Abstract
Purpose
The chromophobe subtype of renal cell carcinoma (chRCC) has generally been associated with a better prognosis than the clear cell type; however, debate continues as to absolute prognosis as well as the significance of certain prognostic variables. We investigated the significance of pathologic stage and a recently proposed chromophobe tumor grading (CTG) scheme in predicting chRCC outcomes.
Materials and Methods
All available chRCCs were identified from our surgical pathology archives from 1987-2010. Original slides were reviewed to verify diagnoses and stage, and each case was graded following a novel chromophobe tumor grade system criteria. Disease status was obtained from a clinical outcome database, and cancer specific deaths and recurrences were recorded.
Results
Eighty-one cases of chRCC were identified, and 73 had adequate follow-up information available. There were only 3 instances of cancer related recurrence or mortality, which included 1 disease specific mortality and 2 disease recurrences. Pathologic stage and CTG 3 were found to be significantly associated with the recurrences or death from chRCC, but there was no association with CTG 1 and CTG 2.
Conclusions
chRCC is associated with a very low rate of cancer specific events (4.1%) even at a tertiary referral center. In our study, pathologic stage and CTG 3, but not CTG 1 or 2, were significantly associated with the development of these events.
Keywords: Nephrectomy, Prognosis, Renal cell carcinoma
INTRODUCTION
Renal cell carcinoma (RCC) is traditionally divided into 5 major subtypes: clear cell, papillary, chromophobe (chRCC), collecting duct, and unclassified types [1]. First described in 1985, chRCC is characterized by recognizable pathognomonic features [2,3]. Although chRCC is generally thought to portend a favorable prognosis when compared with its clear cell counterpart, debate on this issue still exists. In most studies, patients with chRCC have a significantly increased 5-year survival when compared to patients with clear cell RCC; however, the actual 5-year survival estimates vary widely [4,5,6,7,8,9,10,11,12,13,14]. Moreover, in several of these studies, RCC subtype was not shown to be statistically significant in a multivariable analysis of risk [4,9,10,13]. Additionally, grading remains a controversial variable in the prognosis of chRCC. The grading system proposed by Fuhrman in 1982 has long been used to stratify RCC into a four-tiered grading system based on nuclear size, nuclear irregularity, and nucleolar prominence [15]. However, this system was proposed before the current classification scheme of RCCs, and it was recently demonstrated that Fuhrman grading is not useful as a prognostic indicator for chRCC [16]. By definition, chRCC is comprised of tumor cells with irregular nuclei with variation in nuclear size, and as a result, chRCCs would generally be assigned a Fuhrman grade of 3. Because of this issue, Paner et al. [17] recently proposed a three-tiered chromophobe tumor grade (CTG) system, that they report, demonstrates a positive association of CTG with both pathologic stage and outcome (Fig. 1). A subsequent study of 203 patients with chRCC utilized a modified grading scheme similar to that in the Paner et al. study; however, this scheme was not shown to be significantly associated with outcome [18]. Another study of 84 patients with chRCC utilized the CTG system of Paner et al. and found that CTG was not an independent predictor of outcome in multivariable analysis of non-sarcomatoid tumors [19]. Given these continuing controversies in chRCC, we reviewed and analyzed 81 cases of chRCC that were surgically removed at our institution to better understand potential prognostic variables in this specific subtype of RCC.
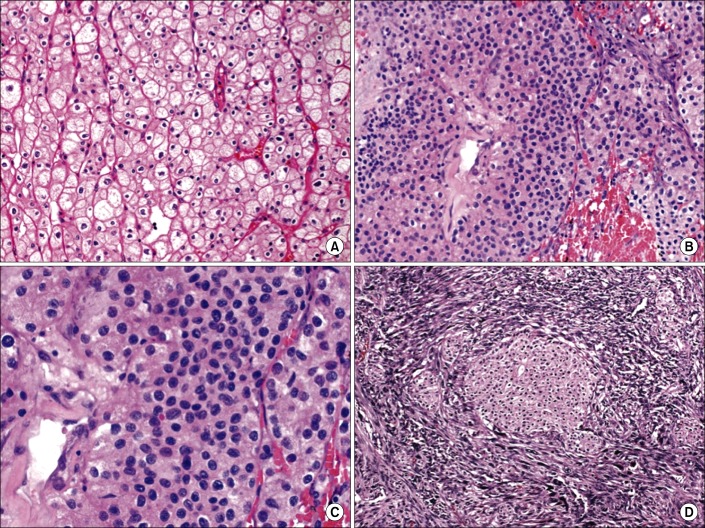
FIG. 1.
Chromophobe tumor grade (CTG) system with hematoxylin and eosin staining (A, ×1,500), classic CTG 1 ChRCC with abundant clear cytoplasm and prominent cell membranes (B, ×1,500), CTG 2 ChRCC characterized by a higher nuclear to cytoplasmic ratio compared to CTG 1 (C, ×3,000), at higher magnification CTG 2, ChRCC has a crowded cellular appearance but does not show the spindling or degree of diffuse anaplasia of CTG 3 (D, ×600). Grade 3 ChRCC with sarcomatoid differentiation characterized by an associated malignant spindle cell proliferation. ChRCC, chromophobe subtype of renal cell carcinoma.
MATERIALS AND METHODS
We evaluated all available chRCCs identified from the surgical pathology archives of our hospital from 1997-2010. Consultation cases and tumors resected at outside hospitals were excluded from further study. The consultation cases of chRCC did not undergo surgery by the providers in our department. Pathology consultation cases (i.e., cases in which the glass slides were sent to our institution by a pathologist with a request to help with histologic classification) were excluded to avoid bias towards cases that were difficult to classify and/or ones with adverse features at the time of diagnosis. Basic demographic data was recorded, and two pathologists (E.P.W. and J.K.M.) reviewed all slides to confirm tumor subtype and pathologic stage. Specifically, slides were individually examined to confirm that the pathologic features were consistent with the diagnosis of chRCC, such as voluminous cytoplasm, prominent cytoplasmic borders/membranes, and nuclei with irregular "raisin-like" nuclear membranes. Eosinophilic variants consisting of abundant, pink, granular cytoplasm and a distinctive perinuclear halo were also recognized as chRCC. Both pathologists were blinded to the clinical outcome of individual cases. Institutional Review Board approval was obtained prior to the initiation of the study. From the database, demographic data, surgical details, disease status, follow-up interval, pathologic stage, and cancer specific outcomes were extracted. The Paner et al. [17] CTG grading was assigned to each case, as was the percentage of tumor comprised of each grade. The hallmark of the CTG system is that it downplays the common nuclear atypia seen in chRCC and instead includes such features such as nuclear crowding, anaplasia, nuclear pleomorphism, and sarcomatoid change. Statistical analysis using Fisher exact test was then performed on the patient cohort with follow-up information available. Fig. 1 illustrates some of the distinctive pathologic features of chRCC and of CTG 1-3.
RESULTS
Eighty-eight cases with available glass slides were identified and reviewed and of these, 81 independent cases of chRCC were found. Seven cases were excluded from further analysis because they did not represent chRCC. These excluded cases comprised of 5 cases that were similar to renal neoplasms that have been reported as "hybrid oncocytic tumor" or "oncocytic renal neoplasm of low/uncertain malignant potential", 1 RCC unclassified type, and 1 clear cell RCC. These 81 cases of chRCC represented 7% of all of the 1,150 total RCC cases diagnosed in that time interval.
Clinicopathologic and demographic characteristics of the cohort are illustrated in Table 1. Twenty one of the eighty one surgical resections (26%) included at least one lymph node for evaluation; of these, only one, at pT3 stage, demonstrated involvement by chRCC. After reassignment of the cases using the proposed CTG system both CTG3 cases were found to have sarcomatoid differentiation. All cases with CTG2 also had foci of CTG1; the percentage of tumor comprised of CTG2 ranged from less than 5% to 60%. Cases presenting at American Joint Committee on Cancer 7th edition [20] stage >3 were as follows: 8/52 CTG1 (15%), 5/27 CTG2 (18.5%), and 2/2 CTG3 (100%).
TABLE 1.
Clinicopathologic and demographic characteristics
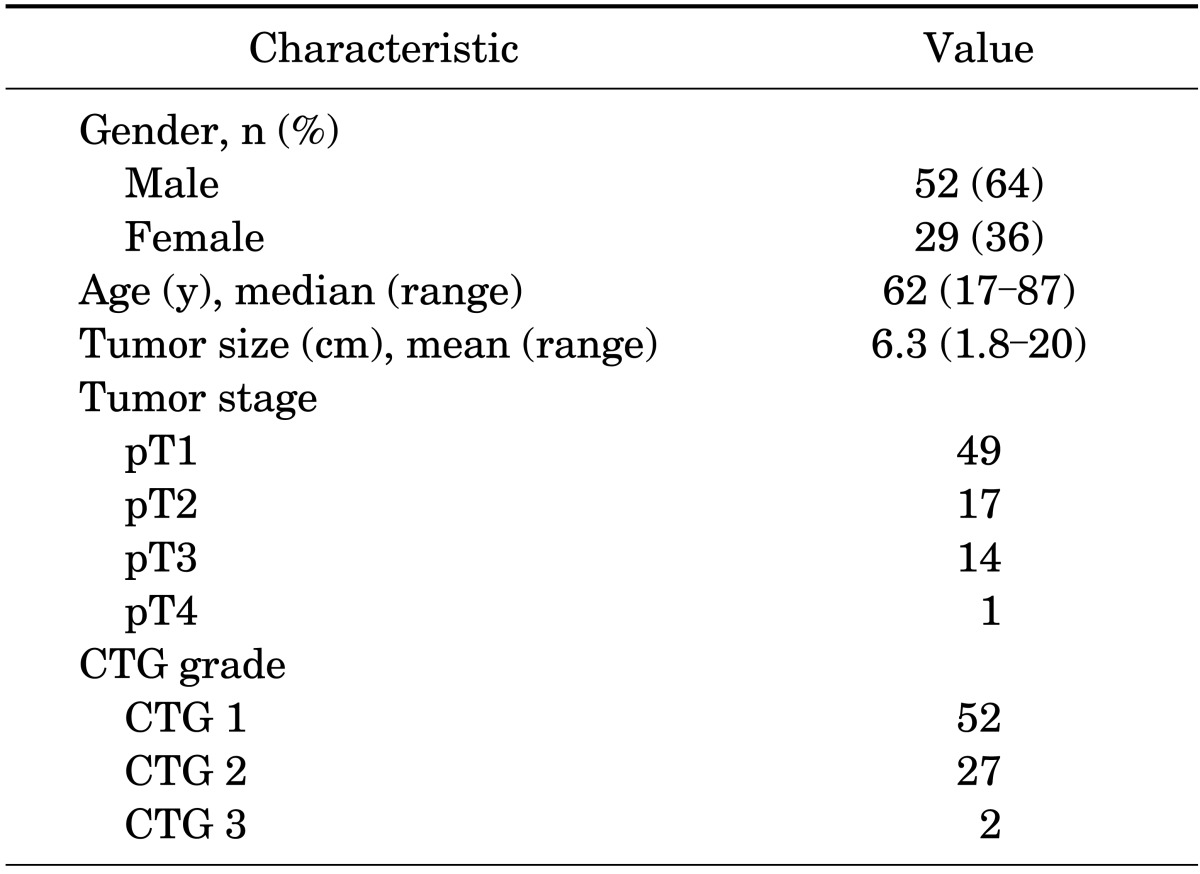
CTG, chromophobe tumor grade.
Table 2 lists the follow-up data for this cohort. Seventy three of the eighty one patients had adequate follow-up postsurgery. Sixteen patients underwent partial nephrectomy, 7 of which were performed laparoscopically or robotically, and 57 underwent radical nephrectomy, 26 of which were performed laparoscopically. Of the 3 patients (4.1%) who experienced cancer specific death or recurrence, 1 died of disease and 2 developed disease recurrences. The patient who died of disease had pT3, CTG3 disease at diagnosis, and died 2.9 years postdiagnosis. One patient who presented with metastatic disease with pT4, CTG2 disease at diagnosis had further disease spread to the liver and is alive at 4.5 years of follow-up. The other patient who developed recurrent disease had pT3, CTG2 disease at diagnosis and developed bony metastasis and aortocaval nodal recurrence and is alive at 3 years of follow-up. Among the remaining 70 patients with follow-up, 59 are alive with no evidence of disease, and 11 have died without evidence of disease. Of these 70 patients, 45 had pT1 disease, 16 had pT2 disease, and 9 had pT3 disease at diagnosis. Additionally, of these 70 patients, 45 had CTG1 tumors and 25 had CTG 2 tumors. Using Fisher exact test, pathologic stage demonstrated a significant association with the occurrence of cancer specific death or recurrence (p=0.001). When CTG1 was compared with CTG2, there was no significant association with recurrence or death (p=0.140) but when CTG1/CTG2 tumors were combined and compared to CTG3 tumors, this association with cancer recurrence or death was significant (p=0.040). When chRCC cancer specific survival was compared to that of clear cell RCC with a control group matched by stage and tumor size, chRCC demonstrated an overall statistically better survival (log rank test, p<0.003).
TABLE 2.
Follow-up data
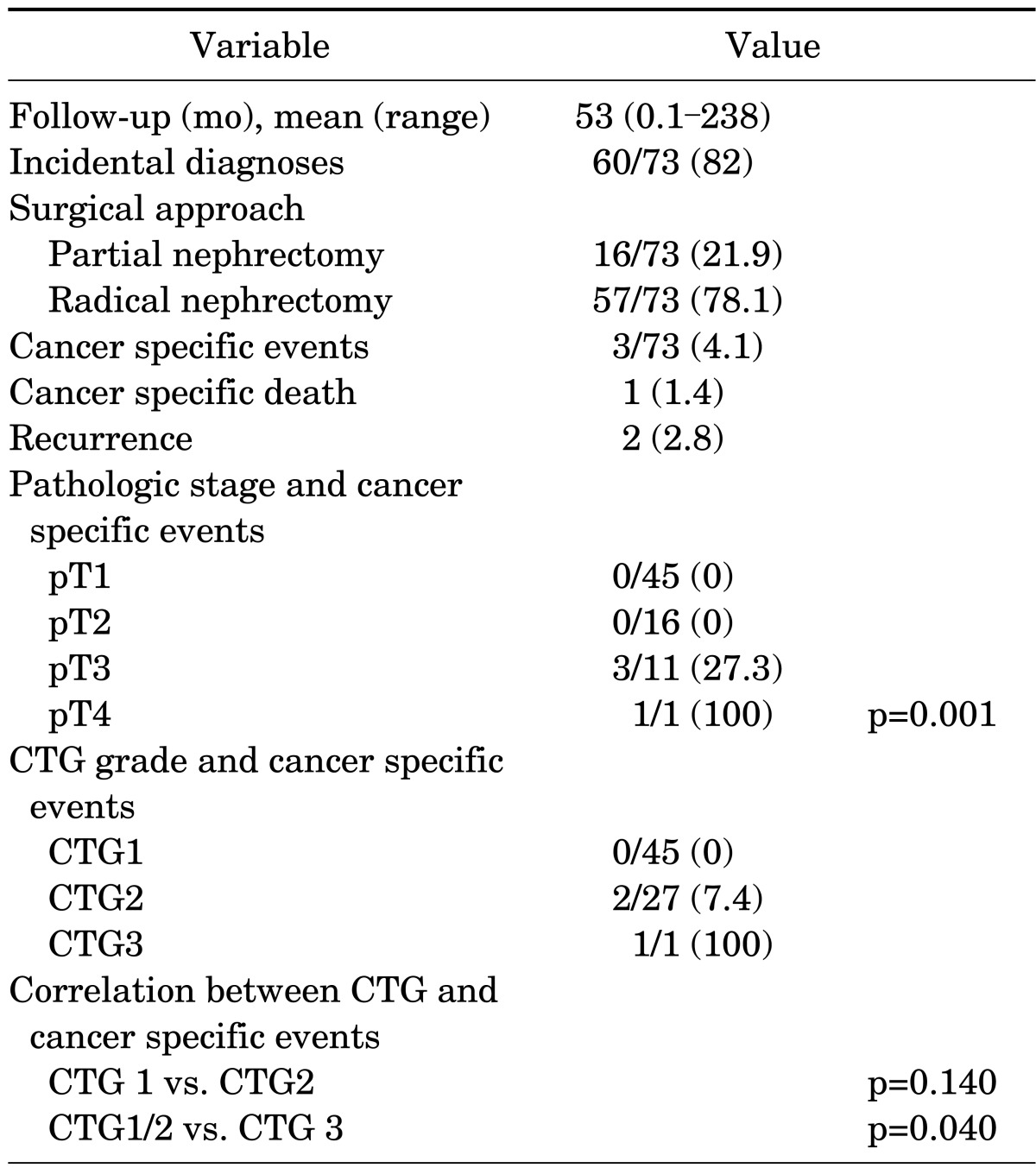
Follow-up data Values are presented as number (%) unless otherwise indicated. CTG, chromophobe tumor grade.
DISCUSSION
ChRCC is a well described subtype of RCC, and has been reported to comprise from 1.6% [6] to 6.5% [21] of all RCCs, behind clear cell and papillary subtypes. In our cohort, we had very few cancer specific deaths or recurrences. This finding has been independently corroborated by previous studies. Similarly, a recent study by Przybycin et al. [18] evaluated 203 consecutive cases of chRCC and determined a similarly low 5-year disease-specific event rate (3.7%). This low rate of disease-specific events is also consistent with several previous studies that demonstrated an increased cancer-specific survival for chRCC over clear cell RCC. In one of the largest studies to date, Cheville et al. [7] reviewed 2,385 RCC cases, including 102 cases of chRCC, and reported improved 5-year cancer specific survivals, respectively, for chRCC versus clear cell RCC for both pT1 stage (100% vs. 88.7%), and pT2 stage (84.1% vs. 65.1%). Also, a large 2009 study by Teloken et al. [14] included 220 cases of chRCC and reported similar 5-year event free survival in favor of chRCC when compared with clear cell RCC (92% vs. 86%, respectively). This difference in outcome has also been corroborated by several smaller studies that demonstrated rates of progression-free and cancer-specific survival for chRCC above 90% for all stages combined [4,8,21,22].
Other studies have corroborated the increased survival of chRCC over clear cell RCC, but with 5-year survivals for chRCC between 80 and 90% [5,11,13]. A 2000 Swiss study by Moch et al. [12] demonstrated a 5-year survival of only 78% for chRCC. However, only 21 of their 31 cases (68%) were organ-confined, whereas in our series, 61 of our 73 cases (84%) with follow-up were organ-confined, suggesting that the decreased survival seen in the Moch study is potentially due to their increased proportion of higher stage disease. The study by Cheville et al. [7] demonstrated that there was no significant difference in 5-year cancer specific survival between chRCC and clear cell RCC for pT3 or pT4 disease, although the number of cases of chRCC at high pT stage was limited. Only one large study, to our knowledge, has demonstrated a 5-year cancer specific survival for chRCC lower than its clear cell counterpart (88.8% vs. 92.2%) [10]. Although this study included 148 reported cases of non-metastatic chRCC, it included a large number of cases of clear cell RCC at pT1 stage (77% of all clear cell RCCs), potentially decreasing the number of events associated with clear cell RCC. Additionally, this study included cases from 26 institutions and lacked central pathology review, potentially limiting its pathologic consistency.
Although many studies have confirmed subtype to be an independent prognostic indicator by multivariable analysis, at least in low stage disease [5,7,11,14,21], several studies have determined subtype to be insignificant in such analysis [4,9,10,23]. Several of these studies, however, were limited by insufficient sample size, lack of central pathologic review, and/or relatively large numbers of chRCC with high stage disease, suggesting that pT3 or pT4 RCC may have a poor prognosis regardless of subtype. Interestingly, although one large-scale study found histologic subtype to be an independent prognostic indicator by multivariable analysis, the incorporation of subtype did not significantly improve the prognostic model [6].
Many of the aforementioned studies that compared RCC subtypes included Fuhrman grading in their analysis. Recently, however, it has been recognized that this grading system is not useful for chRCC, since its nuclei are essentially Fuhrman grade 3 by definition [16]. To address this controversial grading issue, Paner et al. [17] recently developed a three-tiered CTG system which downplays the expected nuclear atypia of chRCC. This new grading system, but not traditional Fuhrman grading, demonstrated a strong positive association with pathologic stage and outcome in their series of 124 chRCCs. Interestingly, in this multivariable analysis of outcome, pathologic stage was no a longer a significant prognostic variable, suggesting that the newly defined CTG and pathologic stage may be interdependent.
Following the introduction of the CTG system, others have tried to validate its findings. A recent 2011 study of 203 patients with chRCC found that outcomes did not show association with a tumor grading scheme similar to the CTG scheme [18]. Their modified grading scheme retained a 4-tiered system and did not take nuclear overcrowding into consideration, which was a major histologic criterion in the grading system proposed by Paner et al. [17] Another recent study of 84 cases of chRCC at University of California, Los Angeles (UCLA) found that, although there was a significant difference in recurrence-free survival between CTG1 and CTG2 tumors (hazard ratio, 5.63), only pathologic stage was retained as a significant independent variable in multivariable analysis of nonsarcomatoid tumors [19]. Some studies have shown that foci of poorly differentiated carcinoma or sarcomatoid differentiation do predict a poor prognosis and the cumulative reported evidence strongly suggests that these high grade chRCCs have prognostic significance. In the Memorial Sloan Kettering series, sarcomatoid differentiation was similarly rare, but was statistically associated with adverse outcome [18]. Similarly, the UCLA study found that 9 of 11 patients with sarcomatoid transformation developed metastatic disease [19]. Indeed, many case reports and series have similarly documented that chRCC with sarcomatoid differentiation is often associated with clinically aggressive disease [24,25]. In our series, we found no significant difference between CTG1 and CTG2 in the occurrence of cancer mortality or recurrence, but there was a significant difference between a combined CTG1/2 group and CTG3. This is not surprising given that CTG3 tumors represent sarcomatoid tumors, which, as mentioned in the above studies, do poorly. Also, our results suggest that those with CTG1 and CTG2 tumors might be better stratified by pathologic stage for prognostic purposes, as all of the cancer specific deaths and recurrences were associated with tumors diagnosed at pT3 stage, and this association of stage with outcome was statistically significant, as has been shown in numerous other studies. Since these histologically high grade/sarcomatoid carcinomas (CTG 3) often present at high stage, it is often difficult to separate the two variables; however, both our and the aggregate data support regarding these chRCCs with sarcomatoid/poorly differentiated areas as high risk carcinomas.
Overall, although our study demonstrated a remarkably low rate of recurrence and death among patients with chRCC, current discrepancies in the literature remain with regard to outcome, grading, and the significance of this subtype. Our study was limited in its prognostic scope by the retrospective nature of the study, the paucity of adverse events, and the presence of few high stage or grade tumors in our series; however, given that our series was comprised of consecutive cases of chRCC surgically excised at a single tertiary referral institution, this paucity may indeed reflect the expected distribution of such variables in the disease. Even the largest study of chRCC to date, which contained 220 cases, included only 9 chRCC-related metastasis or disease-specific death [14]. chRCC may demonstrate such a favorable prognosis that only very few variables, such as pathologic stage or sarcomatoid dedifferentiation, may be important for clinical outcome. Given the low rate of adverse events for chRCC, sufficient statistical power to address these prognostic issues will only be achieved by comparing outcomes of exceedingly large numbers of cases.
CONCLUSIONS
ChRCC is associated with a very favorable clinical course in the majority of cases with a very low incidence of cancer related death and recurrence at 5 years in this series. Similar to recent reports, we found sarcomatoid differentiation to be rare (approximately 2%-3%). We did not find an association between CTG 1 and CTG 2 and clinical outcome. In this study, only pathologic stage and CTG 3 was associated with cancer recurrence or death from chRCC.
Footnotes
The authors have nothing to disclose.
References
- 1.Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Thoenes W, Storkel S, Rumpelt HJ. Human chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;48:207–217. doi: 10.1007/BF02890129. [DOI] [PubMed] [Google Scholar]
- 3.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964–967. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 4.Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, Reuter VE, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–77. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 6.Capitanio U, Cloutier V, Zini L, Isbarn H, Jeldres C, Shariat SF, et al. A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int. 2009;103:1496–1500. doi: 10.1111/j.1464-410X.2008.08259.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V, Martignoni G, Galfano A, Novara G, Gobbo S, Brunelli M, et al. Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol. 2006;50:786–793. doi: 10.1016/j.eururo.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, Einarsson GV. Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. Eur Urol. 2005;48:593–600. doi: 10.1016/j.eururo.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Lee WK, Byun SS, Kim HH, Rha KH, Hwang TK, Sung GT, et al. Characteristics and prognosis of chromophobe non-metastatic renal cell carcinoma: a multicenter study. Int J Urol. 2010;17:898–904. doi: 10.1111/j.1442-2042.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 11.Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–1315. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000;89:604–614. [PubMed] [Google Scholar]
- 13.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Teloken PE, Thompson RH, Tickoo SK, Cronin A, Savage C, Reuter VE, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol. 2009;182:2132–2136. doi: 10.1016/j.juro.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Delahunt B, Sika-Paotonu D, Bethwaite PB, McCredie MR, Martignoni G, Eble JN, et al. Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. Am J Surg Pathol. 2007;31:957–960. doi: 10.1097/01.pas.0000249446.28713.53. [DOI] [PubMed] [Google Scholar]
- 17.Paner GP, Amin MB, Alvarado-Cabrero I, Young AN, Stricker HJ, Moch H, et al. A novel tumor grading scheme for chromophobe renal cell carcinoma: prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol. 2010;34:1233–1240. doi: 10.1097/PAS.0b013e3181e96f2a. [DOI] [PubMed] [Google Scholar]
- 18.Przybycin CG, Cronin AM, Darvishian F, Gopalan A, Al-Ahmadie HA, Fine SW, et al. Chromophobe renal cell carcinoma: a clinicopathologic study of 203 tumors in 200 patients with primary resection at a single institution. Am J Surg Pathol. 2011;35:962–970. doi: 10.1097/PAS.0b013e31821a455d. [DOI] [PubMed] [Google Scholar]
- 19.Finley DS, Shuch B, Said JW, Galliano G, Jeffries RA, Afifi AA, et al. The chromophobe tumor grading system is the preferred grading scheme for chromophobe renal cell carcinoma. J Urol. 2011;186:2168–2174. doi: 10.1016/j.juro.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 21.Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999;36:565–569. doi: 10.1159/000020049. [DOI] [PubMed] [Google Scholar]
- 22.Thoenes W, Storkel S, Rumpelt HJ, Moll R, Baum HP, Werner S. Chromophobe cell renal carcinoma and its variants: a report on 32 cases. J Pathol. 1988;155:277–287. doi: 10.1002/path.1711550402. [DOI] [PubMed] [Google Scholar]
- 23.Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 24.Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435–441. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 25.de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]