Abstract
This report describes a case of a 40-year-old female patient with concurrent invasive ductal carcinoma of the breast and malignant follicular lymphoma, initially suspected to be metastatic breast cancer. During the initial evaluation of invasive ductal carcinoma of right breast, multiple lymphadenopathies were noted throughout the body on ultrasonography and positron emission tomography/computed tomography images. Clinically, metastatic breast cancer was suggested, and the patient was administered chemotherapy, including hormonal therapy. The breast cancer improved slightly, but the lymphadenopathies progressed and excisional biopsy of a cervical lymph node revealed malignant follicular lymphoma.
Keywords: Breast, Follicular lymphoma, Invasive ductal carcinoma
INTRODUCTION
Patients with breast cancer have an increased risk of non-Hodgkin lymphoma following radio/chemotherapy [1,2,3]. However, some authors have reported cases of both breast cancer and lymphoma at initial diagnosis, indicating that lymphoma is not induced by therapy [1,2,3]. Although the coexistence of two tumors in the same lymphatic area is uncommon, the concurrence of breast cancer and malignant lymphoma can result in breast cancer staging errors [4].
We experienced a case of a 40-year-old female patient with concurrent invasive ductal carcinoma of the breast and malignant follicular lymphoma, which was suspected to be metastatic breast cancer during the initial evaluation. We reviewed the diagnostic and therapeutic strategies employed in this case and discuss way to avoiding diagnostic errors at the initial investigation.
CASE REPORT
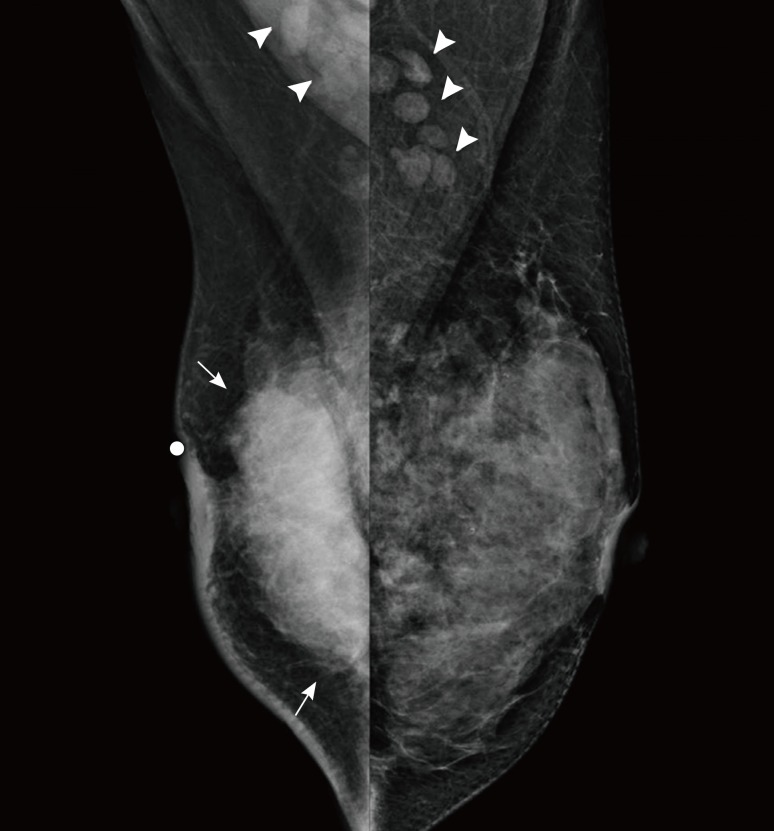
A 40-year-old woman presented with a large palpable lump in the right breast. Mammography revealed a large mass involving the entire breast with nipple retraction and multiple bilateral axillary lymphadenopathies (Figure 1). Ultrasonography (US) images showed an 8×6×3 cm irregular hypoechoic mass in the right breast without any suspicious lesion in the left breast. US of the regional lymph nodes showed multiple enlarged lymph nodes in the axillae on both sides and in the left supraclavicular area (Figure 2). US-guided core needle biopsy (CNB) of the right breast mass indicated invasive ductal carcinoma. Immunohistochemical (IHC) staining showed that the biopsy specimen was positive for estrogen receptor (ER) and progesterone receptor (PR) (4+, 4+, respectively), and fluorescence in situ hybridization (FISH) revealed that the specimen was negative for human epidermal growth factor receptor 2 (HER2) expression. ER and PR expressions were quantified using the Allred score, a semiquantitative system based on the proportion of positive cells (scored from 0 to 5) and staining intensity (scored from 0 to 3) [5]. The proportion and intensity scores were added to yield a total score of 0 to 8. An Allred score >2 was considered positive for ER or PR. HER2 IHC staining was quantified using the HercepTest scoring system (0 and 1+, negative; 2+, equivocal; and 3+, positive). Specimens with an IHC staining score 2+ results were subjected to a second test and were tested with FISH. A HER2-positive tumor was defined as one with an intensity score of 3+ on IHC staining or as a HER2/CEP17 (centromeric probe for chromosome 17) ratio of more than 2.2 on double-probe FISH assays [6].
Figure 1.
Mammograms of both breasts. Bilateral mediolateral oblique view of mammograms show a large high density mass (arrows) and skin thickening on the right breast and bilateral lymphadenopathies (arrowheads).
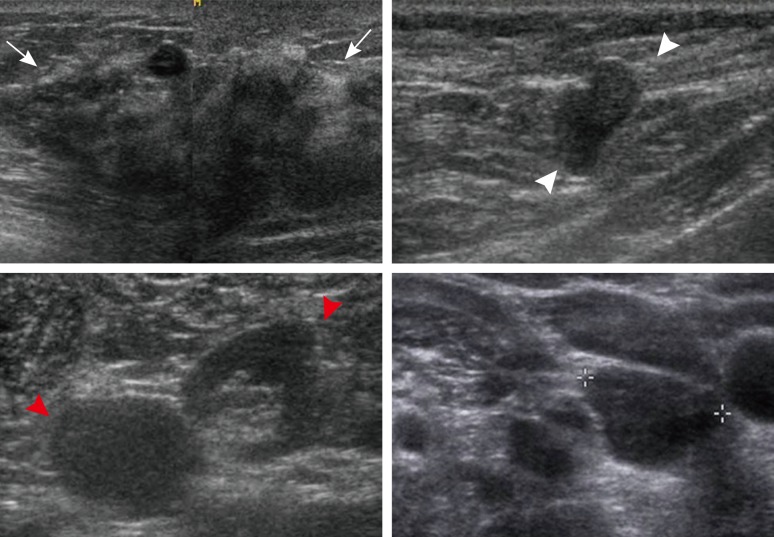
Figure 2.
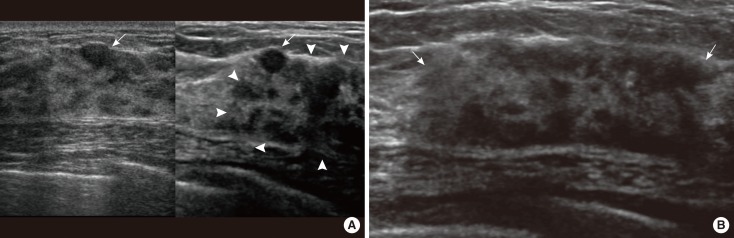
Ultrasonographic (US) images of the right breast cancer and regional lymph nodes. US images reveal an 8 cm irregular hypoechoic mass in the right breast (arrows) and abnormal lymph nodes in the right (white arrowheads) and left axilla (red arrowheads) and the left supraclavicular area (crosses).
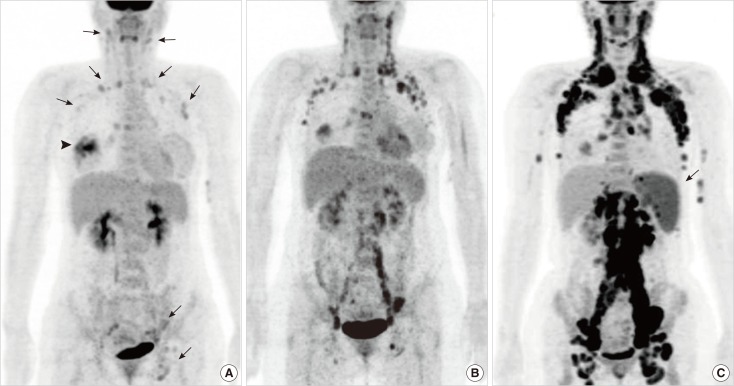
On contrast-enhanced magnetic resonance imaging (CE-MRI), the right breast mass appeared as an irregular mass with initial rapid and late washout enhancement and diffusion restriction. Multiple enlarged lymph nodes were observed in the axillae on both sides, without any suspicious lesion in the left breast. Positron emission tomography/computed tomography (PET/CT) with 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) revealed multiple lymphadenopathies with increased FDG uptake in the axillae on both sides, in the neck, and in the left external iliac and inguinal areas (Figure 3A).
Figure 3.
Maximal intensity projection images of positron emission tomography/computed tomography (PET/CT) scans. (A) Maximal intensity projection image of the initial PET/CT scan shows multiple lymphadenopathies with mildly increased 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) uptake in the axillae on both sides, in the neck, and in the left external iliac and inguinal areas (arrows). The arrowhead indicates high FDG uptake in the right breast mass. (B) PET/CT scan after the discontinuation of chemotherapy shows that the systemic lymphadenopathies are aggravated. (C) Follow-up PET/CT scan demonstrates aggravated lymphadenopathies throughout the torso. Note the newly appeared splenomegaly (arrow). Excisional biopsy of a neck lymph node reveals malignant follicular lymphoma.
We initially considered disseminated metastatic disease from breast cancer, but other pathologies with multiple lymph node involvement such as lymphoproliferative disease and metastasis from another primary malignancy could not be ruled out. US-guided fine needle aspiration (FNA) of an enlarged cervical lymph node and CNB of a left axillary lymph node revealed reactive hyperplasia.
Clinically, metastatic breast cancer was strongly suggested (clinical stage IV, T3N3M1), and therefore initial hormone therapy with zoladex (3.6 mg) and tamoxifen (20 mg) was administered according to the current guidelines for metastatic ER+ breast cancer in a premenopausal woman. One month later, the patient visited another hospital for a second opinion. During the following 8 months, she was enrolled in a clinical trial at another hospital and underwent 8 cycles of maintenance chemotherapy. The regimen comprised 175 mg/m2 paclitaxel, administered intravenously on day 1 and every 21 days thereafter, and 1,250 mg/m2 gemcitabine administered as a 30-minute intravenous infusion on days 1 and 8 and every 21 days thereafter. Postchemotherapy response evaluation showed stable disease, but she refused further chemotherapy because of a poor performance status. Eight months after the discontinuation of chemotherapy, a PET/CT scan at our hospital showed that the primary breast malignancy was stable but that the multiple lymphadenopathies were aggravated, and a new bone metastasis was detected in one thoracic vertebral body (Figure 3B). Therefore, hormone therapy with tamoxifen was initiated. After 1 year of hormone therapy, bilateral neck and right leg swelling developed. A PET/CT scan showed aggravated lymphadenopathies throughout the torso and splenomegaly (Figure 3C), strongly suggesting a lymphoproliferative disease such as lymphoma. Excisional biopsy of a left neck lymph node revealed malignant follicular lymphoma (Figure 4).
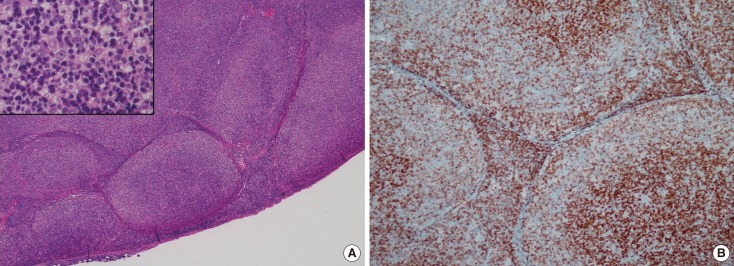
Figure 4.
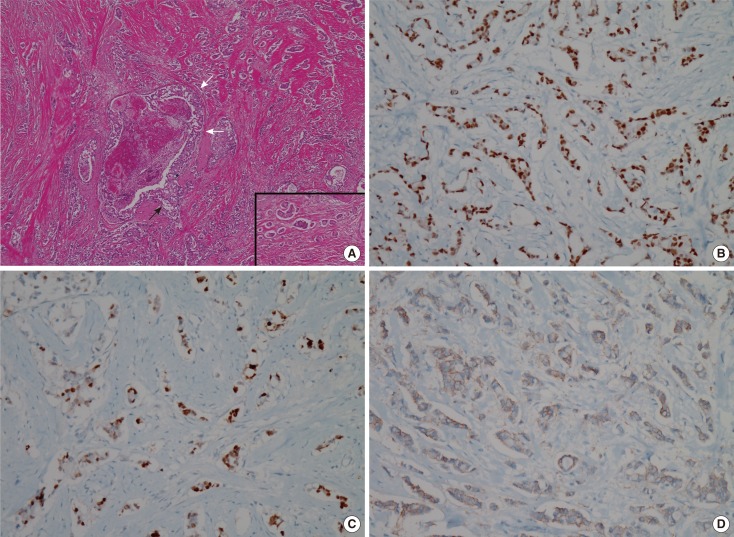
Histologic images of follicular lymphoma. (A) Hematoxyline and eosin staining photomicrograph of a lymph node shows effaced nodal architecture due to closely packed neoplastic follicles (H&E stain, × 40). Inset: The neoplastic follicles show a monotonous population of cells without germinal center and lack any significant mantle zones (H&E stain, × 400). (B) Bcl-2 immunostaining photomicrograph of a lymph node demonstrates positive in follicular cells. Expression of Bcl-2 oncoprotein by follicular cells is a feature of lymphoma and not reactive follicular center cells (Bcl-2 immunostain, × 100).
The lymphoma was diagnosed as clinical stage IV and the patient was administered chemotherapy with rituximab, cyclophosphamide, vincristine, and prednisolone. Four months after chemotherapy, the clinical symptoms had improved and a PET/CT scan showed complete remission of the lymphoma.
Palliative surgery for the breast cancer was planned and mammography, US and MRI were performed. During the evaluation, a new suspicious lesion was found in the upper outer region of the left breast. This lesion showed a 3.5×1.5 cm heterogeneous hypoechoic area on US (Figure 5) and a 4 cm focal area with initial rapid and late plateau enhancement on CE-MRI. US-guided CNB revealed invasive ductal carcinoma. Radical mastectomy with axillary lymph node dissection and a pedicled transverse rectus abdominis muscle flap for chest wall reconstruction was performed for the right-sided breast cancer, and a simple total mastectomy with ipsilateral lymph node sampling was performed for the left-sided breast cancer. Pathology results demonstrated invasive ductal carcinoma (both, modified Bloom and Richardson grade II: tubule and gland formation 3, nuclear grade of pleomorphism 2, mitotic count 1) in both breasts and axillary lymph nodes. On IHC staining, both breast carcinomas was positive for ER and PR (right: 7+ and 7+, respectively; left: 8+ and 5+, respectively) and Ki-67 (right: 2%; left: 5%), and negative for HER2 and epidermal growth factor receptor. Pathologically, the left breast cancer was suspected to have arisen due to metastasis from the right-sided breast cancer (Figure 6 and 7). The postsurgical pathological staging was stage IV (T3N3M1). Maintenance chemotherapy including rituximab for lymphoma was scheduled for 2 years and hormone therapy and palliative radiotherapy were considered for breast cancer.
Figure 5.
Ultrasonographic (US) images of left breast cancer. (A) On comparison of transverse images of the left breast (left: initial; right: follow-up), US images reveal a benign looking ovoid nodule in the initial and follow-up images (arrows) and a new heterogenous hypoechoic area in the follow-up image (arrowheads). (B) Transverse US image shows a heterogenous hypoechoic area in the left upper outer breast (arrows).
Figure 6.
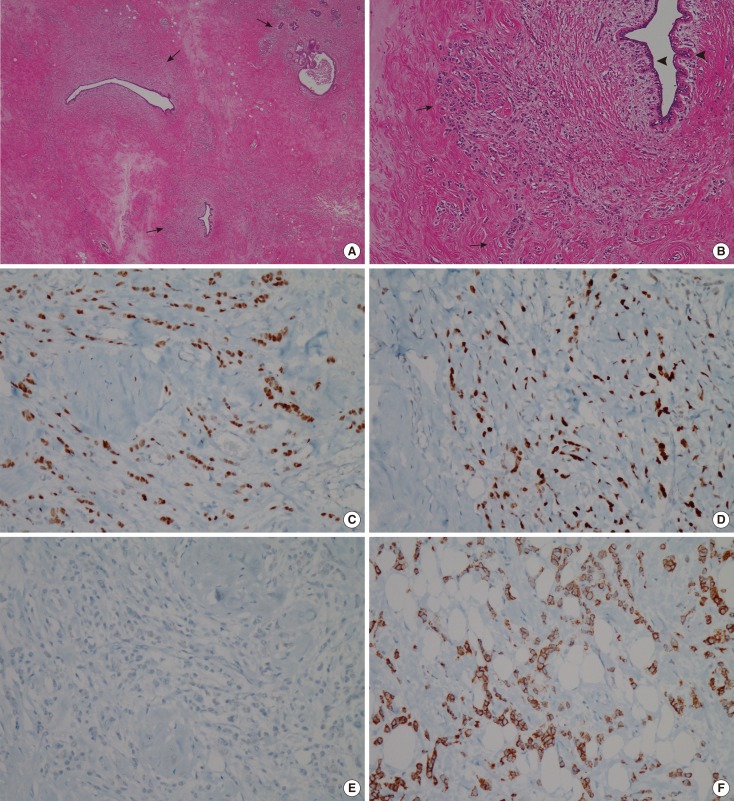
Histologic images of right breast cancer. (A) Hematoxyline and eosin (H&E) staining photomicrograph of right breast cancer shows proliferation of high nuclear grade malignant ductal epithelial cells which represent ductal carcinoma in situ (white arrows) and portions of invasion under basement membrane mean invasive ductal carcinoma (black arrow) (H&E stain, × 40), Inset: Note tumor emboli in lymphatic channels that provide more chance of lymphangitic or hematogenous metastasis (H&E stain, × 40). (B-D) Immunohistochemical staining of surgical specimen is positive for estrogen receptor (×100) (B) and progesterone receptor (×100) (C), equivocal (2+) for human epidermal growth factor receptor 2 (×100) (D). Fluorescence in situ hybridization reveals negativity for HER2 (not shown).
Figure 7.
Histologic images of left breast cancer. (A, B) Hematoxyline and eosin (H&E) staining photomicrographs of left breast cancer demonstrate multifocal infiltrative distribution of tumor cells in periductal area under the basement membrane (arrows). Note that normal ductal epithelium without ductal carcinoma in situ component that often accompanies primary cancer (arrowheads) (H&E stain, A: ×20, B: ×100). (C-E) Immunohistochemistry results are positive for estrogen receptor (×100) (C) and progesterone receptor (×100) (D), and negative for human epidermal growth factor receptor 2 (× 100) (E). (F) Immunoperoxidase staining photomicrograph of left breast cancer shows E-cadherin expression that is lost in lobular carcinoma (Immunohistochemical stain for E-cadherin, × 100).
DISCUSSION
Radiotherapy and chemotherapy for breast cancer increase the risk of non-Hodgkin lymphoma, but the two malignancies rarely coexist [2,3,7]. Since 1947, a small number of cases of synchronous (diagnosed within a 6-month period) and concurrent breast cancer and malignant lymphoma (without evidence of possible induction by therapy) have been described [1,8].
In our case, the initial metastatic work-up after a pathological diagnosis of right breast cancer indicated multiple lymphadenopathies throughout the body. Although the primary right breast cancer was locally advanced, these disseminated lymphadenopathies without evidence of metastatic involvement of other organs were unusual findings for metastatic disease from breast cancer. However, two attempts in the pathological confirmation of suspicious lymph nodes in the left supraclavicular area and axilla using FNA and CNB during the initial investigation demonstrated neither malignant lymphoma nor metastatic breast cancer. Biopsy slide specimens were reviewed retrospectively. Despite the presence of several polymorphous lymphoid cells FNA samples, a diagnosis of lymphoma was difficult to make because of the lack of reliable histologic architecture. For example, a back-to-back nodular growth pattern, which is a helpful histological clue to the diagnosis of follicular lymphoma, was not readily appreciable on cytological smears. Although biopsy samples demonstrated a follicular pattern to some extent, they did not include a germinal center and lacked histological architecture. IHC staining for Bcl-2 (B-cell lymphoma 2) may be helpful in this differentiation; however, it would be difficult to distinguish between follicular lymphoma and reactive hyperplasia of lymph nodes because of the absence of a germinal center.
Two hypotheses were considered to explain this situation; One was that malignant lymphoma was already present at time of breast cancer diagnosis and slowly progressed during follow up and the other was that chemotherapy for breast cancer induced secondary malignant lymphoma that progressed during the 2-year follow-up period [1,2,3]. However multiple lymphadenopathies were noted throughout the body in the initial PET/CT scan and these seemed to gradually increase in size during the follow-up period. Accordingly, the first hypothesis is more likely.
Preoperative evaluation after neoadjuvant chemotherapy revealed a new invasive ductal carcinoma in the contralateral breast, which raised the question of whether this contralateral cancer represented metastasis or a new primary tumor. Metastasis to the contralateral breast may occur via two distinct routes; lymphangitic or hematogenous spread. Furthermore, lymphangitic metastasis to the breast usually occurs across the anterior chest wall (transthoracic or cross-lymphatic metastasis) to the opposite breast [9,10]. On a pathologic review of this case, we considered that metastasis from the primary breast cancer to the left breast cancer was more probable because the left breast mass had no ductal carcinoma in situ (DCIS) component, which often accompanies primary cancer often [11]. And the right breast mass showed more lymphovascular invasion, supporting the notion of lymphangitic or hematogenous metastasis. In addition, the multifocal infiltrative pattern surrounding the normal ducts of the left breast mass, in contrast to the mass forming pattern admixed with the DCIS component in the right breast mass, suggested a metastatic lesion (Figure 7). Moreover, the tumor grade, hormone receptor status, and gene expression status of the two breast specimens were similar. Therefore, we concluded that the left breast cancer represented a metastasis from the right breast rather than a metachronous primary breast cancer.
With regard to the first hypothesis of lymphoma described above, had we been able to prove the lymphadenopathies to be malignant lymphoma during the first investigation, the right breast cancer could have been initially staged appropriately and the lymphadenopathies would not have been confused with disseminated metastatic disease. Accordingly malignant lymphoma and breast cancer could have been treated at a relatively early stage. We evaluated the lymphadenopathies using FNA and CNB. However, these less invasive methods cannot guarantee precise pathology results, although it has been reported that US-guided FNA and CNB have been reported to have higher preoperative staging accuracies thus better ensuring appropriate axillary surgery for individual breast cancer patients [12]. FNA or CNB findings alone are sometimes insufficient for diagnosing other synchronous tumors involving lymph nodes or lymphoproliferative diseases such as malignant lymphoma. Because FNA and CNB provide only small tissue samples of lymph nodes, they cannot accurately represent regional pathological heterogeneities and architecture, and thus, are prone to sampling errors. Therefore, if a suspicious lymphadenopathy in a patient with breast cancer is not diagnosed as malignancy by FNA or CNB and lymph node status is critical for determining the therapeutic strategy, we suggest that excisional lymph node biopsy should be conducted for an accurate diagnosis and treatment decision making.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Wiernik PH, Hu X, Ratech H, Fineberg S, Marino P, Schleider MA, et al. Non-Hodgkin's lymphoma in women with breast cancer. Cancer J. 2000;6:336–342. [PubMed] [Google Scholar]
- 2.Dutta Roy S, Stafford JA, Scally J, Selvachandran SN. A rare case of breast carcinoma co-existing with axillary mantle cell lymphoma. World J Surg Oncol. 2003;1:27. doi: 10.1186/1477-7819-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suresh Attili VS, Dadhich HK, Rao CR, Bapsy PP, Batra U, Anupama G, et al. A case of breast cancer coexisting with B-cell follicular lymphoma. Austral-Asian J Cancer. 2007;6:155–156. [Google Scholar]
- 4.Benoit L, Arnould L, Collin F, Fraisse J, Cuisenier J, Chauffert B. Concurrent lymphoma and metastatic breast carcinoma in the axillary, confounding sentinel lymph-node biopsy. Eur J Surg Oncol. 2004;30:462–463. doi: 10.1016/j.ejso.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 6.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 7.Barranger E, Marpeau O, Uzan S, Antoine M. Axillary sentinel node involvement by breast cancer coexisting with B-cell follicular lymphoma in nonsentinel nodes. Breast J. 2005;11:227–228. doi: 10.1111/j.1075-122X.2005.21697.x. [DOI] [PubMed] [Google Scholar]
- 8.Pandey U, Naraynan M, Karnik U, Sinha B. Carcinoma metastasis to unexpected synchronous lymphoproliferative disorder: report of three cases and review of literature. J Clin Pathol. 2003;56:970–971. doi: 10.1136/jcp.56.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todd MT, Douglas Y. Bilateral breast cancer. In: Singletary SE, Robb GL, Hortobagyi GN, editors. Advanced Therapy of Breast Disease. 2nd ed. Hamilton: B.C. Decker; 2004. pp. 629–630. [Google Scholar]
- 10.Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257–262. doi: 10.7863/jum.2000.19.4.257. [DOI] [PubMed] [Google Scholar]
- 11.Gupta D, Merino MI, Farhood A, Middleton LP. Metastases to breast simulating ductal carcinoma in situ: report of two cases and review of the literature. Ann Diagn Pathol. 2001;5:15–20. doi: 10.1053/adpa.2001.21476. [DOI] [PubMed] [Google Scholar]
- 12.MacNeill M, Arnott I, Thomas J. Fine needle aspiration cytology is a valuable adjunct to axillary ultrasound in the preoperative staging of breast cancer. J Clin Pathol. 2011;64:42–46. doi: 10.1136/jcp.2010.083063. [DOI] [PubMed] [Google Scholar]