlled trials; bid=twice a day; GEM=gemcitabine; CAP=capecitabine; DOC=docetaxel; LET=letrozole.
Quality of individual studies
Among the trials included in the meta-analysis, all trials described the randomization processes and concealed patient allocation, and all of them were multicenter placebo controlled double-blinded trials. Intention-to-treat analyses were all used among the trials included in the meta-analysis (Table 2).
Table 2.
Quality assessment of individual studies in the meta-analysis
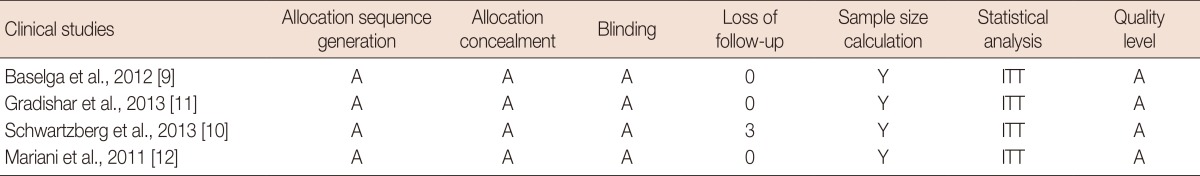
Based on the quality assessment criteria, the quality of the studies was broadly subdivided into the following three categories: (A) All quality criteria were met (adequate): low risk of bias; (B) One or more of the quality criteria were only partly met (unclear): moderate risk of bias; and (C) One or more criteria were not met (inadequate or not used): high risk of bias.
Y=yes; ITT=intention-to-treat analysis.
Efficacy of sorafenib for HER2-negative advanced breast cancer
Four RCTs involving 844 patients (426 in the sorafenib group and 418 in the placebo group) were identified. OS, PFS, TTP, DOR were compared using a HR, and RRs were used for ORR and clinical benefit analysis.
Progression-free survival
Four RCTs were included for total PFS analysis, and three of them were used for subgroup analysis (Figure 2). Subgroup analysis was carried out based on the hormone status. Among the trials included in the analysis, significant differences in total PFS were found in the studies conducted by Baselga et al. [9] and Schwartzberg et al. [10]. In the study by Gradishar et al. [11], the addition of sorafenib to chemotherapy provided a numerical increase in PFS compared to placebo plus chemotherapy, but this was not statistically significant (median, 6.9 months vs. 5.6 months; p=0.09). In the study conducted by Mariani et al. [12], the total PFS was similar between the groups. No significant heterogeneity existed among the trials, and the fixed-effects model was chosen for analysis (p>0.1). Based on the analysis, total PFS was significantly longer in the sorafenib arm (HR, 0.65; 95% CI, 0.56-0.74; p<0.00001). No matter if the hormone status was positive or negative, the PFS was longer with sorafenib combined with chemotherapy compared to placebo with chemotherapy (HR, 0.67, 95% CI, 0.56-0.81, p<0.0001; HR, 0.65, 95% CI, 0.51-0.83, p=0.0005, respectively).
Overall survival
Three RCTs involving 626 patients were included in the meta-analysis (Figure 3). There was no significant heterogeneity between the trials, and the fixed-effects model was used (p=0.72). According to the analysis, the OS did not significantly differ between the groups (HR, 0.95; 95% CI, 0.78-1.16; p=0.60).
Time to progression
Four RCTs involving 844 patients were included in the analysis (Figure 3). Significant heterogeneity existed among the three trials using the random-effects model (p=0.004). According to the analysis, the TTP was significantly longer in the sorafenib plus chemotherapy group (HR, 0.71; 95% CI, 0.54-0.94; p=0.01).
Duration of response
Three RCTs involving 389 patients were included in the analysis (Figure 3). There was no significant heterogeneity between the trials and the fixed-effects model was used (p=0.84). The DOR did not significantly differ between the groups based on the analysis (HR, 0.87; 95% CI, 0.73-1.03; p=0.10).
Overall response rate and clinical benefit
Four RCTs were included in the analysis of ORR, while three of them were included in the clinical benefit analysis (Figure 4). No significant heterogeneity existed among the trials, and the fixed-effects model was chosen for analysis (p=0.53 and p=0.57, respectively). The ORR and clinical benefit rates were significantly higher in the treatment group compared to the placebo group (HR, 1.19, 95% CI, 1.01-1.39, p=0.03; HR, 1.23, 95% CI, 1.03-1.45, p=0.02, respectively).
Safety of sorafenib for HER2-negative advanced breast cancer
Three RCTs were included in the analysis of the safety of sorafenib combined with chemotherapy in the treatment of HER2-negative breast cancer. Adverse effects were compared using RRs. A "fixed-effect" approach was used if heterogeneity was not significant, and a "random-effects" statistical model was chosen if heterogeneity was significant. Based on the analysis, the incidence rates of hand-foot skin reaction/hand-foot syndrome (HFSR/HFS), diarrhea, rash, hypertension, and stomatitis were significantly higher in the sorafenib group. Other adverse effects, including asthenia, fatigue, dyspnea, abdominal pain, vomiting, anemia, headache, nausea, back pain, mucosal inflammation, neutropenia, and pain in extremities were also commonly observed. However, there were no significant differences between the groups (Table 3).
Table 3.
Meta anlaysis of side effects of sorafenib combined with chemotherapy for treatment of HER2-negative advanced breast cancer
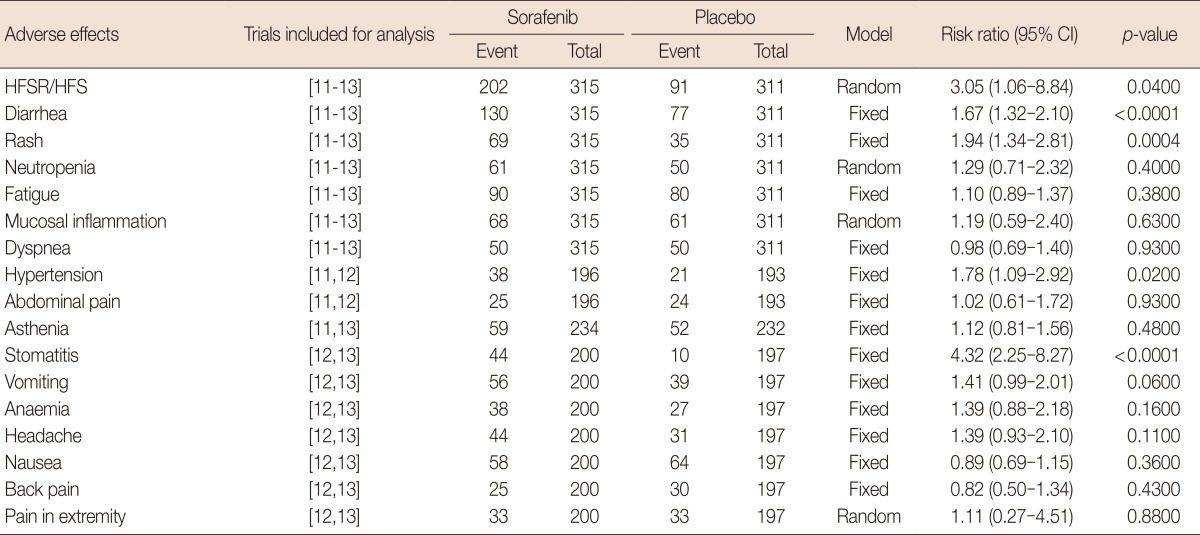
HER2=human epidermal growth factor receptor 2; CI=confidence interval; HFSR/HFS=hand-foot skin reaction/hand-foot syndrome.
DISCUSSION
This study reviewed four contemporary RCTs that included 844 participants [9,10,11,12]. Our meta-analysis suggests that sorafenib combined with chemotherapy is beneficial for HER2-negative advanced breast cancer patients. Although additional toxicities were noticed among the trials, they were manageable after dose interruptions and reductions. Meanwhile, the types of adverse effects were consistent with the known safety profiles of the individual agents. The most frequent toxicity associated with the addition of sorafenib was HFSR/HFS, which is non-life threatening and reversible, but which can reduce the quality of life and necessitate treatment modifications or discontinuation.
Sorafenib is a multikinase inhibitor with both antiproliferative and antiangiogenic activities; it is approved multinationally for unresectable hepatocellular and advanced renal cell carcinoma [7,8]. In breast cancer models, there is evidence that the addition of sorafenib to other systemic therapies may provide additive or synergistic activity, and chemosensitization [16,17,18]. As a monotherapy, sorafenib has demonstrated limited activity in heavily pretreated patients with breast cancer [19,20,21]. However, sorafenib combined with standard chemotherapy was shown to provide clinical benefits in some trials [9,11].
In our analysis, PFS, TTP, OS, DOR, ORR, clinical benefits, and adverse effects were analyzed. The results revealed longer PFS and TTP, and higher ORR and clinical benefit rates in the patients receiving sorafenib plus chemotherapy, whereas the OS and DOR were similar between the two groups.
As a monotherapy, the most commonly reported adverse events (AEs) of sorafenib for treatment of breast cancer are HFSR, rash, and fatigue, but all toxicities are tolerable [21]. In general, we here found that the adverse effects of combinatory sorafenib regimens were manageable, although dose interruptions and reductions were more common in the sorafenib arm than in the placebo arm. The types of AEs experienced by patients in the sorafenib arm were consistent with those associated with the individual agents, with increased rates for some AEs compared with the placebo. Based on the analysis, the most common AE related to sorafenib treatment was HFSR/HFS. The incidence of HFSR/HFS was higher in the sorafenib arm (64.1%, 202/315) compared to in the placebo arm (29.3%, 91/311). The incidence of diarrhea, rash, hypertension, and stomatitis were also significantly higher in the sorafenib group. However, all the toxicities were non-life threatening and reversible, but capable of reducing the quality of life, and necessitating treatment modifications or discontinuation.
As with all meta-analyses, some caveats are pertinent. Publication bias may influence the results, because negative trials are less likely to be published. However, negative trials were also included in the analysis, and the funnel plots do not provide evidence of publication bias (Figure 5).
Although the studies included in the meta-analysis were well designed and the quality reached level A, based on the quality assessment criteria described above; heterogeneity was encountered among these studies. Use of different chemotherapy regimens among the trials and incomplete balanced characteristics may be the reasons for this heterogeneity. In addition, potential selection biases can influence the homogeneity of the groups. When heterogeneity among individual studies is considered, meta-analyses will be crucial to assess the efficacy and safety of combining sorafenib with standard chemotherapy in patients with HER2-negative advanced breast cancer.
In conclusion, the addition of sorafenib to chemotherapy provided PFS and TTP benefits in HER2-negative advanced breast cancer patients. Combination treatment was associated with manageable toxicities, but frequent dose interruptions and reductions were required. Meanwhile, no trials were located in Asia, Africa, and Oceania, so further prospective studies of high quality are still needed to evaluate efficacy and safety of sorafenib for treatment of HER2-negative advanced breast cancer. It should also be noted that all the studies included in the analysis were phase IIB trials, and a phase III trial is still ongoing [22], which we hope will confirm the results of this analysis.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Evans WP. Breast cancer screening: successes and challenges. CA Cancer J Clin. 2012;62:5–9. doi: 10.3322/caac.20137. [DOI] [PubMed] [Google Scholar]
- 2.Gao S, Barber B, Schabert V, Ferrufino C. Tumor hormone/HER2 receptor status and pharmacologic treatment of metastatic breast cancer in Western Europe. Curr Med Res Opin. 2012;28:1111–1118. doi: 10.1185/03007995.2012.694364. [DOI] [PubMed] [Google Scholar]
- 3.Gradishar WJ. Sorafenib in locally advanced or metastatic breast cancer. Expert Opin Investig Drugs. 2012;21:1177–1191. doi: 10.1517/13543784.2012.689824. [DOI] [PubMed] [Google Scholar]
- 4.Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–1790. doi: 10.1200/JCO.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist. 2012;17:1014–1026. doi: 10.1634/theoncologist.2012-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Segalla JG, Roché H, Del Giglio A, Pinczowski H, Ciruelos EM, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzberg LS, Tauer KW, Hermann RC, Makari-Judson G, Isaacs C, Beck JT, et al. Sorafenib or placebo with either gemcitabine or capecitabine in patients with HER-2-negative advanced breast cancer that progressed during or after bevacizumab. Clin Cancer Res. 2013;19:2745–2754. doi: 10.1158/1078-0432.CCR-12-3177. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, Kaklamani V, Sahoo TP, Lokanatha D, Raina V, Bondarde S, et al. A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013;49:312–322. doi: 10.1016/j.ejca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Mariani G, Burdaeva O, Roman L, Staroslawska E, Udovitsa D, Driol P, et al. A double-blind, randomized phase lib study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with docetaxel and/or letrozole in patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur J Cancer. 2011;47(Suppl 2):10. [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.2. Chichester: John Wiley & Sons; 2009. [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Q, Huo L, Yang JY, Xia W, Wei Y, Liao Y, et al. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008;68:6109–6117. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merz M, Komljenovic D, Zwick S, Semmler W, Bäuerle T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur J Cancer. 2011;47:277–286. doi: 10.1016/j.ejca.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Bonelli MA, Fumarola C, Alfieri RR, La Monica S, Cavazzoni A, Galetti M, et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res Treat. 2010;124:79–88. doi: 10.1007/s10549-009-0714-5. [DOI] [PubMed] [Google Scholar]
- 19.Mina LA, Yu M, Johnson C, Burkhardt C, Miller KD, Zon R. A phase II study of combined VEGF inhibitor (bevacizumab+sorafenib) in patients with metastatic breast cancer: Hoosier Oncology Group Study BRE06-109. Invest New Drugs. 2013;31:1307–1310. doi: 10.1007/s10637-013-9976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM, Jr, Wiesenfeld M, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 22.Baselga J, Costa F, Gomez H, Hudis CA, Rapoport B, Roche H, et al. A phase 3 trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (the RESILIENCE study): study protocol for a randomized controlled trial. Trials. 2013;14:228. doi: 10.1186/1745-6215-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]