Abstract
Background:
Nondaily smoking has increased among current U.S. smokers during the past decade and is practiced by a significant percentage of smokers. Although research in nondaily smoking has grown, little is known about levels of exposure to tobacco toxicants among nondaily smokers and their variation across ethnic groups.
Methods:
We examined urinary levels of cotinine and a tobacco-specific nitrosamine (NNAL) in community participants. Associations between the biomarker data and smoking characteristics were evaluated with Spearman’s correlation analysis.
Results:
Participants included 28 Blacks, 4 Latinos, and 25 Whites who smoked at least 1 cigarette on 4–24 days in the past 30 days. Participants averaged 3.3 (SD = 2.1) cigarettes per day (cpd) on days smoked, they smoked an average of 13.0 (SD = 5.4) days in the past month, and they smoked nondaily for 10.5 (SD = 10.5) years. Median levels of creatinine-normalized cotinine and NNAL were 490.9ng/mg and 140.7 pg/mg, respectively. NNAL and cotinine were highly correlated (r = .84); NNAL and cotinine were modestly correlated with cpd (r = .39 and r = .34; all p values <.05). The number of days smoked per month was not associated with any biomarker levels.
Conclusions:
Our findings demonstrate that nondaily smokers are, on average, exposed to significant levels of nicotine and carcinogenic nitrosamines, with exposures of 40%–50% of those seen in daily smokers. This level of exposure suggests a significant health risk. Nicotine and carcinogen exposure is most closely related to number of cigarettes smoked per day but not to number of days per month of smoking.
INTRODUCTION
Cigarette smoking continues to be the leading cause of preventable disease and death in the United States, accounting for over 400,000 preventable deaths per year. Approximately 44,000,000 Americans currently smoke regularly (CDC, 2008; www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a2.htm). However, antismoking legislation and public health efforts have led to lower overall rates of cigarette use and changes in the way that smokers use cigarettes. In fact, the prevalence of nondaily smoking, the practice of smoking on some days but not every day, is increasing (CDC, 2003), with roughly 25%–30% of smoking adults reporting nondaily smoking; this practice appears to be especially significant in the young adult population (CDC, 2007; Schane, Glantz, & Ling, 2009). Additionally, recent data suggest that nondaily smokers initiate cigarette use in response to acute, situational cues, whereas daily smokers act on dependency (Shiffman, Dunbar, Scholl, & Tindle, 2012).
Prior studies have demonstrated differences in tobacco use and exposure between different racial and ethnic groups. For example, Blacks absorb more nicotine per individual cigarette smoked, are less likely to be successful in quit attempts, and have longer lifetime smoking durations compared with White smokers(Benowitz, Dains, Dempsey, Wilson, & Jacob, 2011; Fu et al., 2008). Furthermore, Blacks have a higher risk of smoking-related lung cancer compared with Whites, especially when considering those with lower levels of daily cigarette consumption (Haiman et al., 2006). These findings suggest that substantial differences exist between smokers of different races with regard to exposure, addiction, and biological toxicity.
Given that nondaily smoking is on the rise and racial differences in response to tobacco exposure exist, greater understanding of carcinogen exposure in nondaily cigarette smokers is needed. Urinary cotinine level is widely accepted as an objective marker of actual tobacco exposure that is not subject to the inherent biases of self-reported use. It has a half-life averaging 16hr such that tobacco use can be detected 3–5 days after last nicotine exposure. The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent carcinogen; levels of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), measured in urine, are independently associated with the risk of lung cancer in a large cohort of smokers (Yuan et al., 2009; Yuan, Gao, et al., 2011). Additionally, urine concentration of NNAL has been shown to be predictive of the risk of development of esophageal carcinoma (Yuan, Knezevich, et al., 2011). Urine NNAL has an elimination half-life of 10–18 days, such that tobacco use can be detected for 6–12 weeks after last use (Goniewicz et al., 2009). Urine NNAL would be expected to be a more sensitive measure of intermittent (nondaily) tobacco use(Goniewicz et al., 2011). This study sought to examine urinary cotinine and NNAL among nondaily cigarette smokers in a multiethnic sample.
METHODS
Participants
All procedures were approved by the University of Minnesota Institutional Review Board. Using a convenience sampling approach, potential participants were recruited through advertisements in local newspapers, fliers posted in public places, and in-person invitations at community health fairs. Participants were screened for eligibility using the following criteria: self-identification as Black, White, or Hispanic; age 25 years and older; smoked at least 100 cigarettes in their lifetime, smoked nondaily for at least 6 months, and smoked for at least 1 year. Eligible participants smoked 4–24 days in the past 30 days, the equivalent of at least once a week but less than 25 days per month (Shiffman, Tindle, et al., 2012). Participants who were pregnant, were nursing, or had participated in any smoking cessation treatment in the past 30 days were excluded from participation. To be eligible, participants agreed to provide a urine sample for biomarker analysis and complete a brief survey. All participants attended focus groups scheduled at either 11:30 a.m. or 5:30 p.m., and each received a $50 gift card for participating. Twenty-one participants provided a urine sample between 11:30 a.m. and 1:30 p.m. and 37 between 5:30 p.m. and 7:30 p.m.
Survey Measures
Surveys were administered by trained research assistants to gather self-report demographic and tobacco use behaviors. Demographic variables included age, gender, race, relationship status, educational attainment, employment status, and estimated monthly household income (dichotomized to <$1,800 and ≥$1,800).
Survey items assessing tobacco use included average number of smoked cigarettes per day (cpd) in the last 30 days (on days smoked), number of days smoked in the past 30 days, use of other tobacco products (e.g., cigars, smokeless tobacco, and pipes), and use of mentholated cigarettes (yes, no). Timeline followback was used to assess cigarettes smoked on each day of the past 7 days and today in order to estimate cigarette smoke exposure proximal to obtaining the urine sample (Burgess, Alderman, Evans, Emslie, & Wilson, 1998). The number of total cigarettes smoked in the past 8 days was calculated by summing all the cigarettes smoked in the past week and today. Smoking history included total length of time as a nondaily smoker (reported in years and months) and whether participants had ever smoked daily for 6 months or more (yes/no).
Nicotine dependence was assessed using a single survey item asking time to first cigarette after waking adopted from the Fagerström Test for Nicotine Dependence (Baker et al., 2007; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Responses were dichotomized (smoking < 30min after waking, and smoking > 30min), with smoking within 30min of waking indicative of nicotine dependence.
Participants reported whether they used each of the following forms of tobacco products in the past 30 days: cigars, cigarillos, little cigars, smokeless tobacco, pipes, hand-rolled cigarettes, and hookah. For each form of tobacco used in the past 30 days, participants were asked to provide the number of days used in the past 30 days and average amount each day (on the days used). Users of multiple tobacco products in our sample (n = 25) were compared to those using cigarettes only (n = 32) to address the possibility of confounding from those subjects using multiple products.
Analytic Chemistry
Concentrations of cotinine (unconjugated) and total NNAL (NNAL plus its glucuronide) in urine were determined using liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Jacob et al., 2008, 2011). The limits of quantification were 0.05ng/ml and 0.25 pg/ml for cotinine and NNAL, respectively. Urine nicotine and cotinine concentrations were measured by gas chromatography with nitrogen–phosphorous detection. The limit of quantitation was 10ng/ml for both (Jacob, Wilson, & Benowitz, 1981; Jacob, Yu, Wilson, & Benowitz, 1991).
Data Analysis
Biomarker levels that could not be detected were imputed with values equal to half the limit of quantification for each specific analysis. Descriptive analyses were conducted for all participants and by racial and ethnic group. Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized using means and standard deviations. Because cpd and the biomarker data are not normally distributed, we reported their medians and ranges and conducted nonparametric analyses.
Mann–Whitney U tests were conducted to determine whether there were differences in cpd, total cigarettes in the past week, urinary nicotine, creatinine normalized urinary cotinine, and creatinine normalized urinary NNAL between users of multiple forms of tobacco compared to cigarette-only users and Blacks and Whites. Latinos were not included in the analyses comparing smoking characteristics and biomarker levels by race due to the small number of Latino participants (n =4). Spearman’s correlations were calculated for cpd, total cigarettes in the past week, and biomarker levels. All analyses were conducted using SPSS 20.0.0 for Windows (SPSS Inc.). The level of statistical significance for all analyses was .05.
RESULTS
A total of 58 participants provided urine samples for biomarker analysis; however, at the time of the survey, one participant reported smoking daily for the past 30 days and was excluded from this study. Participants were 28 (49.1%) Blacks, 4 (7.0%) Latinos, and 25 (43.9%) Whites. The majority of participants were male (56%), and the mean age was 42 (SD = 10). Eligible participants smoked 4–24 days in the past 30 days, the equivalent of at least once a week but less than 25 days per month. Participants averaged 3.3 (SD = 2.2) cpd on days smoked, smoked an average of 13.0 (SD = 5.4) days in the past 30 days, and had been smoking nondaily for 10.7 (SD = 10.5) years. Sixteen percent smoked within 30min of waking and 44% had used other forms of tobacco (e.g., hand-rolled cigarettes, cigars, and cigarillos) in the past 30 days. Among polyproduct users, the average number of products used was 1.4 (SD = 0.9) and the average number of days in the past 30 days participants reported using other tobacco products was 5.2 (SD = 4.6).
Cigarette use and urinary biomarker levels are summarized in Table 1. Cotinine was detected in 52 of 56 nondaily smokers (one participant provided insufficient urine for the cotinine and nicotine analysis), NNAL was detected in 54 of the 57 nondaily smokers, and nicotine was detected in 48 of 56 smokers. Participants with cotinine levels (n = 4) below the limit of quantification smoked on 9.0 days (SD = 7.5) in the past 30 and reported a mean of 1.5 cpd (SD = 0.6) on the days smoked; the 3 participants with NNAL levels below the limit of quantification smoked on 6.7 days (SD = 0.6) and smoked 3.0 cpd (SD = 2.6); and the 8 participants whose urinary nicotine levels were below the limit of quantitation smoked on 8.9 days (SD = 5.0) and smoked 2.1 cpd (SD = 1.0). When examining biomarkers by race (only Blacks and Whites were included in analyses of median differences), the differences on levels for NNAL and nicotine were nonsignificant (ps = .13 and .40, respectively), but cotinine trended toward significant differences (p = .05). Although not statistically significant in this small sample, notable findings include differing median levels of NNAL between ethnic groups: 40.7 pg/mg for Whites, 64.6 pg/mg for Latinos, and 187.6 pg/mg for Blacks. Additionally, the median cotinine level for all participants was 490.9ng/mg with medians of 125.2ng/mg for Latinos, 264.8ng/mg for Whites, and 807.8ng/mg for Blacks. Median nicotine levels were 240.46ng/mg with the lowest median for Latinos (28.2ng/ml), followed by 103.4ng/mg for Whites and 408.0ng/mg for Blacks (data not shown in tables/figures). There were no differences between Blacks and Whites on cpd and number of days smoked per month. To address the possibility of confounding resulting from some subjects using multiple tobacco products, comparison of NNAL, cotinine, and nicotine in those using multiple tobacco products with those using cigarettes was performed. This revealed median normalized NNAL, cotinine, and nicotine levels in polyproduct users and users of cigarettes only to be 111.8 pg/mg, 353.4ng/mg, and 259.5ng/mg versus 151.5 pg/mg, 524.7ng/mg, and 216.3ng/mg, respectively (data not shown in tables/figures). These values were not significantly different (p = .68 for NNAL, p = .59 for cotinine, and p = 1.0 for nicotine). There were no differences on cpd or number of days smoked between users of other tobacco products versus cigarettes-only users.
Table 1.
Cigarette Use and Urinary Biomarker Levels
| Mean (SD) | Median | Range | N | |
|---|---|---|---|---|
| Cigarettes per day on days smoked | 3.26 (2.15) | 3.0 | 1–11 | 56 |
| Total cigarettes/8 days | 17.07 (13.83) | 13.0 | 1–52 | 57 |
| Days smoked/30 days | 12.96 (5.41) | 15.0 | 1–24 | 57 |
| Urinary creatinine, mg/ml | 1.19 (0.71) | 1.0 | 0.14–3.15 | 57 |
| Normalized urinary nicotine, ng/mg creatine | 914.93 (2137.08) | 240.46 | 2.7–15062 | 56 |
| Normalized urinary NNAL, pg/mg creatine | 268.13 (422.83) | 140.74 | 0.77–2305.68 | 57 |
| Normalized urinary cotinine, ng/mg creatine | 804.40 (917.76) | 490.85 | 3.21–3743 | 56 |
Note. NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Urinary nicotine, NNAL, and cotinine were creatinine normalized. Ns ranged from 56 to 57 due to missing values.
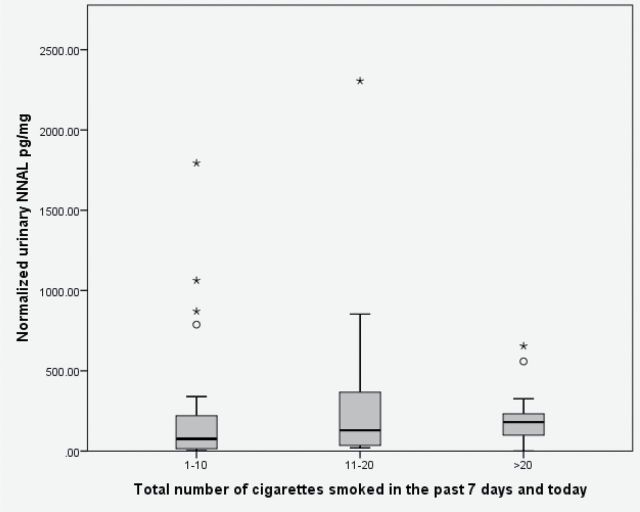
Figure 1 shows comparison of NNAL levels in those who smoked 1–10, 11–20, or >20 cigarettes over the last 7 days and today. Median NNAL levels were 76.2, 33129.1, and 180.0 pg/mg, respectively. The chosen cutoff points between these groups enabled the generation of similar sized groups. Comparison of these three groups by medians and distribution (Kruskal–Wallis test) did not reveal significant differences.
Figure 1.
Box plot showing total number of cigarettes smoked in the previous 7 days and today and urinary creatinine-normalized NNAL, pg/mg. * = significant outlier; ο = moderate outlier; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Additionally, associations between the biomarker data and smoking characteristics were evaluated using Spearman’s rank correlation analysis correlational coefficients (see Table 2). This revealed strong, positive correlations between NNAL, cotinine, and nicotine. Urinary NNAL and cotinine were correlated (r = .84); NNAL was correlated with cpd (r = .39) (Table 2), cotinine with cpd, (r = .34), nicotine with NNAL (r = .70), nicotine with cotinine (r = .76), and nicotine with cpd (.30) (all p values <.05). Total numbers of cigarettes smoked in the past 7 days and today were positively associated with nicotine (r = .32, p < .05) and cotinine (r = .33, p < .05) but not NNAL (p > .05) (Table 2). No associations were identified between biomarker levels and number of days smoked in the past 30 days.
Table 2.
Biomarker Intercorrelations
| Normalized urinary nicotine, ng/mg | Normalized urinary cotinine, ng/mg | Normalized urinary NNAL, pg/mg | |
|---|---|---|---|
| Cigarettes per day on days smoked | 0.30* | 0.34* | 0.39** |
| Total cigarettes/8 days | 0.32* | 0.33* | 0.23 |
| Normalized urinary nicotine, ng/ml | – | 0.76** | 0.70** |
| Normalized urinary cotinine, ng/mg | – | 1.0** | 0.84** |
Note. NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Spearman’s Rho correlations are reported.
*p < .05. **p < .01.
DISCUSSION
Nicotine and Carcinogen Exposure in Nondaily Smokers
Nondaily smoking is a growing phenomenon, the implications of which are only recently beginning to be understood. This pattern of cigarette use appears to be more prevalent among younger people, and smoking tends to be associated with social cues. A significant percentage of those who engage in nondaily smoking expect that their risk of smoking-related diseases is lessened compared with daily smoking (Carpenter et al., 2009). Further, it is likely that clinicians caring for nondaily smokers may feel less compelled to aggressively push nondaily smokers to quit due to an erroneous perception of decreased risk.
The data presented here are among the first to examine exposure to nicotine and tobacco-specific carcinogens in nondaily smokers. Cotinine is the major proximate metabolite of nicotine and is a biomarker of daily nicotine intake. The subjects in this study had a median urine cotinine of 490ng/mg creatinine. In comparison, a study of 128 daily smokers found a geometric mean cotinine of 1,323ng/mg creatinine (Benowitz et al., 2011). Thus, our nondaily smoker subjects had an average of 37% daily intake of daily smokers.
NNAL, a metabolite of NNK, is a carcinogen itself and was found at substantial levels among the nondaily smokers studied here. We found a median urine NNAL level of 140 pg/mg creatinine. In comparison, two studies of daily smokers found a geometric mean of 230 pg/mg creatinine (Benowitz et al., 2011; Joseph et al., 2005), whereas another studying 27 daily smokers found mean normalized NNAL of 300.0 pg/mg (Berg, Schauer, Ahluwalia, & Benowitz, 2012). Thus, our nondaily smokers had an average 46%–59% of the intake of NNK of daily smokers. Interestingly, subjects reported median cigarettes per day of only 3 during days when cigarettes were used. Nevertheless, this seemingly low level of cigarette smoking is, based on our findings, still enough to expose users to substantial levels of nitrosamines. A separate analysis in our study compared NNAL levels in those who smoked 0–10, 11–20, or >20 cigarettes over the last 7 days and today (Figure 1). Here, we did not see any significant differences in NNAL levels between groups. With the caveat that our sample size is small, this finding may work to further strengthen our interpretation of this data: nondaily smokers are exposed to nontrivial amounts of NNAL and the degree of exposure changes less than expected as one increases cigarettes moderately.
Berg et al. (2012) noted that cpd on smoking days correlated with NNAL levels among nondaily smokers and that a NNAL cutoff point (81.6 pg/ml/g Cr) could be used to differentiate daily from nondaily smokers. Of note, our sample revealed higher levels of NNAL compared to the college students (and the determined cutoff point) studied by Berg although the cpd on days smoked was similar in both studies (mean cpd = 3.3 in our dataset vs. mean cpd = 2.8 in Berg et al.). The mean days smoked in the last 30 was 13 in our study compared with 12.3 in the study by Berg et al. However, Berg et al.’s study also included polyproduct users to a varying degree. Thus, in determining the health-related risks among nondaily smokers, researchers should attend to demographic factors, such as brand/type of product used or polyproduct use, that may influence overall intake and exposure.
Analysis of correlations between variables in our dataset showed that cpd correlated significantly with both cotinine and NNAL. Previous data have shown a similar correlation in smokers who use their products daily (Joseph et al., 2005). We find it notable that this correlation also exists in nondaily smokers, which is consistent with our main conclusion that nondaily smokers are subjected to tobacco carcinogens at levels close to those seen in daily smokers. Furthermore, the correlation data demonstrate that cotinine and NNAL correlate with cpd despite NNAL having a longer half-life than cotinine. This would suggest that cotinine may act as a surrogate biomarker for NNK exposure in nondaily smokers. Lastly, we did not see correlation between number of days per month smoked and the tobacco biomarkers. Although we would expect NNAL to demonstrate correlation, it is possible that the variability in our population with regard to number of days smoked per month was high enough that this finding could not reach significance. Certainly, this warrants further exploration and future, larger studies of nondaily smokers.
Interethnic Variations
Our sample is notable in that it is composed entirely of nondaily smokers and also includes smokers of three racial/ethnic groups. This allows an opportunity to examine differences in exposure that may exist between these groups. Previous data have shown that differences are present between Whites and Blacks such that self-reported cpd correlates poorly with nitrosamine (NNAL) and polycyclic aromatic hydrocarbon (PAH) levels in Blacks compared with Whites (Benowitz et al., 2011). Additionally, Blacks and Hispanics are more likely to smoke intermittently than Whites (Trinidad et al., 2009). We identified a trend toward significant differences between races with respect to normalized nicotine, NNAL, and cotinine. With a larger study sample, we are confident that a difference will be positively identified. Although Hispanic representation in our study is small, we noted that NNAL and cotinine were highest among Black nondaily smokers compared with Hispanics and Whites despite similar self-reported cpd. The Hispanics in our sample also smoked fewer days per month compared with the Blacks and Whites. This exploratory finding requires further investigation and a larger sample size for confirmation.
Study Limitations
The main limitations of this study include a small sample size that limits generalizability, especially with regard to data in Hispanics. Additionally, self-reported smoking behaviors were used for inclusion as nondaily smokers and thus the inadvertent inclusion of daily smokers would introduce error. Still, despite the small sample size, the findings reported here serve as a foundation for larger studies to confirm and further examine carcinogen exposure among nondaily smokers as well as associated interethnic variation.
CONCLUSIONS
We report two novel findings based on analysis of a sample of nondaily smokers. First, exposure to NNK as determined by NNAL quantification is significant among smokers who smoke less than every other day and is on average only slightly less than that seen in daily smokers. This finding is significant in that nondaily smokers may be smoking under the mistaken assumption that nondaily use mitigates exposure and risk to carcinogens. Second, although not statistically significant, we identified a trend suggesting higher normalized cotinine and NNAL in Blacks compared with Whites. This observation is likely due to slower metabolism of cotinine in Blacks (Pérez-Stable, Herrera, Jacob, & Benowitz, 1998), but we cannot exclude racial difference in the rate of NNAL clearance as well. Both of these findings warrant further study in larger samples so that the implications of nondaily smoking and interethnic variability on health risks may be better understood. This information would be critical in informing nondaily smokers of the risks associated with this practice.
FUNDING
This project is funded by Pfizer’s Global Research Awards for Nicotine Dependence (Ahluwalia). Dr. Ahluwalia is also supported in part by the National Institute for Minority Health Disparities (NCMHD/NIH-1P60MD003422).
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Baker T. B., Piper M. E., McCarthy D. E., Bolt D. M., Smith S. S., Kim S. Y. … Toll B. A. (2007). Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. [Research Support, N.I.H., Extramural]. Nicotine Tobacco Research, 9, S555–S570. 10.1080/14622200701673480 [DOI] [PMC free article] [PubMed]
- Benowitz N. L., Dains K. M., Dempsey D., Wilson M., Jacob P. (2011). Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine & Tobacco Research, 13, 772–783. 10.1093/ntr/ntr072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. J., Schauer G. L., Ahluwalia J. S., Benowitz N. L. (2012). Correlates of NNAL levels among nondaily and daily smokers in the college student population. Current Biomarker Findings, 2, 87–94. http//dx..org/10.2147/CBF.S34642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. W., Alderman N., Evans J., Emslie H., Wilson B. A. (1998). The ecological validity of tests of executive function. Journal of the International Neuropsychological Society, 4, 547–558 [DOI] [PubMed] [Google Scholar]
- Carpenter M. J., Garrett-Mayer E., Vitoc C., Cartmell K., Biggers S., Alberg A. J. (2009). Adolescent nondaily smokers: Favorable views of tobacco yet receptive to cessation. Nicotine & Tobacco Research, 11, 348–355. 10.1093/ntr/ntp023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2003). Prevalence of current cigarette smoking among adults and changes in prevalence of current and some day smoking—United States, 1996–2001. MMWR Morbidity and Mortality Weekly Report, 52, 303–304, 306–307 [PubMed] [Google Scholar]
- CDC. (2007). Cigarette smoking among adults—United States, 2006. MMWR Morbidity and Mortality Weekly Report, 56, 1157–1161 [PubMed] [Google Scholar]
- CDC. (2008). Cigarette smoking among adults—United States, 2007. MMWR Morbidity and Mortality Weekly Report, 57, 1221–1226 Retrieved from www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a2.htm [PubMed] [Google Scholar]
- Fu S. S., Kodl M. M., Joseph A. M., Hatsukami D. K., Johnson E. O., Breslau N. … Bierut L. (2008). Racial/Ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiology, Biomarkers & Prevention, 17, 1640–1647. 10.1158/1055–9965.EPI-07-2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Eisner M. D., Lazcano-Ponce E., Zielinska-Danch W., Koszowski B., Sobczak A. … Benowitz N. L. (2011). Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine & Tobacco Research, 13, 202–208. 10.1093/ntr/ntq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Havel C. M., Peng M. W., Jacob P., 3rd, Dempsey D., Yu L. … Benowitz N. L. (2009). Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiology, Biomarkers & Prevention, 18, 3421–3425. 10.1158/1055–9965.EPI-09-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman C. A., Stram D. O., Wilkens L. R., Pike M. C., Kolonel L. N., Henderson B. E., Le Marchand L. (2006). Ethnic and racial differences in the smoking-related risk of lung cancer. The New England Journal of Medicine, 354, 333–342. 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerstrom K. O. (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Jacob P., 3rd, Havel C., Lee D. H., Yu L., Eisner M. D., Benowitz N. L. (2008). Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Analytical Chemistry, 80, 8115–8121. 10.1021/ac8009005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P., 3rd, Wilson M., Benowitz N. L. (1981). Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromatography, 222, 61–70 [DOI] [PubMed] [Google Scholar]
- Jacob P., 3rd, Yu L., Duan M., Ramos L., Yturralde O., Benowitz N. L. (2011). Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 879, 267–276. 10.1016/j.jchromb.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P., 3rd, Yu L., Wilson M., Benowitz N. L. (1991). Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: Absence of an isotope effect in the clearance of (S)-nicotine-3’,3’-d2 in humans. Biological Mass Spectrometry, 20, 247–252. 10.1002/bms.1200200503 [DOI] [PubMed] [Google Scholar]
- Joseph A. M., Hecht S. S., Murphy S. E., Carmella S. G., Le C. T., Zhang Y. … Hatsukami D. K. (2005). Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiology, Biomarkers & Prevention, 14, 2963–2968. 10.1158/1055-9965.EPI-04-0768 [DOI] [PubMed] [Google Scholar]
- Pérez-Stable E. J., Herrera B., Jacob P., 3rd, Benowitz N. L. (1998). Nicotine metabolism and intake in black and white smokers. The Journal of the American Medical Association, 280, 152–156. 10.1001/jama.280.2.152 [DOI] [PubMed] [Google Scholar]
- Schane R. E., Glantz S. A., Ling P. M. (2009). Nondaily and social smoking: An increasingly prevalent pattern. Archives of Internal Medicine, 169, 1742–1744. 10.1001/archinternmed.2009.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Dunbar M. S., Scholl S. M., Tindle H. A. (2012). Smoking motives of daily and non-daily smokers: A profile analysis. Drug and Alcohol Dependence, 126, 362–368. 10.1016/j.drugalcdep.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Tindle H., Li X., Scholl S., Dunbar M., Mitchell-Miland C. (2012). Characteristics and smoking patterns of intermittent smokers. Experimental and Clinical Psychopharmacology, 20, 264–277. 10.1037/a0027546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad D. R., Pérez-Stable E. J., Emery S. L., White M. M., Grana R. A., Messer K. S. (2009). Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine & Tobacco Research, 11, 203–210. 10.1093/ntr/ntn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. M., Gao Y. T., Murphy S. E., Carmella S. G., Wang R., Zhong Y. … Hecht S. S. (2011). Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Research, 71, 6749–6757. 10.1158/0008-5472.CAN-11–0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. M., Knezevich A. D., Wang R., Gao Y. T., Hecht S. S., Stepanov I. (2011). Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis, 32, 1366–1371. 10.1093/carcin/bgr125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. M., Koh W. P., Murphy S. E., Fan Y., Wang R., Carmella S. G. … Hecht S. S. (2009). Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Research, 69, 2990–2995. 0008-5472.CAN-08-4330 [pii] 10.1158/0008-5472.CAN-08-4330 [DOI] [PMC free article] [PubMed] [Google Scholar]